Peptides Bearing Multiple Post-Translational Modifications as Antigenic Targets for Severe Rheumatoid Arthritis Patients
Abstract
:1. Introduction
2. Results
2.1. Selection of the Home-Designed ELISA Methodology
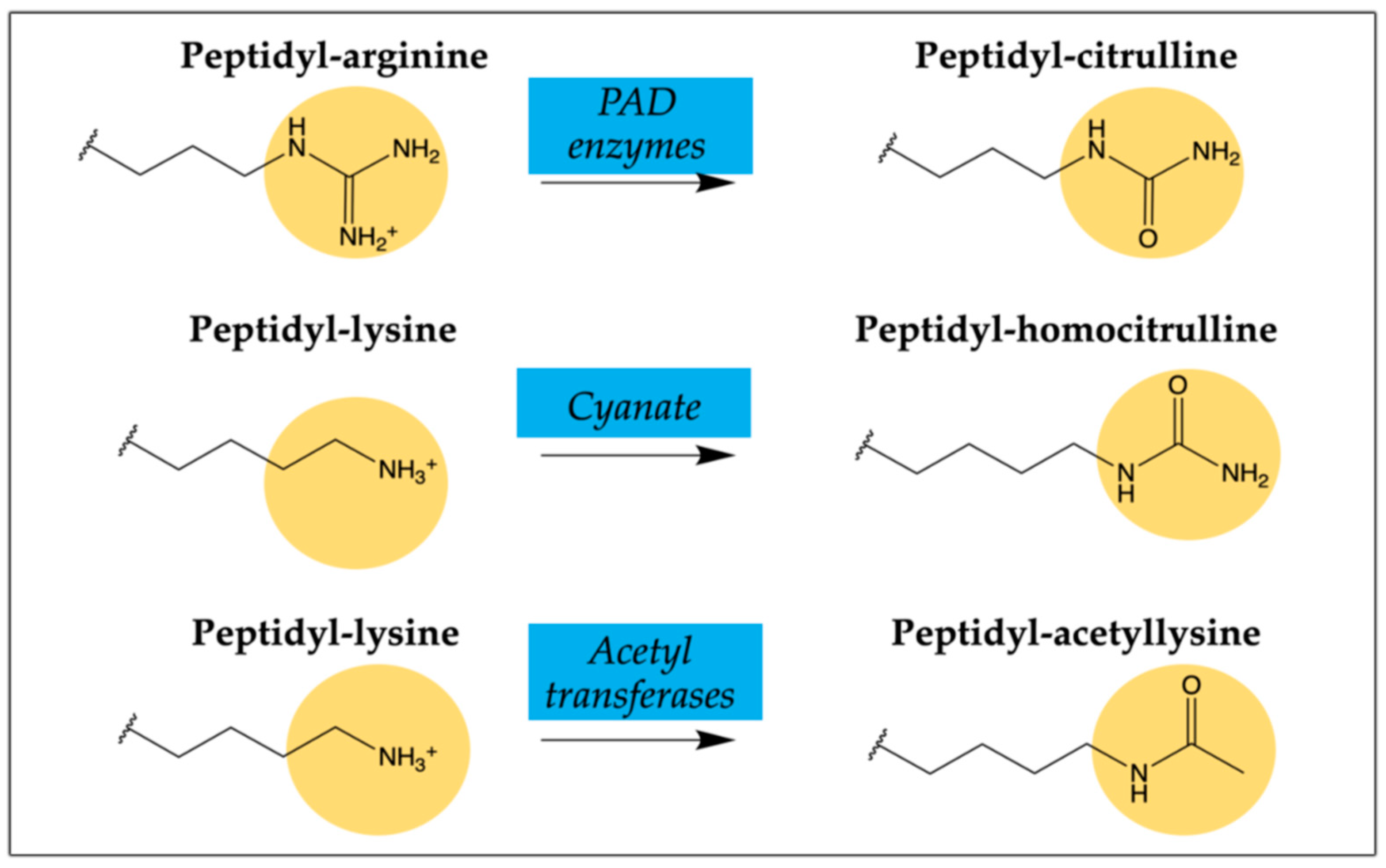
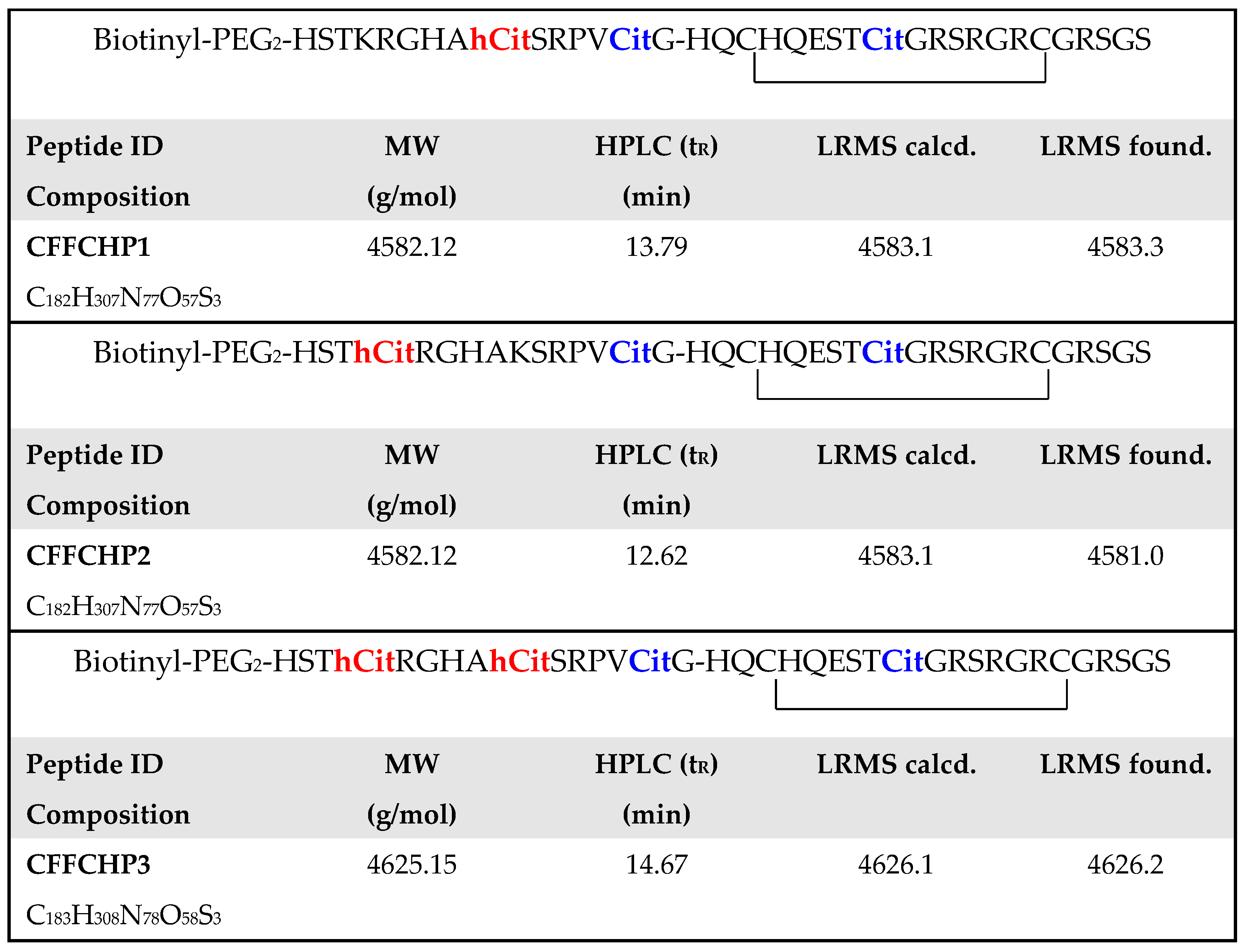
2.2. Citrullinated/Homocitrullinated Chimeric Peptides
2.3. Anti-AMPA and RA-ILD
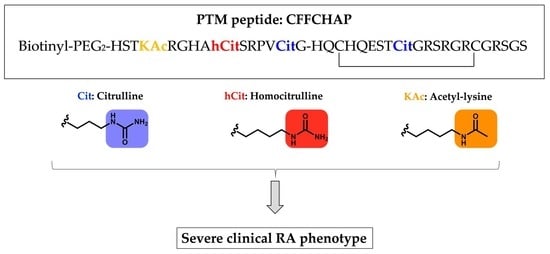
2.4. Citrullinated/Homocitrullinated/Acetylated Chimeric Peptide
2.5. Association of Anti-Chimeric Peptide Autoantibodies with the Severity of Articular Disease (Joint Destruction)
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Synthesis of Cyclic Chimeric Peptides
4.3. Study Design
4.4. ELISA Assays
4.4.1. Home-Designed ELISA Assay with Peptides Covalently Bound to Microtiter Plates
4.4.2. Home-Designed ELISA Assay with Biotinyl-Peptides Bound to Neutravidin Derivatized Microtiter Plates
4.5. Statistical Analysis
4.6. Ethics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Acm | acetamidomethyl |
| ACPAs | anti-citrullinated protein/peptide antibodies |
| AMPAs | anti-modified protein/peptide antibodies |
| anti-APA | antibodies against acetylated proteins |
| anti-CarP | antibodies against homocitrullinated (carbamylated) peptides/proteins |
| AU | arbitrary units |
| Boc | tert-butoxycarbonyl |
| BSA | bovine serum albumin |
| CFFCP | chimeric fibrin-filaggrin citrullinated peptide |
| CFFCHP | chimeric fibrin-filaggrin citrullinated/homocitrullinated peptide |
| CFFCHAP | chimeric fibrin/filaggrin citrullinated/homocitrullinated/acetylated peptide |
| CV | coefficient of variation |
| DIPCDI | N,N′-diisopropylcarbodiimide |
| DIPEA | diisopropylethylamine |
| DMF | dimethylformamide |
| ELISA | enzyme-linked immunosorbent assay |
| ESI-MS | electrospray ionization mass spectrometry |
| FBS | fetal bovine serum |
| FCS | fetal calf serum |
| Fmoc | 9-fluorenyl-methoxycarbonyl |
| HATU | 2-(1H-7-azabenzotriazole-1-yl)-1,1,3,3- tetramethyluronium hexafluorophosphate methanaminium |
| HOBt | 1-hydroxybenzotriazole |
| HPLC | high-performance liquid chromatography |
| ILD | interstitial lung disease |
| IQR | interquartile range |
| LRMS | low-resolution mass spectrometry |
| MW | molecular weight |
| OD | optical density |
| OPD | o-phenylenediamine dihydrochloride |
| PAD | peptidylarginine deiminase |
| PBS | phosphate-buffered saline |
| Pmc | 2,2,5,7,8-pentamethyl-chroman-6-sulfonyl |
| PTMs | post-translational modifications |
| PyBOP | benzotriazole-1-yloxytris(pirrolidino) phosphonium hexafluorophosphate |
| RA | rheumatoid arthritis |
| ROC | receiver operating characteristic |
| RP-HPLC | reverse-phase high-performance liquid chromatography |
| SDS | sodium dodecyl sulfate |
| SEM | standard error of the mean |
| SPPS | solid-phase peptide synthesis |
| tBu | tert-butyl |
| TFA | trifluroacetic acid |
| TIS | triisopropylsilane |
| TRIS | tris(hydroxymethyl)-aminomethane |
| Trt | triphenylmethyl |
References
- Haro, I.; Sanmarti, R. Rheumatoid arthritis: Current advances in pathogenesis, diagnosis and therapy. Curr. Top. Med. Chem. 2013, 13, 697. [Google Scholar] [CrossRef]
- Mueller, A.L.; Payandeh, Z.; Mohammadkhani, N.; Mubarak, S.M.H.; Zakeri, A.; Bahrami, A.A.; Brockmueller, A.; Shakibaei, M. Recent Advances in Understanding the Pathogenesis of Rheumatoid Arthritis: New Treatment Strategies. Cells 2021, 10, 3017. [Google Scholar] [CrossRef]
- Kwon, E.J.; Ju, J.H. Impact of Posttranslational modification in pathogenesis of rheumatoid arthritis: Focusing on citrullination, carbamylation, and acetylation. Int. J. Mol. Sci. 2021, 22, 10576. [Google Scholar] [CrossRef]
- Scherer, H.U.; Haupl, T.; Burmester, G.R. The etiology of rheumatoid arthritis. J. Autoimmun. 2020, 110, 102400. [Google Scholar] [CrossRef]
- Darrah, E.; Andrade, F. Rheumatoid arthritis and citrullination. Curr. Opin. Rheumatol. 2018, 30, 72–78. [Google Scholar] [CrossRef]
- Sebbag, M.; Moinard, N.; Auger, I.; Clavel, C.; Arnaud, J.; Nogueira, L.; Roudier, J.; Serre, G. Epitopes of human fibrin recognized by the rheumatoid arthritis-specific autoantibodies to citrullinated proteins. Eur. J. Immunol. 2006, 36, 2250–2263. [Google Scholar] [CrossRef] [PubMed]
- van Delft, M.A.M.; Huizinga, T.W.J. An overview of autoantibodies in rheumatoid arthritis. J. Autoimmun. 2020, 110, 102392. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Nicholls, S.J.; Rodriguez, E.R.; Kummu, O.; Hörkkö, S.; Barnard, J.; Reynolds, W.F.; Topol, E.J.; DiDonato, J.A.; Hazen, S.L. Protein carbamylation links inflammation, smoking, uremia and atherogenesis. Nat. Med. 2007, 13, 1176–1184. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Knevel, R.; Suwannalai, P.; van der Linden, M.P.; Janssen, G.M.C.; van Veelen, P.A.; Levarht, N.E.W.; van der Helm-van Mil, A.H.M.; Cerami, A.; Huizinga, T.W.J.; et al. Autoantibodies recognizing carbamylated proteins are present in sera of patients with rheumatoid arthritis and predict joint damage. Proc. Natl. Acad. Sci. USA 2011, 108, 17372–17377. [Google Scholar] [CrossRef] [Green Version]
- Ajeganova, S.; van Steenbergen, H.W.; Verheul, M.K.; Forslind, K.; Hafström, I.; Toes, R.E.M.; Huizinga, T.W.J.; Svensson, B.; Trouw, L.A.; van der Helm-van Mil, A.H.M. The association between anti-carbamylated protein (anti-CarP) antibodies and radiographic progression in early rheumatoid arthritis: A study exploring replication and the added value to ACPA and rheumatoid factor. Ann. Rheum. Dis. 2017, 76, 112–118. [Google Scholar] [CrossRef]
- Thiele, G.M.; Duryee, M.J.; Anderson, D.R.; Klassen, L.W.; Mohring, S.M.; Young, K.A.; Benissan-Messan, D.; Sayles, H.; Dusad, A.; Hunter, C.D.; et al. Malondialdehyde-acetaldehyde adducts and anti-malondialdehyde-acetaldehyde antibodies in rheumatoid arthritis. Arthritis Rheumatol. 2015, 67, 645–655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Juarez, M.; Bang, H.; Hammar, F.; Reimer, U.; Dyke, B.; Sahbudin, I.; Buckley, C.D.; Fisher, B.; Filer, A.; Raza, K. Identification of novel antiacetylated vimentin antibodies in patients with early inflammatory arthritis. Ann. Rheum. Dis. 2016, 75, 1099–1107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muller, S. Synthetic peptides as tools for diagnosis and therapeutic strategies to treat systemic lupus erythematous. Autoimmun. Rev. 2012, 11, 799–800. [Google Scholar] [CrossRef] [PubMed]
- Nuti, F.; Peroni, E.; Real-Fernández, F.; Bonache, M.A.; Le Chevalier-Isaad, A.; Chelli, M.; Lubin-Germain, N.; Uziel, J.; Rovero, P.; Lolli, F.; et al. Posttranslationally modified peptides efficiently mimicking neoantigens: A challenge for theragnostics of autoimmune diseases. Biopolymers 2010, 94, 791–799. [Google Scholar] [CrossRef] [PubMed]
- Papini, A.M. The use of post-translationally modified peptides for detection of biomarkers of immune-mediated diseases. J. Pept. Sci. 2009, 15, 621–628. [Google Scholar] [CrossRef]
- Perez, M.L.; Gomara, M.J.; Ercilla, G.; Sanmarti, R.; Haro, I. Antibodies to citrullinated human fibrinogen synthetic peptides in diagnosing rheumatoid arthritis. J. Med. Chem. 2007, 50, 3573–3584. [Google Scholar] [CrossRef]
- Malakoutikhah, M.; Gomara, M.J.; Gomez-Puerta, J.A.; Sanmarti, R.; Haro, I. The use of chimeric vimentin citrullinated peptides for the diagnosis of rheumatoid arthritis. J. Med. Chem. 2011, 54, 7486–7492. [Google Scholar] [CrossRef]
- Garcia-Moreno, C.; Gomara, M.J.; Bleda, M.J.; Sanmarti, R.; Haro, I. Development of a multiplex assay based on chimeric citrullinated peptides as proof of concept for diagnosis of rheumatoid arthritis. PLoS ONE 2019, 14, e0215927. [Google Scholar] [CrossRef] [Green Version]
- Sanmarti, R.; Graell, E.; Perez, M.L.; Ercilla, G.; Vinas, O.; Gomez-Puerta, J.A.; Gratacos, J.; Balsa, A.; Gomara, M.J.; Larrosa, M.; et al. Diagnostic and prognostic value of antibodies against chimeric fibrin/filaggrin citrullinated synthetic peptides in rheumatoid arthritis. Arthritis Res. Ther. 2009, 11, R135. [Google Scholar] [CrossRef] [Green Version]
- Hyldgaard, C.; Hilberg, O.; Pedersen, A.B.; Ulrichsen, S.P.; Løkke, A.; Bendstrup, E.; Ellingsen, T. A population-based cohort study of rheumatoid arthritis-associated interstitial lung disease: Comorbidity and mortality. Ann. Rheum. Dis. 2017, 76, 1700–1706. [Google Scholar] [CrossRef]
- Kuwana, M.; Gil-Vila, A.; Selva-O’Callaghan, A. Role of autoantibodies in the diagnosis and prognosis of interstitial lung disease in autoimmune rheumatic disorders. Ther. Adv. Musculoskelet. Dis. 2021, 13, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Castellanos-Moreira, R.; Rodriguez-Garcia, S.C.; Gomara, M.J.; Ruiz-Esquide, V.; Cuervo, A.; Casafont-Sole, I.; Ramirez, J.; Holgado, S.; Gomez-puerta, J.A.; Cañete, J.D.; et al. Anti-carbamylated proteins antibody repertoire in rheumatoid arthritis: Evidence of a new autoantibody linked to interstitial lung disease. Ann. Rheum. Dis. 2020, 79, 587–594. [Google Scholar] [CrossRef]
- Bastarache, J.A.; Koyama, T.; Wickersham, N.E.; Mitchell, D.B.; Mernaugh, R.L.; Ware, L.B. Accuracy and reproducibility of a multiplex immunoassay platform: A validation study. J. Immunol. Methods 2011, 367, 33–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanneman, S.K.; Cox, C.D.; Green, K.E.; Kang, D.H. Estimating intra- and inter-assay variability in salivary cortisol. Biol. Res. Nurs. 2011, 13, 243–250. [Google Scholar] [CrossRef]
- European Medicines Agency; Committee for Medicinal Products for Human Use. Guideline on Bioanalytical Method Validation; European Medicines Agency: London, UK, 2011; Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-bioanalytical-method-validation_en.pdf (accessed on 21 July 2011).
- Solomon, J.J.; Matson, S.; Kelmenson, L.B.; Chung, J.H.; Hobbs, S.B.; Rosas, I.O.; Dellaripa, P.F.; Doyle, T.J.; Poli, S.; Esposito, A.J.; et al. IgA antibodies directed against citrullinated protein antigens are elevated in patients with idiopathic pulmonary fibrosis. Chest 2020, 157, 1513–1521. [Google Scholar] [CrossRef]
- Harlow, L.; Rosas, I.O.; Gochuico, B.R.; Mikuls, T.R.; Dellaripa, P.F.; Oddis, C.V.; Ascherman, D.P. Identification of citrullinated Hsp90 isoforms as novel autoantigens in rheumatoid arthritis-associated interstitial lung disease. Arthritis Rheum. 2013, 65, 869–879. [Google Scholar] [CrossRef]
- Alunno, A.; Bistoni, O.; Pratesi, F.; La Paglia, G.M.C.; Puxeddu, I.; Migliorini, P.; Gerli, R. Anti-citrullinated alpha enolase antibodies, interstitial lung disease and bone erosion in rheumatoid arthritis. Rheumatology 2018, 57, 850–855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- England, B.R.; Duryee, M.J.; Roul, P.; Mahajan, T.D.; Singh, N.; Poole, J.A.; Ascherman, D.P.; Caplan, L.; Demoruelle, M.K.; Deane, K.D.; et al. Malondialdehyde–Acetaldehyde adducts and antibody responses in rheumatoid arthritis–associated interstitial lung disease. Arthritis Rheumatol. 2019, 71, 1483–1493. [Google Scholar] [CrossRef]
- Kirwan, J.R. Using the Larsen index to assess radiographic progression in rheumatoid arthritis. J. Rheumatol. 2000, 27, 264–268. [Google Scholar]
- Wada, T.T.; Araki, Y.; Sato, K.; Aizaki, Y.; Yokota, K.; Kim, Y.T.; Oda, H.; Kurokawa, R.; Mimura, T. Aberrant histone acetylation contributes to elevated interleukin-6 production in rheumatoid arthritis synovial fibroblasts. Biochem. Biophys. Res. Commun. 2014, 444, 682–686. [Google Scholar] [CrossRef]
- Jilani, A.A.; Mackworth-Young, C.G. The role of citrullinated protein antibodies in predicting erosive disease in rheumatoid arthritis: A systematic literature review and meta-analysis. Int. J. Rheumatol. 2015, 2015, 728610. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Li, S.; Chen, B.; Zhu, Q.; Xu, L.; Li, F. Serum anti-citrullinated protein antibodies and rheumatoid factor increase the risk of rheumatoid arthritis-related interstitial lung disease: A meta-analysis. Clin. Rheumatol. 2021, 40, 4533–4543. [Google Scholar] [CrossRef] [PubMed]
- Truchetet, M.E.; Dublanc, S.; Barnetche, T.; Vittecoq, O.; Mariette, X.; Richez, C.; Blanco, P.; Mahler, M.; Contin-Bordes, C.; Schaeverbeke, T.; et al. Association of the Presence of Anti-Carbamylated Protein Antibodies in Early Arthritis with a Poorer Clinical and Radiologic Outcome: Data From the French ESPOIR Cohort. Arthritis Rheumatol. 2017, 69, 2292–2302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nijjar, J.S.; Morton, F.R.; Bang, H.; Buckley, C.D.; van der Heijde, D.; Gilmour, A.; Paterson, C.; McInnes, I.B.; Porter, D.; Raza, K.; et al. The impact of autoantibodies against citrullinated, carbamylated and acetylated peptides on radiographic progression in patients with new-onset rheumatoid arthritis: An observational cohort study. Lancet Rheumatol. 2021, 3, e284–e293. [Google Scholar] [CrossRef]

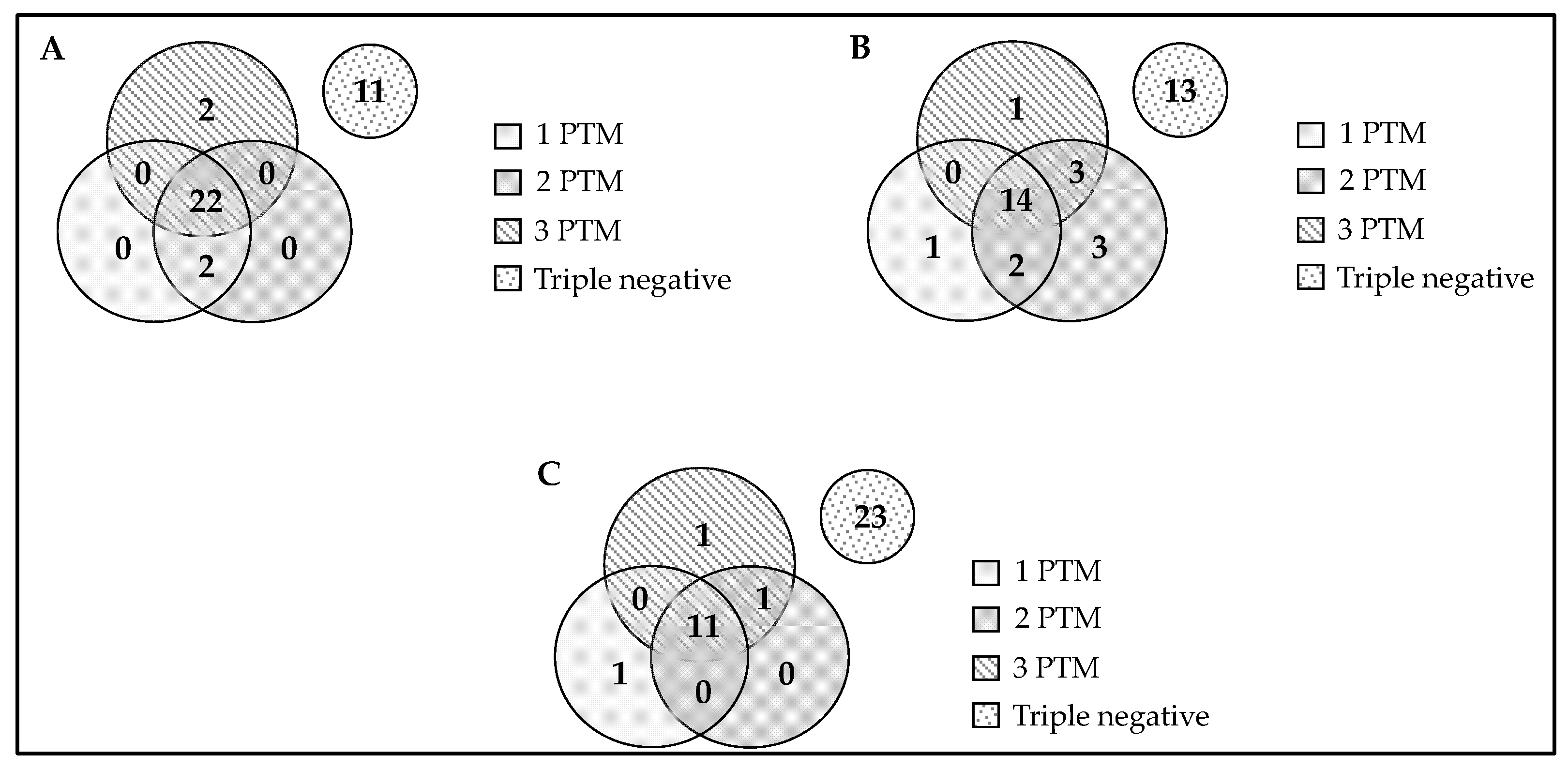
| RA Population (n = 178) | α-IgG | α-IgA | α-IgM |
|---|---|---|---|
| anti-CFFCP1-positive (%) | 101 (56.7) | 60 (33.7) | 40 (22.5) |
| median titer anti-CFFCP1 AU/mL (IQR) | 45 (456) | 0 (55) | 0 (48) |
| anti-CFFCHP1-positive (%) | 104 (58.4) | 64 (36.0) | 37 (21.3) |
| median titer anti-CFFCHP1 AU/mL (IQR) | 55 (469) | 6 (52) | 0 (40) |
| anti-CFFCHP2-positive (%) | 103 (57.8) | 70 (39.3) | 41 (23.0) |
| median titer anti-CFFCHP2AU/mL (IQR) | 52 (590) | 12 (66) | 0 (52) |
| anti-CFFCHP3-positive (%) | 102 (57.3) | 64 (36.0) | 41 (23.0) |
| median titer anti-CFFCHP3AU/mL (IQR) | 69 (503) | 18 (54) | 0 (73) |
| RA-ILD (n = 37) | RA-non-ILD (n = 141) | p-Value | |
|---|---|---|---|
| CFFCHP1 | |||
| anti-CFFCHP1-IgG-positive (%) | 24 (64.8) | 80 (56.7) | NS |
| median titer anti-CFFCHP1 AU/mL (IQR) | 113 (2525) | 49 (292) | NS |
| anti-CFFCHP1-IgA-positive (%) | 22 (59.4) | 42 (29.8) | <0.005 |
| median titer anti-CFFCHP1 AU/mL (IQR) | 31 (497) | 3 (34) | <0.005 |
| anti-CFFCHP1-IgM-positive (%) | 12 (32.4) | 25 (17.7) | 0.05 |
| median titer anti-CFFCHP1 AU/mL (IQR) | 0 (537) | 0 (33) | 0.04 |
| CFFCHP2 | |||
| anti-CFFCHP2-IgG-positive (%) | 25 (67.6) | 78 (55.3) | NS |
| median titer anti-CFFCHP2 AU/mL (IQR) | 134 (2126) | 43 (377) | NS |
| anti-CFFCHP2-IgA-positive (%) | 21 (56.7) | 49 (34.8) | 0.02 |
| median titer anti-CFFCHP2 AU/mL (IQR) | 24 (374) | 5 (51) | <0.005 |
| anti-CFFCHP2-IgM-positive (%) | 13 (35.1) | 28 (19.8) | 0.05 |
| median titer anti-CFFCHP2 AU/mL (IQR) | 10 (359) | 0 (39) | 0.01 |
| CFFCHP3 | |||
| anti-CFFCHP3-IgG-positive (%) | 25 (67.6) | 77 (54.6) | NS |
| median titer anti-CFFCHP3 AU/mL (IQR) | 135 (2826) | 54 (351) | NS |
| anti-CFFCHP3-IgA-positive (%) | 19 (51.3) | 45 (31.9) | 0.03 |
| median titer anti-CFFCHP3 AU/mL (IQR) | 27 (308) | 16 (31) | 0.009 |
| anti-CFFCHP3-IgM-positive (%) | 13 (35.1) | 28 (19.8) | 0.05 |
| median titer anti-CFFCHP3 AU/mL (IQR) | 22 (440) | 0 (46) | 0.007 |
| RA-ILD (n = 37) | RA-Non-ILD (n = 141) | p-Value | |
|---|---|---|---|
| CFFCHAP | |||
| anti-CFFCHAP-IgG-positive (%) | 24 (64.9) | 71 (50.4) | NS |
| median titer anti-CFFCHAP AU/mL (IQR) | 67 (3123) | 23 (211) | 0.008 |
| anti-CFFCHAP-IgA-positive (%) | 18 (48.6) | 40 (28.4) | 0.02 |
| median titer anti-CFFCHAP AU/mL (IQR) | 18 (319) | 0 (33) | 0.01 |
| anti-CFFCHAP-IgM-positive (%) | 13 (35.1) | 28 (19.8) | 0.05 |
| median titer anti-CFFCHAP AU/mL (IQR) | 17 (267) | 0 (24) | 0.02 |
| Median Larsen Score 18 | Larsen < 18 (n = 88) | Larsen ≥ 18 (n = 90) | p-Value |
|---|---|---|---|
| CFFCHP1 | |||
| anti-CFFCHP1-IgG-positive (%) | 46 (52.3) | 58 (64.4) | NS |
| median titer anti-CFFCHP1 AU/mL (IQR) | 34 (181) | 101 (764) | 0.02 |
| anti-CFFCHP1-IgA-positive (%) | 24 (27.3) | 40 (44.4) | 0.02 |
| median titer anti-CFFCHP1 AU/mL (IQR) | 0 (26) | 11 (127) | 0.008 |
| anti-CFFCHP1-IgM-positive (%) | 13 (14.8%) | 24 (26.7) | 0.05 |
| median titer anti-CFFCHP1 AU/mL (IQR) | 0 (17) | 0 (109) | 0.04 |
| CFFCHP2 | |||
| anti-CFFCHP2-IgG-positive (%) | 46 (52.3) | 57 (63.3) | NS |
| median titer anti-CFFCHP2 AU/mL (IQR) | 28 (166) | 86 (1164) | 0.03 |
| anti-CFFCHP2-IgA-positive (%) | 28 (30.7) | 42 (46.7) | 0.04 |
| median titer anti-CFFCHP2 AU/mL (IQR) | 4 (33) | 18 (143) | 0.008 |
| anti-CFFCHP2-IgM-positive (%) | 14 (15.9) | 27 (30.0) | 0.03 |
| median titer anti-CFFCHP2 AU/mL (IQR) | 0 (25) | 0 (94) | 0.02 |
| CFFCHP3 | |||
| anti-CFFCHP3-IgG-positive (%) | 45 (51.1) | 57 (63.3) | NS |
| median titer anti-CFFCHP3 AU/mL (IQR) | 24 (233) | 107 (807) | 0.02 |
| anti-CFFCHP3-IgA-positive (%) | 28 (30.7) | 36 (40.0) | NS |
| median titer anti-CFFCHP3 AU/mL (IQR) | 16 (29) | 20 (134) | 0.05 |
| anti-CFFCHP3-IgM-positive (%) | 14 (15.9) | 27 (30.0) | 0.03 |
| median titer anti-CFFCHP3 AU/mL (IQR) | 0 (34) | 0 (111) | 0.01 |
| CFFCHAP | |||
| anti-CFFCHAP-IgG-positive (%) | 40 (45.4) | 55 (61.1) | 0.036 |
| median titer anti-CFFCHAP AU/mL (IQR) | 18 (109) | 55 (891) | 0.01 |
| anti-CFFCHAP-IgA-positive (%) | 21 (23.9) | 37 (41.1) | 0.01 |
| median titer anti-CFFCHAP AU/mL (IQR) | 0 (23) | 18 (133) | <0.005 |
| anti-CFFCHAP-IgM-positive (%) | 16 (18.2) | 25 (27.8) | NS |
| median titer anti-CFFCHAP AU/mL (IQR) | 0 (20) | 0 (45) | 0.03 |
| IgG | IgA | IgM | |
|---|---|---|---|
| Anti-CFFCP1 | ≥11.5 | ≥8.5 | ≥55.0 |
| Anti-CFFCHP1 | ≥18.0 | ≥20.5 | ≥52.5 |
| Anti-CFFCHP2 | ≥15.0 | ≥21.5 | ≥60.0 |
| Anti-CFFCHP3 | ≥17.5 | ≥26.5 | ≥84.5 |
| Anti-CFFCHAP | ≥19.5 | ≥25.5 | ≥42.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Moreno, C.; Gómara, M.J.; Castellanos-Moreira, R.; Sanmartí, R.; Haro, I. Peptides Bearing Multiple Post-Translational Modifications as Antigenic Targets for Severe Rheumatoid Arthritis Patients. Int. J. Mol. Sci. 2021, 22, 13290. https://doi.org/10.3390/ijms222413290
García-Moreno C, Gómara MJ, Castellanos-Moreira R, Sanmartí R, Haro I. Peptides Bearing Multiple Post-Translational Modifications as Antigenic Targets for Severe Rheumatoid Arthritis Patients. International Journal of Molecular Sciences. 2021; 22(24):13290. https://doi.org/10.3390/ijms222413290
Chicago/Turabian StyleGarcía-Moreno, Cristina, María J. Gómara, Raúl Castellanos-Moreira, Raimon Sanmartí, and Isabel Haro. 2021. "Peptides Bearing Multiple Post-Translational Modifications as Antigenic Targets for Severe Rheumatoid Arthritis Patients" International Journal of Molecular Sciences 22, no. 24: 13290. https://doi.org/10.3390/ijms222413290
APA StyleGarcía-Moreno, C., Gómara, M. J., Castellanos-Moreira, R., Sanmartí, R., & Haro, I. (2021). Peptides Bearing Multiple Post-Translational Modifications as Antigenic Targets for Severe Rheumatoid Arthritis Patients. International Journal of Molecular Sciences, 22(24), 13290. https://doi.org/10.3390/ijms222413290