Lactate Suppresses Retroviral Transduction in Cervical Epithelial Cells through DNA-PKcs Modulation
Abstract
1. Introduction
2. Results
2.1. L- and D-Lactate Stimulate DNA-PKcs Nuclear Translocation in Cancer Cervical Epithelial Cells
2.2. L- and D-Lactate Suppress Lentiviral Transduction in Cancer Cervical Epithelial Cells
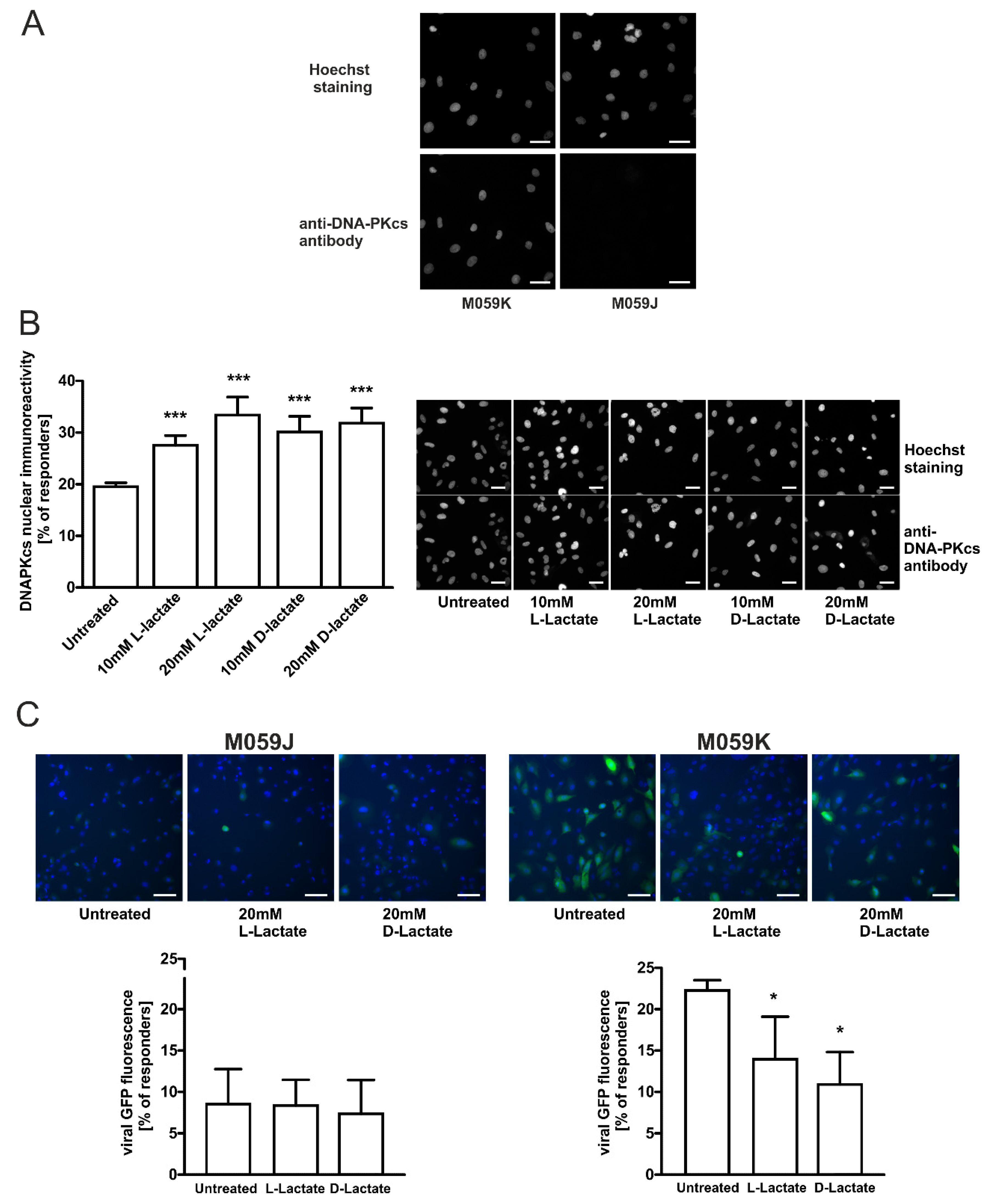
2.3. DNA-PKcs-Proficient Glioma Cells Are Prone to Lactate-Driven Modulation of DNA-PKcs and Lentivirus Transduction Efficacy but Not DNA-PKcs-Deficient Counterpart
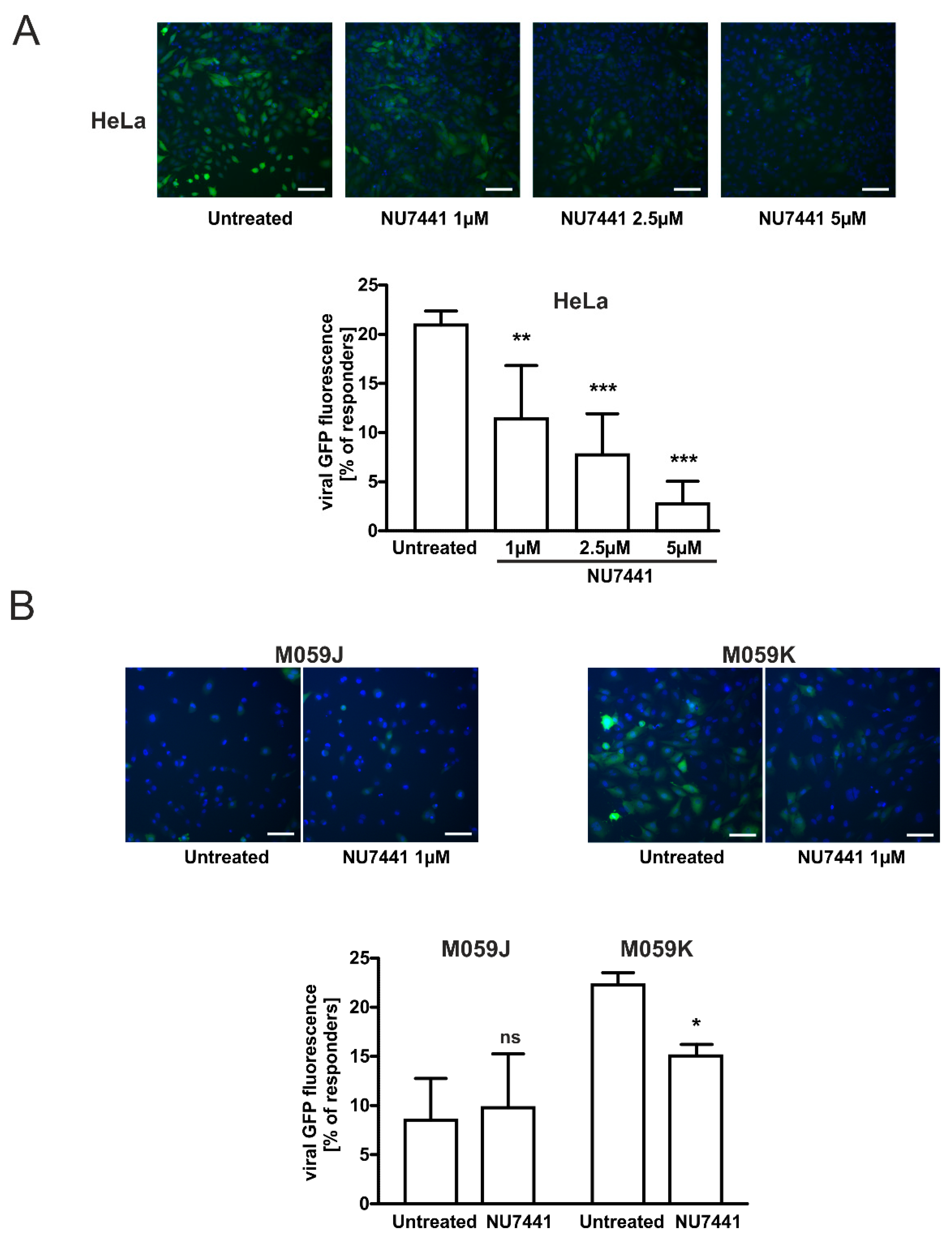
2.4. Specific DNA-PKcs Inhibitor NU7441 Affects Lentivirus Transduction Efficacy
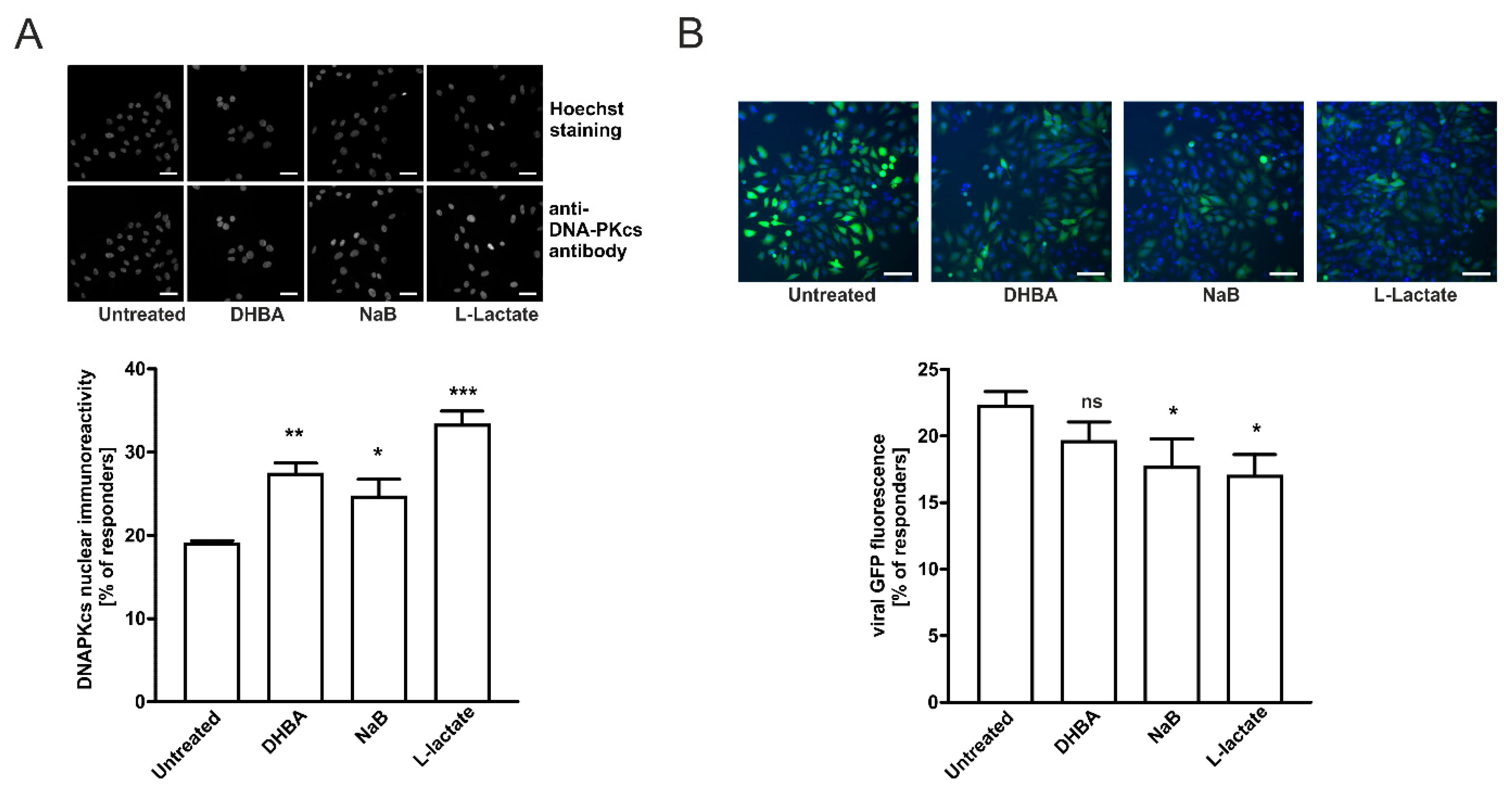
2.5. HCA1 and HDAC Activities Are Required for Lactate-Mediated Enhancement of DNA-PKcs Nuclear Localization in HeLa Cells
2.6. Enhancement of DNA-PKcs Nuclear Translocation by Intra/Extracellular Lactate Is Related to Monocarboxylate Transporters Activity
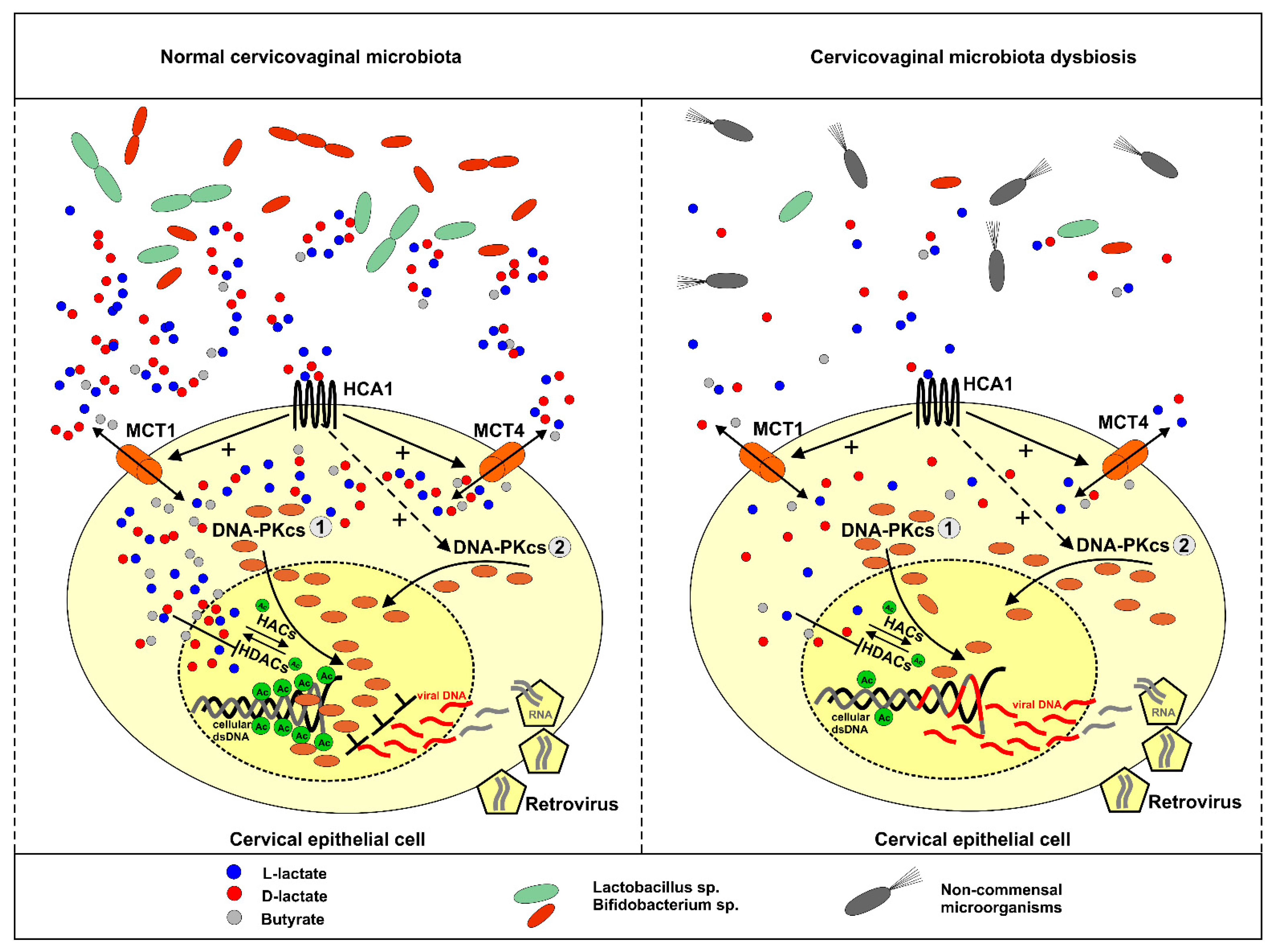
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Cell Culture
4.3. DNA-PKcs Immunocytochemistry
4.4. Western Blot Analysis
4.5. Single Round Transduction Assay
4.6. Population Characterization (Responders) Principles
4.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Boskey, E.R.; Cone, R.A.; Whaley, K.J.; Moench, T.R. Origins of vaginal acidity: High D/L lactate ratio is consistent with bacteria being the primary source. Hum. Reprod. 2001, 16, 1809–1813. [Google Scholar] [CrossRef]
- Wagner, W.; Ciszewski, W.M.; Kania, K.D. L- and D-lactate enhance DNA repair and modulate the resistance of cervical carcinoma cells to anticancer drugs via histone deacetylase inhibition and hydroxycarboxylic acid receptor 1 activation. Cell Commun. Signal. 2015, 13, 36. [Google Scholar] [CrossRef]
- Wagner, W.; Ciszewski, W.; Kania, K.; Dastych, J. Lactate Stimulates IL-4 and IL-13 Production in Activated HuT-78 T Lymphocytes Through a Process That Involves Monocarboxylate Transporters and Protein Hyperacetylation. J. Interferon Cytokine Res. 2016, 36, 317–327. [Google Scholar] [CrossRef]
- Li, G.; Wang, H.Q.; Wang, L.H.; Chen, R.P.; Liu, J.P. Distinct pathways of ERK1/2 activation by hydroxy-carboxylic acid receptor-1. PLoS ONE 2014, 9, e93041. [Google Scholar] [CrossRef]
- Brown, T.P.; Ganapathy, V. Lactate/GPR81 signaling and proton motive force in cancer: Role in angiogenesis, immune escape, nutrition, and Warburg phenomenon. Pharmacol. Ther. 2020, 206, 107451. [Google Scholar] [CrossRef] [PubMed]
- Ranganathan, P.; Shanmugam, A.; Swafford, D.; Suryawanshi, A.; Bhattacharjee, P.; Hussein, M.S.; Koni, P.A.; Prasad, P.D.; Kurago, Z.B.; Thangaraju, M.; et al. GPR81, a Cell-Surface Receptor for Lactate, Regulates Intestinal Homeostasis and Protects Mice from Experimental Colitis. J. Immunol. 2018, 200, 1781–1789. [Google Scholar] [CrossRef] [PubMed]
- de Castro Abrantes, H.; Briquet, M.; Schmuziger, C.; Restivo, L.; Puyal, J.; Rosenberg, N.; Rocher, A.B.; Offermanns, S.; Chatton, J.Y. The Lactate Receptor HCAR1 Modulates Neuronal Network Activity through the Activation of Gα and Gβγ Subunits. J. Neurosci. 2019, 39, 4422–4433. [Google Scholar] [CrossRef]
- Latham, T.; Mackay, L.; Sproul, D.; Karim, M.; Culley, J.; Harrison, D.J.; Hayward, L.; Langridge-Smith, P.; Gilbert, N.; Ramsahoye, B.H. Lactate, a product of glycolytic metabolism, inhibits histone deacetylase activity and promotes changes in gene expression. Nucleic Acids Res. 2012, 40, 4794–4803. [Google Scholar] [CrossRef] [PubMed]
- Payen, V.L.; Mina, E.; Van Hée, V.F.; Porporato, P.E.; Sonveaux, P. Monocarboxylate transporters in cancer. Mol. Metab. 2020, 33, 48–66. [Google Scholar] [CrossRef]
- Wagner, W.; Kania, K.D.; Ciszewski, W.M. Stimulation of lactate receptor (HCAR1) affects cellular DNA repair capacity. DNA Repair 2017, 52, 49–58. [Google Scholar] [CrossRef]
- Scully, R.; Panday, A.; Elango, R.; Willis, N.A. DNA double-strand break repair-pathway choice in somatic mammalian cells. Nat. Rev. Mol. Cell Biol. 2019, 20, 698–714. [Google Scholar] [CrossRef] [PubMed]
- Hristova, D.B.; Lauer, K.B.; Ferguson, B.J. Viral interactions with non-homologous end-joining: A game of hide-and-seek. J. Gen. Virol. 2020, 101, 1133–1144. [Google Scholar] [CrossRef] [PubMed]
- Daniel, R.; Katz, R.A.; Skalka, A.M. A role for DNA-PK in retroviral DNA integration. Science 1999, 284, 644–647. [Google Scholar] [CrossRef] [PubMed]
- Baekelandt, V.; Claeys, A.; Cherepanov, P.; De Clercq, E.; De Strooper, B.; Nuttin, B.; Debyser, Z. DNA-Dependent protein kinase is not required for efficient lentivirus integration. J. Virol. 2000, 74, 11278–11285. [Google Scholar] [CrossRef]
- Knyazhanskaya, E.; Anisenko, A.; Shadrina, O.; Kalinina, A.; Zatsepin, T.; Zalevsky, A.; Mazurov, D.; Gottikh, M. NHEJ pathway is involved in post-integrational DNA repair due to Ku70 binding to HIV-1 integrase. Retrovirology 2019, 16, 30. [Google Scholar] [CrossRef]
- Anisenko, A.; Kan, M.; Shadrina, O.; Brattseva, A.; Gottikh, M. Phosphorylation Targets of DNA-PK and Their Role in HIV-1 Replication. Cells 2020, 9, 1907. [Google Scholar] [CrossRef]
- Zhang, S.M.; Zhang, H.; Yang, T.Y.; Ying, T.Y.; Yang, P.X.; Liu, X.D.; Tang, S.J.; Zhou, P.K. Interaction between HIV-1 Tat and DNA-PKcs modulates HIV transcription and class switch recombination. Int. J. Biol. Sci. 2014, 10, 1138–1149. [Google Scholar] [CrossRef]
- Chee, W.J.Y.; Chew, S.Y.; Than, L.T.L. Vaginal microbiota and the potential of Lactobacillus derivatives in maintaining vaginal health. Microb. Cell Factories 2020, 19, 203. [Google Scholar] [CrossRef] [PubMed]
- Moench, T.R.; Chipato, T.; Padian, N.S. Preventing disease by protecting the cervix: The unexplored promise of internal vaginal barrier devices. AIDS 2001, 15, 1595–1602. [Google Scholar] [CrossRef]
- Wu, Z.; Chen, Z.; Phillips, D.M. Human genital epithelial cells capture cell-free human immunodeficiency virus type 1 and transmit the virus to CD4+ Cells: Implications for mechanisms of sexual transmission. J. Infect. Dis. 2003, 188, 1473–1482. [Google Scholar] [CrossRef]
- Yasen, A.; Herrera, R.; Rosbe, K.; Lien, K.; Tugizov, S.M. HIV internalization into oral and genital epithelial cells by endocytosis and macropinocytosis leads to viral sequestration in the vesicles. Virology 2018, 515, 92–107. [Google Scholar] [CrossRef]
- Gokavi, J.; Sadawarte, S.; Shelke, A.; Kulkarni-Kale, U.; Thakar, M.; Saxena, V. Inhibition of miR-155 Promotes TGF- Mediated Suppression of HIV Release in the Cervical Epithelial Cells. Viruses 2021, 13, 2266. [Google Scholar] [CrossRef] [PubMed]
- Howell, A.L.; Edkins, R.D.; Rier, S.E.; Yeaman, G.R.; Stern, J.E.; Fanger, M.W.; Wira, C.R. Human immunodeficiency virus type 1 infection of cells and tissues from the upper and lower human female reproductive tract. J. Virol. 1997, 71, 3498–3506. [Google Scholar] [CrossRef]
- Micsenyi, A.M.; Zony, C.; Alvarez, R.A.; Durham, N.D.; Chen, B.K.; Klotman, M.E. Postintegration HIV-1 infection of cervical epithelial cells mediates contact-dependent productive infection of T cells. J. Infect. Dis. 2013, 208, 1756–1767. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hughes, K.; Akturk, G.; Gnjatic, S.; Chen, B.; Klotman, M.; Blasi, M. Proliferation of HIV-infected renal epithelial cells following virus acquisition from infected macrophages. AIDS 2020, 34, 1581–1591. [Google Scholar] [CrossRef]
- Blasi, M.; Balakumaran, B.; Chen, P.; Negri, D.R.; Cara, A.; Chen, B.K.; Klotman, M.E. Renal epithelial cells produce and spread HIV-1 via T-cell contact. AIDS 2014, 28, 2345–2353. [Google Scholar] [CrossRef] [PubMed]
- Devadoss, D.; Singh, S.P.; Acharya, A.; Do, K.C.; Periyasamy, P.; Manevski, M.; Mishra, N.; Tellez, C.S.; Ramakrishnan, S.; Belinsky, S.A.; et al. HIV-1 Productively Infects and Integrates in Bronchial Epithelial Cells. Front. Cell Infect. Microbiol. 2021, 10, 12360. [Google Scholar] [CrossRef]
- Liu, R.; Huang, L.; Li, J.; Zhou, X.; Zhang, H.; Zhang, T.; Lei, Y.; Wang, K.; Xie, N.; Zheng, Y.; et al. HIV Infection in gastric epithelial cells. J. Infect. Dis. 2013, 208, 1221–1230. [Google Scholar] [CrossRef]
- Anisenko, A.N.; Knyazhanskaya, E.S.; Isaguliants, M.G.; Gottikh, M.B. A qPCR assay for measuring the post-integrational DNA repair in HIV-1 replication. J. Virol. Methods 2018, 262, 12–19. [Google Scholar] [CrossRef]
- Quanz, M.; Bender, E.; Kopitz, C.; Grünewald, S.; Schlicker, A.; Schwede, W.; Eheim, A.; Toschi, L.; Neuhaus, R.; Richter, C.; et al. Preclinical Efficacy of the Novel Monocarboxylate Transporter 1 Inhibitor BAY-8002 and Associated Markers of Resistance. Mol. Cancer Ther. 2018, 17, 2285–2296. [Google Scholar] [CrossRef]
- Li, L.; Olvera, J.M.; Yoder, K.E.; Mitchell, R.S.; Butler, S.L.; Lieber, M.; Martin, S.L.; Bushman, F.D. Role of the non-homologous DNA end joining pathway in the early steps of retroviral infection. EMBO J. 2001, 20, 3272–3281. [Google Scholar] [CrossRef] [PubMed]
- Bouquet, F.; Ousset, M.; Biard, D.; Fallone, F.; Dauvillier, S.; Frit, P.; Salles, B.; Muller, C. A DNA-dependent stress response involving DNA-PK occurs in hypoxic cells and contributes to cellular adaptation to hypoxia. J. Cell Sci. 2011, 124 (Pt 11), 1943–1951. [Google Scholar] [CrossRef]
- Huston, E.; Lynch, M.J.; Mohamed, A.; Collins, D.M.; Hill, E.V.; MacLeod, R.; Krause, E.; Baillie, G.S.; Houslay, M.D. EPAC and PKA allow cAMP dual control over DNA-PK nuclear translocation. Proc. Natl. Acad. Sci. USA 2008, 105, 12791–12796. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Baeza, Y.; Sandoval, P.Y.; Alarcón, R.; Galaz, A.; Cortés-Molina, F.; Alegría, K.; Baeza-Lehnert, F.; Arce-Molina, R.; Guequén, A.; Flores, C.A.; et al. Monocarboxylate transporter 4 (MCT4) is a high affinity transporter capable of exporting lactate in high-lactate microenvironments. J. Biol. Chem. 2019, 294, 20135–20147. [Google Scholar] [CrossRef]
- Miranda-Gonçalves, V.; Honavar, M.; Pinheiro, C.; Martinho, O.; Pires, M.M.; Pinheiro, C.; Cordeiro, M.; Bebiano, G.; Costa, P.; Palmeirim, I.; et al. Monocarboxylate transporters (MCTs) in gliomas: Expression and exploitation as therapeutic targets. Neuro-Oncol. 2013, 15, 172–188. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wagner, W.; Sobierajska, K.; Kania, K.D.; Paradowska, E.; Ciszewski, W.M. Lactate Suppresses Retroviral Transduction in Cervical Epithelial Cells through DNA-PKcs Modulation. Int. J. Mol. Sci. 2021, 22, 13194. https://doi.org/10.3390/ijms222413194
Wagner W, Sobierajska K, Kania KD, Paradowska E, Ciszewski WM. Lactate Suppresses Retroviral Transduction in Cervical Epithelial Cells through DNA-PKcs Modulation. International Journal of Molecular Sciences. 2021; 22(24):13194. https://doi.org/10.3390/ijms222413194
Chicago/Turabian StyleWagner, Waldemar, Katarzyna Sobierajska, Katarzyna Dominika Kania, Edyta Paradowska, and Wojciech Michał Ciszewski. 2021. "Lactate Suppresses Retroviral Transduction in Cervical Epithelial Cells through DNA-PKcs Modulation" International Journal of Molecular Sciences 22, no. 24: 13194. https://doi.org/10.3390/ijms222413194
APA StyleWagner, W., Sobierajska, K., Kania, K. D., Paradowska, E., & Ciszewski, W. M. (2021). Lactate Suppresses Retroviral Transduction in Cervical Epithelial Cells through DNA-PKcs Modulation. International Journal of Molecular Sciences, 22(24), 13194. https://doi.org/10.3390/ijms222413194







