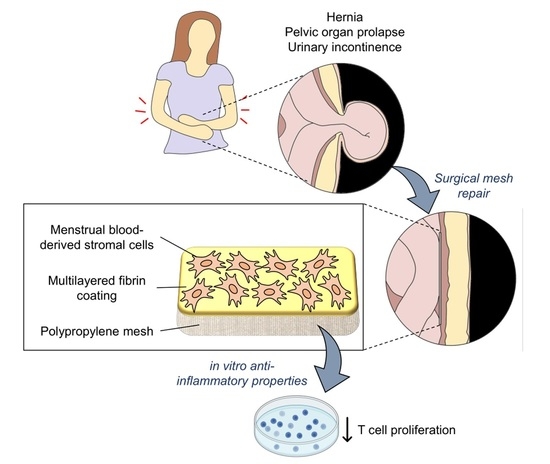
A Fibrin Coating Method of Polypropylene Meshes Enables the Adhesion of Menstrual Blood-Derived Mesenchymal Stromal Cells: A New Delivery Strategy for Stem Cell-Based Therapies
Abstract
1. Introduction
2. Results
2.1. Fibrin Sealant Components Characterization: Fibrin Clotting Test
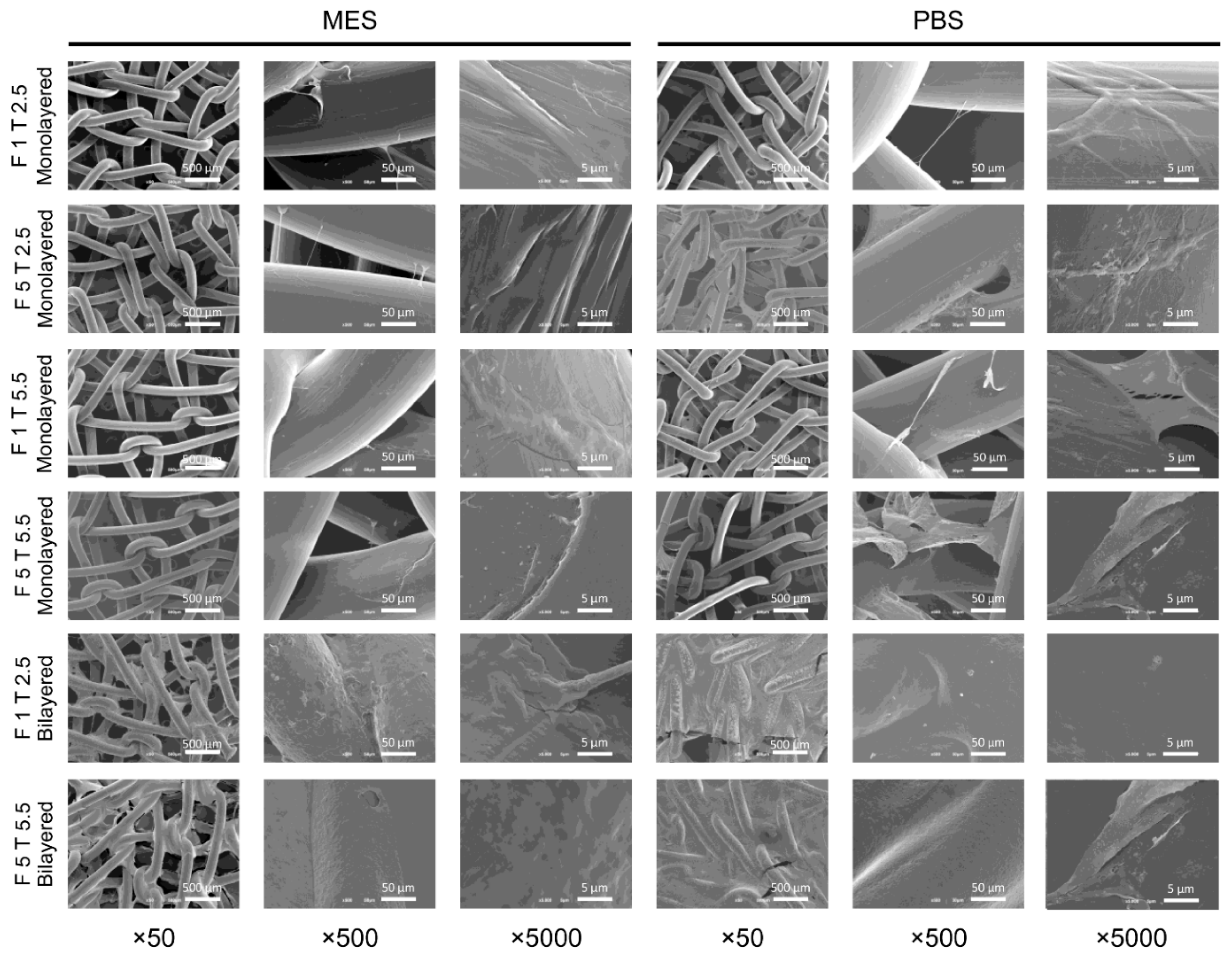
2.2. Characterization of Plasma-Treated and Fibrin-Coated Meshes: Scanning Electron Microscopy Analysis
2.3. Mechanical Strength of Fibrin-Coated PP Meshes
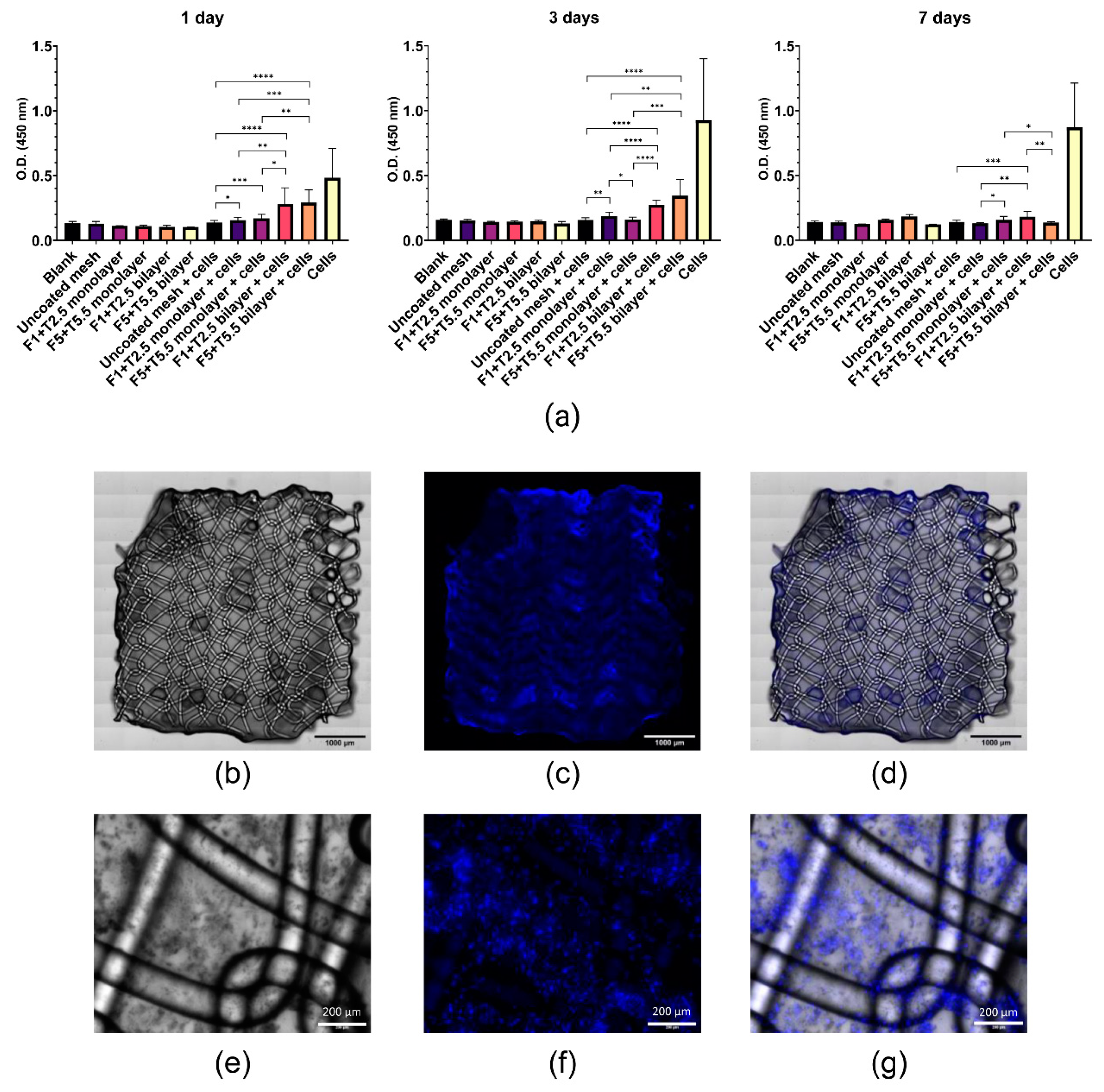
2.4. Viability and Adhesion of MenSCs Seeded on Fibrin-Coated PP Meshes
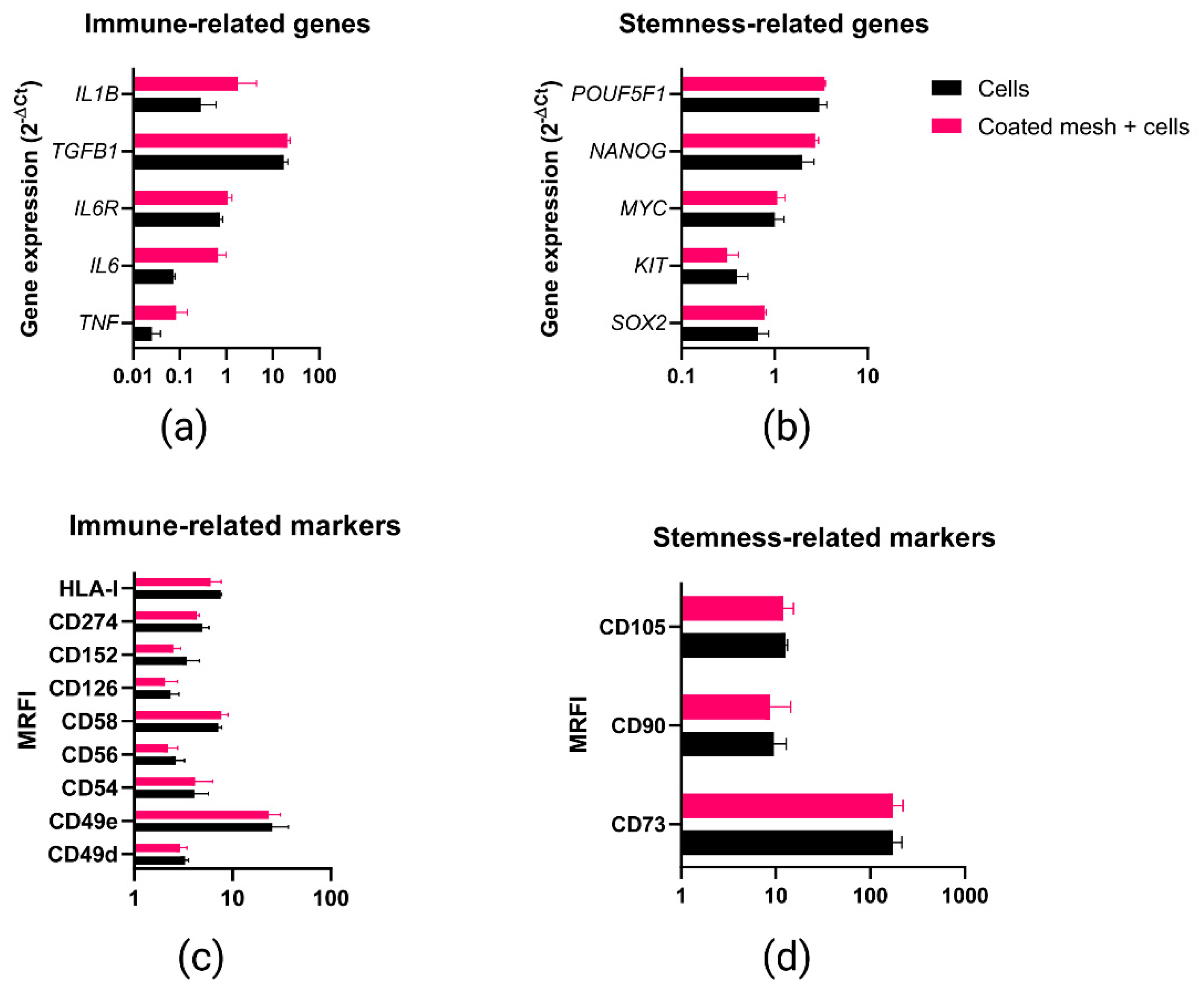
2.5. Phenotypic and Gene Expression Response of MenSCs to the Fibrin Coating
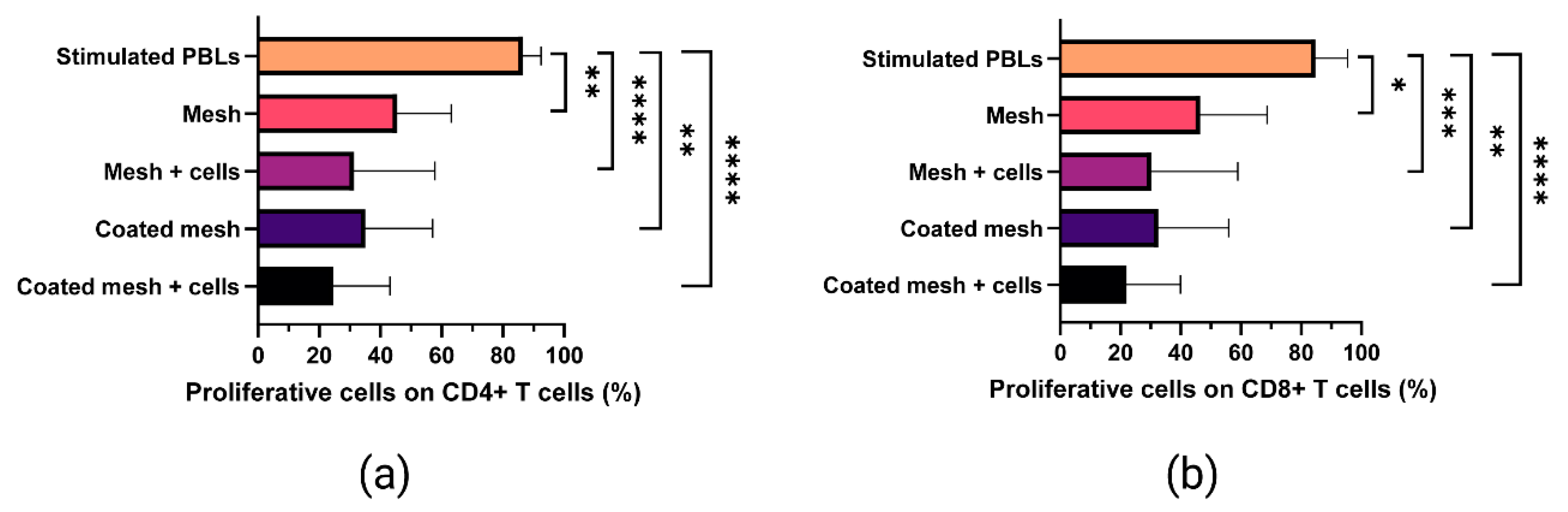
2.6. Proliferative Ability of In Vitro-Stimulated T Cells Co-Cultured with Fibrin-Coated Meshes Seeded with MenSCs
3. Discussion
4. Materials and Methods
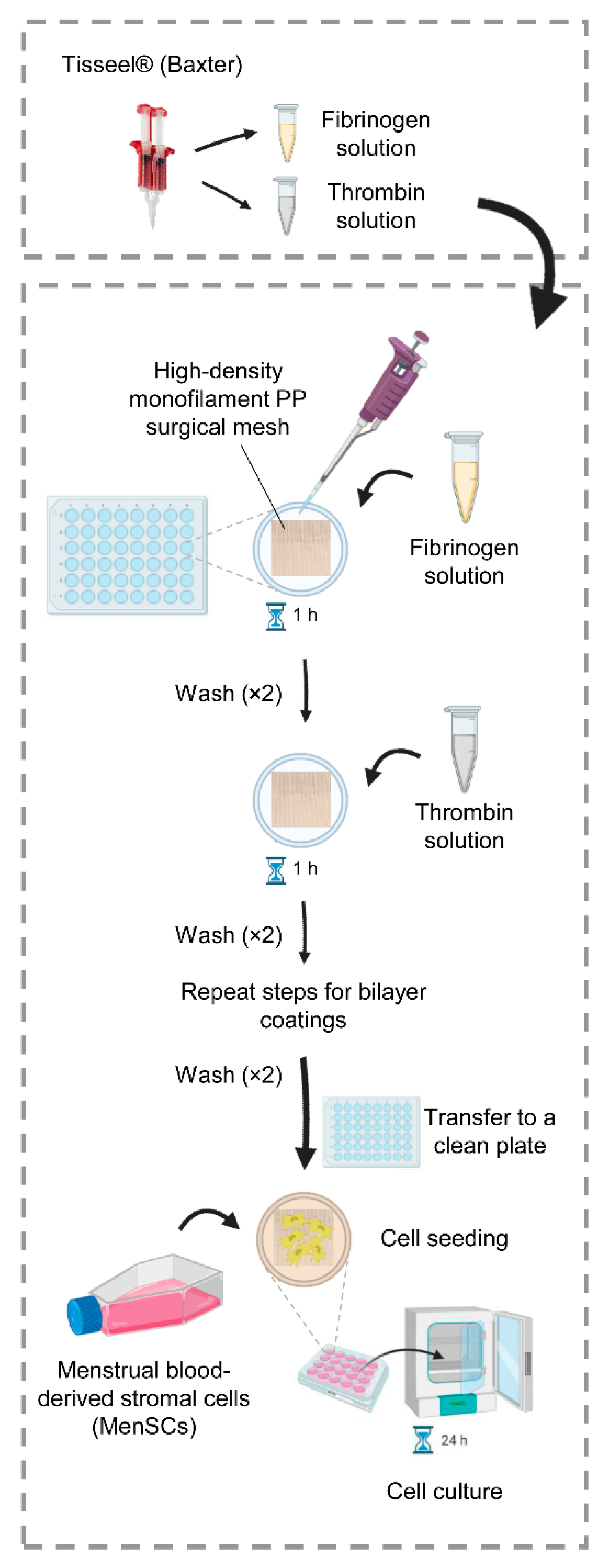
4.1. Optimization of the Fibrin Coating
4.2. Plasma Treatment of the PP Meshes
4.3. Fibrin Coating of PP Meshes
4.4. Uniaxial Tensile Test
4.5. Cell Seeding and CCK-8 Assay
4.6. Hoechst Staining and Fluorescence Microscopy
4.7. Phenotypic and Gene Expression Analyses
4.8. Immunomodulatory Assay
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Claverie, J.P.; Schaper, F. Ziegler-Natta Catalysis: 50 Years after the Nobel Prize. MRS Bull. 2013, 38, 213–218. [Google Scholar] [CrossRef]
- Clayman, H.M. Polypropylene. Ophthalmology 1981, 88, 959–964. [Google Scholar] [CrossRef]
- Rosen, D.A.; Rosen, K.R.; Silvasi, D.L. In Vitro Variability in Fentanyl Absorption by Different Membrane Oxygenators. J. Cardiothorac. Anesth. 1990, 4, 332–335. [Google Scholar] [CrossRef]
- Beiler, B.; Barraud, D.; Vigneron, J.; Demoré, B. Physicochemical Stability of an Admixture of Lidocaine and Ketamine in Polypropylene Syringe Used in Opioid-Free Anaesthesia. Eur. J. Hosp. Pharm. 2020, 27, e79–e83. [Google Scholar] [CrossRef]
- Kusaka, M.; Miyamoto, N.; Akimoto, M. Repairing Iridodialysis by Riveting with a Double-Flanged Polypropylene Suture. J. Cataract. Refract. Surg. 2019, 45, 1531–1534. [Google Scholar] [CrossRef]
- Pande, T.; Naidu, C.S. Mesh Infection in Cases of Polypropylene Mesh Hernioplasty. Hernia 2020, 24, 849–856. [Google Scholar] [CrossRef]
- Marinaro, F.; Sánchez-Margallo, F.M.; Álvarez, V.; López, E.; Tarazona, R.; Brun, M.V.; Blázquez, R.; Casado, J.G. Meshes in a Mess: Mesenchymal Stem Cell-Based Therapies for Soft Tissue Reinforcement. Acta Biomater. 2019, 85, 60–74. [Google Scholar] [CrossRef] [PubMed]
- Chapple, C.R.; Cruz, F.; Deffieux, X.; Milani, A.L.; Arlandis, S.; Artibani, W.; Bauer, R.M.; Burkhard, F.; Cardozo, L.; Castro-Diaz, D.; et al. Consensus Statement of the European Urology Association and the European Urogynaecological Association on the Use of Implanted Materials for Treating Pelvic Organ Prolapse and Stress Urinary Incontinence. Eur. Urol. 2017, 72, 424–431. [Google Scholar] [CrossRef] [PubMed]
- Mangir, N.; Dikici, B.A.; Chapple, C.R.; MacNeil, S. Landmarks in Vaginal Mesh Development: Polypropylene Mesh for Treatment of SUI and POP. Nat. Rev. Urol. 2019, 16, 675–689. [Google Scholar] [CrossRef] [PubMed]
- Houshyar, S.; Sarker, A.; Jadhav, A.; Kumar, G.S.; Bhattacharyya, A.; Nayak, R.; Shanks, R.A.; Saha, T.; Rifai, A.; Padhye, R.; et al. Polypropylene-Nanodiamond Composite for Hernia Mesh. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 111, 110780. [Google Scholar] [CrossRef] [PubMed]
- Gentile, P.; Bernini, M.; Orzalesi, L.; Sordi, S.; Meattini, I.; Lessi, F.; Kothari, A.; Calabrese, C. Titanium-Coated Polypropylene Mesh as Innovative Bioactive Material in Conservatives Mastectomies and Pre-Pectoral Breast Reconstruction. Bioact. Mater. 2021, 6, 4640–4653. [Google Scholar] [CrossRef]
- Giuntoli, G.; Muzio, G.; Actis, C.; Ganora, A.; Calzone, S.; Bruno, M.; Ciardelli, G.; Carmagnola, I.; Tonda-Turo, C. In-Vitro Characterization of a Hernia Mesh Featuring a Nanostructured Coating. Front. Bioeng. Biotechnol. 2020, 8, 589223. [Google Scholar] [CrossRef]
- Fernández-Gutiérrez, M.; Pérez-Köhler, B.; Benito-Martínez, S.; García-Moreno, F.; Pascual, G.; García-Fernández, L.; Aguilar, M.R.; Vázquez-Lasa, B.; Bellón, J.M. Development of Biocomposite Polymeric Systems Loaded with Antibacterial Nanoparticles for the Coating of Polypropylene Biomaterials. Polymers 2020, 12, 1829. [Google Scholar] [CrossRef]
- Serafim, A.; Cecoltan, S.; Olăreț, E.; Dragusin, D.-M.; Vasile, E.; Popescu, V.; Mastalier, B.S.M.; Iovu, H.; Stancu, I.-C. Bioinspired Hydrogel Coating Based on Methacryloyl Gelatin Bioactivates Polypropylene Meshes for Abdominal Wall Repair. Polymers 2020, 12, 1677. [Google Scholar] [CrossRef]
- Qiao, Y.; Li, Y.; Zhang, Q.; Wang, Q.; Gao, J.; Wang, L. Dopamine-Mediated Zwitterionic Polyelectrolyte-Coated Polypropylene Hernia Mesh with Synergistic Anti-Inflammation Effects. Langmuir 2020, 36, 5251–5261. [Google Scholar] [CrossRef]
- Yang, D.; Song, Z.; Shen, J.; Song, H.; Yang, J.; Zhang, P.; Gu, Y. Regenerated Silk Fibroin (RSF) Electrostatic Spun Fibre Composite with Polypropylene Mesh for Reconstruction of Abdominal Wall Defects in a Rat Model. Artif. Cells. Nanomed. Biotechnol. 2020, 48, 425–434. [Google Scholar] [CrossRef]
- Wolf, M.T.; Carruthers, C.A.; Dearth, C.L.; Crapo, P.M.; Huber, A.; Burnsed, O.A.; Londono, R.; Johnson, S.A.; Daly, K.A.; Stahl, E.C.; et al. Polypropylene Surgical Mesh Coated with Extracellular Matrix Mitigates the Host Foreign Body Response. J. Biomed. Mater. Res. A 2014, 102, 234–246. [Google Scholar] [CrossRef]
- Guillaume, O.; Pérez-Köhler, B.; Schädl, B.; Keibl, C.; Saxenhuber, N.; Heimel, P.; Priglinger, E.; Wolbank, S.; Redl, H.; Petter-Puchner, A.; et al. Stromal Vascular Fraction Cells as Biologic Coating of Mesh for Hernia Repair. Hernia 2020, 24, 1233–1243. [Google Scholar] [CrossRef]
- Berney, C.R.; Descallar, J. Review of 1000 Fibrin Glue Mesh Fixation during Endoscopic Totally Extraperitoneal (TEP) Inguinal Hernia Repair. Surg. Endosc. 2016, 30, 4544–4552. [Google Scholar] [CrossRef] [PubMed]
- Blázquez, R.; Sánchez-Margallo, F.M.; Álvarez, V.; Usón, A.; Marinaro, F.; Casado, J.G. Fibrin Glue Mesh Fixation Combined with Mesenchymal Stem Cells or Exosomes Modulates the Inflammatory Reaction in a Murine Model of Incisional Hernia. Acta Biomater. 2018, 71, 318–329. [Google Scholar] [CrossRef]
- Marinaro, F.; Casado, J.G.; Blázquez, R.; Brun, M.V.; Marcos, R.; Santos, M.; Duque, F.J.; López, E.; Álvarez, V.; Usón, A.; et al. Laparoscopy for the Treatment of Congenital Hernia: Use of Surgical Meshes and Mesenchymal Stem Cells in a Clinically Relevant Animal Model. Front. Pharmacol. 2020, 11, 01332. [Google Scholar] [CrossRef]
- Gnecchi, M.; Danieli, P.; Malpasso, G.; Ciuffreda, M.C. Paracrine Mechanisms of Mesenchymal Stem Cells in Tissue Repair. In Mesenchymal Stem Cells: Methods and Protocols; Gnecchi, M., Ed.; Methods in Molecular Biology; Springer: New York, NY, USA, 2016; pp. 123–146. ISBN 978-1-4939-3584-0. [Google Scholar]
- Zhou, Y.; Yamamoto, Y.; Xiao, Z.; Ochiya, T. The Immunomodulatory Functions of Mesenchymal Stromal/Stem Cells Mediated via Paracrine Activity. J. Clin. Med. 2019, 8, 1025. [Google Scholar] [CrossRef]
- Bozorgmehr, M.; Gurung, S.; Darzi, S.; Nikoo, S.; Kazemnejad, S.; Zarnani, A.-H.; Gargett, C.E. Endometrial and Menstrual Blood Mesenchymal Stem/Stromal Cells: Biological Properties and Clinical Application. Front. Cell Dev. Biol. 2020, 8, 497. [Google Scholar] [CrossRef]
- Spitzer, T.L.B.; Rojas, A.; Zelenko, Z.; Aghajanova, L.; Erikson, D.W.; Barragan, F.; Meyer, M.; Tamaresis, J.S.; Hamilton, A.E.; Irwin, J.C.; et al. Perivascular Human Endometrial Mesenchymal Stem Cells Express Pathways Relevant to Self-Renewal, Lineage Specification, and Functional Phenotype. Biol. Reprod. 2012, 86, 58. [Google Scholar] [CrossRef]
- Chen, X.; Kong, X.; Liu, D.; Gao, P.; Zhang, Y.; Li, P.; Liu, M. In Vitro Differentiation of Endometrial Regenerative Cells into Smooth Muscle Cells: A Potential Approach for the Management of Pelvic Organ Prolapse. Int. J. Mol. Med. 2016, 38, 95–104. [Google Scholar] [CrossRef]
- Emmerson, S.; Mukherjee, S.; Melendez-Munoz, J.; Cousins, F.; Edwards, S.L.; Karjalainen, P.; Ng, M.; Tan, K.S.; Darzi, S.; Bhakoo, K.; et al. Composite Mesh Design for Delivery of Autologous Mesenchymal Stem Cells Influences Mesh Integration, Exposure and Biocompatibility in an Ovine Model of Pelvic Organ Prolapse. Biomaterials 2019, 225, 119495. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, D.; Edwards, S.L.; Su, K.; Tan, K.S.; White, J.F.; Ramshaw, J.A.M.; Lo, C.; Rosamilia, A.; Werkmeister, J.A.; Gargett, C.E. Human Endometrial Mesenchymal Stem Cells Modulate the Tissue Response and Mechanical Behavior of Polyamide Mesh Implants for Pelvic Organ Prolapse Repair. Tissue Eng. Part A 2014, 20, 785–798. [Google Scholar] [CrossRef] [PubMed]
- Edwards, S.L.; Ulrich, D.; White, J.F.; Su, K.; Rosamilia, A.; Ramshaw, J.A.M.; Gargett, C.E.; Werkmeister, J.A. Temporal Changes in the Biomechanical Properties of Endometrial Mesenchymal Stem Cell Seeded Scaffolds in a Rat Model. Acta Biomater. 2015, 13, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Darzi, S.; Deane, J.A.; Nold, C.A.; Edwards, S.E.; Gough, D.J.; Mukherjee, S.; Gurung, S.; Tan, K.S.; Vashi, A.V.; Werkmeister, J.A.; et al. Endometrial Mesenchymal Stem/Stromal Cells Modulate the Macrophage Response to Implanted Polyamide/Gelatin Composite Mesh in Immunocompromised and Immunocompetent Mice. Sci. Rep. 2018, 8, 6554. [Google Scholar] [CrossRef]
- Mukherjee, S.; Darzi, S.; Rosamilia, A.; Kadam, V.; Truong, Y.; Werkmeister, J.A.; Gargett, C.E. Blended Nanostructured Degradable Mesh with Endometrial Mesenchymal Stem Cells Promotes Tissue Integration and Anti-Inflammatory Response in Vivo for Pelvic Floor Application. Biomacromolecules 2019, 20, 454–468. [Google Scholar] [CrossRef]
- Paul, K.; Darzi, S.; McPhee, G.; Del Borgo, M.P.; Werkmeister, J.A.; Gargett, C.E.; Mukherjee, S. 3D Bioprinted Endometrial Stem Cells on Melt Electrospun Poly ε-Caprolactone Mesh for Pelvic Floor Application Promote Anti-Inflammatory Responses in Mice. Acta Biomater. 2019, 97, 162–176. [Google Scholar] [CrossRef]
- Regis, S.; Jassal, M.; Mukherjee, N.; Bayon, Y.; Scarborough, N.; Bhowmick, S. Altering Surface Characteristics of Polypropylene Mesh via Sodium Hydroxide Treatment. J. Biomed. Mater. Res. Part A 2012, 100A, 1160–1167. [Google Scholar] [CrossRef] [PubMed]
- Peng, Q.; Gong, B.; Parsons, G.N. Making Inert Polypropylene Fibers Chemically Responsive by Combining Atomic Layer Deposition and Vapor Phase Chemical Grafting. Nanotechnology 2011, 22, 155601. [Google Scholar] [CrossRef]
- Saha, T.; Houshyar, S.; Sarker, S.R.; Ghosh, S.; Dekiwadia, C.; Padhye, R.; Wang, X. Surface-Functionalized Polypropylene Surgical Mesh for Enhanced Performance and Biocompatibility. ACS Appl. Bio. Mater. 2019, 2, 5905–5915. [Google Scholar] [CrossRef]
- Gomathi, N.; Rajasekar, R.; Babu, R.R.; Mishra, D.; Neogi, S. Development of Bio/Blood Compatible Polypropylene through Low Pressure Nitrogen Plasma Surface Modification. Mater. Sci. Eng. C 2012, 32, 1767–1778. [Google Scholar] [CrossRef]
- Hachim, D.; Brown, B.N. Surface Modification of Polypropylene for Enhanced Layer-by-Layer Deposition of Polyelectrolytes. J. Biomed. Mater. Res. Part A 2018, 106, 2078–2085. [Google Scholar] [CrossRef]
- Baker, S.C.; Atkin, N.; Gunning, P.A.; Granville, N.; Wilson, K.; Wilson, D.; Southgate, J. Characterisation of Electrospun Polystyrene Scaffolds for Three-Dimensional in Vitro Biological Studies. Biomaterials 2006, 27, 3136–3146. [Google Scholar] [CrossRef] [PubMed]
- Yoo, H.S.; Kim, T.G.; Park, T.G. Surface-Functionalized Electrospun Nanofibers for Tissue Engineering and Drug Delivery. Adv. Drug Deliv. Rev. 2009, 61, 1033–1042. [Google Scholar] [CrossRef]
- Sanbhal, N.; Mao, Y.; Sun, G.; Xu, R.F.; Zhang, Q.; Wang, L. Surface Modification of Polypropylene Mesh Devices with Cyclodextrin via Cold Plasma for Hernia Repair: Characterization and Antibacterial Properties. Appl. Surf. Sci. 2018, 439, 749–759. [Google Scholar] [CrossRef]
- Labay, C.; Canal, J.M.; Modic, M.; Cvelbar, U.; Quiles, M.; Armengol, M.; Arbos, M.A.; Gil, F.J.; Canal, C. Antibiotic-Loaded Polypropylene Surgical Meshes with Suitable Biological Behaviour by Plasma Functionalization and Polymerization. Biomaterials 2015, 71, 132–144. [Google Scholar] [CrossRef]
- Zahedi, L.; Beigi, P.G.; Shafiee, M.; Zare, F.; Mahdikia, H.; Abdouss, M.; Abdollahifar, M.-A.; Shokri, B. Development of Plasma Functionalized Polypropylene Wound Dressing for Betaine Hydrochloride Controlled Drug Delivery on Diabetic Wounds. Sci. Rep. 2021, 11, 9641. [Google Scholar] [CrossRef]
- Lanzalaco, S.; Valle, L.J.D.; Turon, P.; Weis, C.; Estrany, F.; Alemán, C.; Armelin, E. Polypropylene Mesh for Hernia Repair with Controllable Cell Adhesion/de-Adhesion Properties. J. Mater. Chem. B 2020, 8, 1049–1059. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Zhang, Z.; Lu, S.; Zhang, T.; Zhou, N.; Ren, P.; Wang, F.; Yang, Y.; Ji, Z. Assembled Anti-Adhesion Polypropylene Mesh with Self-Fixable and Degradable in Situ Mussel-Inspired Hydrogel Coating for Abdominal Wall Defect Repair. Biomater. Sci. 2018, 6, 3030–3041. [Google Scholar] [CrossRef]
- Hianik, T.; Ostatná, V.; Sonlajtnerova, M.; Grman, I. Influence of Ionic Strength, PH and Aptamer Configuration for Binding Affinity to Thrombin. Bioelectrochemistry 2007, 70, 127–133. [Google Scholar] [CrossRef]
- Protopopova, A.D.; Barinov, N.A.; Zavyalova, E.G.; Kopylov, A.M.; Sergienko, V.I.; Klinov, D.V. Visualization of Fibrinogen AC Regions and Their Arrangement during Fibrin Network Formation by High-Resolution AFM. J. Thromb. Haemost. 2015, 13, 570–579. [Google Scholar] [CrossRef] [PubMed]
- Motlagh, D.; Amrani, D.L.; Diorio, J.P. Use of Scaffold Comprising Fibrin for Delivery of Stem Cells. U.S. Patent Application No 12/500,582, 2010. [Google Scholar]
- Bacakova, M.; Musilkova, J.; Riedel, T.; Stranska, D.; Brynda, E.; Zaloudkova, M.; Bacakova, L. The Potential Applications of Fibrin-Coated Electrospun Polylactide Nanofibers in Skin Tissue Engineering. Int. J. Nanomed. 2016, 11, 771–789. [Google Scholar] [CrossRef]
- Collen, A.; Koolwijk, P.; Kroon, M.; van Hinsbergh, V.W. Influence of Fibrin Structure on the Formation and Maintenance of Capillary-like Tubules by Human Microvascular Endothelial Cells. Angiogenesis 1998, 2, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Eyrich, D.; Wiese, H.; Maier, G.; Skodacek, D.; Appel, B.; Sarhan, H.; Tessmar, J.; Staudenmaier, R.; Wenzel, M.M.; Goepferich, A.; et al. In Vitro and in Vivo Cartilage Engineering Using a Combination of Chondrocyte-Seeded Long-Term Stable Fibrin Gels and Polycaprolactone-Based Polyurethane Scaffolds. Tissue Eng. 2007, 13, 2207–2218. [Google Scholar] [CrossRef] [PubMed]
- Janmey, P.A.; Winer, J.P.; Weisel, J.W. Fibrin Gels and Their Clinical and Bioengineering Applications. J. R. Soc. Interface 2009, 6, 1–10. [Google Scholar] [CrossRef]
- Gamboa-Martínez, T.C.; Ribelles, J.L.G.; Ferrer, G.G. Fibrin Coating on Poly (L-Lactide) Scaffolds for Tissue Engineering. J. Bioact. Compat. Polym. 2011, 26, 464–477. [Google Scholar] [CrossRef]
- Baxter International Inc. Highlights of Prescribing Information-TISSEEL [Fibrin Sealant] for Topical Use Only. Available online: https://www.baxterpi.com/pi-pdf/Tisseel_PI.pdf (accessed on 10 December 2021).
- Buen, E.P.; Orozco-Mosqueda, A.; Leal-Cortés, C.; Vázquez-Camacho, G.; Fuentes-Orozco, C.; Alvarez-Villaseñor, A.S.; Macías-Amezcua, M.D.; González-Ojeda, A. Fibrinogen and Thrombin Concentrations Are Critical for Fibrin Glue Adherence in Rat High-Risk Colon Anastomoses. Clinics 2014, 69, 259–264. [Google Scholar] [CrossRef]
- Murakami, M.; Tono, T.; Okada, K.; Yano, H.; Monden, T. Fibrin Glue Injection Method with Diluted Thrombin for Refractory Postoperative Digestive Fistula. Am. J. Surg. 2009, 198, 715–719. [Google Scholar] [CrossRef] [PubMed]
- Use of Fibrin Tissue Adhesive in Conjunctival, Corneal, Cataract, and Refractive Surgery. US Ophthalmic Rev. 2007, 2, 42–44. [CrossRef]
- Goessl, A.; Redl, H. Optimized Thrombin Dilution Protocol for a Slowly Setting Fibrin Sealant in Surgery. Eur. Surg. 2005, 37, 43–51. [Google Scholar] [CrossRef]
- Pasternak, T.P.; Prinsen, E.; Ayaydin, F.; Miskolczi, P.; Potters, G.; Asard, H.; Van Onckelen, H.A.; Dudits, D.; Fehér, A. The Role of Auxin, PH, and Stress in the Activation of Embryogenic Cell Division in Leaf Protoplast-Derived Cells of Alfalfa. Plant Physiol. 2002, 129, 1807–1819. [Google Scholar] [CrossRef]
- Du, X.; Yuan, Q.; Qu, Y.; Zhou, Y.; Bei, J. Endometrial Mesenchymal Stem Cells Isolated from Menstrual Blood by Adherence. Stem Cells Int. 2016, 2016, 3573846. [Google Scholar] [CrossRef]
- de Pedro, M.Á.; Gómez-Serrano, M.; Marinaro, F.; López, E.; Pulido, M.; Preußer, C.; von Strandmann, E.P.; Sánchez-Margallo, F.M.; Álvarez, V.; Casado, J.G. IFN-Gamma and TNF-Alpha as a Priming Strategy to Enhance the Immunomodulatory Capacity of Secretomes from Menstrual Blood-Derived Stromal Cells. Int. J. Mol. Sci. 2021, 22, 12177. [Google Scholar] [CrossRef]
- Marinaro, F.; Gómez-Serrano, M.; Jorge, I.; Silla-Castro, J.C.; Vázquez, J.; Sánchez-Margallo, F.M.; Blázquez, R.; López, E.; Álvarez, V.; Casado, J.G. Unraveling the Molecular Signature of Extracellular Vesicles From Endometrial-Derived Mesenchymal Stem Cells: Potential Modulatory Effects and Therapeutic Applications. Front. Bioeng Biotechnol. 2019, 7, 431. [Google Scholar] [CrossRef]
- Qi, L.; Knapton, E.K.; Zhang, X.; Zhang, T.; Gu, C.; Zhao, Y. Pre-Culture Sudan Black B Treatment Suppresses Autofluorescence Signals Emitted from Polymer Tissue Scaffolds. Sci. Rep. 2017, 7, 8361. [Google Scholar] [CrossRef]
- Macasev, D.; Diorio, J.P.; Gugerell, A.; Goppelt, A.; Gulle, H.; Bittner, M. Cell Compatibility of Fibrin Sealants: In Vitro Study with Cells Involved in Soft Tissue Repair. J. Biomater. Appl. 2011, 26, 129–149. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.; Lee, S.K.; Yoon, J.I.; Kim, D.E.; Kim, M.; Ha, H. Fibrin Glue Improves the Therapeutic Effect of MSCs by Sustaining Survival and Paracrine Function. Tissue Eng. Part A 2013, 19, 2373–2381. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Olmo, D.; Herreros, D.; Pascual, I.; Pascual, J.A.; Del-Valle, E.; Zorrilla, J.; De-La-Quintana, P.; Garcia-Arranz, M.; Pascual, M. Expanded Adipose-Derived Stem Cells for the Treatment of Complex Perianal Fistula: A Phase II Clinical Trial. Dis. Colon Rectum 2009, 52, 79–86. [Google Scholar] [CrossRef]
- McGrath, A.M.; Brohlin, M.; Kingham, P.J.; Novikov, L.N.; Wiberg, M.; Novikova, L.N. Fibrin Conduit Supplemented with Human Mesenchymal Stem Cells and Immunosuppressive Treatment Enhances Regeneration after Peripheral Nerve Injury. Neurosci. Lett. 2012, 516, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K.; Ichihara, Y.; Tano, N.; Fields, L.; Murugesu, N.; Ito, T.; Ikebe, C.; Lewis, F.; Yashiro, K.; Shintani, Y.; et al. Fibrin Glue-Aided, Instant Epicardial Placement Enhances the Efficacy of Mesenchymal Stromal Cell-Based Therapy for Heart Failure. Sci. Rep. 2018, 8, 9448. [Google Scholar] [CrossRef] [PubMed]
- Ludwicka, K.; Kolodziejczyk, M.; Gendaszewska-Darmach, E.; Chrzanowski, M.; Jedrzejczak-Krzepkowska, M.; Rytczak, P.; Bielecki, S. Stable Composite of Bacterial Nanocellulose and Perforated Polypropylene Mesh for Biomedical Applications. J. Biomed. Mater. Res. Part B Appl. Biomater. 2019, 107, 978–987. [Google Scholar] [CrossRef]
- Abdulagatov, I.M.; Ragimov, R.M.; Khamidov, М.А.; Maksumova, A.M.; Abdullaeva, N.M. ALD Coated Polypropylene Hernia Meshes for Prevention of Mesh-Related Post-Surgery Complications: An Experimental Study in Animals. Biomed. Mater. 2021, 17. [Google Scholar] [CrossRef]
- Benito-Martínez, S.; Pérez-Köhler, B.; Rodríguez, M.; García-Moreno, F.; Gómez-Gil, V.; Pascual, G.; Bellón, J.M. Antibacterial Biopolymer Gel Coating on Meshes Used for Abdominal Hernia Repair Promotes Effective Wound Repair in the Presence of Infection. Polymers 2021, 13, 2371. [Google Scholar] [CrossRef]
- Qiao, Y.; Zhang, Q.; Wang, Q.; Lin, J.; Wang, J.; Li, Y.; Wang, L. Synergistic Anti-Inflammatory Coating “Zipped Up” on Polypropylene Hernia Mesh. ACS Appl. Mater. Interfaces 2021, 13, 35456–35468. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, V.; Sánchez-Margallo, F.M.; Macías-García, B.; Gómez-Serrano, M.; Jorge, I.; Vázquez, J.; Blázquez, R.; Casado, J.G. The Immunomodulatory Activity of Extracellular Vesicles Derived from Endometrial Mesenchymal Stem Cells on CD4+ T Cells Is Partially Mediated by TGFbeta. J. Tissue Eng. Regen. Med. 2018, 12, 2088–2098. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An Open-Source Platform for Biological-Image Analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Blázquez, R.; Sánchez-Margallo, F.M.; Reinecke, J.; Álvarez, V.; López, E.; Marinaro, F.; Casado, J.G. Conditioned Serum Enhances the Chondrogenic and Immunomodulatory Behavior of Mesenchymal Stem Cells. Front. Pharmacol. 2019, 10, 699. [Google Scholar] [CrossRef] [PubMed]


| Breaking Load [N] | Elongation at Break [%] | Young Modulus (E) [MPa] | |
|---|---|---|---|
| Uncoated meshes | 345.81 | 123.36 | 35.07 |
| Monolayered coatings | 258.37 | 110.53 | 26.48 |
| Bilayered coatings | 344.8 | 116.31 | 35.22 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marinaro, F.; Silva, J.M.; Barros, A.A.; Aroso, I.M.; Gómez-Blanco, J.C.; Jardin, I.; Lopez, J.J.; Pulido, M.; de Pedro, M.Á.; Reis, R.L.; et al. A Fibrin Coating Method of Polypropylene Meshes Enables the Adhesion of Menstrual Blood-Derived Mesenchymal Stromal Cells: A New Delivery Strategy for Stem Cell-Based Therapies. Int. J. Mol. Sci. 2021, 22, 13385. https://doi.org/10.3390/ijms222413385
Marinaro F, Silva JM, Barros AA, Aroso IM, Gómez-Blanco JC, Jardin I, Lopez JJ, Pulido M, de Pedro MÁ, Reis RL, et al. A Fibrin Coating Method of Polypropylene Meshes Enables the Adhesion of Menstrual Blood-Derived Mesenchymal Stromal Cells: A New Delivery Strategy for Stem Cell-Based Therapies. International Journal of Molecular Sciences. 2021; 22(24):13385. https://doi.org/10.3390/ijms222413385
Chicago/Turabian StyleMarinaro, Federica, Joana M. Silva, Alexandre A. Barros, Ivo M. Aroso, Juan C. Gómez-Blanco, Isaac Jardin, Jose J. Lopez, María Pulido, María Ángeles de Pedro, Rui L. Reis, and et al. 2021. "A Fibrin Coating Method of Polypropylene Meshes Enables the Adhesion of Menstrual Blood-Derived Mesenchymal Stromal Cells: A New Delivery Strategy for Stem Cell-Based Therapies" International Journal of Molecular Sciences 22, no. 24: 13385. https://doi.org/10.3390/ijms222413385
APA StyleMarinaro, F., Silva, J. M., Barros, A. A., Aroso, I. M., Gómez-Blanco, J. C., Jardin, I., Lopez, J. J., Pulido, M., de Pedro, M. Á., Reis, R. L., Sánchez-Margallo, F. M., Casado, J. G., & López, E. (2021). A Fibrin Coating Method of Polypropylene Meshes Enables the Adhesion of Menstrual Blood-Derived Mesenchymal Stromal Cells: A New Delivery Strategy for Stem Cell-Based Therapies. International Journal of Molecular Sciences, 22(24), 13385. https://doi.org/10.3390/ijms222413385