Conditioned Medium from Bone Marrow Mesenchymal Stem Cells Restored Oxidative Stress-Related Impaired Osteogenic Differentiation
Abstract
:1. Introduction
2. Results
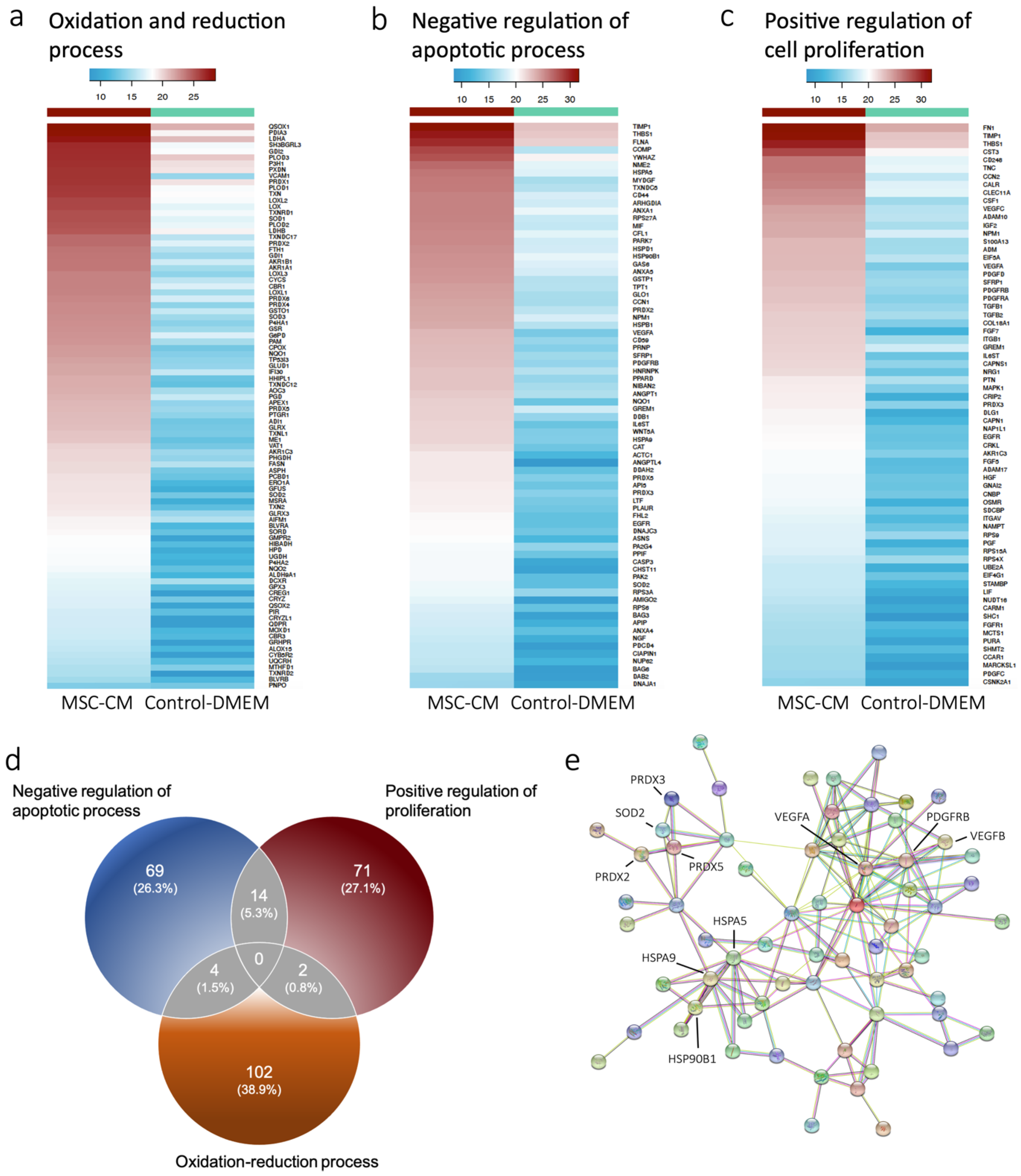
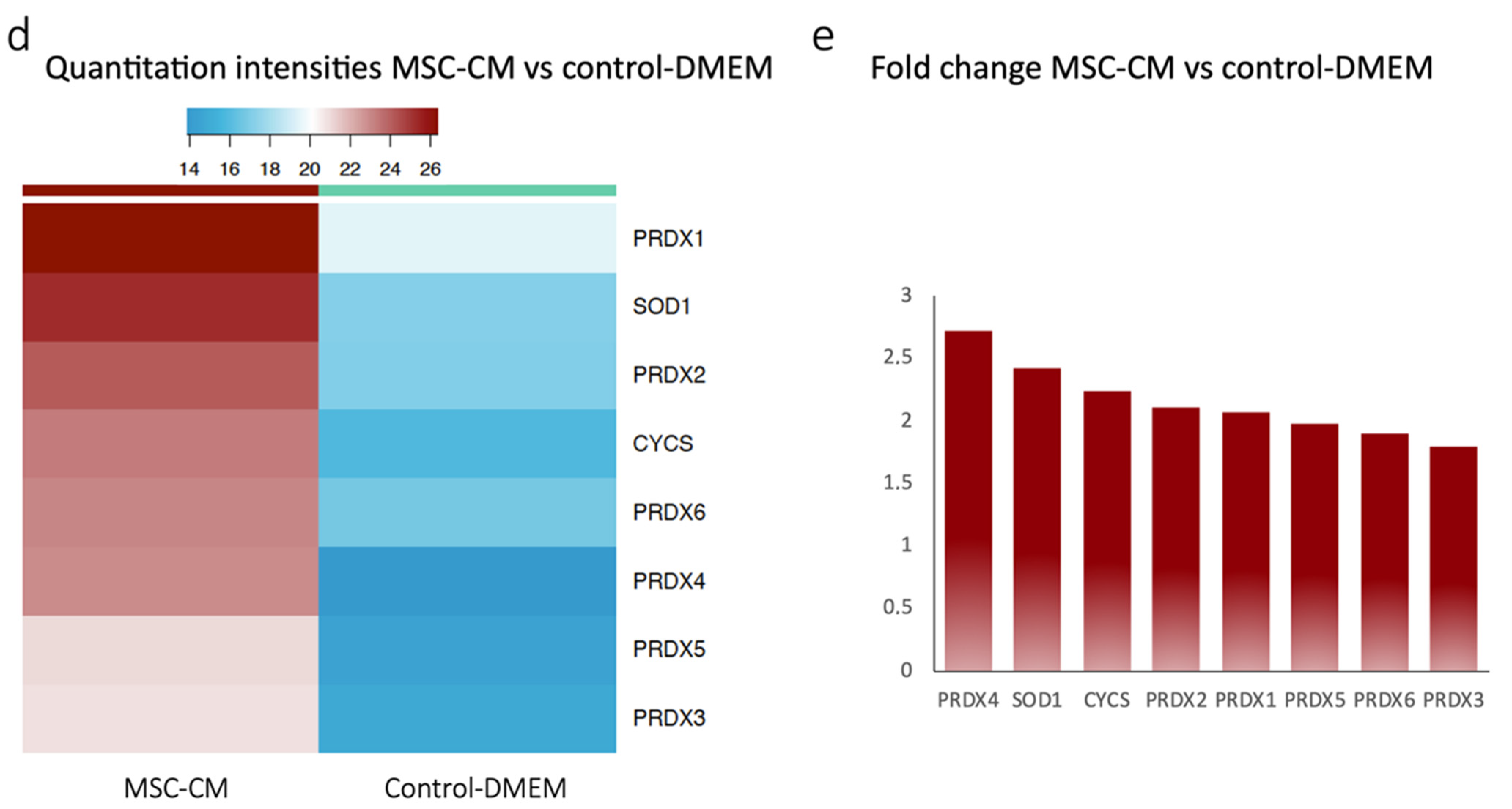
2.1. Proteomics Revealed Expression of Antioxidant Proteins in MSC-CM
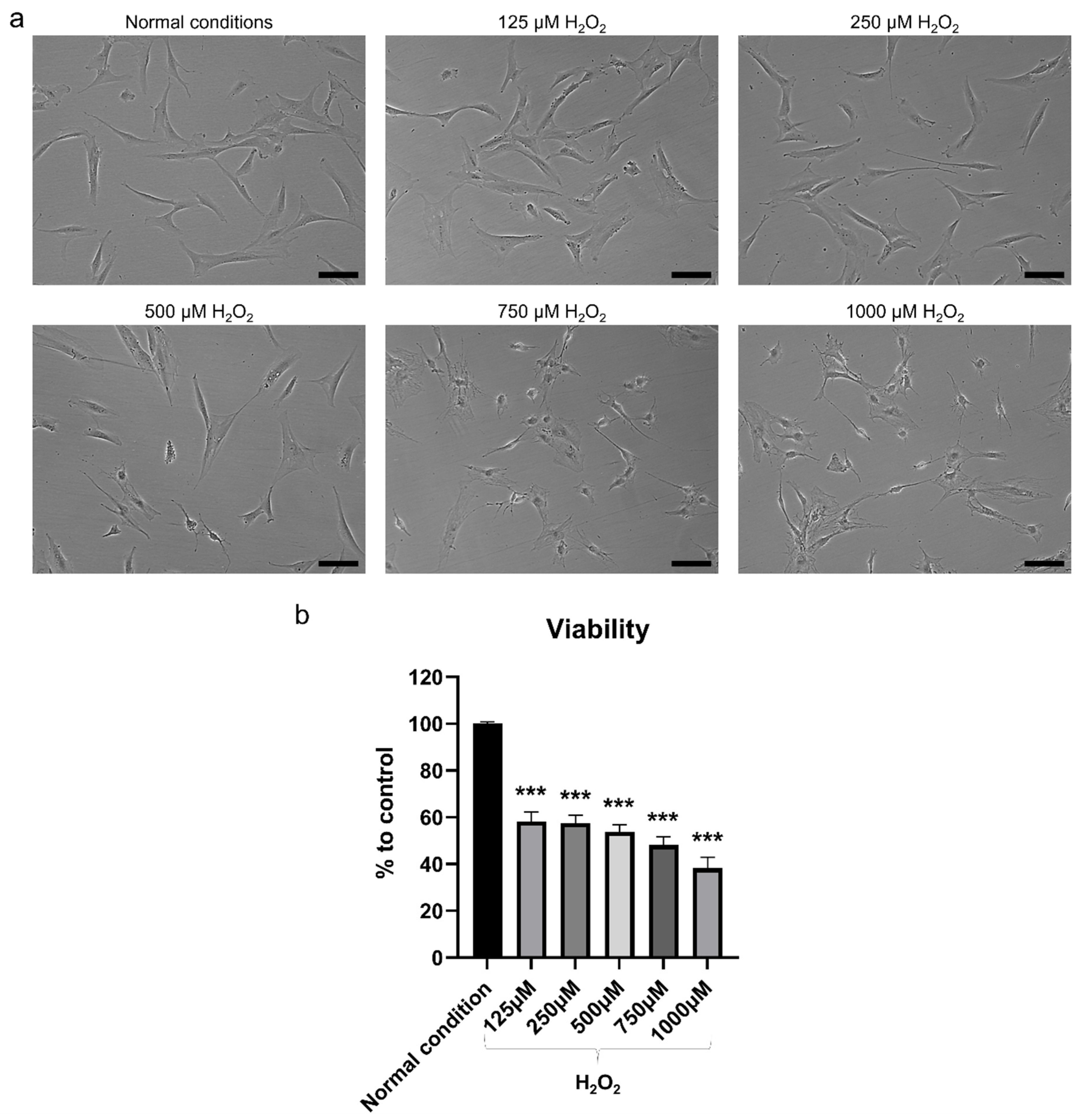
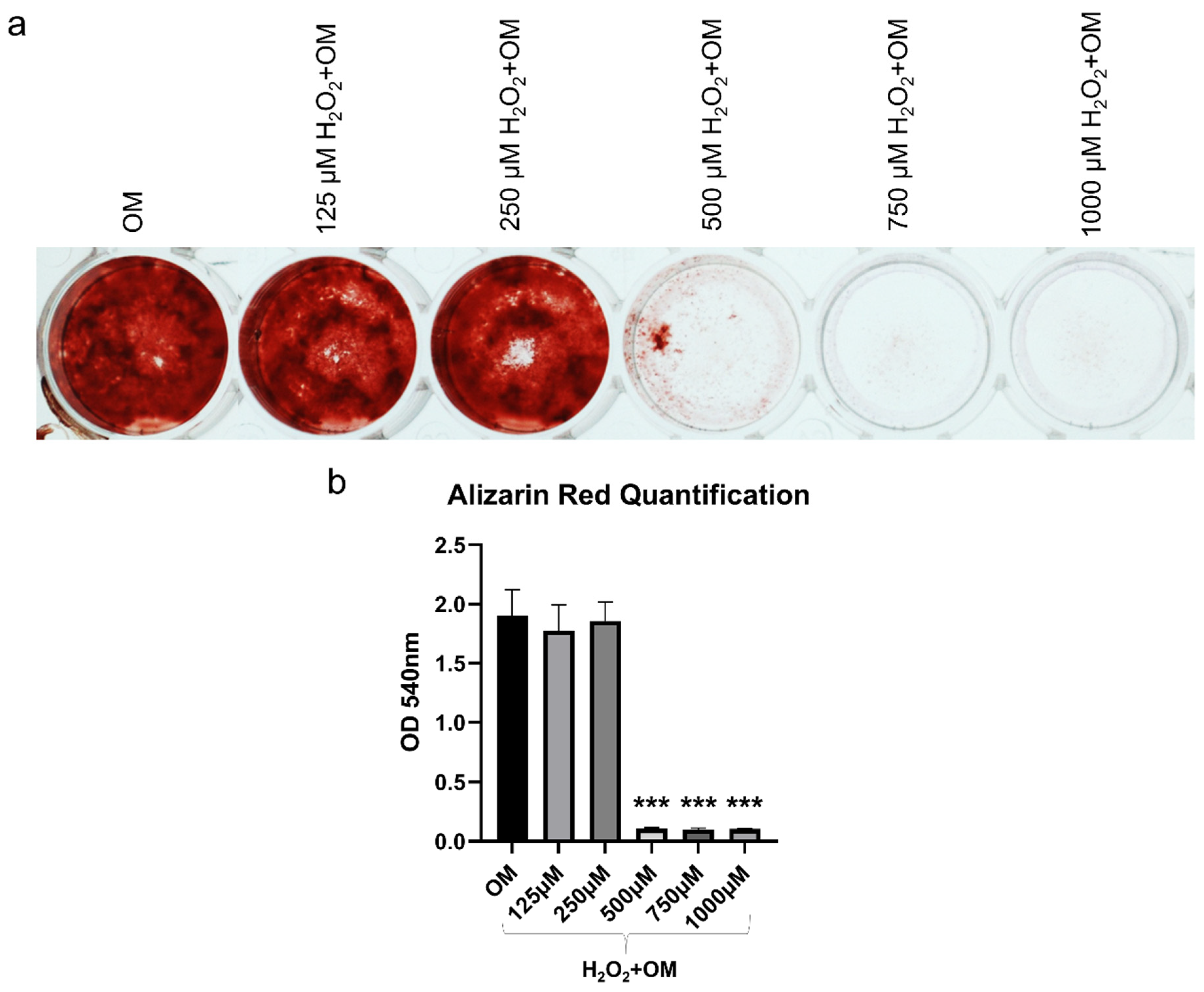
2.2. H2O2 Had Detrimental Effects on hBMSC
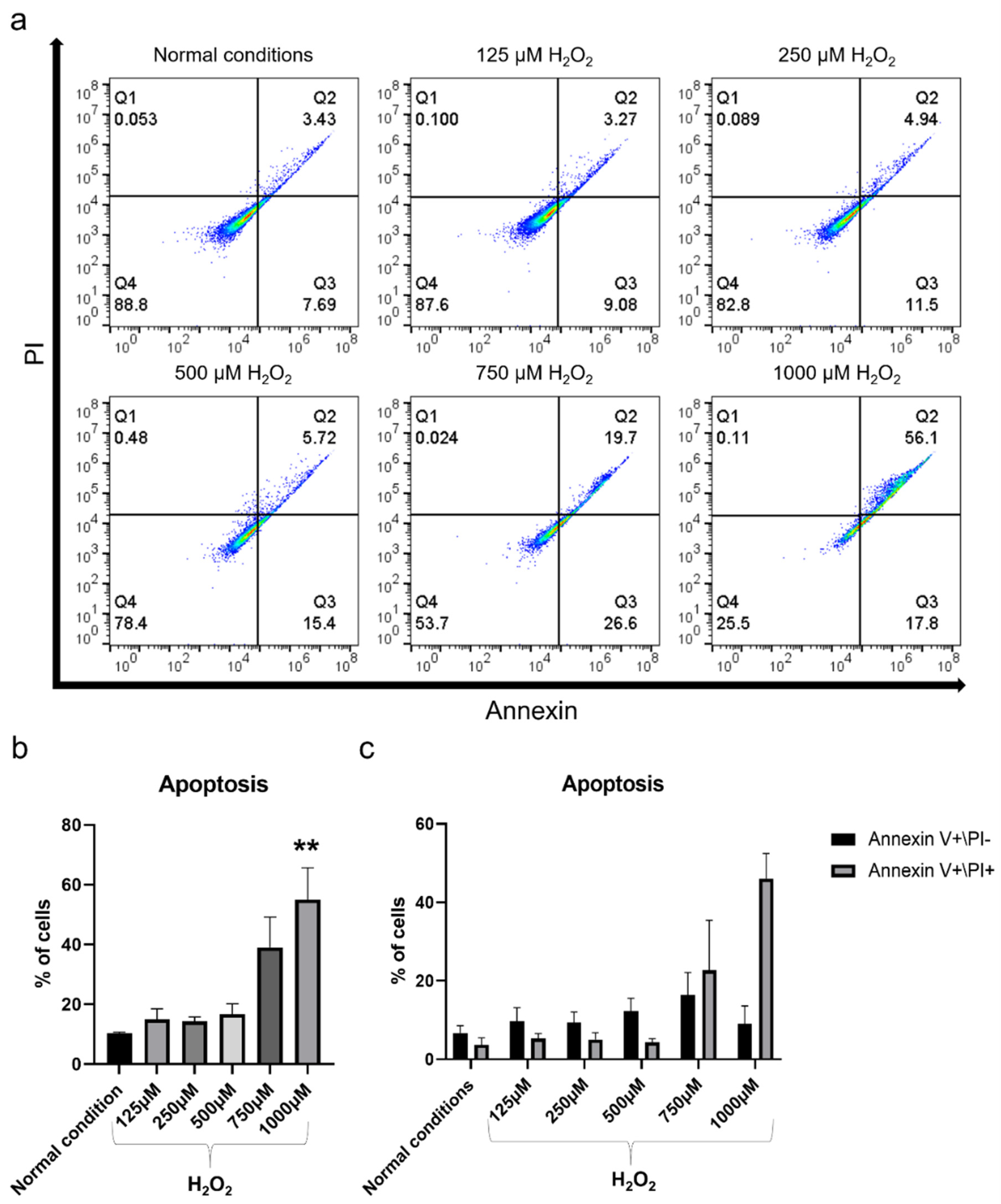
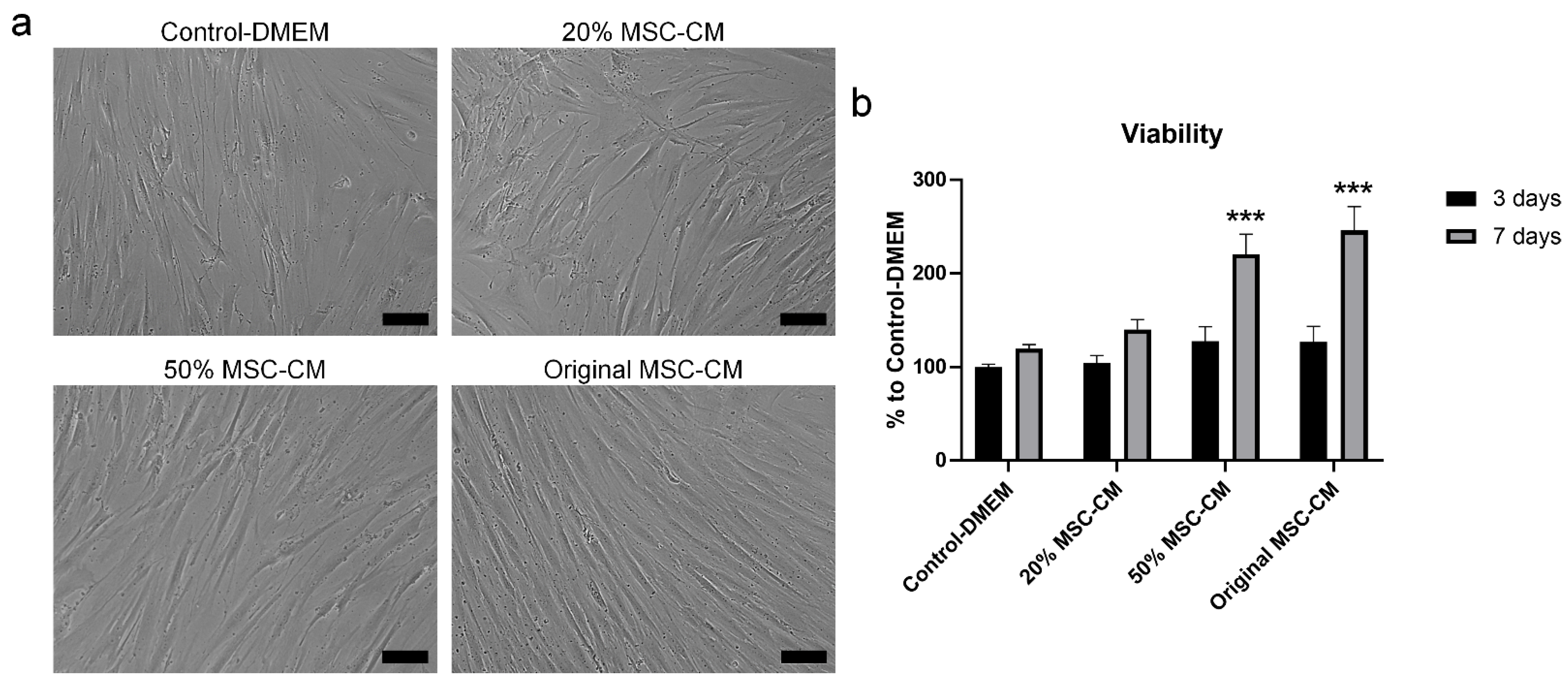
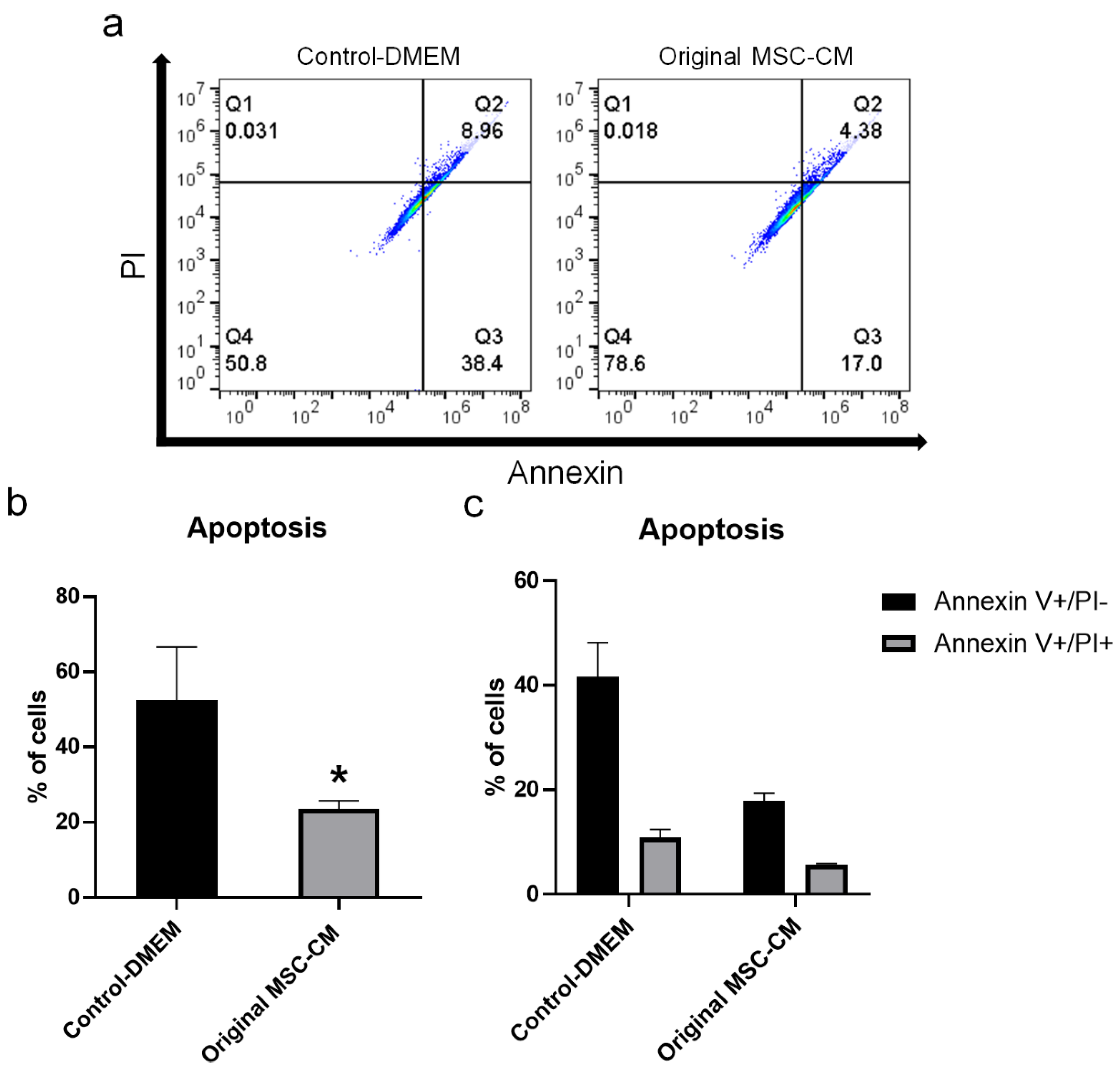
2.3. MSC-CM Reduced H2O2 Adverse Effects on hBMSC Viability and Apoptosis
2.4. MSC-CM Exerted Therapeutic Effects by Increasing the mRNA Expression Level of the Anti-Apoptotic Gene BCL-2, Reducing ROS Generation, and Increasing the Production of the Antioxidant Enzyme-Sod
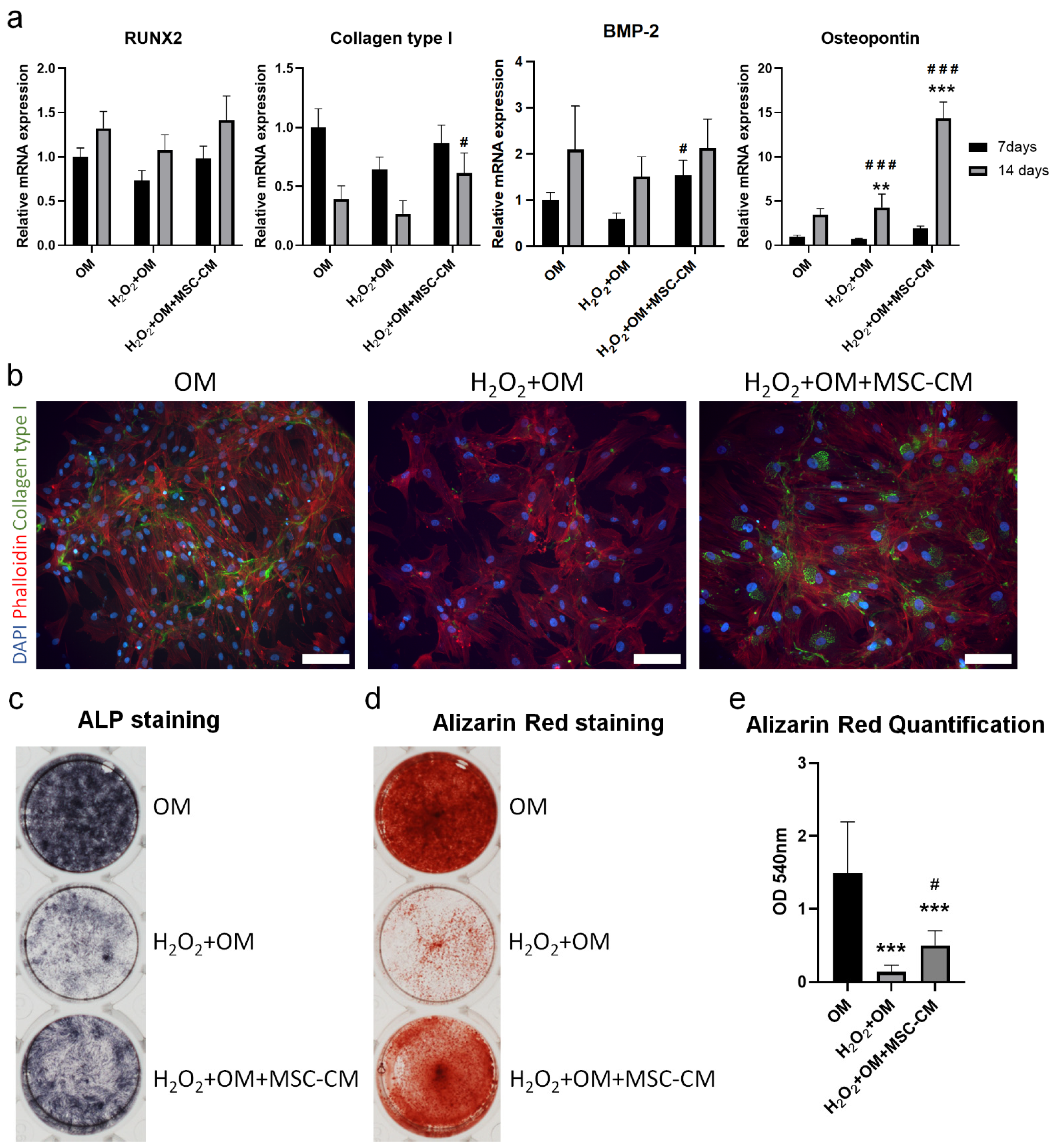
2.5. MSC-CM Restored the H2O2 Induced Inhibition of Osteogenic Differentiation of hBMSC
3. Discussion
4. Materials and Methods
4.1. Expansion and Characterization of hBMSC
4.2. Preparation of MSC-CM
4.3. Liquid Chromatography with Tandem Mass Spectrometry (LC-MS/MS) Analysis
4.4. Bioinformatic Analysis and the Antioxidant Protein Database
4.5. Exposure to H2O2 and MSC-CM Treatments
4.6. Cell Viability Assay
4.7. Flow Cytometry Analysis of Cell Apoptosis
4.8. Intracellular ROS Assay
4.9. Superoxide Dismutase (SOD) Assay
4.10. Osteogenic Differentiation
4.11. Real-Time Quantitative Polymerase Chain Reaction (qPCR)
4.12. Immunofluorescence Staining
4.13. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Omolehinwa, T.T.; Akintoye, S.O. Chemical and Radiation-Associated Jaw Lesions. Dent. Clin. N. Am. 2016, 60, 265–277. [Google Scholar] [CrossRef] [Green Version]
- Willey, J.S.; Lloyd, S.A.J.; Nelson, G.A.; Bateman, T.A. Ionizing Radiation and Bone Loss: Space Exploration and Clinical Therapy Applications. Clin. Rev. Bone Miner. Metab. 2011, 9, 54–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmad, J.; Akhtar, N.; Beg, M.; Sharma, T.; Islam, N. Correlation between bone mineral density and oxidative stress in postmenopausal women. Indian J. Endocrinol. Metab. 2015, 19, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Philippe, C.; Wittrant, Y. Oxidative stress in musculoskeletal disorders—Bone disease. In Systems Biology of Free Radicals and Antioxidants; Springer: Singapore, 2014; pp. 2951–2959. [Google Scholar]
- Domazetovic, V.; Marcucci, G.; Iantomasi, T.; Brandi, M.L.; Vincenzini, M.T. Oxidative stress in bone remodeling: Role of antioxidants. Clin. Cases Miner. Bone Metab. 2017, 14, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Gjerde, C.; Mustafa, K.; Hellem, S.; Rojewski, M.; Gjengedal, H.; Yassin, M.A.; Feng, X.; Skaale, S.; Berge, T.; Rosen, A.; et al. Cell therapy induced regeneration of severely atrophied mandibular bone in a clinical trial. Stem Cell Res. Ther. 2018, 9, 1–15. [Google Scholar] [CrossRef]
- Sauerbier, S.; Rickert, D.; Gutwald, R.; Nagursky, H.; Oshima, T.; Xavier, S.P.; Christmann, J.; Kurz, P.; Menne, D.; Vissink, A.; et al. Bone Marrow Concentrate and Bovine Bone Mineral for Sinus Floor Augmentation: A Controlled, Randomized, Single-Blinded Clinical and Histological Trial—Per-Protocol Analysis. Tissue Eng. Part A 2011, 17, 2187–2197. [Google Scholar] [CrossRef]
- Scaglione, M.; Fabbri, L.; Dell’Omo, D.; Gambini, F.; Guido, G. Long bone nonunions treated with autologous concentrated bone marrow-derived cells combined with dried bone allograft. Musculoskelet. Surg. 2014, 98, 101–106. [Google Scholar] [CrossRef]
- Vizoso, F.J.; Eiro, N.; Cid, S.; Schneider, J.; Perez-Fernandez, R. Mesenchymal Stem Cell Secretome: Toward Cell-Free Therapeutic Strategies in Regenerative Medicine. Int. J. Mol. Sci. 2017, 18, 1852. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, M.; Griffith, L.G.; Wells, A. Growth factor regulation of proliferation and survival of multipotential stromal cells. Stem Cell Res. Ther. 2010, 1, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Cantinieaux, D.; Quertainmont, R.; Blacher, S.; Rossi, L.; Wanet, T.; Noël, A.; Brook, G.; Schoenen, J.; Franzen, R. Conditioned medium from bone marrow-derived mesenchymal stem cells improves recovery after spinal cord injury in rats: An original strategy to avoid cell transplantation. PLoS ONE 2013, 8, e69515. [Google Scholar] [CrossRef]
- Fujio, M.; Xing, Z.; Sharabi, N.; Xue, Y.; Yamamoto, A.; Hibi, H.; Ueda, M.; Fristad, I.; Mustafa, K. Conditioned media from hypoxic-cultured human dental pulp cells promotes bone healing during distraction osteogenesis. J. Tissue Eng. Regen. Med. 2017, 11, 2116–2126. [Google Scholar] [CrossRef] [Green Version]
- Al-Sharabi, N.; Xue, Y.; Fujio, M.; Ueda, M.; Gjerde, C.; Mustafa, K.; Fristad, I. Bone Marrow Stromal Cell Paracrine Factors Direct Osteo/Odontogenic Differentiation of Dental Pulp Cells. Tissue Eng. Part A 2014, 20, 3063–3072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Sharabi, N.; Xue, Y.; Mustafa, K.; Fristad, I. Influence of bone marrow stromal cell secreted molecules on pulpal and periodontal healing in re-planted immature rat molars. Dent. Traumatol. 2016, 32, 231–239. [Google Scholar] [CrossRef]
- Ogata, K.; Katagiri, W.; Osugi, M.; Kawai, T.; Sugimura, Y.; Hibi, H.; Nakamura, S.; Ueda, M. Evaluation of the therapeutic effects of conditioned media from mesenchymal stem cells in a rat bisphosphonate-related osteonecrosis of the jaw-like model. Bone 2015, 74, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Huang, Y.; Huang, D.; Wu, Z.; Li, Y.; Zhou, C.; Wei, G. Protective effect of gigantol against hydrogen peroxide-induced apoptosis in rat bone marrow mesenchymal stem cells through the PI3K/Akt pathway. Mol. Med. Rep. 2017, 17, 3267–3273. [Google Scholar] [CrossRef] [Green Version]
- Wei, H.; Li, Z.; Hu, S.; Chen, X.; Cong, X. Apoptosis of mesenchymal stem cells induced by hydrogen peroxide concerns both endoplasmic reticulum stress and mitochondrial death pathway through regulation of caspases, p38 and JNK. J. Cell. Biochem. 2010, 111, 967–978. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Yin, L.; Liu, X.; Lv, B.; Xie, Z.; Wang, X.; Yu, B.; Zhang, Y. Geraniin protects bone marrow derived mesenchymal stem cells against hydrogen peroxide induced cellu-lar oxidative stress in vitro. Int. J. Mol. Med. 2018, 41, 739–748. [Google Scholar] [PubMed] [Green Version]
- Khatlani, T.; Algudiri, D.; Alenzi, R.; Al Subayyil, A.M.; Abomaray, F.M.; Bahattab, E.; AlAskar, A.S.; Kalionis, B.; El-Muzaini, M.F.; Abumaree, M.H. Preconditioning by Hydrogen Peroxide Enhances Multiple Properties of Human Decidua Basalis Mesen-chymal Stem/Multipotent Stromal Cells. Stem. Cells Int. 2018, 2018, 6480793. [Google Scholar] [CrossRef] [Green Version]
- Pan, Q.; Qiu, W.-Y.; Huo, Y.-N.; Yao, Y.-F.; Lou, M.F. Low Levels of Hydrogen Peroxide Stimulate Corneal Epithelial Cell Adhesion, Migration, and Wound Healing. Investig. Opthalmol. Vis. Sci. 2011, 52, 1723–1734. [Google Scholar] [CrossRef]
- DAVID Bioinformatics Resources. Available online: https://david.ncifcrf.gov/tools.jsp (accessed on 24 November 2021).
- STRING—Functional Protein Association Networks. Available online: http://string-db.org/ (accessed on 25 November 2021).
- Feng, P.; Ding, H.; Lin, H.; Chen, W. AOD: The antioxidant protein database. Sci. Rep. 2017, 7, 7449. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, A.; Nakajima, H.; Uchida, K.; Takeura, N.; Honjoh, K.; Watanabe, S.; Kitade, M.; Kokubo, Y.; Johnson, W.; Matsumine, A. Comparison of Mesenchymal Stromal Cells Isolated from Murine Adipose Tissue and Bone Marrow in the Treatment of Spinal Cord Injury. Cell Transplant. 2018, 27, 1126–1139. [Google Scholar] [CrossRef] [Green Version]
- Fan, X.-L.; Zhang, Y.; Li, X.; Fu, Q.-L. Mechanisms underlying the protective effects of mesenchymal stem cell-based therapy. Cell. Mol. Life Sci. 2020, 77, 2771–2794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oryan, A.; Kamali, A.; Moshiri, A.; Eslaminejad, M.B. Role of Mesenchymal Stem Cells in Bone Regenerative Medicine: What Is the Evidence? Cells Tissues Organs 2017, 204, 59–83. [Google Scholar] [CrossRef]
- Niu, Y.; Xia, X.; Song, P.; Fang, H.; Dong, F.; Tao, H.; Yang, C.; Shen, C. Bone mesenchymal stem cell-conditioned medium attenuates the effect of oxidative stress injury on NSCs by inhibiting the Notch1 signaling pathway. Cell Biol. Int. 2019, 43, 1267–1275. [Google Scholar] [CrossRef] [PubMed]
- Forman, H.J.; Zhang, H. Targeting oxidative stress in disease: Promise and limitations of antioxidant therapy. Nat. Rev. Drug Discov. 2021, 20, 689–709, Erratum in 2021, 20, 652. [Google Scholar] [CrossRef] [PubMed]
- Stavely, R.; Nurgali, K. The emerging antioxidant paradigm of mesenchymal stem cell therapy. Stem Cells Transl. Med. 2020, 9, 985–1006. [Google Scholar] [CrossRef]
- Liang, X.; Ding, Y.; Zhang, Y.; Tse, H.F.; Lian, Q. Paracrine Mechanisms of Mesenchymal Stem Cell-Based Therapy: Current Status and Perspectives. Cell Transplant. 2014, 23, 1045–1059. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pires, A.O.; Pinheiro, B.; Teixeira, F.G.; Anjo, S.; Samy, S.; Gomes, E.; Serra, S.; Silva, N.; Manadas, B.; Sousa, N.; et al. Unveiling the Differences of Secretome of Human Bone Marrow Mesenchymal Stem Cells, Adipose Tissue-Derived Stem Cells, and Human Umbilical Cord Perivascular Cells: A Proteomic Analysis. Stem Cells Dev. 2016, 25, 1073–1083. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brentnall, M.; Rodriguez-Menocal, L.; De Guevara, R.L.; Cepero, E.; Boise, L.H. Caspase-9, caspase-3 and caspase-7 have distinct roles during intrinsic apoptosis. BMC Cell Biol. 2013, 14, 32. [Google Scholar] [CrossRef] [Green Version]
- Siddiqui, W.A.; Ahad, A.; Ahsan, H. The mystery of BCL2 family: Bcl-2 proteins and apoptosis: An update. Arch. Toxicol. 2015, 89, 289–317. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Li, D.; Li, X.; Shi, Q.; Ju, X. Mesenchymal stem cell conditioned medium alleviates oxidative stress injury induced by hydrogen peroxide via regulating miR143 and its target protein in hepatocytes. BMC Immunol. 2017, 18, 51. [Google Scholar] [CrossRef]
- Johnson, T.V.; DeKorver, N.W.; Levasseur, V.A.; Osborne, A.; Tassoni, A.; Lorber, B.; Heller, J.P.; Villasmil, R.; Bull, N.D.; Martin, K.R.; et al. Identification of retinal ganglion cell neuroprotection conferred by platelet-derived growth factor through analysis of the mesenchymal stem cell secretome. Brain 2014, 137, 503–519. [Google Scholar] [CrossRef]
- Pankajakshan, D.; Agrawal, D.K. Mesenchymal Stem Cell Paracrine Factors in Vascular Repair and Regeneration. J. Biomed. Technol. Res. 2014, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ikwegbue, P.C.; Masamba, P.; Oyinloye, B.E.; Kappo, A.P. Roles of Heat Shock Proteins in Apoptosis, Oxidative Stress, Human Inflammatory Diseases, and Cancer. Pharmaceuticals 2017, 11, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maggio, D.; Barabani, M.; Pierandrei, M.; Polidori, M.C.; Catani, M.; Mecocci, P.; Senin, U.; Pacifici, R.; Cherubini, A. Marked Decrease in Plasma Antioxidants in Aged Osteoporotic Women: Results of a Cross-Sectional Study. J. Clin. Endocrinol. Metab. 2003, 88, 1523–1527. [Google Scholar] [CrossRef] [Green Version]
- Lee, N.K.; Choi, Y.G.; Baik, J.Y.; Han, S.Y.; Jeong, D.-W.; Bae, Y.S.; Kim, N.; Lee, S.Y. A crucial role for reactive oxygen species in RANKL-induced osteoclast differentiation. Blood 2005, 106, 852–859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bai, X.C.; Lu, D.; Bai, J.; Zheng, H.; Ke, Z.Y.; Li, X.M.; Luo, S.Q. Oxidative stress inhibits osteoblastic differentiation of bone cells by ERK and NF-kappaB. Biochem. Biophys. Res. Commun. 2004, 314, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Katagiri, W.; Watanabe, J.; Toyama, N.; Osugi, M.; Sakaguchi, K.; Hibi, H. Clinical Study of Bone Regeneration by Conditioned Medium From Mesenchymal Stem Cells After Maxillary Sinus Floor Elevation. Implant. Dent. 2017, 26, 607–612. [Google Scholar] [CrossRef]
- Wyles, C.C.; Houdek, M.T.; Crespo-Diaz, R.J.; Norambuena, G.A.; Stalboerger, P.G.; Terzic, A.; Behfar, A.; Sierra, R.J. Adipose-derived Mesenchymal Stem Cells Are Phenotypically Superior for Regeneration in the Setting of Osteonecrosis of the Femoral Head. Clin. Orthop. Relat. Res. 2015, 473, 3080–3090. [Google Scholar] [CrossRef] [Green Version]
- Lian, J.B.; Stein, G.S. Development of the osteoblast phenotype: Molecular mechanisms mediating osteoblast growth and differentiation. Iowa Orthop. J. 1995, 15, 118–140. [Google Scholar]
- Liang, M.; Liu, W.; Peng, Z.; Lv, S.; Guan, Y.; An, G.; Zhang, Y.; Huang, T.; Wang, Y. The therapeutic effect of secretome from human umbilical cord-derived mesenchymal stem cells in age-related osteoporosis. Artif. Cells Nanomed. Biotechnol. 2019, 47, 1357–1366. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.; Zhang, Y.; Ni, C.-Y.; Chen, C.-Y.; Rao, S.-S.; Yin, H.; Huang, J.; Tan, Y.-J.; Wang, Z.-X.; Cao, J.; et al. Human umbilical cord mesenchymal stromal cells-derived extracellular vesicles exert potent bone protective effects by CLEC11A-mediated regulation of bone metabolism. Theranostics 2020, 10, 2293–2308. [Google Scholar] [CrossRef]
- Tamaoka, J.; Takaoka, K.; Hattori, H.; Ueta, M.; Maeda, H.; Yamamura, M.; Yamanegi, K.; Noguchi, K.; Kishimoto, H. Osteonecrosis of the jaws caused by bisphosphonate treatment and oxidative stress in mice. Exp. Ther. Med. 2018, 17, 1440–1448. [Google Scholar] [CrossRef]
- Stegen, S.; van Gastel, N.; Carmeliet, G. Bringing new life to damaged bone: The importance of angiogenesis in bone re-pair and regeneration. Bone 2015, 70, 19–27. [Google Scholar] [CrossRef]
- Mohamed-Ahmed, S.; Fristad, I.; Lie, S.A.; Suliman, S.; Mustafa, K.; Vindenes, H.; Idris, S.B. Adipose-derived and bone marrow mesenchymal stem cells: A donor-matched comparison. Stem Cell Res. Ther. 2018, 9, 1–15. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Krause, D.S.; Deans, R.J.; Keating, A.; Prockop, D.J.; Horwitz, E.M. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Aasebø, E.; Birkeland, E.; Selheim, F.; Berven, F.; Brenner, A.K.; Bruserud, Ø. The Extracellular Bone Marrow Microenvironment-A Proteomic Comparison of Constitutive Protein Re-lease by In Vitro Cultured Osteoblasts and Mesenchymal Stem Cells. Cancers 2020, 13, 62. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saleem, R.; Mohamed-Ahmed, S.; Elnour, R.; Berggreen, E.; Mustafa, K.; Al-Sharabi, N. Conditioned Medium from Bone Marrow Mesenchymal Stem Cells Restored Oxidative Stress-Related Impaired Osteogenic Differentiation. Int. J. Mol. Sci. 2021, 22, 13458. https://doi.org/10.3390/ijms222413458
Saleem R, Mohamed-Ahmed S, Elnour R, Berggreen E, Mustafa K, Al-Sharabi N. Conditioned Medium from Bone Marrow Mesenchymal Stem Cells Restored Oxidative Stress-Related Impaired Osteogenic Differentiation. International Journal of Molecular Sciences. 2021; 22(24):13458. https://doi.org/10.3390/ijms222413458
Chicago/Turabian StyleSaleem, Ragda, Samih Mohamed-Ahmed, Rammah Elnour, Ellen Berggreen, Kamal Mustafa, and Niyaz Al-Sharabi. 2021. "Conditioned Medium from Bone Marrow Mesenchymal Stem Cells Restored Oxidative Stress-Related Impaired Osteogenic Differentiation" International Journal of Molecular Sciences 22, no. 24: 13458. https://doi.org/10.3390/ijms222413458
APA StyleSaleem, R., Mohamed-Ahmed, S., Elnour, R., Berggreen, E., Mustafa, K., & Al-Sharabi, N. (2021). Conditioned Medium from Bone Marrow Mesenchymal Stem Cells Restored Oxidative Stress-Related Impaired Osteogenic Differentiation. International Journal of Molecular Sciences, 22(24), 13458. https://doi.org/10.3390/ijms222413458




