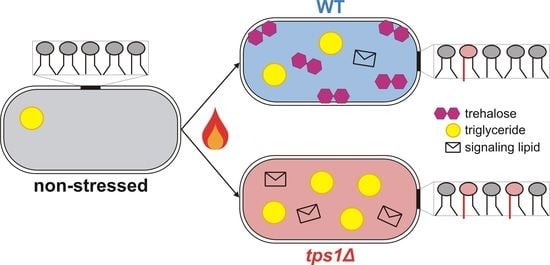
Lipids and Trehalose Actively Cooperate in Heat Stress Management of Schizosaccharomyces pombe
Abstract
1. Introduction
2. Results
2.1. Trehalose Metabolism Mutants
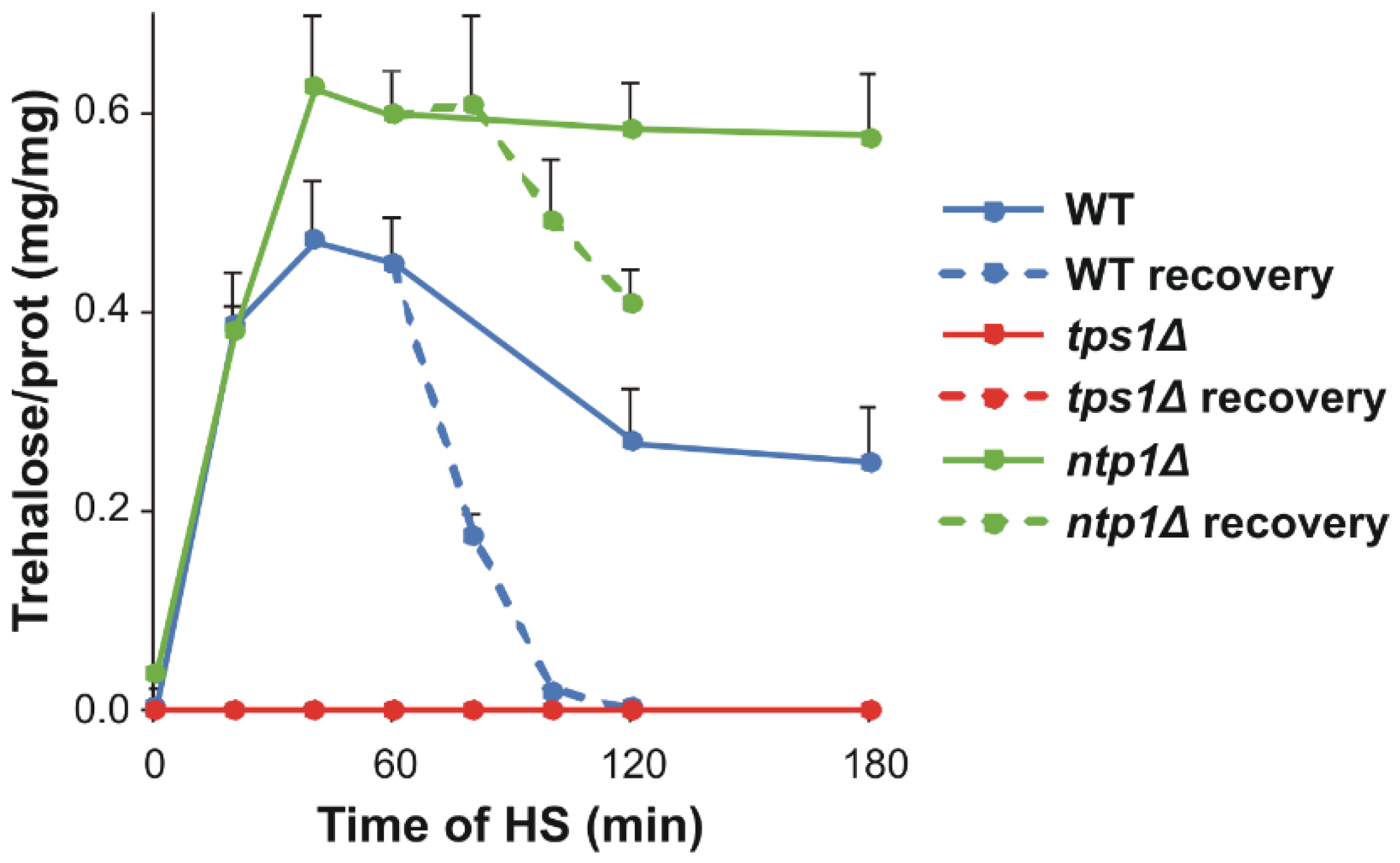
2.2. Trehalose Accumulation Due to Heat Stress
2.3. Comparative Shotgun Lipidomics
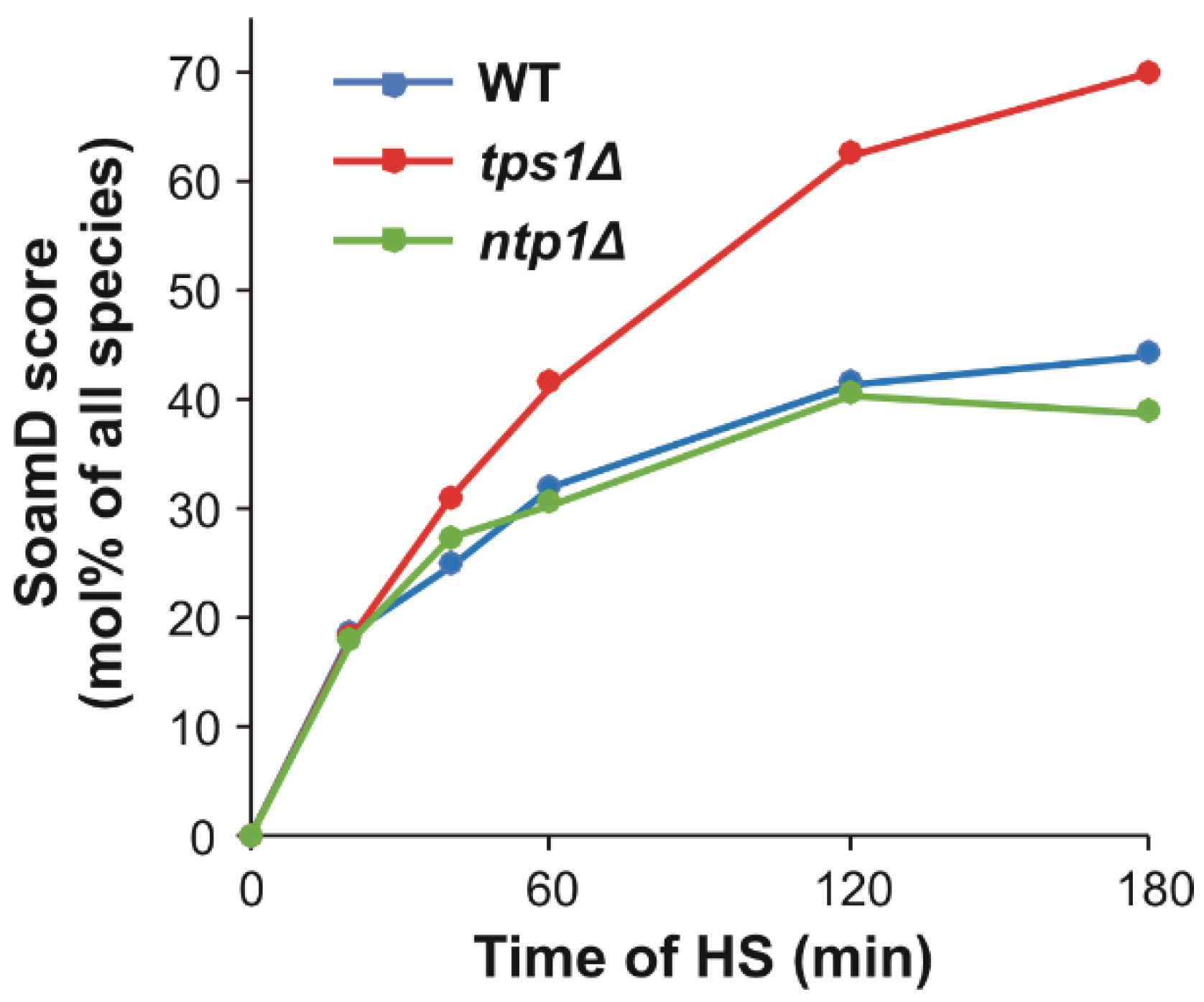
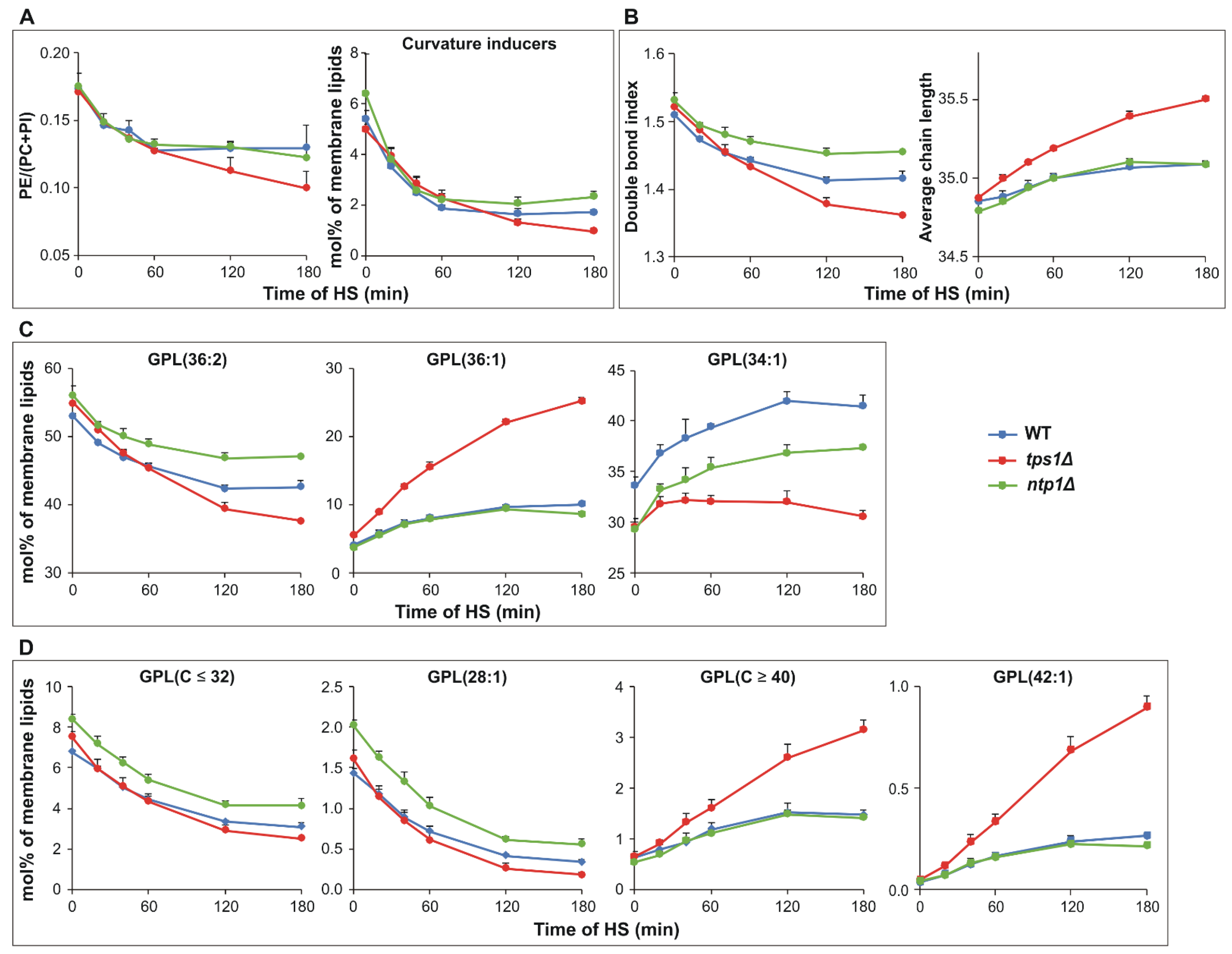
2.4. Trehalose Deficiency Induces More Pronounced Membrane Lipid Remodeling during HS
2.5. HS-Induced Signaling Lipid Generation Is Enhanced and Sustained in Trehalose-Deficient Cells
2.6. Intensified Triglyceride Synthesis in the Absence of Trehalose upon HS
2.7. De novo Fatty Acid Synthesis and Elongation Have Priority in Heat-Stressed Trehalose-Deficient Cells
2.8. Glycerophosphocholine Induction in the Absence of Trehalose
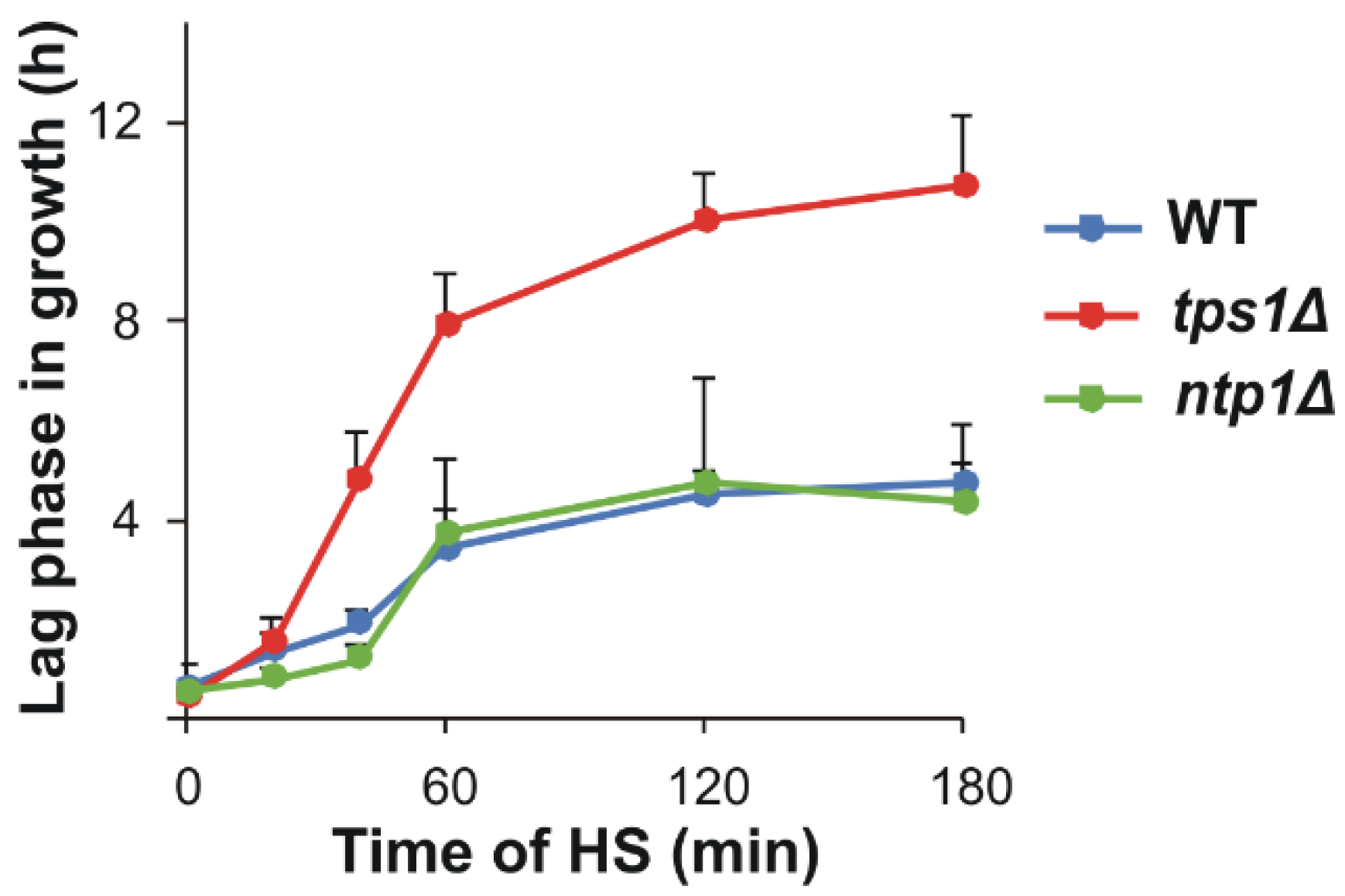
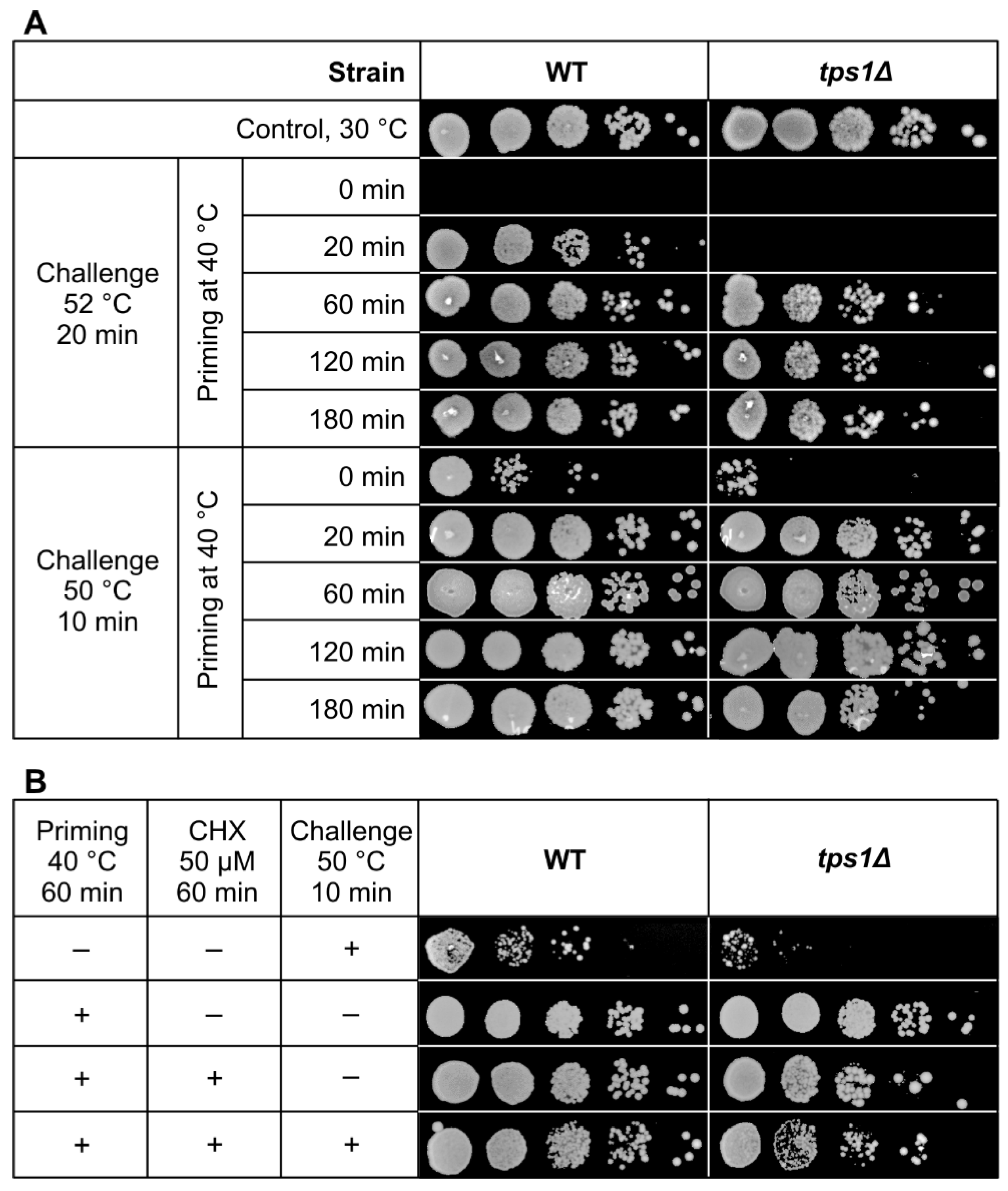
2.9. Impaired but Sizeable Acquired Thermotolerance in the Absence of Trehalose
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Yeast Strains and Culture
4.3. Heat Stress
4.4. Trehalose and Protein Quantitation
4.5. Acquisition of Thermotolerance
4.6. Mass Spectrometry-Based Lipidomics
4.7. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Morenilla-Palao, C.; Pertusa, M.; Meseguer, V.; Cabedo, H.; Viana, F. Lipid Raft Segregation Modulates TRPM8 Channel Activity. J. Biol. Chem. 2009, 284, 9215. [Google Scholar] [CrossRef]
- Yatvin, M.B.; Cramp, W.A. Role of cellular membranes in hyperthermia: Some observations and theories reviewed. Int. J. Hyperth. 1993, 9, 165–185. [Google Scholar] [CrossRef]
- Vígh, L.; Török, Z.; Balogh, G.; Glatz, A.; Piotto, S.; Horváth, I. Membrane-regulated stress response: A theoretical and practical approach. In Molecular Aspects of the Stress Response: Chaperones, Membranes and Networks. Advances in Experimental Medicine and Biology; Csermely, P., Vígh, L., Eds.; Springer: New York, NY, USA, 2007; Volume 594, pp. 114–131. ISBN 9780387399744. [Google Scholar]
- Atwoli, L.; Baqui, A.H.; Benfield, T.; Bosurgi, R.; Godlee, F.; Hancocks, S.; Horton, R.; Laybourn-Langton, L.; Monteiro, C.A.; Norman, I.; et al. Call for Emergency Action to Limit Global Temperature Increases, Restore Biodiversity, and Protect Health. N. Engl. J. Med. 2021, 385, 1134–1137. [Google Scholar] [CrossRef] [PubMed]
- Verghese, J.; Abrams, J.; Wang, Y.; Morano, K.A. Biology of the Heat Shock Response and Protein Chaperones: Budding Yeast (Saccharomyces cerevisiae) as a Model System. Microbiol. Mol. Biol. Rev. 2012, 76, 115–158. [Google Scholar] [CrossRef]
- Mühlhofer, M.; Berchtold, E.; Stratil, C.G.; Csaba, G.; Kunold, E.; Bach, N.C.; Sieber, S.A.; Haslbeck, M.; Zimmer, R.; Buchner, J. The Heat Shock Response in Yeast Maintains Protein Homeostasis by Chaperoning and Replenishing Proteins. Cell Rep. 2019, 29, 4593–4607.e8. [Google Scholar] [CrossRef] [PubMed]
- Radanović, T.; Reinhard, J.; Ballweg, S.; Pesek, K.; Ernst, R. An Emerging Group of Membrane Property Sensors Controls the Physical State of Organellar Membranes to Maintain Their Identity. BioEssays 2018, 40, 250. [Google Scholar] [CrossRef] [PubMed]
- Cowart, L.A.; Hannun, Y.A. Selective substrate supply in the regulation of yeast de novo sphingolipid synthesis. J. Biol. Chem. 2007, 282, 12330–12340. [Google Scholar] [CrossRef] [PubMed]
- De Kroon, A.I.P.M.; Rijken, P.J.; De Smet, C.H. Checks and balances in membrane phospholipid class and acyl chain homeostasis, the yeast perspective. Prog. Lipid Res. 2013, 52, 374–394. [Google Scholar] [CrossRef] [PubMed]
- Pan, D.; Wiedemann, N.; Kammerer, B. Heat stress-induced metabolic remodeling in Saccharomyces cerevisiae. Metabolites 2019, 9, 266. [Google Scholar] [CrossRef]
- Péter, M.; Glatz, A.; Gudmann, P.; Gombos, I.; Török, Z.; Horváth, I.; Vígh, L.; Balogh, G. Metabolic crosstalk between membrane and storage lipids facilitates heat stress management in Schizosaccharomyces pombe. PLoS ONE 2017, 12, 3739. [Google Scholar] [CrossRef]
- Crowe, J.H.; Crowe, L.M.; Jackson, S.A. Preservation of structural and functional activity in lyophilized sarcoplasmic reticulum. Arch. Biochem. Biophys. 1983, 220, 477–484. [Google Scholar] [CrossRef]
- Crowe, J.H. Trehalose as a “chemical chaperone”: Fact and fantasy. Adv. Exp. Med. Biol. 2007, 594, 143–158. [Google Scholar] [CrossRef]
- Gibney, P.A.; Schieler, A.; Chen, J.C.; Rabinowitz, J.D.; Botstein, D. Characterizing the in vivo role of trehalose in Saccharomyces cerevisiae using the AGT1 transporter. Proc. Natl. Acad. Sci. USA 2015, 112, 6116–6121. [Google Scholar] [CrossRef]
- Arai, C.; Suyama, A.; Arai, S.; Arai, N.; Yoshizane, C.; Koya-Miyata, S.; Mizote, A.; Endo, S.; Ariyasu, T.; Mitsuzumi, H.; et al. Trehalose itself plays a critical role on lipid metabolism: Trehalose increases jejunum cytoplasmic lipid droplets which negatively correlated with mesenteric adipocyte size in both HFD-fed trehalase KO and WT mice. Nutr. Metab. 2020, 17, 1–12. [Google Scholar] [CrossRef]
- Yoshizane, C.; Mizote, A.; Arai, C.; Arai, N.; Ogawa, R.; Endo, S.; Mitsuzumi, H.; Ushio, S. Daily consumption of one teaspoon of trehalose can help maintain glucose homeostasis: A double-blind, randomized controlled trial conducted in healthy volunteers. Nutr. J. 2020, 19, 586. [Google Scholar] [CrossRef] [PubMed]
- Eleutherio, E.; Panek, A.; De Mesquita, J.F.; Trevisol, E.; Magalhães, R. Revisiting yeast trehalose metabolism. Curr. Genet. 2015, 61, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Singer, M.A.; Lindquist, S. Thermotolerance in Saccharomyces cerevisiae: The Yin and Yang of trehalose. Trends Biotechnol. 1998, 16, 460–468. [Google Scholar] [CrossRef]
- Magalhães, R.S.S.; Popova, B.; Braus, G.H.; Outeiro, T.F.; Eleutherio, E.C.A. The trehalose protective mechanism during thermal stress in Saccharomyces cerevisiae: The roles of Ath1 and Agt1. FEMS Yeast Res. 2018, 18, 66. [Google Scholar] [CrossRef] [PubMed]
- De Virgilio, C.; Hottiger, T.; Dominguez, J.; Boller, T.; Wiemken, A. The role of trehalose synthesis for the acquisition of thermotolerance in yeast: I. Genetic evidence that trehalose is a thermoprotectant. Eur. J. Biochem. 1994, 219, 179–186. [Google Scholar] [CrossRef]
- De Virgilio, C.; Simmen, U.; Hottiger, T.; Boiler, T.; Wiemken, A. Heat shock induces enzymes of trehalose metabolism, trehalose accumulation, and thermotolerance in Schizosaccharomyces pombe, even in the presence of cycloheximide. FEBS Lett. 1990, 273, 107–110. [Google Scholar] [CrossRef]
- Ribeiro, M.J.S.; Reinders, A.; Boller, T.; Wiemken, A.; De Virgilio, C. Trehalose synthesis is important for the acquisition of thermotolerance in Schizosaccharomyces pombe. Mol. Microbiol. 1997, 25, 571–581. [Google Scholar] [CrossRef]
- Hottiger, T.; De Virgilio, C.; Hall, M.N.; Boller, T.; Wiemken, A. The role of trehalose synthesis for the acquisition of thermotolerance in yeast: II. Physiological concentrations of trehalose increase the thermal stability of proteins in vitro. Eur. J. Biochem. 1994, 219, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Dupont, S.; Beney, L.; Ritt, J.F.; Lherminier, J.; Gervais, P. Lateral reorganization of plasma membrane is involved in the yeast resistance to severe dehydration. Biochim. Biophys. Acta-Biomembr. 2010, 1798, 975–985. [Google Scholar] [CrossRef] [PubMed]
- Soto, T.; Fernández, J.; Vicente-Soler, J.; Cansado, J.; Gacto, M. Accumulation of trehalose by overexpression of tps1, coding for trehalose-6-phosphate synthase, causes increased resistance to multiple stresses in the fission yeast Schizosaccharomyces pombe. Appl. Environ. Microbiol. 1999, 65, 2020–2024. [Google Scholar] [CrossRef]
- Singer, M.A.; Lindquist, S. Multiple effects of trehalose on protein folding in vitro and in vivo. Mol. Cell 1998, 1, 639–648. [Google Scholar] [CrossRef]
- Wera, S.; Schrijver, E.; De Geyskens, I.; Nwaka, S.; Thevelein, J.M. Opposite roles of trehalase activity in heat-shock recovery and heat-shock survival in Saccharomyces cerevisiae. Biochem. J. 1999, 343, 621. [Google Scholar] [CrossRef] [PubMed]
- Sinensky, M. Homeoviscous adaptation: A homeostatic process that regulates the viscosity of membrane lipids in Escherichia coli. Proc. Natl. Acad. Sci. USA 1974, 71, 522–525. [Google Scholar] [CrossRef]
- Guschina, I.A.; Harwood, J.L. Mechanisms of temperature adaptation in poikilotherms. FEBS Lett. 2006, 580, 5477–5483. [Google Scholar] [CrossRef] [PubMed]
- Klug, L.; Daum, G. Yeast lipid metabolism at a glance. FEMS Yeast Res. 2014, 14, 369–388. [Google Scholar] [CrossRef]
- Renne, M.F.; Bao, X.; De Smet, C.H.; de Kroon, A.I.P.M. Lipid acyl chain remodeling in yeast. Lipid Insights 2015, 2015, 33–40. [Google Scholar] [CrossRef]
- Ernst, R.; Ballweg, S.; Levental, I. Cellular mechanisms of physicochemical membrane homeostasis. Curr. Opin. Cell Biol. 2018, 53, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Ernst, R.; Ejsing, C.S.; Antonny, B. Homeoviscous Adaptation and the Regulation of Membrane Lipids. J. Mol. Biol. 2016, 428, 4776–4791. [Google Scholar] [CrossRef]
- Ejsing, C.S.; Sampaio, J.L.; Surendranath, V.; Duchoslav, E.; Ekroos, K.; Klemm, R.W.; Simons, K.; Shevchenko, A. Global analysis of the yeast lipidome by quantitative shotgun mass spectrometry. Proc. Natl. Acad. Sci. USA 2009, 106, 2136–2141. [Google Scholar] [CrossRef] [PubMed]
- Glatz, A.; Pilbat, A.M.; Németh, G.L.; Vince-Kontár, K.; Jósvay, K.; Hunya, Á.; Udvardy, A.; Gombos, I.; Péter, M.; Balogh, G.; et al. Involvement of small heat shock proteins, trehalose, and lipids in the thermal stress management in Schizosaccharomyces pombe. Cell Stress Chaperones 2016, 21, 327–338. [Google Scholar] [CrossRef]
- Jenkins, G.M.; Ashley Cowart, L.; Signorelli, P.; Pettus, B.J.; Chalfant, C.E.; Hannun, Y.A. Acute activation of de novo sphingolipid biosynthesis upon heat shock causes an accumulation of ceramide and subsequent dephosphorylation of SR proteins. J. Biol. Chem. 2002, 277, 42572–42578. [Google Scholar] [CrossRef]
- Hannun, Y.A.; Obeid, L.M. Principles of bioactive lipid signalling: Lessons from sphingolipids. Nat. Rev. Mol. Cell Biol. 2008, 9, 139–150. [Google Scholar] [CrossRef]
- Dickson, R.C. New insights into sphingolipid metabolism and function in budding yeast. J. Lipid Res. 2008, 49, 909–921. [Google Scholar] [CrossRef]
- Peksel, B.; Gombos, I.; Péter, M.; Vigh, L.; Tiszlavicz, Á.; Brameshuber, M.; Balogh, G.; Schütz, G.J.; Horváth, I.; Veigh, L.; et al. Mild heat induces a distinct “eustress” response in Chinese Hamster Ovary cells but does not induce heat shock protein synthesis. Sci. Rep. 2017, 7, 15821. [Google Scholar] [CrossRef]
- Balogh, G.; Péter, M.; Glatz, A.; Gombos, I.; Török, Z.; Horváth, I.; Harwood, J.L.; Vígh, L. Key role of lipids in heat stress management. FEBS Lett. 2013, 587, 1970–1980. [Google Scholar] [CrossRef]
- Dickson, R.C.; Sumanasekera, C.; Lester, R.L. Functions and metabolism of sphingolipids in Saccharomyces cerevisiae. Prog. Lipid Res. 2006, 45, 447–465. [Google Scholar] [CrossRef]
- Hannun, Y.A.; Luberto, C. Ceramide in the eukaryotic stress response. Trends Cell Biol. 2000, 10, 73–80. [Google Scholar] [CrossRef]
- Blázquez, M.A.; Stucka, R.; Feldmann, H.; Gancedo, C. Trehalose-6-P synthase is dispensable for growth on glucose but not for spore germination in Schizosaccharomyces pombe. J. Bacteriol. 1994, 176, 3895. [Google Scholar] [CrossRef] [PubMed]
- Tarasov, K.; Stefanko, A.; Casanovas, A.; Surma, M.A.; Berzina, Z.; Hannibal-Bach, H.K.; Ekroos, K.; Ejsing, C.S. High-content screening of yeast mutant libraries by shotgun lipidomics. Mol. Biosyst. 2014, 10, 1364–1376. [Google Scholar] [CrossRef] [PubMed]
- Obeid, L.M.; Okamoto, Y.; Mao, C. Yeast sphingolipids: Metabolism and biology. Biochim. Biophys. Acta-Mol. Cell Biol. Lipids 2002, 1585, 163–171. [Google Scholar] [CrossRef]
- Eichmann, T.O.; Lass, A. DAG tales: The multiple faces of diacylglycerol-Stereochemistry, metabolism, and signaling. Cell. Mol. Life Sci. 2015, 72, 3931–3952. [Google Scholar] [CrossRef] [PubMed]
- Olzmann, J.A.; Carvalho, P. Dynamics and functions of lipid droplets. Nat. Rev. Mol. Cell Biol. 2019, 20, 137–155. [Google Scholar] [CrossRef]
- Anthoni, U.; Christophersen, C.; Hougaard, L.; Nielsen, P.H. Quaternary ammonium compounds in the biosphere-An example of a versatile adaptive strategy. Comp. Biochem. Physiol.—Part B Biochem. 1991, 99, 1–18. [Google Scholar] [CrossRef]
- Piper, P.W. Molecular events associated with acquisition of heat tolerance by the yeast Saccharomyces cerevisiae. FEMS Microbiol. Rev. 1993, 11, 339–355. [Google Scholar] [CrossRef]
- Parsell, D.A.; Kowal, A.S.; Singer, M.A.; Lindquist, S. Protein disaggregation mediated by heat-shock protein Hspl04. Nature 1994, 372, 475–478. [Google Scholar] [CrossRef]
- Erdbrügger, P.; Fröhlich, F. The role of very long chain fatty acids in yeast physiology and human diseases. Biol. Chem. 2020, 402, 25–38. [Google Scholar] [CrossRef]
- Schneiter, R.; Brügger, B.; Amann, C.M.; Prestwich, G.D.; Epand, R.F.; Zellnig, G.; Wieland, F.T.; Epand, R.M. Identification and biophysical characterization of a very-long-chain-fatty- acid-substituted phosphatidylinositol in yeast subcellular membranes. Biochem. J. 2004, 381, 941–949. [Google Scholar] [CrossRef]
- Řezanka, T.; Sigler, K. Odd-numbered very-long-chain fatty acids from the microbial, animal and plant kingdoms. Prog. Lipid Res. 2009, 48, 206–238. [Google Scholar] [CrossRef] [PubMed]
- Cerbón, J.; Falcon, A.; Hernández-Luna, C.; Segura-Cobos, D. Inositol phosphoceramide synthase is a regulator of intracellular levels of diacylglycerol and ceramide during the G1 to S transition in Saccharomyces cerevisiae. Biochem. J. 2005, 388, 169–176. [Google Scholar] [CrossRef]
- Low, C.P.; Shui, G.; Liew, L.P.; Buttner, S.; Madeo, F.; Dawes, I.W.; Wenk, M.R.; Yang, H.R. Caspase-dependent and -independent lipotoxic cell-death pathways in fission yeast. J. Cell Sci. 2008, 121, 2671–2684. [Google Scholar] [CrossRef]
- Kiewietdejonge, A.; Pitts, M.; Cabuhat, L.; Sherman, C.; Kladwang, W.; Miramontes, G.; Floresvillar, J.; Chan, J.; Ramirez, R.M. Hypersaline stress induces the turnover of phosphatidylcholine and results in the synthesis of the renal osmoprotectant glycerophosphocholine in Saccharomyces cerevisiae. FEMS Yeast Res. 2006, 6, 205–217. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kwon, E.D.; Kyu, Y.J.; Edsall, L.C.; Kim, H.Y.; Garcia-Perez, A.; Burg, M.B. Osmotic regulation of synthesis of glycerophosphocholine from phosphatidylcholine in MDCK cells. Am. J. Physiol.-Cell Physiol. 1995, 268, 402. [Google Scholar] [CrossRef] [PubMed]
- Szabó, E.R.; Plangár, I.; Tokés, T.; Mán, I.; Polanek, R.; Kovács, R.; Fekete, G.; Szabó, Z.; Csenki, Z.; Baska, F.; et al. L-alpha glycerylphosphorylcholine as a potential radioprotective agent in zebrafish embryo model. Zebrafish 2016, 13, 481–488. [Google Scholar] [CrossRef]
- Mitra, J. Genetics and genetic improvement of drought resistance in crop plants. Curr. Sci. 2001, 80, 758–763. [Google Scholar]
- Lavoie, J.N.; Gingras-Breton, G.; Tanguay, R.M.; Landry, J. Induction of Chinese hamster HSP27 gene expression in mouse cells confers resistance to heat shock. HSP27 stabilization of the microfilament organization. J. Biol. Chem. 1993, 268, 3420–3429. [Google Scholar] [CrossRef]
- Quinn, P.J.; Joo, F.; Vigh, L. The role of unsaturated lipids in membrane structure and stability. Prog. Biophys. Mol. Biol. 1989, 53, 71–103. [Google Scholar] [CrossRef]
- Makarova, M.; Peter, M.; Balogh, G.; Glatz, A.; MacRae, J.I.; Lopez Mora, N.; Booth, P.; Makeyev, E.; Vigh, L.; Oliferenko, S. Delineating the Rules for Structural Adaptation of Membrane-Associated Proteins to Evolutionary Changes in Membrane Lipidome. Curr. Biol. 2020, 30, 367–380.e8. [Google Scholar] [CrossRef]
- Smith, P.; Owen, D.M.; Lorenz, C.D.; Makarova, M. Asymmetric glycerophospholipids impart distinctive biophysical properties to lipid bilayers. Biophys. J. 2021, 120, 1746–1754. [Google Scholar] [CrossRef]
- Hazell, B.W.; Nevalainen, H.; Attfield, P.V. Evidence that the Saccharomyces cerevisiae CIF1 (GGS1/TPS1) gene modulates heat shock response positively. FEBS Lett. 1995, 377, 457–460. [Google Scholar] [CrossRef] [PubMed]
- Crowe, J.H.; Crowe, L.M.; Chapman, D. Preservation of membranes in anhydrobiotic organisms: The role of trehalose. Science 1984, 223, 701–703. [Google Scholar] [CrossRef] [PubMed]
- Luzardo, M.C.; Amalfa, F.; Nuñez, A.M.; Díaz, S.; Lopez, A.C.B.; De Disalvo, E.A. Effect of trehalose and sucrose on the hydration and dipole potential of lipid bilayers. Biophys. J. 2000, 78, 2452. [Google Scholar] [CrossRef]
- Ohtake, S.; Schebor, C.; de Pablo, J.J. Effects of trehalose on the phase behavior of DPPC-cholesterol unilamellar vesicles. Biochim. Biophys. Acta-Biomembr. 2006, 1758, 65–73. [Google Scholar] [CrossRef][Green Version]
- Pereira, C.S.; Lins, R.D.; Chandrasekhar, I.; Freitas, L.C.G.; Hünenberger, P.H. Interaction of the Disaccharide Trehalose with a Phospholipid Bilayer: A Molecular Dynamics Study. Biophys. J. 2004, 86, 2273–2285. [Google Scholar] [CrossRef]
- Doxastakis, M.; Sum, A.K.; De Pablo, J.J. Modulating membrane properties: The effect of trehalose and cholesterol on a phospholipid bilayer. J. Phys. Chem. B 2005, 109, 24173–24181. [Google Scholar] [CrossRef]
- Mensonides, F.I.C.; Brul, S.; Klis, F.M.; Hellingwerf, K.J.; De Mattos, M.J.T. Activation of the protein kinase C1 pathway upon continuous heat stress in Saccharomyces cerevisiae is triggered by an intracellular increase in osmolarity due to trehalose accumulation. Appl. Environ. Microbiol. 2005, 71, 4531–4538. [Google Scholar] [CrossRef][Green Version]
- Török, Z.; Goloubinoff, P.; Horváth, I.; Tsvetkova, N.M.; Glatz, A.; Balogh, G.; Varvasovszki, V.; Los, D.A.; Vierling, E.; Crowe, J.H.; et al. Synechocystis HSP17 is an amphitropic protein that stabilizes heat-stressed membranes and binds denatured proteins for subsequent chaperone-mediated refolding. Proc. Natl. Acad. Sci. USA 2001, 98, 3098–3103. [Google Scholar] [CrossRef]
- Balogi, Z.; Multhoff, G.; Jensen, T.K.; Lloyd-Evans, E.; Yamashima, T.; Jäättelä, M.; Harwood, J.L.; Vígh, L. Hsp70 interactions with membrane lipids regulate cellular functions in health and disease. Prog. Lipid Res. 2019, 74, 18–30. [Google Scholar] [CrossRef]
- Horváth, I.; Multhoff, G.; Sonnleitner, A.; Vígh, L. Membrane-associated stress proteins: More than simply chaperones. Biochim. Biophys. Acta-Biomembr. 2008, 1778, 1653–1664. [Google Scholar] [CrossRef] [PubMed]
- Cansado, J.; Soto, T.; Fernandez, J.; Vicente-Soler, J.; Gacto, M. Characterization of mutants devoid of neutral trehalase activity in the fission yeast Schizosaccharomyces pombe: Partial protection from heat shock and high-salt stress. J. Bacteriol. 1998, 180, 1342–1345. [Google Scholar] [CrossRef]
- Forsburg, S.L. Growth and Manipulation of S. pombe. Curr. Protoc. Mol. Biol. 2003, 13, 64. [Google Scholar] [CrossRef] [PubMed]
- Herzog, R.; Schwudke, D.; Schuhmann, K.; Sampaio, J.L.; Bornstein, S.R.; Schroeder, M.; Shevchenko, A. A novel informatics concept for high-throughput shotgun lipidomics based on the molecular fragmentation query language. Genome Biol. 2011, 12, 8. [Google Scholar] [CrossRef] [PubMed]
- Fahy, E.; Subramaniam, S.; Murphy, R.C.; Nishijima, M.; Raetz, C.R.H.; Shimizu, T.; Spener, F.; Meer, G.; van Wakelam, M.J.O.; Dennis, E.A. Update of the LIPID MAPS comprehensive classification system for lipids. J. Lipid Res. 2009, 50, S9. [Google Scholar] [CrossRef]
- Liebisch, G.; Vizcaíno, J.A.; Köfeler, H.; Trötzmüller, M.; Griffiths, W.J.; Schmitz, G.; Spener, F.; Wakelam, M.J.O. Shorthand notation for lipid structures derived from mass spectrometry. J. Lipid Res. 2013, 54, 1523. [Google Scholar] [CrossRef]
- Storey, J.D.; Tibshirani, R. Statistical significance for genomewide studies. Proc. Natl. Acad. Sci. USA 2003, 100, 9440–9445. [Google Scholar] [CrossRef]
- Xia, J.; Wishart, D.S. Web-based inference of biological patterns, functions and pathways from metabolomic data using MetaboAnalyst. Nat. Protoc. 2011, 6, 743–760. [Google Scholar] [CrossRef] [PubMed]
- Öksüz, H.B.; Buzrul, S. MONTE CARLO ANALYSIS FOR MICROBIAL GROWTH CURVES. J. Microbiol. Biotechnol. food Sci. 2020, 10, 418–423. [Google Scholar] [CrossRef]
- Baranyi, J.; Roberts, T.A. A dynamic approach to predicting bacterial growth in food. Int. J. Food Microbiol. 1994, 23, 277–294. [Google Scholar] [CrossRef]
- Kvalseth, T.O. Cautionary Note about R 2. Am. Stat. 1985, 39, 279. [Google Scholar] [CrossRef]
- Sekova, V.Y.; Dergacheva, D.I.; Isakova, E.P.; Gessler, N.N.; Tereshina, V.M.; Deryabina, Y.I. Soluble sugar and lipid readjustments in the yarrowia lipolytica yeast at various temperatures and ph. Metabolites 2019, 9, 307. [Google Scholar] [CrossRef]
- Delorge, I.; Janiak, M.; Carpentier, S.; Van Dijck, P. Fine tuning of trehalose biosynthesis and hydrolysis as novel tools for the generation of abiotic stress tolerant plants. Front. Plant Sci. 2014, 5, 147. [Google Scholar] [CrossRef]
- Rasulova, M.; Zečić, A.; Moreno, J.M.M.; Vandemeulebroucke, L.; Dhondt, I.; Braeckman, B.P. Elevated trehalose levels in c. Elegans daf-2 mutants increase stress resistance, not lifespan. Metabolites 2021, 11, 105. [Google Scholar] [CrossRef] [PubMed]
- Van Laere, A. Trehalose, reserve and/or stress metabolite? FEMS Microbiol. Lett. 1989, 63, 201–209. [Google Scholar] [CrossRef]
- Kieliszek, M.; Bierla, K.; Jiménez-Lamana, J.; Kot, A.M.; Alcántara-Durán, J.; Piwowarek, K.; Błażejak, S.; Szpunar, J. Metabolic response of the yeast candida utilis during enrichment in Selenium. Int. J. Mol. Sci. 2020, 21, 5287. [Google Scholar] [CrossRef] [PubMed]
- Keuenhof, K.S.; Larsson Berglund, L.; Malmgren Hill, S.; Schneider, K.L.; Widlund, P.O.; Nyström, T.; Höög, J.L. Large organellar changes occur during mild heat shock in yeast. J. Cell Sci. 2022, 135, 8325. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Péter, M.; Gudmann, P.; Kóta, Z.; Török, Z.; Vígh, L.; Glatz, A.; Balogh, G. Lipids and Trehalose Actively Cooperate in Heat Stress Management of Schizosaccharomyces pombe. Int. J. Mol. Sci. 2021, 22, 13272. https://doi.org/10.3390/ijms222413272
Péter M, Gudmann P, Kóta Z, Török Z, Vígh L, Glatz A, Balogh G. Lipids and Trehalose Actively Cooperate in Heat Stress Management of Schizosaccharomyces pombe. International Journal of Molecular Sciences. 2021; 22(24):13272. https://doi.org/10.3390/ijms222413272
Chicago/Turabian StylePéter, Mária, Péter Gudmann, Zoltán Kóta, Zsolt Török, László Vígh, Attila Glatz, and Gábor Balogh. 2021. "Lipids and Trehalose Actively Cooperate in Heat Stress Management of Schizosaccharomyces pombe" International Journal of Molecular Sciences 22, no. 24: 13272. https://doi.org/10.3390/ijms222413272
APA StylePéter, M., Gudmann, P., Kóta, Z., Török, Z., Vígh, L., Glatz, A., & Balogh, G. (2021). Lipids and Trehalose Actively Cooperate in Heat Stress Management of Schizosaccharomyces pombe. International Journal of Molecular Sciences, 22(24), 13272. https://doi.org/10.3390/ijms222413272