The Pathophysiologic Role of Gelsolin in Chronic Kidney Disease: Focus on Podocytes
Abstract
:1. Introduction
2. Results
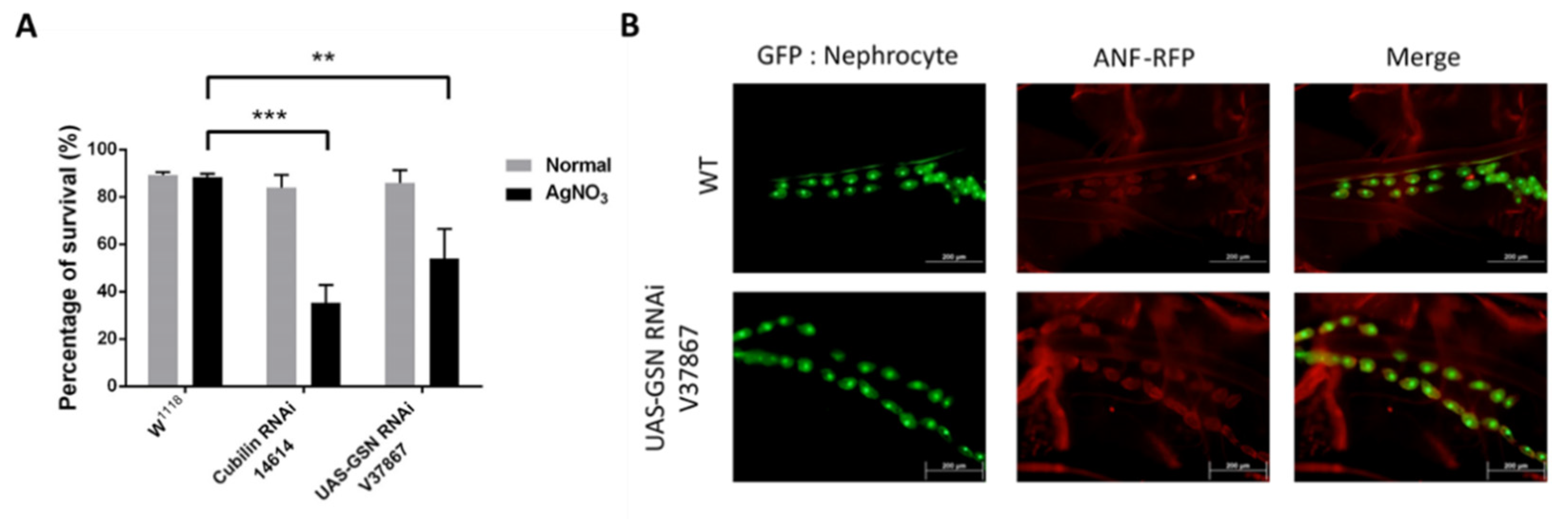
2.1. Gelsolin Is Required for Maintaining Nephrocyte Function in Drosophila
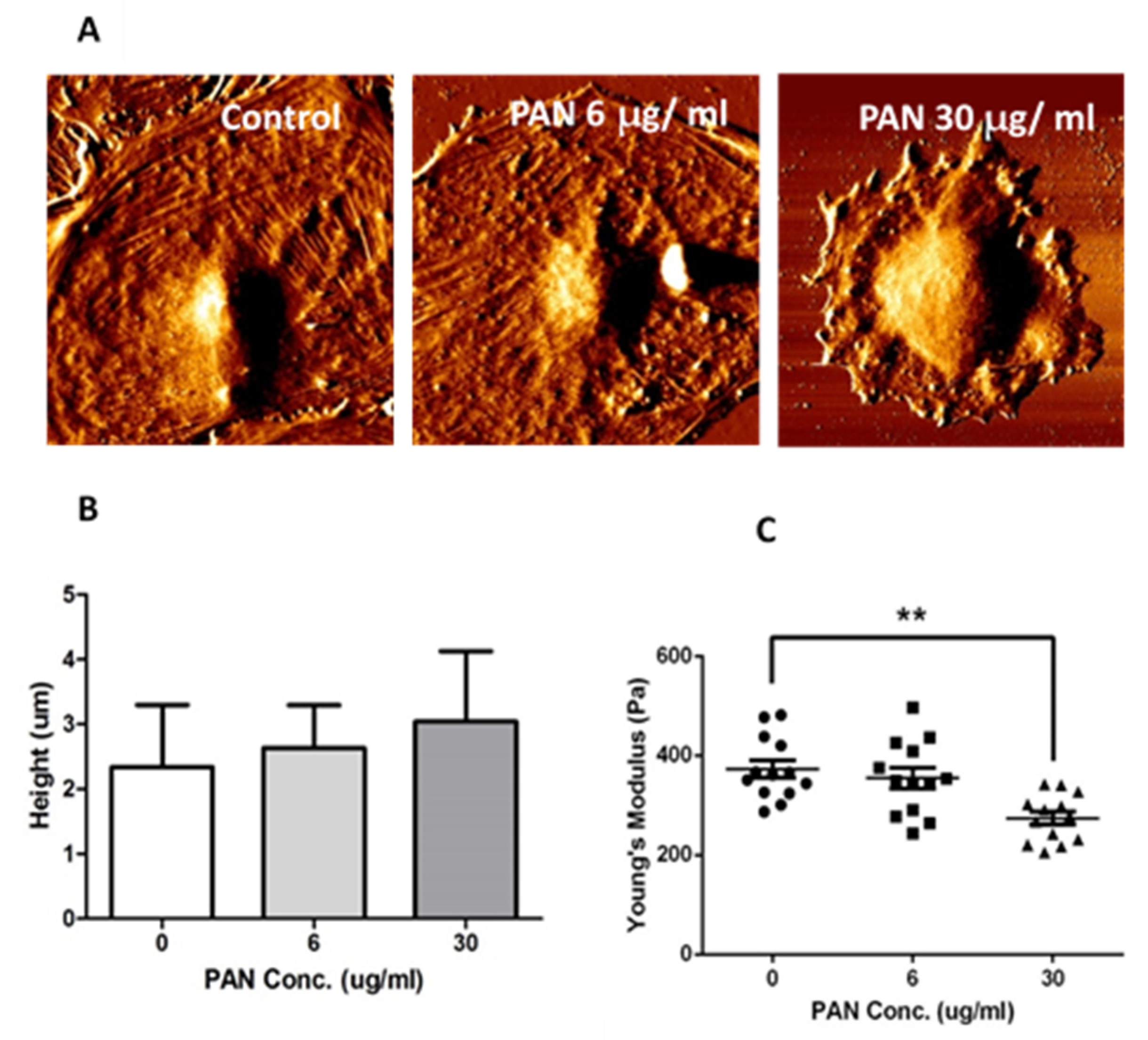
2.2. Podocyte Cell-Height and Stiffness were Altered by PAN Exposure
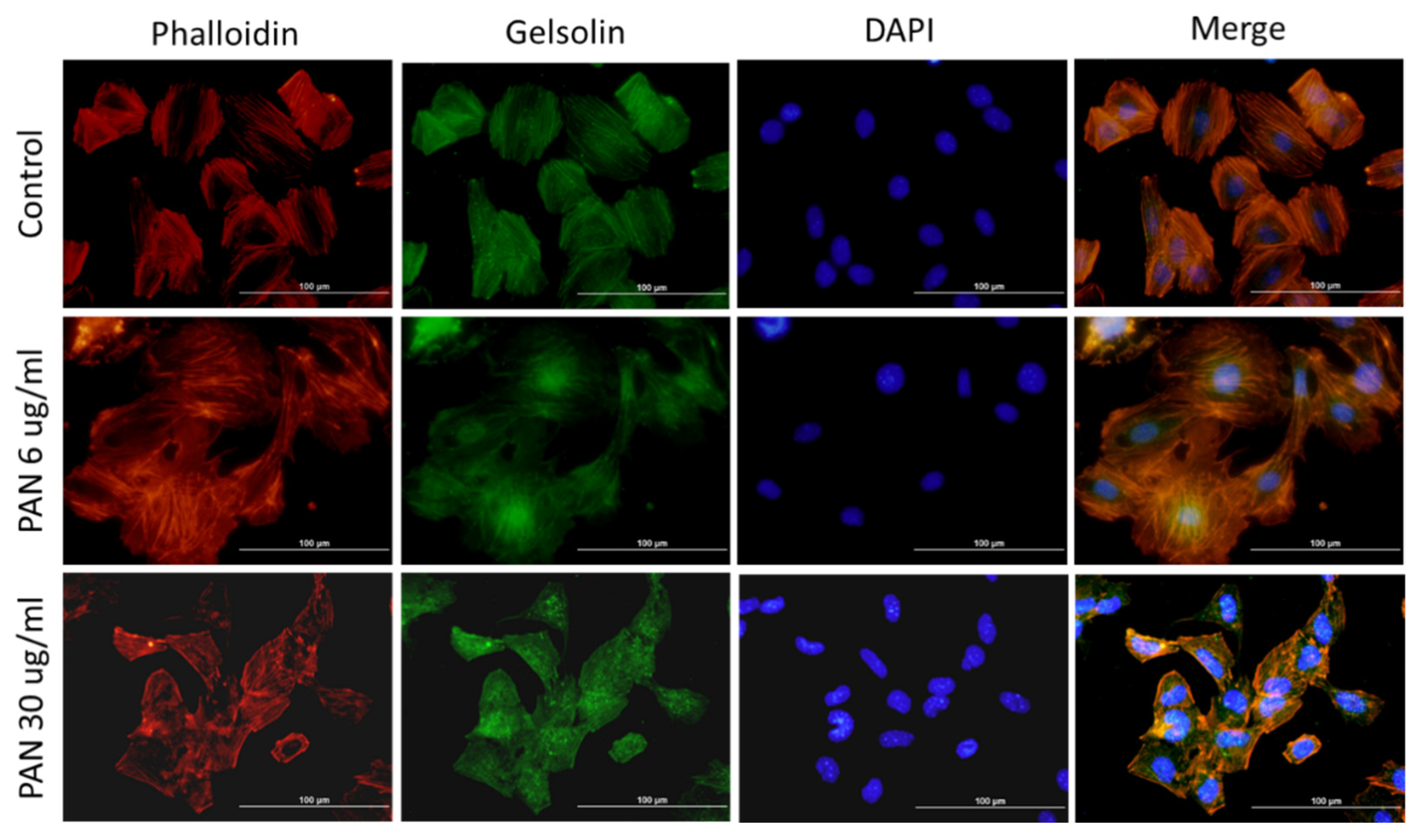
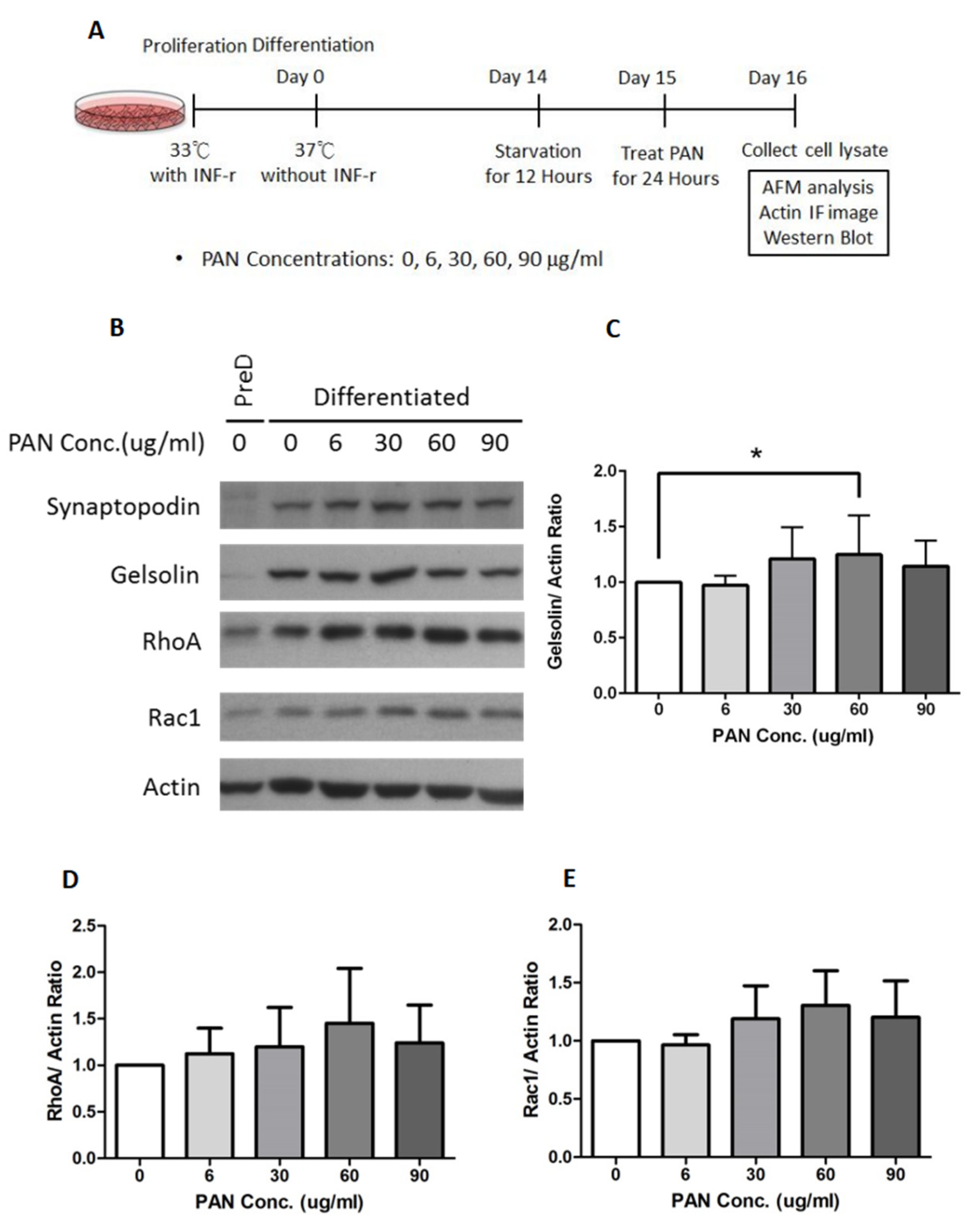
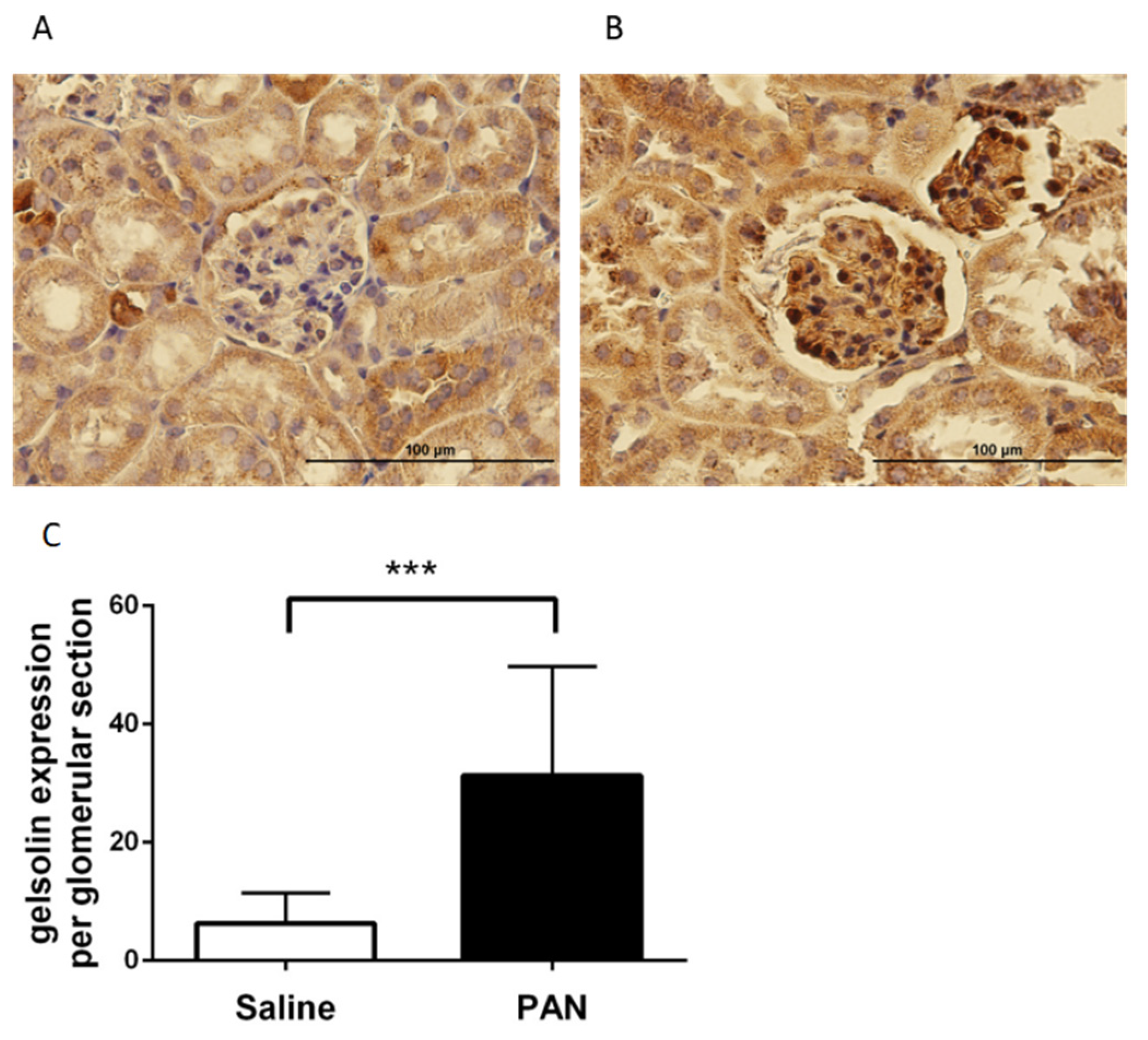
2.3. Gelsolin Expression Increased in PAN-Damaged Podocytes
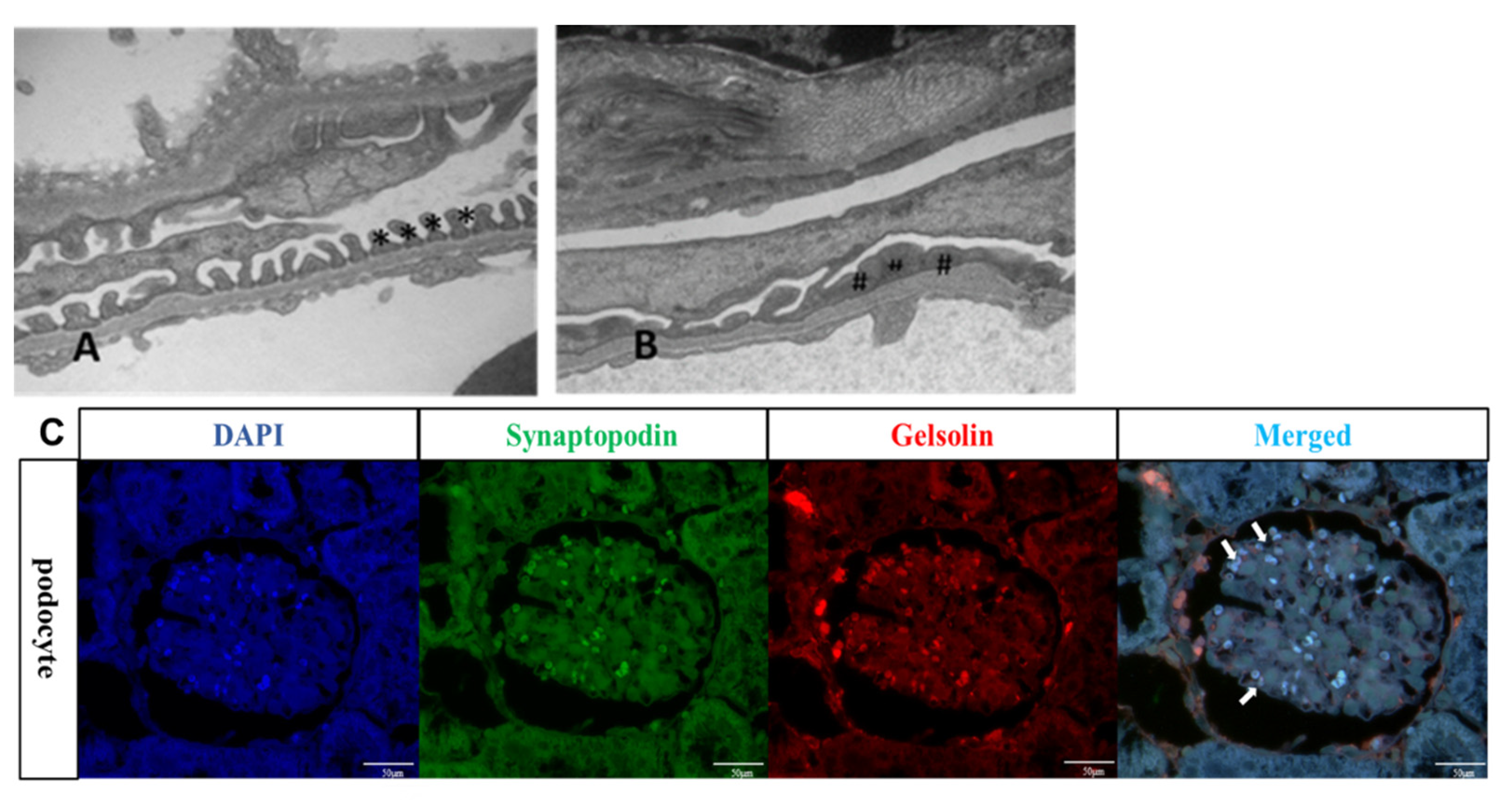
2.4. PAN-Induced CKD Mice Exhibited Effacement of the Podocyte Foot Process
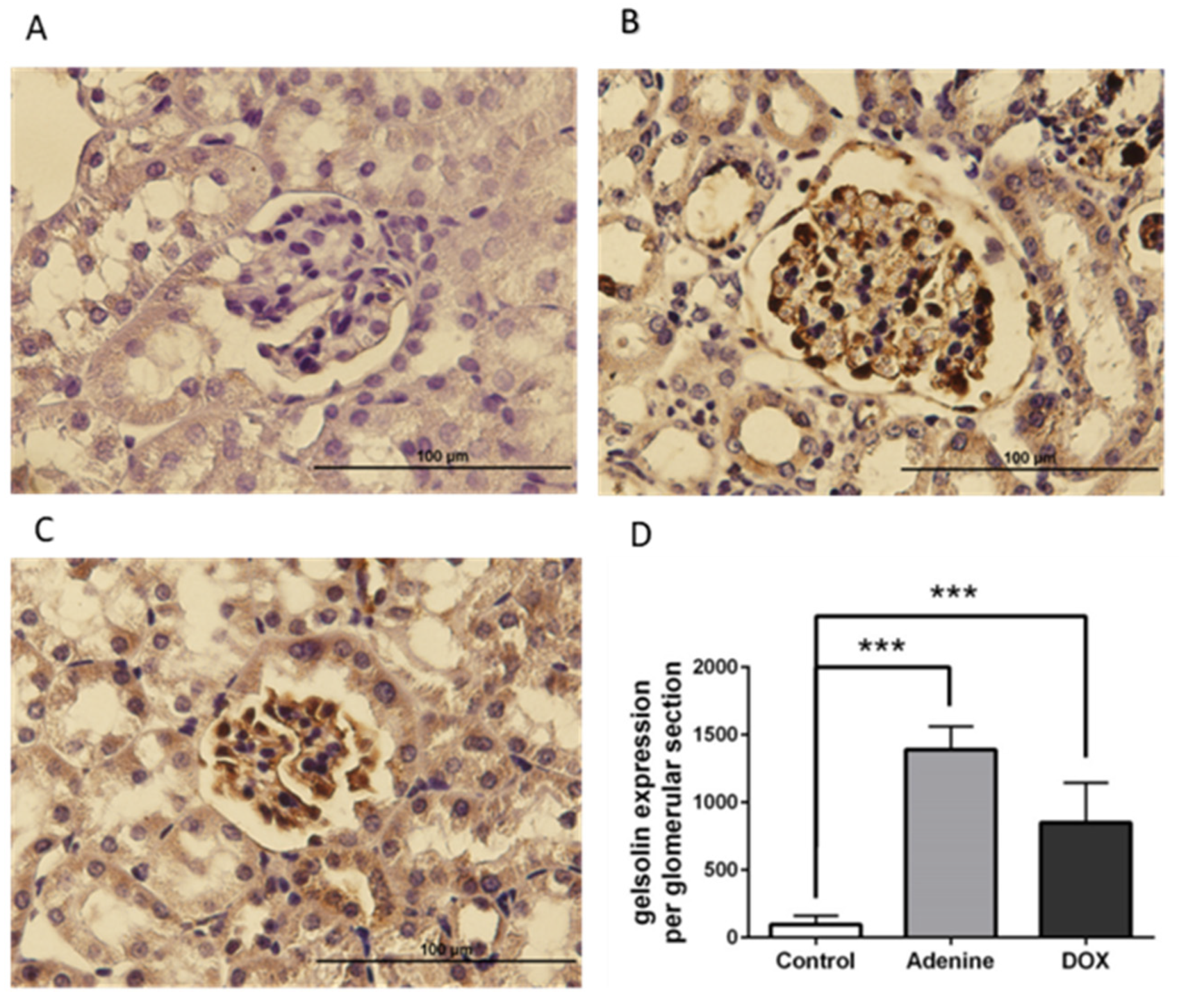
2.5. Gelsolin was Abundantly Expressed in the Glomeruli of Chronic Kidney Disease (CKD) Mice Models
3. Discussion
4. Materials and Methods
4.1. Fly Strains
4.2. AgNO3 Toxin Assay for Nephrocyte Function
4.3. Nephrocyte Dissection
4.4. Culture of Podocyte Cells
4.5. Bicinchoninic Acid (BCA) Protein Assay for Quantification of Protein Concentration
4.6. Western Blot Analysis of Gelsolin’s Effect on Podocytes
4.7. Phalloidin Actin Staining
4.8. Determination of Podocyte Cell Height and Stiffness by Atom Force Microscopy (AFM)
4.9. PAN-Induced Effacement of the Podocyte Foot Process
4.10. Immunohistochemistry Staining of Kidney Sections
4.11. Transmission Electron Microscopy (TEM) of Kidney Sections
4.12. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- De Carvalho Ribeiro, P.; Lojudice, F.H.; Fernandes-Charpiot, I.M.M.; Baptista, M.A.S.F.; de Almeida Araújo, S.; Mendes, G.E.F.; Sogayar, M.C.; Abbud-Filho, M.; Caldas, H.C. Therapeutic Potential of Human Induced Pluripotent Stem Cells and Renal Progenitor Cells in Experimental Chronic Kidney Disease. Stem Cell Res. Ther. 2020, 11, 530, 1–10. [Google Scholar] [CrossRef]
- Miner, J.H. Podocyte Biology in 2015: New Insights into the Mechanisms of Podocyte Health. Nat. Rev. Nephrol. 2016, 12, 63–64. [Google Scholar] [CrossRef]
- Liu, M.C.; Lee, Y.W.; Lee, P.T.; Chang, C.S.; Tai, Y.L.; Yu, J.R.; Su, X.T.; Hsu, L.W.; Lin, S.H.; Wu, C.H.; et al. Cyclophilin A Is Associated with Peripheral Artery Disease and Chronic Kidney Disease in Geriatrics: The Tianliao Old People (TOP) Study. Sci. Rep. 2015, 5, 9937. [Google Scholar] [CrossRef] [Green Version]
- Garg, P. A Review of Podocyte Biology. Rev. Artic. Am. J. Nephrol. 2018, 47, 3–13. [Google Scholar] [CrossRef]
- Lu, C.-C.; Wang, G.-H.; Lu, J.; Chen, P.-P.; Zhang, Y.; Hu, Z.-B.; Ma, K.-L. Role of Podocyte Injury in Glomerulosclerosis. Adv. Exp. Med. Biol. 2019, 1165, 195–232. [Google Scholar] [CrossRef]
- Blaine, J.; Dylewski, J. Regulation of the Actin Cytoskeleton in Podocytes. Cells 2020, 9, 1700. [Google Scholar] [CrossRef] [PubMed]
- Miyaki, T.; Kawasaki, Y.; Matsumoto, A.; Kakuta, S.; Sakai, T.; Ichimura, K. Nephrocytes Are Part of the Spectrum of Filtration Epithelial Diversity. Cell Tissue Res. 2020, 382, 609–625. [Google Scholar] [CrossRef] [PubMed]
- Kimbrell, D.A.; Hice, C.; Bolduc, C.; Kleinhesselink, K.; Beckingham, K. The Dorothy Enhancer Has Tinman Binding Sites and Drives Hopscotch-Induced Tumor Formation. Genesis 2002, 34, 23–28. [Google Scholar] [CrossRef]
- Zhang, F.; Zhao, Y.; Han, Z. An In Vivo Functional Analysis System for Renal Gene Discovery in Drosophila Pericardial Nephrocytes. J. Am. Soc. Nephrol. 2013, 24, 191–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heidings, J.B.; Demosthene, B.; Merlino, T.R.; Castaneda, N.; Kang, E.H. Gelsolin-Mediated Actin Filament Severing in Crowded Environments. Biochem. Biophys. Res. Commun. 2020, 532, 548–554. [Google Scholar] [CrossRef]
- Jiang, L.; Cui, H.; Ding, J.; Yang, A.; Zhang, Y. Puromycin Aminonucleoside-Induced Podocyte Injury Is Ameliorated by the Smad3 Inhibitor SIS3. FEBS Open Bio 2020, 10, 1601–1611. [Google Scholar] [CrossRef]
- Wu, J.; Zheng, C.; Wang, X.; Yun, S.; Zhao, Y.; Liu, L.; Lu, Y.; Ye, Y.; Zhu, X.; Zhang, C.; et al. MicroRNA-30 Family Members Regulate Calcium/Calcineurin Signaling in Podocytes. J. Clin. Investig. 2015, 125, 4091–4106. [Google Scholar] [CrossRef] [Green Version]
- Nielsen, R.; Christensen, E.I.; Birn, H. Megalin and Cubilin in Proximal Tubule Protein Reabsorption: From Experimental Models to Human Disease. Kidney Int. 2016, 89, 58–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, Y.-W.; Tang, H.-K.; Lin, M.-J.; Yeh, H.-H. The Influence of Physical and Physiological Cues on Atomic Force Microscopy-Based Cell Stiffness Assessment. PLoS ONE 2013, 8, 77384. [Google Scholar] [CrossRef]
- Yu, H.; Kistler, A.; Hafeez Faridi, M.; Otto Meyer, J.; Tryniszewska, B.; Mehta, D.; Yue, L.; Dryer, S.; Reiser, J. Synaptopodin Limits TRPC6 Podocyte Surface Expression and Attenuates Proteinuria. J. Am. Soc. Nephrol. 2016, 27, 3308–3319. [Google Scholar] [CrossRef]
- Asano-Matsuda, K.; Ibrahim, S.; Takano, T.; Matsuda, J.; Ungefroren, H. Molecular Sciences Role of Rho GTPase Interacting Proteins in Subcellular Compartments of Podocytes. Int. J. Mol. Sci. 2021, 22, 3656. [Google Scholar] [CrossRef] [PubMed]
- Saleem, M.A.; Welsh, G.I.; Seattle, S.A.; Usa, W. Open Peer Review Podocyte RhoGTPases: New Therapeutic Targets for Nephrotic Syndrome? F1000Research 2019, 8. [Google Scholar] [CrossRef] [PubMed]
- Ogawa-Akiyama, A.; Sugiyama Id, H.; Kitagawa, M.; Tanaka, K.; Kano, Y.; Mise, K.; Otaka, N.; Tanabe Id, K.; Morinaga, H.; Kinomura, M.; et al. Podocyte Autophagy Is Associated with Foot Process Effacement and Proteinuria in Patients with Minimal Change Nephrotic Syndrome. PLoS ONE 2020, 15, e0228337. [Google Scholar] [CrossRef]
- Piktel, E.; Levental, I.; Durna´s, B.D.; Janmey, P.A.; Bucki, R. Molecular Sciences Plasma Gelsolin: Indicator of Inflammation and Its Potential as a Diagnostic Tool and Therapeutic Target. Int. J. Mol. Sci. 2018, 19, 2516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Z.; Ting, T.; Chiou, Y.; Stossel, T.P.; Kobzik, L. Plasma Gelsolin Improves Lung Host Defense against Pneumonia by Enhancing Macrophage NOS3 Function. Am. J. Physiol. Lung Cell Mol. Physiol. 2015, 309, 11–16. [Google Scholar] [CrossRef]
- Coleman, J.R.; Moore, E.E.; Freeman, K.; Grubinger, N.D.; Hennig, G.W.; Cohen, M.J.; Samuels, J.M.; Hansen, K. Actin Is Associated with Tissue Injury in Trauma Patients and Produces a Hypercoagulable Profile In Vitro. J. Trauma Acute Care Surg. 2020, 89, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Dinsdale, R.J.; Hazeldine, J.; Al Tarrah, K.; Hampson, P.; Devi, A.; Ermogenous, C.; Bamford, A.L.; Bishop, J.; Watts, S.; Kirkman, E.; et al. Dysregulation of the Actin Scavenging System and Inhibition of DNase Activity Following Severe Thermal Injury. Br. J. Surg. 2020, 107, 391–401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flores Gama, C.; Rosales, L.M.; Ouellet, G.; Dou, Y.; Thijssen, S.; Usvyat, L.; Zhang, H.; Kuntsevich, V.; Levin, N.W.; Kotanko, P. Plasma Gelsolin and Its Association with Mortality and Hospitalization in Chronic Hemodialysis Patients. Blood Purif. 2017, 43, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Audley, J.; Gliniewicz, E.F.; Zarember, K.A.; Hong, H.S.; Wald, G.; Kuhns, D.B.; Kang, E.; Malech, H.L.; Suffredini, A.F.; Noveck, R.J.; et al. Low Plasma Gelsolin Concentrations in Chronic Granulomatous Disease. Inflammation 2021, 44, 1, 270–277. [Google Scholar] [CrossRef]
- Asare-Werehene, M.; Nakka, K.; Reunov, A.; Chiu, C.-T.; Lee, W.-T.; Abedini, M.R.; Wang, P.-W.; Shieh, D.-B.; Jeffrey Dilworth, F.; Carmona, E.; et al. The Exosome-Mediated Autocrine and Paracrine Actions of Plasma Gelsolin in Ovarian Cancer Chemoresistance. Oncogene 2020, 39, 1600–1616. [Google Scholar] [CrossRef] [Green Version]
- Shimo, T.; Adachi, Y.; Yamanouchi, S.; Tsuji, S.; Kimata, T.; Umezawa, K.; Okigaki, M.; Takaya, J.; Ikehara, S.; Kaneko, K. A Novel Nuclear Factor ΚB Inhibitor, Dehydroxymethylepoxyquinomicin, Ameliorates Puromycin Aminonucleoside-Induced Nephrosis in Mice. Am. J. Nephrol. 2013, 37, 302–309. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, C.-J.; Damaiyanti, D.W.; Yan, S.-J.; Wu, C.-H.; Tang, M.-J.; Shieh, D.-B.; Liu, P.P.; Liu, P.-Y. The Pathophysiologic Role of Gelsolin in Chronic Kidney Disease: Focus on Podocytes. Int. J. Mol. Sci. 2021, 22, 13281. https://doi.org/10.3390/ijms222413281
Yu C-J, Damaiyanti DW, Yan S-J, Wu C-H, Tang M-J, Shieh D-B, Liu PP, Liu P-Y. The Pathophysiologic Role of Gelsolin in Chronic Kidney Disease: Focus on Podocytes. International Journal of Molecular Sciences. 2021; 22(24):13281. https://doi.org/10.3390/ijms222413281
Chicago/Turabian StyleYu, Chia-Jung, Dian W. Damaiyanti, Shian-Jang Yan, Chih-Hsing Wu, Ming-Jer Tang, Dar-Bin Shieh, Peter P. Liu, and Ping-Yen Liu. 2021. "The Pathophysiologic Role of Gelsolin in Chronic Kidney Disease: Focus on Podocytes" International Journal of Molecular Sciences 22, no. 24: 13281. https://doi.org/10.3390/ijms222413281
APA StyleYu, C.-J., Damaiyanti, D. W., Yan, S.-J., Wu, C.-H., Tang, M.-J., Shieh, D.-B., Liu, P. P., & Liu, P.-Y. (2021). The Pathophysiologic Role of Gelsolin in Chronic Kidney Disease: Focus on Podocytes. International Journal of Molecular Sciences, 22(24), 13281. https://doi.org/10.3390/ijms222413281





