Effects of Pesticides on Longevity and Bioenergetics in Invertebrates—The Impact of Polyphenolic Metabolites
Abstract
1. Introduction
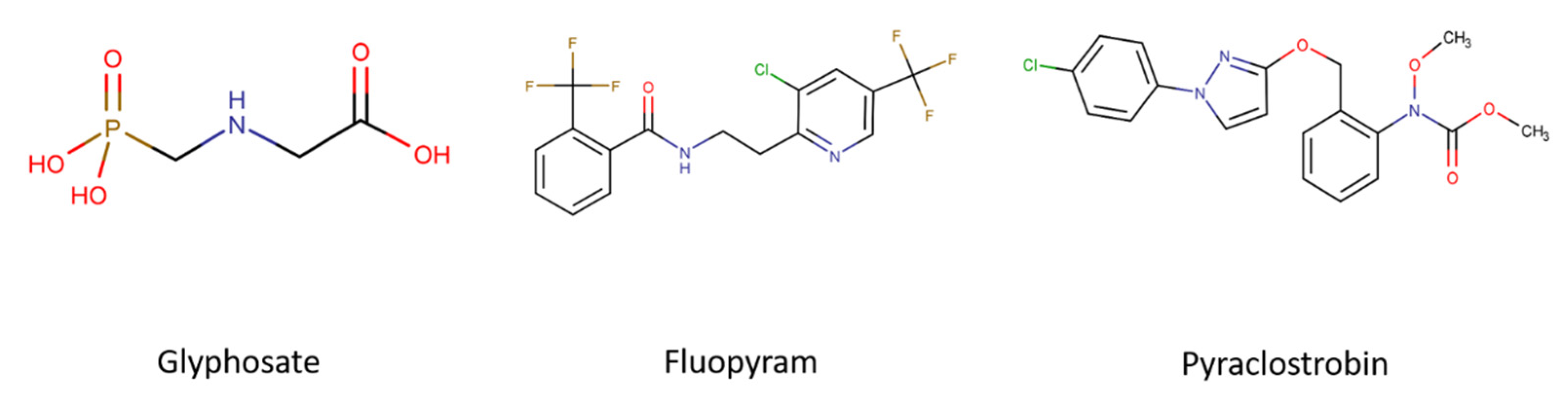
| Pesticide | Target | Acute Toxicity | Literature |
|---|---|---|---|
| Glyphosate | EPSPS | LD50 Oral: Rat—4.873 mg/kg LD50 Dermal: Rabbit—2.000 mg/kg | [41,42,43,44,45,46,47] |
| Fluopyram | Succinate dehydrogenase (complex II) | LD50 Oral: Rat—>2.000 mg/kg LD50 Dermal Rat—>2.000 mg/kg | [17,19,48,49] |
| Pyraclostrobin | cytochrome c–oxidoreductase (complex III) | LD50 Oral: Rat—>5.000 mg/kg LC50 Inhalation: Rat—4 h—0.31—1.07 mg/L LD50 Dermal: Rat—>2.000 mg/kg | [50,51,52,53,54,55] |
2. Material and Methods
2.1. Chemicals
2.2. Cells
2.3. Nematode and Bacterial Strain
2.4. Cultivation and Treatment
2.5. Heat Stress Survival Assay
2.6. Chemotaxis Assay
2.7. Nematode Homogenization
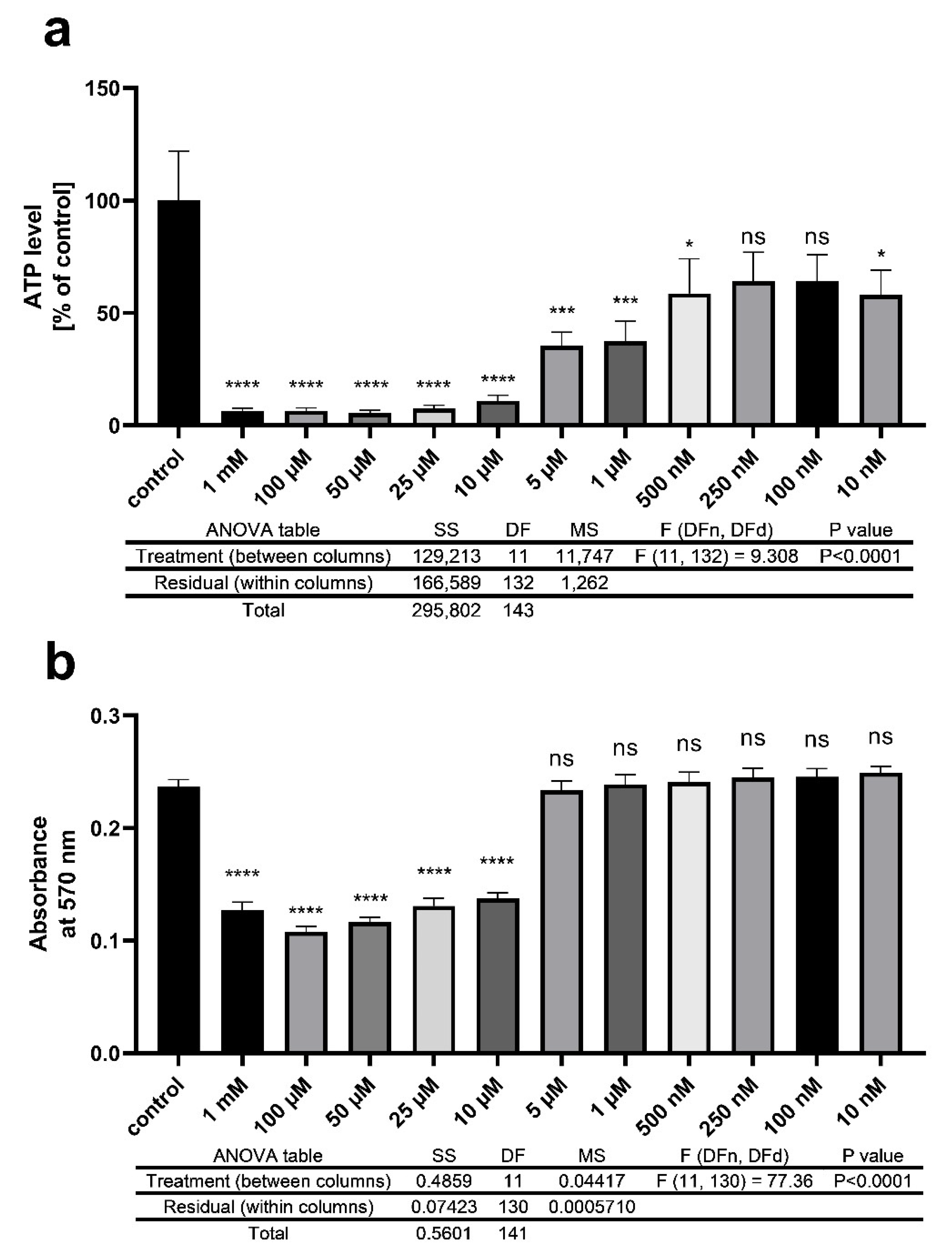
2.8. ATP Measurement
2.9. Colorimetric Assessment of Lactate and Pyruvate Content
2.10. Protein Quantification
2.11. MTT Cell Viability Test
2.12. Quantitative Real-Time PCR
2.13. Statistics
3. Results
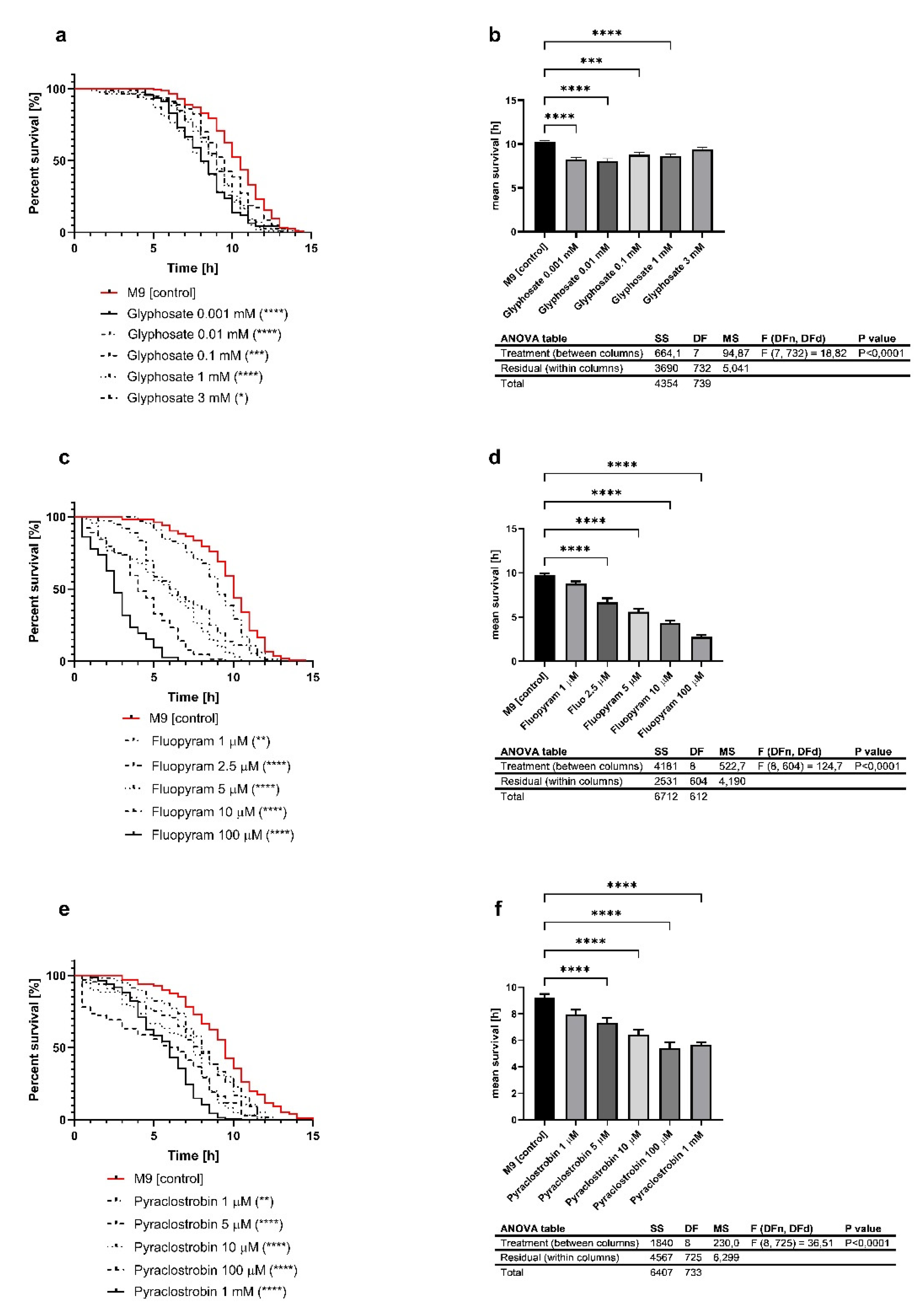
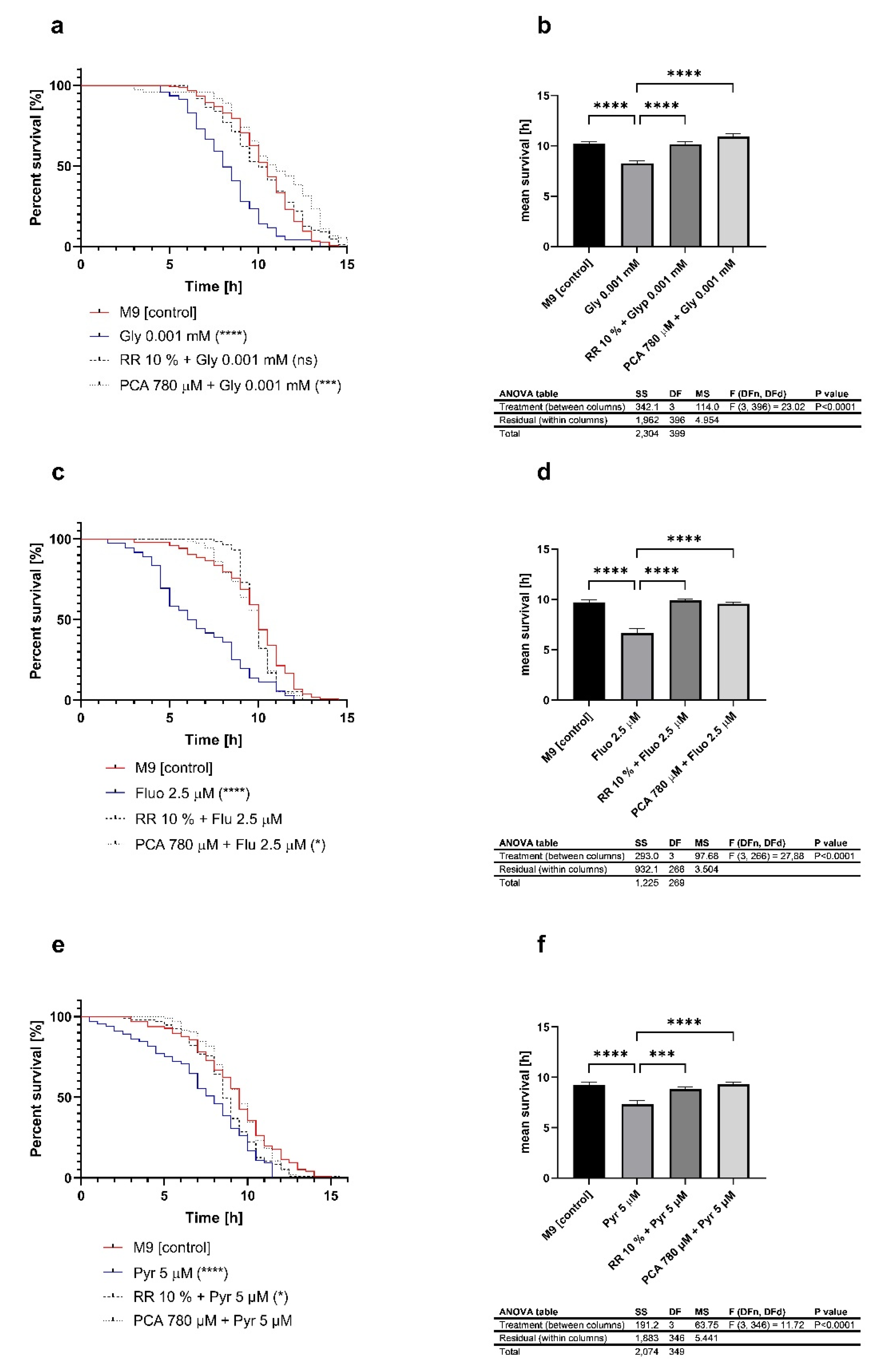
3.1. Pesticides Reduce the Survival under Heat Stress at 37 °C
3.2. Chemotaxis Is Not Altered after Exposure to Pesticides
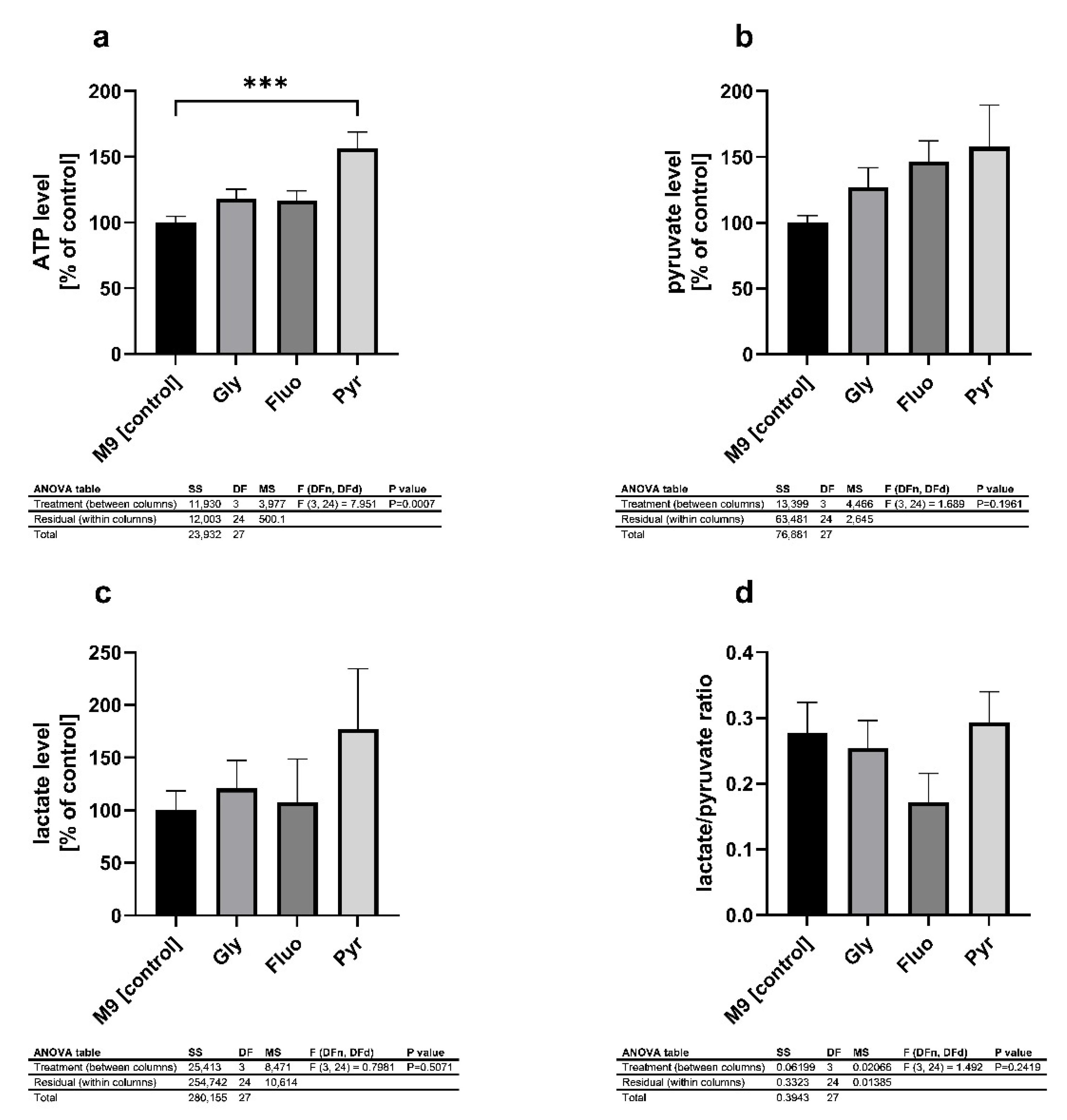
3.3. Energy Metabolites Were Altered in Different Ways by Pesticides
3.4. Effects of Pesticides on mRNA Expression
3.5. Heat Stress Resistance Is Restored after Treatment with Phenolic Metabolites
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Aktar, M.W.; Sengupta, D.; Chowdhury, A. Impact of pesticides use in agriculture: Their benefits and hazards. Interdiscip. Toxicol. 2009, 2, 1–12. [Google Scholar] [CrossRef]
- Boxall, R.A. Post-harvest losses to insects—A world overview. Int. Biodeterior. Biodegrad. 2001, 48, 137–152. [Google Scholar] [CrossRef]
- Oerke, E.-C.; Dehne, H.-W. Safeguarding production—Losses in major crops and the role of crop protection. Crop. Prot. 2004, 23, 275–285. [Google Scholar] [CrossRef]
- Cooper, J.; Dobson, H. The benefits of pesticides to mankind and the environment. Crop Prot. 2007, 26, 1337–1348. [Google Scholar] [CrossRef]
- Sana, S.; Qadir, A.; Mumtaz, M.; Evans, N.P.; Ahmad, S.R. Spatial trends and human health risks of organochlorinated pesticides from bovine milk; a case study from a developing country, Pakistan. Chemosphere 2021, 276, 130110. [Google Scholar] [CrossRef]
- Gilden, R.C.; Huffling, K.; Sattler, B. Pesticides and health risks. J. Obstet. Gynecol. Neonatal Nurs. 2010, 39, 103–110. [Google Scholar] [CrossRef]
- Alengebawy, A.; Abdelkhalek, S.T.; Qureshi, S.R.; Wang, M.-Q. Heavy Metals and Pesticides Toxicity in Agricultural Soil and Plants: Ecological Risks and Human Health Implications. Toxics 2021, 9, 42. [Google Scholar] [CrossRef]
- Anguiano-Vega, G.A.; Cazares-Ramirez, L.H.; Rendon-Von Osten, J.; Santillan-Sidon, A.P.; Vazquez-Boucard, C.G. Risk of genotoxic damage in schoolchildren exposed to organochloride pesticides. Sci. Rep. 2020, 10, 17584. [Google Scholar] [CrossRef]
- Arévalo-Jaramillo, P.; Idrobo, A.; Salcedo, L.; Cabrera, A.; Vintimilla, A.; Carrión, M.; Bailon-Moscoso, N. Biochemical and genotoxic effects in women exposed to pesticides in Southern Ecuador. Environ. Sci. Pollut. Res. Int. 2019, 26, 24911–24921. [Google Scholar] [CrossRef]
- Yu, Y.; Yang, Y.; Zhao, X.; Liu, X.; Xue, J.; Zhang, J.; Yang, A. Exposure to the mixture of organophosphorus pesticides is embryotoxic and teratogenic on gestational rats during the sensitive period. Environ. Toxicol. 2017, 32, 139–146. [Google Scholar] [CrossRef]
- Schwingl, P.J.; Lunn, R.M.; Mehta, S.S. A tiered approach to prioritizing registered pesticides for potential cancer hazard evaluations: Implications for decision making. Environ. Health 2021, 20, 13. [Google Scholar] [CrossRef]
- Anke, T.; Oberwinkler, F.; Steglich, W.; Schramm, G. The strobilurins—New antifungal antibiotics from the basidiomycete Strobilurus tenacellus. J. Antibiot. 1977, 30, 806–810. [Google Scholar] [CrossRef]
- Herms, S.; Seehaus, K.; Koehle, H.; Conrath, U. A strobilurin fungicide enhances the resistance of tobacco against tobacco mosaic virus and Pseudomonas syringae pv tabaci. Plant Physiol. 2002, 130, 120–127. [Google Scholar] [CrossRef]
- Köhle, H.; Grossmann, K.; Jabs, T.; Gerhard, M.; Kaiser, W.; Glaab, J.; Conrath, U.; Seehaus, K.; Herms, S. Modern Fungicides and Antifungal Compounds III; Dehne, H.W., Gisi, U., Juck, K.H., Russel, P.E., Lyr, H., Eds.; Agroconcept GmbH: Bonn, Gernamy, 2002; pp. 61–74. [Google Scholar]
- Sauter, H.; Steglich, W.; Anke, T. Strobilurins: Evolution of a New Class of Active Substances. Angew. Chem. Int. Ed. 1999, 38, 1328–1349. [Google Scholar] [CrossRef]
- Labourdette, G.; Lachaise, H.; Rieck, H.; Steiger, D. Fluopyram: Efficacy and beyond on problematic diseases. In Modern fungicides and antifungal compounds VI, Proceedings of the 16th International Reinhardsbrunn Symposium, Friedrichroda, Germany, 25–29 April 2010; Deutsche Phytomedizinische Gesellschaft eV Selbstverlag: Braunschweig, Germany, 2011; pp. 75–80. [Google Scholar]
- Veloukas, T.; Karaoglanidis, G.S. Biological activity of the succinate dehydrogenase inhibitor fluopyram against Botrytis cinerea and fungal baseline sensitivity. Pest Manag. Sci. 2012, 68, 858–864. [Google Scholar] [CrossRef]
- Fraaije, B.A.; Bayon, C.; Atkins, S.; Cools, H.J.; Lucas, J.A.; Fraaije, M.W. Risk assessment studies on succinate dehydrogenase inhibitors, the new weapons in the battle to control Septoria leaf blotch in wheat. Mol. Plant Pathol. 2012, 13, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Ishii, H.; Miyamoto, T.; Ushio, S.; Kakishima, M. Lack of cross-resistance to a novel succinate dehydrogenase inhibitor, fluopyram, in highly boscalid-resistant isolates of Corynespora cassiicola and Podosphaera xanthii. Pest Manag. Sci. 2011, 67, 474–482. [Google Scholar] [CrossRef]
- Vargas-Pérez, M.; Egea González, F.J.; Garrido Frenich, A. Dissipation and residue determination of fluopyram and its metabolites in greenhouse crops. J. Sci. Food Agric. 2020, 100, 4826–4833. [Google Scholar] [CrossRef]
- Molin, W.T. Glyphosate, a Unique Global Herbicide. J. E. Franz, M.K. Mao, and J. A. Sikorski, ACS Monograph 189, 1997. 653 pp. Weed Technol. 1998, 12, 564–565. [Google Scholar] [CrossRef]
- Duke, S.O.; Powles, S.B. Glyphosate: A once-in-a-century herbicide. Pest Manag. Sci. 2008, 64, 319–325. [Google Scholar] [CrossRef]
- Grossbard, E. (Ed.) The Herbicide Glyphosate; Butterworths: London, UK, 1985; ISBN 0408111534. [Google Scholar]
- Steinrücken, H.C.; Amrhein, N. The herbicide glyphosate is a potent inhibitor of 5-enolpyruvylshikimic acid-3-phosphate synthase. Biochem. Biophys. Res. Commun. 1980, 94, 1207–1212. [Google Scholar] [CrossRef]
- Maeda, H.; Dudareva, N. The shikimate pathway and aromatic amino Acid biosynthesis in plants. Annu. Rev. Plant Biol. 2012, 63, 73–105. [Google Scholar] [CrossRef]
- Heck, G.R.; CaJacob, C.A.; Padgette, S.R. Discovery, Development, and Commercialization of Roundup Ready® Crops. In Plant Biotechnology 2002 and Beyond; Vasil, I.K., Ed.; Springer: Dordrecht, The Netherlands, 2003; pp. 139–142. ISBN 978-90-481-6220-8. [Google Scholar]
- Andreotti, G.; Koutros, S.; Hofmann, J.N.; Sandler, D.P.; Lubin, J.H.; Lynch, C.F.; Lerro, C.C.; de Roos, A.J.; Parks, C.G.; Alavanja, M.C.; et al. Glyphosate Use and Cancer Incidence in the Agricultural Health Study. J. Natl. Cancer Inst. 2018, 110, 509–516. [Google Scholar] [CrossRef]
- Stur, E.; Aristizabal-Pachon, A.F.; Peronni, K.C.; Agostini, L.P.; Waigel, S.; Chariker, J.; Miller, D.M.; Thomas, S.D.; Rezzoug, F.; Detogni, R.S.; et al. Glyphosate-based herbicides at low doses affect canonical pathways in estrogen positive and negative breast cancer cell lines. PLoS ONE 2019, 14, e0219610. [Google Scholar] [CrossRef]
- Thongprakaisang, S.; Thiantanawat, A.; Rangkadilok, N.; Suriyo, T.; Satayavivad, J. Glyphosate induces human breast cancer cells growth via estrogen receptors. Food Chem. Toxicol. 2013, 59, 129–136. [Google Scholar] [CrossRef]
- Mitchell, P. Coupling of phosphorylation to electron and hydrogen transfer by a chemi-osmotic type of mechanism. Nature 1961, 191, 144–148. [Google Scholar] [CrossRef]
- Hatefi, Y. The mitochondrial electron transport and oxidative phosphorylation system. Annu. Rev. Biochem. 1985, 54, 1015–1069. [Google Scholar] [CrossRef]
- Saraste, M. Oxidative phosphorylation at the fin de siècle. Science 1999, 283, 1488–1493. [Google Scholar] [CrossRef]
- Niccoli, T.; Partridge, L. Ageing as a risk factor for disease. Curr. Biol. 2012, 22, R741–R752. [Google Scholar] [CrossRef]
- Farkhondeh, T.; Mehrpour, O.; Forouzanfar, F.; Roshanravan, B.; Samarghandian, S. Oxidative stress and mitochondrial dysfunction in organophosphate pesticide-induced neurotoxicity and its amelioration: A review. Environ. Sci. Pollut. Res. Int. 2020, 27, 24799–24814. [Google Scholar] [CrossRef]
- Gomez, C.; Bandez, M.J.; Navarro, A. Pesticides and impairment of mitochondrial function in relation with the parkinsonian syndrome. Front. Biosci. 2007, 12, 1079–1093. [Google Scholar] [CrossRef]
- Jenner, P. Parkinson’s disease, pesticides and mitochondrial dysfunction. Trends Neurosci. 2001, 24, 245–246. [Google Scholar] [CrossRef]
- Ko, E.; Choi, M.; Shin, S. Bottom-line mechanism of organochlorine pesticides on mitochondria dysfunction linked with type 2 diabetes. J. Hazard. Mater. 2020, 393, 122400. [Google Scholar] [CrossRef]
- Hunt, P.R. The C. elegans model in toxicity testing. J. Appl. Toxicol. 2017, 37, 50–59. [Google Scholar] [CrossRef] [PubMed]
- van der Bliek, A.M.; Sedensky, M.M.; Morgan, P.G. Cell Biology of the Mitochondrion. Genetics 2017, 207, 843–871. [Google Scholar] [CrossRef] [PubMed]
- Kirstein-Miles, J.; Morimoto, R.I. Caenorhabditis elegans as a model system to study intercompartmental proteostasis: Interrelation of mitochondrial function, longevity, and neurodegenerative diseases. Dev. Dyn. 2010, 239, 1529–1538. [Google Scholar] [CrossRef] [PubMed]
- Duke, S.O. Herbicide-Resistant Crops: Agricultural, Economic, Environmental, Regulatory, and Technological Aspects; CRC Press: Milton, MA, USA, 2018; ISBN 9781351073196. [Google Scholar]
- McVey, K.A.; Snapp, I.B.; Johnson, M.B.; Negga, R.; Pressley, A.S.; Fitsanakis, V.A. Exposure of C. elegans eggs to a glyphosate-containing herbicide leads to abnormal neuronal morphology. Neurotoxicol. Teratol. 2016, 55, 23–31. [Google Scholar] [CrossRef]
- Kronberg, M.F.; Clavijo, A.; Moya, A.; Rossen, A.; Calvo, D.; Pagano, E.; Munarriz, E. Glyphosate-based herbicides modulate oxidative stress response in the nematode Caenorhabditis elegans. Comp. Biochem. Physiol. C. Toxicol. Pharmacol. 2018, 214, 1–8. [Google Scholar] [CrossRef]
- Bailey, D.C.; Todt, C.E.; Burchfield, S.L.; Pressley, A.S.; Denney, R.D.; Snapp, I.B.; Negga, R.; Traynor, W.L.; Fitsanakis, V.A. Chronic exposure to a glyphosate-containing pesticide leads to mitochondrial dysfunction and increased reactive oxygen species production in Caenorhabditis elegans. Environ. Toxicol. Pharmacol. 2018, 57, 46–52. [Google Scholar] [CrossRef]
- García-Espiñeira, M.; Tejeda-Benitez, L.; Olivero-Verbel, J. Toxicity of atrazine- and glyphosate-based formulations on Caenorhabditis elegans. Ecotoxicol. Environ. Saf. 2018, 156, 216–222. [Google Scholar] [CrossRef]
- Negga, R.; Rudd, D.A.; Davis, N.S.; Justice, A.N.; Hatfield, H.E.; Valente, A.L.; Fields, A.S.; Fitsanakis, V.A. Exposure to Mn/Zn ethylene-bis-dithiocarbamate and glyphosate pesticides leads to neurodegeneration in Caenorhabditis elegans. Neurotoxicology 2011, 32, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ezemaduka, A.N.; Li, Z.; Chen, Z.; Song, C. Joint Toxicity of Arsenic, Copper and Glyphosate on Behavior, Reproduction and Heat Shock Protein Response in Caenorhabditis elegans. Bull. Environ. Contam. Toxicol. 2017, 98, 465–471. [Google Scholar] [CrossRef]
- Liu, G.; Lin, X.; Xu, S.; Liu, G.; Liu, Z.; Liu, F.; Mu, W. Efficacy of fluopyram as a candidate trunk-injection agent against Bursaphelenchus xylophilus. Eur. J. Plant Pathol. 2020, 157, 403–411. [Google Scholar] [CrossRef]
- Xu, C.; Li, M.; Zhou, Z.; Li, J.; Chen, D.; Duan, Y.; Zhou, M. Impact of Five Succinate Dehydrogenase Inhibitors on DON Biosynthesis of Fusarium asiaticum, Causing Fusarium Head Blight in Wheat. Toxins 2019, 11, 272. [Google Scholar] [CrossRef]
- Da Costa Domingues, C.E.; Bello Inoue, L.V.; Da Silva-Zacarin, E.C.M.; Malaspina, O. Fungicide pyraclostrobin affects midgut morphophysiology and reduces survival of Brazilian native stingless bee Melipona scutellaris. Ecotoxicol. Environ. Saf. 2020, 206, 111395. [Google Scholar] [CrossRef] [PubMed]
- Da Eduardo Costa Domingues, C.; Bello Inoue, L.V.; Da Mathias Silva-Zacarin, E.C.; Malaspina, O. Foragers of Africanized honeybee are more sensitive to fungicide pyraclostrobin than newly emerged bees. Environ. Pollut. 2020, 266, 115267. [Google Scholar] [CrossRef]
- Jiang, J.; Wu, S.; Lv, L.; Liu, X.; Chen, L.; Zhao, X.; Wang, Q. Mitochondrial dysfunction, apoptosis and transcriptomic alterations induced by four strobilurins in zebrafish (Danio rerio) early life stages. Environ. Pollut. 2019, 253, 722–730. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhao, F.; Cao, F.; Teng, M.; Yang, Y.; Qiu, L. Mitochondrial dysfunction-based cardiotoxicity and neurotoxicity induced by pyraclostrobin in zebrafish larvae. Environ. Pollut. 2019, 251, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Nicodemo, D.; Mingatto, F.E.; de Carvalho, A.; Bizerra, P.F.V.; Tavares, M.A.; Balieira, K.V.B.; Bellini, W.C. Pyraclostrobin Impairs Energetic Mitochondrial Metabolism and Productive Performance of Silkworm (Lepidoptera: Bombycidae) Caterpillars. J. Econ. Entomol. 2018, 111, 1369–1375. [Google Scholar] [CrossRef]
- Tadei, R.; Domingues, C.E.C.; Malaquias, J.B.; Camilo, E.V.; Malaspina, O.; Silva-Zacarin, E.C.M. Late effect of larval co-exposure to the insecticide clothianidin and fungicide pyraclostrobin in Africanized Apis mellifera. Sci. Rep. 2019, 9, 3277. [Google Scholar] [CrossRef]
- Brenner, S. The genetics of Caenorhabditis elegans. Genetics 1974, 77, 71–94. [Google Scholar] [CrossRef] [PubMed]
- Stiernagle, T. Maintenance of C. elegans. WormBook 2006, 11, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Fitzenberger, E.; Deusing, D.J.; Marx, C.; Boll, M.; Lüersen, K.; Wenzel, U. The polyphenol quercetin protects the mev-1 mutant of Caenorhabditis elegans from glucose-induced reduction of survival under heat stress depending on SIR-2.1, DAF-12, and proteasomal activity. Mol. Nutr. Food Res. 2014, 58, 984–994. [Google Scholar] [CrossRef]
- Margie, O.; Palmer, C.; Chin-Sang, I. C. elegans chemotaxis assay. J. Vis. Exp. 2013, e50069. [Google Scholar] [CrossRef]
- Dilberger, B.; Passon, M.; Asseburg, H.; Silaidos, C.V.; Schmitt, F.; Schmiedl, T.; Schieber, A.; Eckert, G.P. Polyphenols and Metabolites Enhance Survival in Rodents and Nematodes-Impact of Mitochondria. Nutrients 2019, 11, 1886. [Google Scholar] [CrossRef] [PubMed]
- Septembre-Malaterre, A.; Remize, F.; Poucheret, P. Fruits and vegetables, as a source of nutritional compounds and phytochemicals: Changes in bioactive compounds during lactic fermentation. Food Res. Int. 2018, 104, 86–99. [Google Scholar] [CrossRef] [PubMed]
- Murota, K.; Nakamura, Y.; Uehara, M. Flavonoid metabolism: The interaction of metabolites and gut microbiota. Biosci. Biotechnol. Biochem. 2018, 82, 600–610. [Google Scholar] [CrossRef]
- Song, J.; He, Y.; Luo, C.; Feng, B.; Ran, F.; Xu, H.; Ci, Z.; Xu, R.; Han, L.; Zhang, D. New progress in the pharmacology of protocatechuic acid: A compound ingested in daily foods and herbs frequently and heavily. Pharmacol. Res. 2020, 161, 105109. [Google Scholar] [CrossRef]
- Fitsanakis, V.A. Caenorhabditis elegans: An Emerging Model System for Pesticide Neurotoxicity. J. Environ. Anal. Toxicol. S 2012, 4, 2161-0525. [Google Scholar] [CrossRef]
- Ruan, Q.-L.; Ju, J.-J.; Li, Y.-H.; Liu, R.; Pu, Y.-P.; Yin, L.-H.; Wang, D.-Y. Evaluation of pesticide toxicities with differing mechanisms using Caenorhabditis elegans. J. Toxicol. Environ. Health A 2009, 72, 746–751. [Google Scholar] [CrossRef]
- Tejeda-Benitez, L.; Olivero-Verbel, J. Caenorhabditis elegans, a Biological Model for Research in Toxicology. Rev. Environ. Contam. Toxicol. 2016, 237, 1–35. [Google Scholar] [CrossRef] [PubMed]
- Boyd, W.A.; Smith, M.V.; Kissling, G.E.; Freedman, J.H. Medium- and high-throughput screening of neurotoxicants using C. elegans. Neurotoxicol. Teratol. 2010, 32, 68–73. [Google Scholar] [CrossRef]
- Richardson, J.R.; Fitsanakis, V.; Westerink, R.H.S.; Kanthasamy, A.G. Neurotoxicity of pesticides. Acta Neuropathol. 2019, 138, 343–362. [Google Scholar] [CrossRef]
- Meyer, D.; Williams, P.L. Toxicity testing of neurotoxic pesticides in Caenorhabditis elegans. J. Toxicol. Environ. Health B Crit. Rev. 2014, 17, 284–306. [Google Scholar] [CrossRef]
- Lai, C.H.; Chou, C.Y.; Ch’ang, L.Y.; Liu, C.S.; Lin, W. Identification of novel human genes evolutionarily conserved in Caenorhabditis elegans by comparative proteomics. Genome Res. 2000, 10, 703–713. [Google Scholar] [CrossRef] [PubMed]
- Holden-Dye, L.; Walker, R.J. Anthelmintic drugs and nematicides: Studies in Caenorhabditis elegans. WormBook 2014, 16, 1–29. [Google Scholar] [CrossRef]
- Lewis, J.A.; Gehman, E.A.; Baer, C.E.; Jackson, D.A. Alterations in gene expression in Caenorhabditis elegans associated with organophosphate pesticide intoxication and recovery. BMC Genom. 2013, 14, 291. [Google Scholar] [CrossRef]
- Lewis, J.A.; Szilagyi, M.; Gehman, E.; Dennis, W.E.; Jackson, D.A. Distinct patterns of gene and protein expression elicited by organophosphorus pesticides in Caenorhabditis elegans. BMC Genom. 2009, 10, 202. [Google Scholar] [CrossRef] [PubMed]
- Viñuela, A.; Snoek, L.B.; Riksen, J.A.G.; Kammenga, J.E. Genome-wide gene expression analysis in response to organophosphorus pesticide chlorpyrifos and diazinon in C. elegans. PLoS ONE 2010, 5, e12145. [Google Scholar] [CrossRef]
- Cuhra, M.; Traavik, T.; Bøhn, T. Clone- and age-dependent toxicity of a glyphosate commercial formulation and its active ingredient in Daphnia magna. Ecotoxicology 2013, 22, 251–262. [Google Scholar] [CrossRef]
- Bridi, D.; Altenhofen, S.; Gonzalez, J.B.; Reolon, G.K.; Bonan, C.D. Glyphosate and Roundup® alter morphology and behavior in zebrafish. Toxicology 2017, 392, 32–39. [Google Scholar] [CrossRef] [PubMed]
- King, J.J.; Wagner, R.S. Toxic Effects of the Herbicide Roundup® Regular on Pacific Northwestern Amphibians. Northwestern Nat. 2010, 91, 318–324. [Google Scholar] [CrossRef]
- Morrison, S.A.; McMurry, S.T.; Smith, L.M.; Belden, J.B. Acute toxicity of pyraclostrobin and trifloxystrobin to Hyalella azteca. Environ. Toxicol. Chem. 2013, 32, 1516–1525. [Google Scholar] [CrossRef]
- Ruszkiewicz, J.A.; Pinkas, A.; Miah, M.R.; Weitz, R.L.; Lawes, M.J.A.; Akinyemi, A.J.; Ijomone, O.M.; Aschner, M. C. elegans as a model in developmental neurotoxicology. Toxicol. Appl. Pharmacol. 2018, 354, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Opperman, C.H.; Chang, S. Effects of Aldicarb and Fenamiphos on Acetycholinesterase and Motility of Caenorhabditis elegans. J. Nematol. 1991, 23, 20–27. [Google Scholar]
- Jadiya, P.; Nazir, A. Environmental toxicants as extrinsic epigenetic factors for parkinsonism: Studies employing transgenic C. elegans model. CNS Neurol. Disord. Drug Targets 2012, 11, 976–983. [Google Scholar] [CrossRef]
- Ebrahimi, C.M.; Rankin, C.H. Early patterned stimulation leads to changes in adult behavior and gene expression in C. elegans. Genes Brain Behav. 2007, 6, 517–528. [Google Scholar] [CrossRef]
- Sokolova, I.M.; Sokolov, E.P.; Ponnappa, K.M. Cadmium exposure affects mitochondrial bioenergetics and gene expression of key mitochondrial proteins in the eastern oyster Crassostrea virginica Gmelin (Bivalvia: Ostreidae). Aquat. Toxicol. 2005, 73, 242–255. [Google Scholar] [CrossRef]
- Ivanina, A.V.; Sokolov, E.P.; Sokolova, I.M. Effects of cadmium on anaerobic energy metabolism and mRNA expression during air exposure and recovery of an intertidal mollusk Crassostrea virginica. Aquat. Toxicol. 2010, 99, 330–342. [Google Scholar] [CrossRef]
- Bradbury, D.A.; Simmons, T.D.; Slater, K.J.; Crouch, S. Measurement of the ADP:ATP ratio in human leukaemic cell lines can be used as an indicator of cell viability, necrosis and apoptosis. J. Immunol. Methods 2000, 240, 79–92. [Google Scholar] [CrossRef]
- Babczyńska, A.; Wilczek, G.; Wilczek, P.; Szulińska, E.; Witas, I. Metallothioneins and energy budget indices in cadmium and copper exposed spiders Agelena labyrinthica in relation to their developmental stage, gender and origin. Comp. Biochem. Physiol. C. Toxicol. Pharmacol. 2011, 154, 161–171. [Google Scholar] [CrossRef]
- Matin, M.A.; Husain, K. Cerebral glycogenolysis and glycolysis in malathion-treated hyperglycaemic animals. Biochem. Pharmacol. 1987, 36, 1815–1817. [Google Scholar] [CrossRef]
- Ferrando, M.D.; Andreu-Moliner, E. Effects of lindane on fish carbohydrate metabolism. Ecotoxicol. Environ. Saf. 1991, 22, 17–23. [Google Scholar] [CrossRef]
- Huckabee, W.E. Relationships of pyruvate and lactate during anaerobic metabolism. I. Effects of infusion of pyruvate or glucose and of hyperventilation. J. Clin. Investig. 1958, 37, 244–254. [Google Scholar] [CrossRef]
- Kumar, P.; Nagarajan, A.; Uchil, P.D. Analysis of Cell Viability by the MTT Assay. Cold Spring Harb. Protoc. 2018, 2018, pdb. prot095505. [Google Scholar] [CrossRef]
- Becker, W.F.; von Jagow, G.; Anke, T.; Steglich, W. Oudemansin, strobilurin A, strobilurin B and myxothiazol: New inhibitors of the bc1 segment of the respiratory chain with an E-β-methoxyacrylate system as common structural element. FEBS Lett. 1981, 132, 329–333. [Google Scholar] [CrossRef]
- Vankoningsloo, S.; Piens, M.; Lecocq, C.; Gilson, A.; de Pauw, A.; Renard, P.; Demazy, C.; Houbion, A.; Raes, M.; Arnould, T. Mitochondrial dysfunction induces triglyceride accumulation in 3T3-L1 cells: Role of fatty acid beta-oxidation and glucose. J. Lipid Res. 2005, 46, 1133–1149. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Wen, X.; Esser, L.; Quinn, B.; Yu, L.; Yu, C.-A.; Xia, D. Structural basis for the quinone reduction in the bc1 complex: A comparative analysis of crystal structures of mitochondrial cytochrome bc1 with bound substrate and inhibitors at the Qi site. Biochemistry 2003, 42, 9067–9080. [Google Scholar] [CrossRef]
- Zhang, Z.; Huang, L.; Shulmeister, V.M.; Chi, Y.I.; Kim, K.K.; Hung, L.W.; Crofts, A.R.; Berry, E.A.; Kim, S.H. Electron transfer by domain movement in cytochrome bc1. Nature 1998, 392, 677–684. [Google Scholar] [CrossRef] [PubMed]
- Luz, A.L.; Kassotis, C.D.; Stapleton, H.M.; Meyer, J.N. The high-production volume fungicide pyraclostrobin induces triglyceride accumulation associated with mitochondrial dysfunction, and promotes adipocyte differentiation independent of PPARγ activation, in 3T3-L1 cells. Toxicology 2018, 393, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Falk, M.J.; Zhang, Z.; Rosenjack, J.R.; Nissim, I.; Daikhin, E.; Sedensky, M.M.; Yudkoff, M.; Morgan, P.G. Metabolic pathway profiling of mitochondrial respiratory chain mutants in C. elegans. Mol. Genet. Metab. 2008, 93, 388–397. [Google Scholar] [CrossRef] [PubMed]
- Morgan, P.G.; Higdon, R.; Kolker, N.; Bauman, A.T.; Ilkayeva, O.; Newgard, C.B.; Kolker, E.; Steele, L.M.; Sedensky, M.M. Comparison of proteomic and metabolomic profiles of mutants of the mitochondrial respiratory chain in Caenorhabditis elegans. Mitochondrion 2015, 20, 95–102. [Google Scholar] [CrossRef][Green Version]
- Pulliam, D.A.; Deepa, S.S.; Liu, Y.; Hill, S.; Lin, A.-L.; Bhattacharya, A.; Shi, Y.; Sloane, L.; Viscomi, C.; Zeviani, M.; et al. Complex IV-deficient Surf1(-/-) mice initiate mitochondrial stress responses. Biochem. J. 2014, 462, 359–371. [Google Scholar] [CrossRef]
- Zečić, A.; Braeckman, B.P. DAF-16/FoxO in Caenorhabditis elegans and Its Role in Metabolic Remodeling. Cells 2020, 9, 109. [Google Scholar] [CrossRef]
- Uno, M.; Nishida, E. Lifespan-regulating genes in C. elegans. NPJ Aging Mech. Dis. 2016, 2, 16010. [Google Scholar] [CrossRef]
- Berdichevsky, A.; Viswanathan, M.; Horvitz, H.R.; Guarente, L. C. elegans SIR-2.1 interacts with 14-3-3 proteins to activate DAF-16 and extend life span. Cell 2006, 125, 1165–1177. [Google Scholar] [CrossRef]
- Apfeld, J.; O’Connor, G.; McDonagh, T.; DiStefano, P.S.; Curtis, R. The AMP-activated protein kinase AAK-2 links energy levels and insulin-like signals to lifespan in C. elegans. Genes Dev. 2004, 18, 3004–3009. [Google Scholar] [CrossRef] [PubMed]
- Burchfield, S.L.; Bailey, D.C.; Todt, C.E.; Denney, R.D.; Negga, R.; Fitsanakis, V.A. Acute exposure to a glyphosate-containing herbicide formulation inhibits Complex II and increases hydrogen peroxide in the model organism Caenorhabditis elegans. Environ. Toxicol. Pharmacol. 2019, 66, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Harris, G.; Eschment, M.; Orozco, S.P.; McCaffery, J.M.; Maclennan, R.; Severin, D.; Leist, M.; Kleensang, A.; Pamies, D.; Maertens, A.; et al. Toxicity, recovery, and resilience in a 3D dopaminergic neuronal in vitro model exposed to rotenone. Arch. Toxicol. 2018, 92, 2587–2606. [Google Scholar] [CrossRef]
- Yakes, F.M.; van Houten, B. Mitochondrial DNA damage is more extensive and persists longer than nuclear DNA damage in human cells following oxidative stress. Proc. Natl. Acad. Sci. USA 1997, 94, 514–519. [Google Scholar] [CrossRef]
- Ma, J.; Cheng, C.; Du, Z.; Li, B.; Wang, J.; Wang, J.; Wang, Z.; Zhu, L. Toxicological effects of pyraclostrobin on the antioxidant defense system and DNA damage in earthworms (Eisenia fetida). Ecol. Indic. 2019, 101, 111–116. [Google Scholar] [CrossRef]
- Copeland, J.M.; Cho, J.; Lo, T.; Hur, J.H.; Bahadorani, S.; Arabyan, T.; Rabie, J.; Soh, J.; Walker, D.W. Extension of Drosophila life span by RNAi of the mitochondrial respiratory chain. Curr. Biol. 2009, 19, 1591–1598. [Google Scholar] [CrossRef]
- Dillin, A.; Hsu, A.-L.; Arantes-Oliveira, N.; Lehrer-Graiwer, J.; Hsin, H.; Fraser, A.G.; Kamath, R.S.; Ahringer, J.; Kenyon, C. Rates of behavior and aging specified by mitochondrial function during development. Science 2002, 298, 2398–2401. [Google Scholar] [CrossRef] [PubMed]
- van Raamsdonk, J.M.; Meng, Y.; Camp, D.; Yang, W.; Jia, X.; Bénard, C.; Hekimi, S. Decreased energy metabolism extends life span in Caenorhabditis elegans without reducing oxidative damage. Genetics 2010, 185, 559–571. [Google Scholar] [CrossRef]
- Ng, L.F.; Ng, L.T.; van Breugel, M.; Halliwell, B.; Gruber, J. Mitochondrial DNA Damage Does Not Determine C. elegans Lifespan. Front. Genet. 2019, 10, 311. [Google Scholar] [CrossRef] [PubMed]
- Castellani, C.A.; Longchamps, R.J.; Sumpter, J.A.; Newcomb, C.E.; Lane, J.A.; Grove, M.L.; Bressler, J.; Brody, J.A.; Floyd, J.S.; Bartz, T.M.; et al. Mitochondrial DNA copy number can influence mortality and cardiovascular disease via methylation of nuclear DNA CpGs. Genome Med. 2020, 12, 84. [Google Scholar] [CrossRef]
- Dang, S.; Qu, Y.; Wei, J.; Shao, Y.; Yang, Q.; Ji, M.; Shi, B.; Hou, P. Low copy number of mitochondrial DNA (mtDNA) predicts worse prognosis in early-stage laryngeal cancer patients. Diagn. Pathol. 2014, 9, 28. [Google Scholar] [CrossRef]
- Mei, H.; Sun, S.; Bai, Y.; Chen, Y.; Chai, R.; Li, H. Reduced mtDNA copy number increases the sensitivity of tumor cells to chemotherapeutic drugs. Cell Death Dis. 2015, 6, e1710. [Google Scholar] [CrossRef] [PubMed]
- Marco, M.L.; Heeney, D.; Binda, S.; Cifelli, C.J.; Cotter, P.D.; Foligné, B.; Gänzle, M.; Kort, R.; Pasin, G.; Pihlanto, A.; et al. Health benefits of fermented foods: Microbiota and beyond. Curr. Opin. Biotechnol. 2017, 44, 94–102. [Google Scholar] [CrossRef]
- Hippeli, S.; Janisch, K.; Kern, S.; Ölschläger, C.; Treutter, D.; May, C.; Elstner, E.F. Antioxidant and immune modulatory activities of fruit and vegetable extracts after―Cascade fermentation. Curr. Topics Biochem. Res 2007, 9, 83–97. [Google Scholar]
- Semaming, Y.; Sripetchwandee, J.; Sa-Nguanmoo, P.; Pintana, H.; Pannangpetch, P.; Chattipakorn, N.; Chattipakorn, S.C. Protocatechuic acid protects brain mitochondrial function in streptozotocin-induced diabetic rats. Appl. Physiol. Nutr. Metab. 2015, 40, 1078–1081. [Google Scholar] [CrossRef]
- Dilberger, B.; Baumanns, S.; Schmitt, F.; Schmiedl, T.; Hardt, M.; Wenzel, U.; Eckert, G.P. Mitochondrial Oxidative Stress Impairs Energy Metabolism and Reduces Stress Resistance and Longevity of C. elegans. Oxid. Med. Cell. Longev. 2019, 2019, 6840540. [Google Scholar] [CrossRef] [PubMed]
- Link, P.; Wink, M. Isoliquiritigenin exerts antioxidant activity in Caenorhabditis elegans via insulin-like signaling pathway and SKN-1. Phytomedicine 2019, 55, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Friedman, J.R.; Nunnari, J. Mitochondrial form and function. Nature 2014, 505, 335–343. [Google Scholar] [CrossRef]
- Palikaras, K.; Lionaki, E.; Tavernarakis, N. Coordination of mitophagy and mitochondrial biogenesis during ageing in C. elegans. Nature 2015, 521, 525–528. [Google Scholar] [CrossRef]

| Primer | Sequence | Product Size (bp) |
|---|---|---|
| aak-2 NM_001029697.6 | 5′-tgcttcaccatatgctctgc-3′ 5′-gtggatcatctcccagcaat-3′ | 219 |
| ama-1 NM_068122.9 | 5′-ccaggaacttcggctcagta-3′ 5′-tgtatgatggtgaagctggcg-3′ | 85 |
| act-2 NM_001383398.2 | 5′-cccactcaatccaaaggcta-3′ 5′-gggactgtgtgggtaacacc-3′ | 168 |
| atfs-1 NM_074114.7 | 5′-tcggcgatcgatcagctaac-3′ 5′-agaatcagttcttggattagggga-3′ | 75 |
| atp-2 NM_065710.8 | 5′-tccaagtcgctgaggtgttc-3′ 5′-aggtggtcgagttctcctga-3′ | 151 |
| daf-16 NM_001026422.6 | 5′-tcctcattcactcccgattc-3′ 5′-ccggtgtattcatgaacgtg-3′ | 175 |
| sir-2.1 NM_001268555.5 | 5′-tggctgacgattcgatggat-3′ 5′-atgagcagaaatcgcgacac-3′ | 179 |
| skn-1 NM_171345.6 | 5′-acagggtggaaaaagcaagg-3′ 5′-caggccaaacgccaatgac-3′ | 246 |
| M9 (Control) | Gly 0.001 mM | M9 (Control) | Fluo 2.5 µM | M9 (Control) | Pyr 5 µM | |
|---|---|---|---|---|---|---|
| daf-16 | 100.0 ± 11.34 | 81.37 ± 6.095 | 100.0 ± 5.940 | 103.2 ± 11.74 | 100.0 ± 2.323 | 34.42 ± 4.417 **** p < 0.0001 |
| sir-2.1 | 100.0 ± 5.070 | 114.9 ± 5.327 | 100.0 ± 6.891 | 86.87 ± 4.198 | 100.0 ± 16.06 | 28.68 ± 13.85 ** p = 0.0072 |
| aak-2 | 100.0 ± 15.92 | 91.36 ± 8.859 | 100.0 ± 12.00 | 86.56 ± 11.17 | 100.0 ± 13.20 | 20.20 ± 6.315 *** p < 0.0007 |
| atp-2 | 100.0 ± 6.574 | 93.64 ± 4.589 | 100.0 ± 12.47 | 160.5 ± 19.61 * p = 0.0263 | 100.0 ± 9.440 | 13.54 ± 3.302 **** p < 0.0001 |
| skn-1 | 100.0 ± 15.80 | 74.41 ± 7.508 | 100.0 ± 12.92 | 95.47 ± 7.471 | 100.0 ± 7.316 | 14.72 ± 5.783 **** p < 0.0001 |
| atfs-1 | 100.0 ± 13.29 | 68.17 ± 6.165 | 100.0 ± 8.947 | 83.77 ± 5.508 | 100.0 ± 6.257 | 39.34 ± 9.921 *** p < 0.0004 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schmitt, F.; Babylon, L.; Dieter, F.; Eckert, G.P. Effects of Pesticides on Longevity and Bioenergetics in Invertebrates—The Impact of Polyphenolic Metabolites. Int. J. Mol. Sci. 2021, 22, 13478. https://doi.org/10.3390/ijms222413478
Schmitt F, Babylon L, Dieter F, Eckert GP. Effects of Pesticides on Longevity and Bioenergetics in Invertebrates—The Impact of Polyphenolic Metabolites. International Journal of Molecular Sciences. 2021; 22(24):13478. https://doi.org/10.3390/ijms222413478
Chicago/Turabian StyleSchmitt, Fabian, Lukas Babylon, Fabian Dieter, and Gunter P. Eckert. 2021. "Effects of Pesticides on Longevity and Bioenergetics in Invertebrates—The Impact of Polyphenolic Metabolites" International Journal of Molecular Sciences 22, no. 24: 13478. https://doi.org/10.3390/ijms222413478
APA StyleSchmitt, F., Babylon, L., Dieter, F., & Eckert, G. P. (2021). Effects of Pesticides on Longevity and Bioenergetics in Invertebrates—The Impact of Polyphenolic Metabolites. International Journal of Molecular Sciences, 22(24), 13478. https://doi.org/10.3390/ijms222413478






