1. Introduction
Serotonin (5-HT) and 5-HT1A receptor (5-HT1AR) signaling plays important roles in the pathology and treatment of depression, anxiety, and related mood disorders [
1]. For example, mice constitutively lacking 5-HT1ARs are more anxious [
2] and 5-HT1AR agonists are used as anxiolytic drugs. Furthermore, the desensitization (reduced responsiveness) of 5-HT1ARs is essential for the therapeutic effects of antidepressants [
3,
4]. Depression affects 1 in 6 Americans, but only 2/3 of those patients are effectively treated with current oral medication approaches [
5]. Women are almost twice as likely to suffer from depression as men, and fluctuations in estrogens contribute to depression and other mood disorders [
6]. Estrogens can regulate 5-HT1AR signaling [
7]. Our previous study demonstrated that the estrogen 17β-estradiol benzoate (EB) causes a partial desensitization of 5-HT1AR signaling [
8] and the combination of the antidepressant fluoxetine and EB produces a robust and rapid desensitization [
9]. However, the mechanisms of this regulation are unclear.
Understanding the mechanisms underlying the regulation of 5-HT1ARs will inform the development of more effective therapeutic approaches. We previously demonstrated that 5-HT1ARs are SUMOylated by SUMO1 (SUMO1-5-HT1ARs) in the rat brain [
10]. SUMOylation is a post-translational modification whereby a small ubiquitin-like modifier protein (SUMO) is covalently conjugated to a target protein in a manner similar to ubiquitination. SUMOylation can alter protein function, protein to protein interactions, and protein localization [
11]. We demonstrated that the agonist stimulation of 5-HT1ARs increases this SUMOylation and EB further enhances the SUMOylation of 5-HT1ARs. Using discontinuous gradient centrifugation and receptor binding assays with (+)8-hydroxy-2-dipropylaminotetralin (8-OH-DPAT), a 5-HT1A/7R agonist, we found that SUMO1-5-HT1ARs have a low capacity for agonist binding [
10]. Furthermore, using discontinuous gradient centrifugation and Western blotting, SUMO1-5-HT1ARs did not co-localize with Gαz, a Gα to which the receptor couples [
10,
12]. Taken together, these results suggest SUMO1-5-HT1ARs are not active receptors and that SUMOylation contributes to the desensitization of 5-HT1ARs caused by treatment with 8-OH-DPAT and EB.
SUMO proteins are conjugated to their substrates via an isopeptide bond between the di-glycines at the C-terminus of SUMO and a lysine residue in the substrate protein. In mammals, the process of SUMOylation occurs through a series of enzymatic reactions analogous to ubiquitination [
11,
13]. SUMOylation does not occur in vivo for most substrates without SUMO E3 ligases, which facilitate the transfer of the SUMO group to the target protein. The protein inhibitor of activated STAT (PIAS) family proteins has E3 ligase activity and mammalian cells contain four members of the PIAS family, PIAS1, PIAS3, and PIASy, as well as splice variants PIASxα and PIASxβ [
11,
14]. Different PIAS proteins have preferences for different substrates and have selectivity for different SUMO proteins. E3 ligase activity has been demonstrated for several other proteins, such as RanBP2, Polycomb protein 2, and histone deacetylase 4, but a very limited number of substrates have been identified as E3 ligases to date.
DeSUMOylation is catalyzed primarily by sentrin-specific proteases known as SENPs [
15]. There are six mammalian SENPs that are involved in deSUMOylation, SENPs 1–3 and 5–7. SENPs act not only to deconjugate the SUMO moiety from substrate proteins, but also act in the maturation of SUMO proteins by exposing the C-terminal diglycine moiety on SUMO proteins to initiate the SUMOylation process. Different SENPs have different activities in deconjugation and maturation processing, as well as substrate specificity. SENP1 displays little specificity for the deconjugation of SUMO2 and SUMO3, while it is essential for the deconjugation of SUMO1-modified proteins [
16,
17]. SENP2 catalyzes deconjugation better than maturation processing and deconjugates SUMO-1, SUMO-2 and SUMO-3 from SUMOylated Ran GTPase-activating protein 1 with similar efficiency. In contrast, SENP2 possesses different substrate specificities during processing; for example, it processes SUMO2 more efficiently than SUMO1 and SUMO3 [
11]. SENP3 and SENP5 show both maturation processing and deconjugating activities for SUMO2 and SUMO3 but possess neither for SUMO1. SENP6 and SENP7 have deconjugating activities for poly SUMO2/3 chains, while exerting very weak processing and deconjugating activity on monomeric SUMO modified substrates [
11].
The goal of this study was to determine the mechanisms involved in the regulation of the SUMOylation of 5-HT1ARs. To address this question, we first investigated which PIAS and SENP proteins regulate the SUMOylation of 5-HT1ARs. Then we used two drug treatment strategies shown to increase the SUMOylation of 5-HT1ARs, agonist stimulation using 8-OH-DPAT and the combination of EB and 8-OH-DPAT, which further increased SUMOylation. A previous study suggested that local concentrations of the PIAS E3 ligases play a role in determining which E3 ligase catalyzes the SUMOylation of a target protein [
18]. Furthermore, the study demonstrated that the translocation of the E3 ligase to the substrate plays a role in regulating the SUMOylation of the substrate. Based on these observations, we hypothesized that the PIAS protein(s) catalyzing the SUMOylation of 5-HT1ARs would be expressed in the cell membrane and drug treatments that increased the SUMOylation of 5-HT1AR would increase the membrane localization of the PIAS protein. To test this hypothesis, we ectopically expressed different PIAS proteins in mouse neuroblastoma 2a (N2a) cells to determine their effects on SUMO1-5-HT1ARs and measured the levels of PIAS proteins following the treatment of rats with 8-OH-DPAT or a co-treatment of EB and 8-OH-DPAT. Additionally, a decrease in SENP proteins in the membrane could result in less deSUMOylation, thereby increasing the SUMOylation of 5-HT1ARs. Therefore, we over-expressed SENPs1, 2, and 6 to determine which SENP deSUMOylates 5-HT1ARs and then measured the levels of SENP2 following drug treatment.
We found that overexpression of PIASxα increased SUMOylation and SENP2 decreased SUMOylation of 5-HT1ARs in N2a cells. The treatment of rats with the 5-HT1A/7R agonist 8-OH-DPAT decreased SENP2 proteins in the membrane. In contrast, the combined treatment with 8-OH-DPAT and EB on rats increased PIASxα proteins in the membrane. These results provide a further understanding of the mechanisms by which agonists and estrogens regulate the SUMOylation of 5-HT1ARs and thereby the sensitivity of 5-HT1AR signaling.
3. Discussion
Protein SUMOylation is an important post-translational modification that has recently gained attention. Although the vast majority of studies on SUMOylation focus on the regulation of nuclear proteins, studies have revealed that SUMOylation can also play an important role in regulating the function of extra-nuclear proteins [
19]. In the last several years, a role for SUMOylation in neurodegenerative diseases, cerebral ischemia, and synaptic function has become apparent [
20,
21,
22,
23].
We previously reported that 5-HT1ARs are SUMOylated, and that agonist stimulation increases the SUMOylation of 5-HT1ARs in the frontal cortex and hypothalamus [
10]. EB facilitates the effect of agonist stimulation of the receptor, resulting in significantly more SUMO1–5-HT1ARs than a treatment with agonist alone [
10]. The purpose of the current studies is to elucidate the mechanisms regulating the SUMOylation of 5-HT1ARs. To this end, we first focused on the PIAS E3 ligases which facilitate SUMOylation and then SENP proteins which deSUMOylate proteins. Previous studies demonstrated that local concentrations of PIAS E3 ligases and SENPs can regulate the SUMOylation of target proteins [
18,
24], so we examined the levels of these enzymes in the membrane and cytosol fractions to determine if a change in the membrane levels is related to an increase in the amount of SUMOylated 5-HT1ARs, since SUMOylated 5-HT1ARs exist only in the membrane fractions [
10]. Although previous studies reported PIAS and SENP proteins distributed primarily in the nucleus and cytosol of cells [
11,
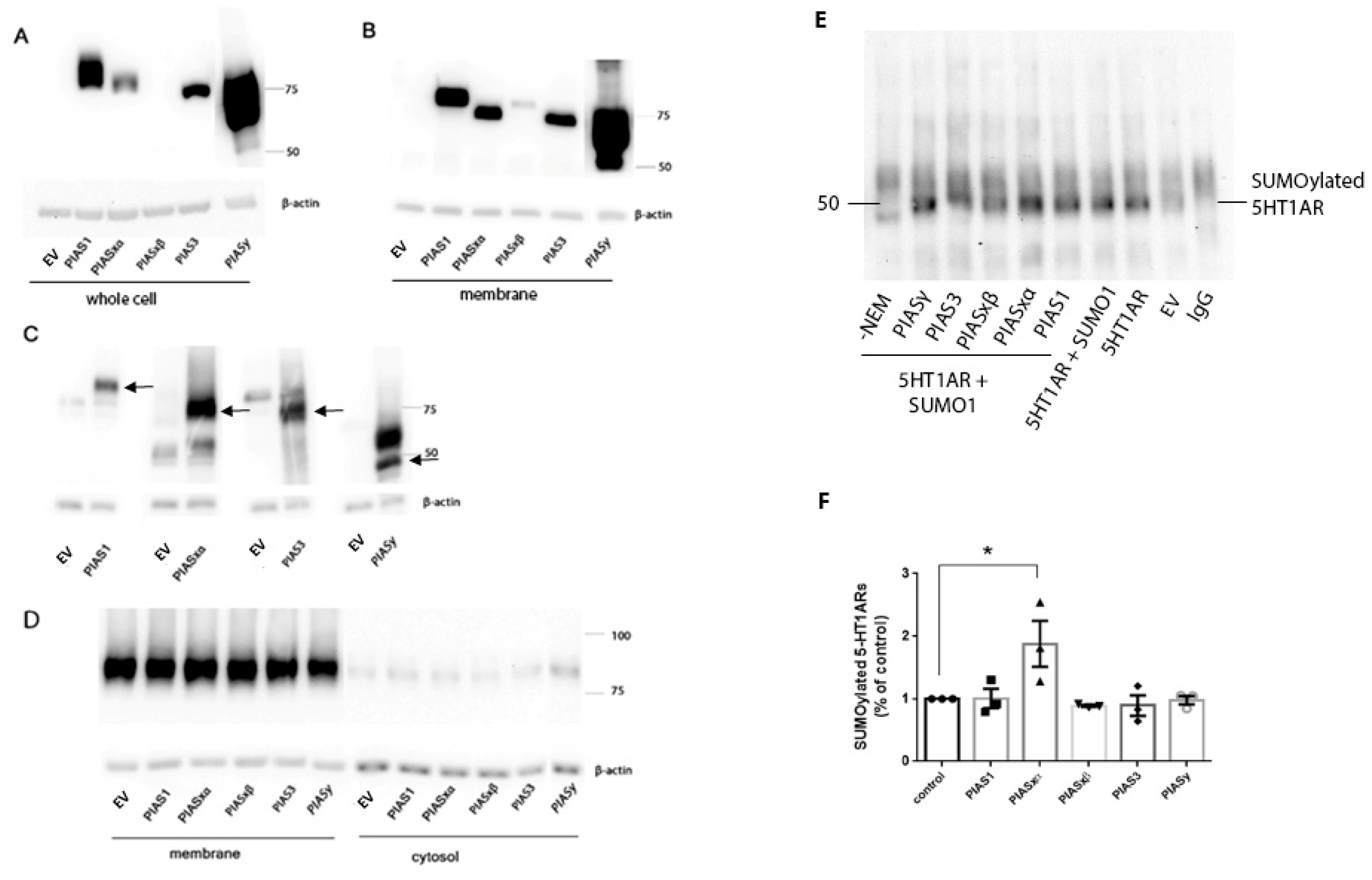
25], our results show PIAS and SENP proteins distributed in the membrane fraction of N2a cells and the rat PVN and frontal cortex. Transfected PIASxα proteins were expressed at low levels in the whole cell lysate, but a higher proportion of PIASxα was expressed in the membrane fraction than in the whole cell lysate, suggesting PIASxα is positioned to participate in the SUMOylation of 5-HT1ARs which only exist in the membrane fraction [
10]. Although there are low levels of endogenous PIAS proteins in the membrane fraction (
Figure 1C), the increase in PIASxα in the membrane following transfection resulted in increased SUMO1–5-HT1ARs. Based on the transfection data, our interpretation is that PIASxα is the primary PIAS protein that catalyzes SUMO1 binding to 5-HT1AR and that low levels of endogenous PIASxα catalyze the SUMOylation of 5-HT1AR, as seen in the vector transfected cells. PIASxα selectively increases SUMO1–5-HT1ARs even though the expression level of transfected PIASxα is low compared to other transfected PIAS proteins. Despite much higher amounts of PIAS1 and PIASy proteins in the whole cell lysate preparation as well as the membrane fraction of N2a cells after transfection, high PIAS1 and PIASy did not increase SUMOylation of 5-HT1ARs over the levels that occur with the endogenous expression of PIAS proteins.
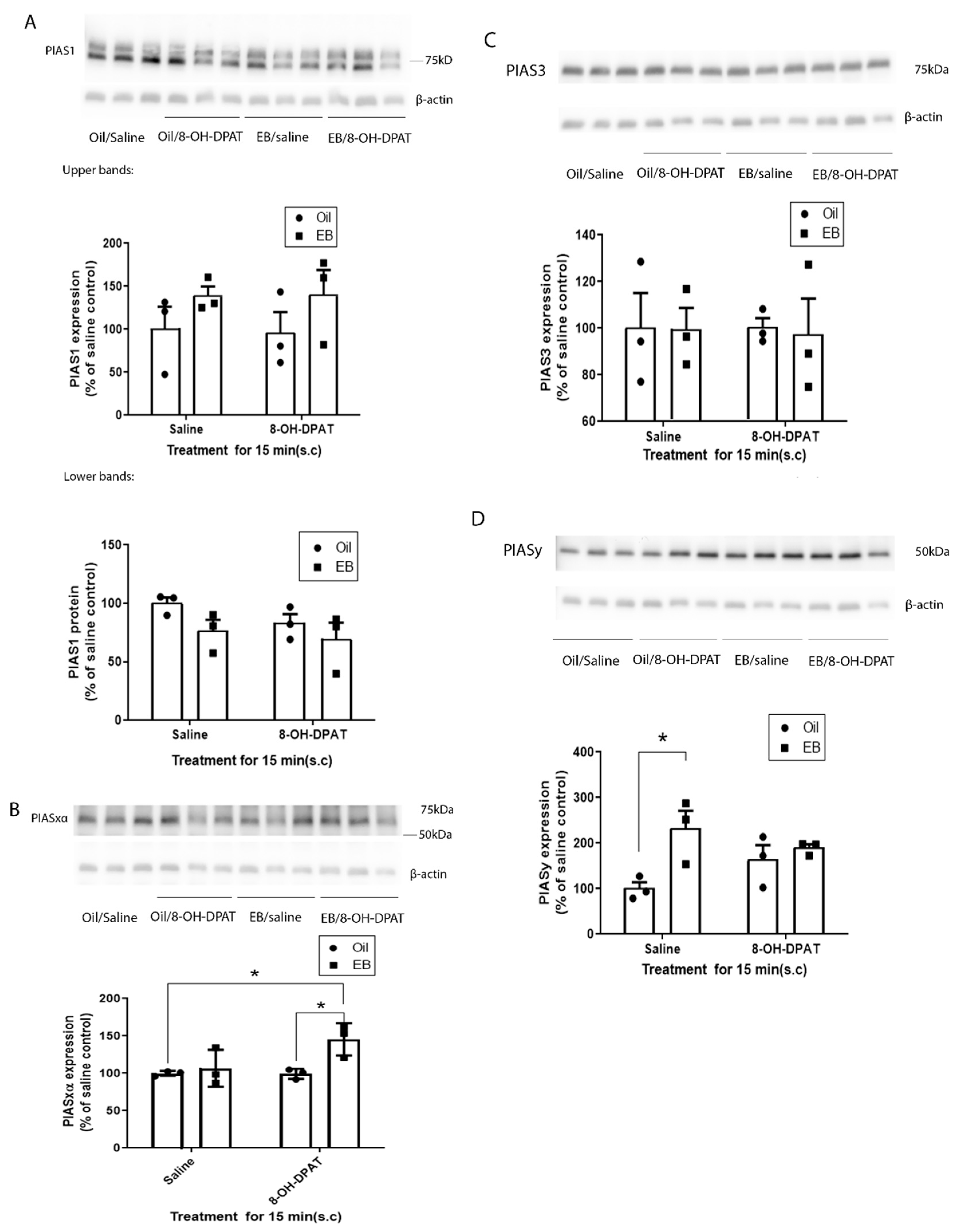
In this study, we found that PIASxα is increased in the membrane fraction of PVN in rats treated with EB and 8-OH-DPAT, suggesting that this increase in PIASxα is responsible for the increase in 5-HT1ARs SUMOylation induced by EB and 8-OH-DPAT [
10]. It is important to note that the EB treatment was once daily for two days and then the agonist treatment was 5–15 min before sacrifice in the experiments examining PIASxα and those demonstrating increased 5-HT1AR SUMOylation. The two days of EB administration is the minimum treatment time for EB to reduce 5-HT1AR and hypothalamic pituitary adrenal (HPA) axis signaling, an important biomarker for depression [
8]. Both 5-HT1ARs and estrogens are known to impact mood, especially in depression and anxiety. Numerous studies demonstrate that estrogens reduce 5-HT1AR signaling not only in the HPA axis [
9,
10] but also in the hypothalamus, hippocampus, dorsal raphe, and cortex [
7,
26,
27]. In this study we focused on the frontal cortex and PVN due to the importance of these brain regions in depression and anxiety, as well as our previous experiments demonstrating the effects of 8-OH-DPAT and the combination of 8-OH-DPAT on the frontal cortex and hypothalamus, respectively. One of the mechanisms by which estrogens could reduce 5-HT1AR signaling is via SUMOylation, as our previous data suggests that the SUMOylation of 5-HT1ARs could reduce signaling via a reduced interaction with Gα proteins [
10]. Mize et al. [
27] also reported a reduction in G-protein coupling to 5-HT1ARs after EB treatment. The SUMOylation of proteins often alters interactions with their binding partners consistent with SUMOylation causing a change in receptor G-protein coupling. The effects of EB on 5-HT1AR SUMOylation and signaling may contribute to the increased incidence of mood disorders in women with fluctuations in estrogens such as during the perimenopausal period.
The administration of EB alone did not have significant effects on the levels of PIASxα, a finding consistent with our previous results, that the treatment of EB alone did not increase the SUMOylation of 5-HT1ARs. EB treatment alone did significantly increase the expression level of PIASy in the membrane fraction of the rat PVN. Since PIASy did not alter the SUMOylation of 5-HT1ARs, the change in PIASy levels may be involved in EB-induced SUMOylation of other proteins such as RGSz1 [
28]. RGSz1 can be SUMOylated and there are numerous isoforms of SUMOylated RGSz1 at approximately 35 kDa, 45 kDa, 50 kDa, 90 kDa, and 135 kDa in the rat cortex; EB treatment increased the 135-kDa RGSz1 in the PVN membrane fraction [
28].
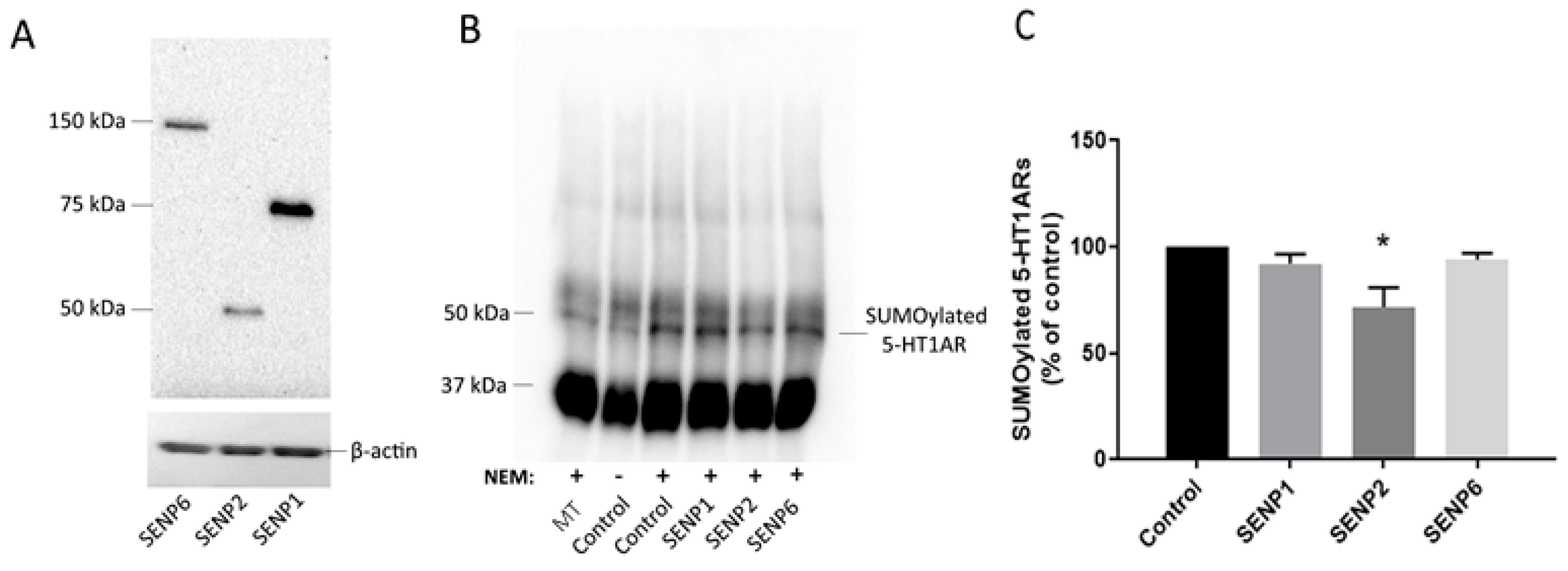
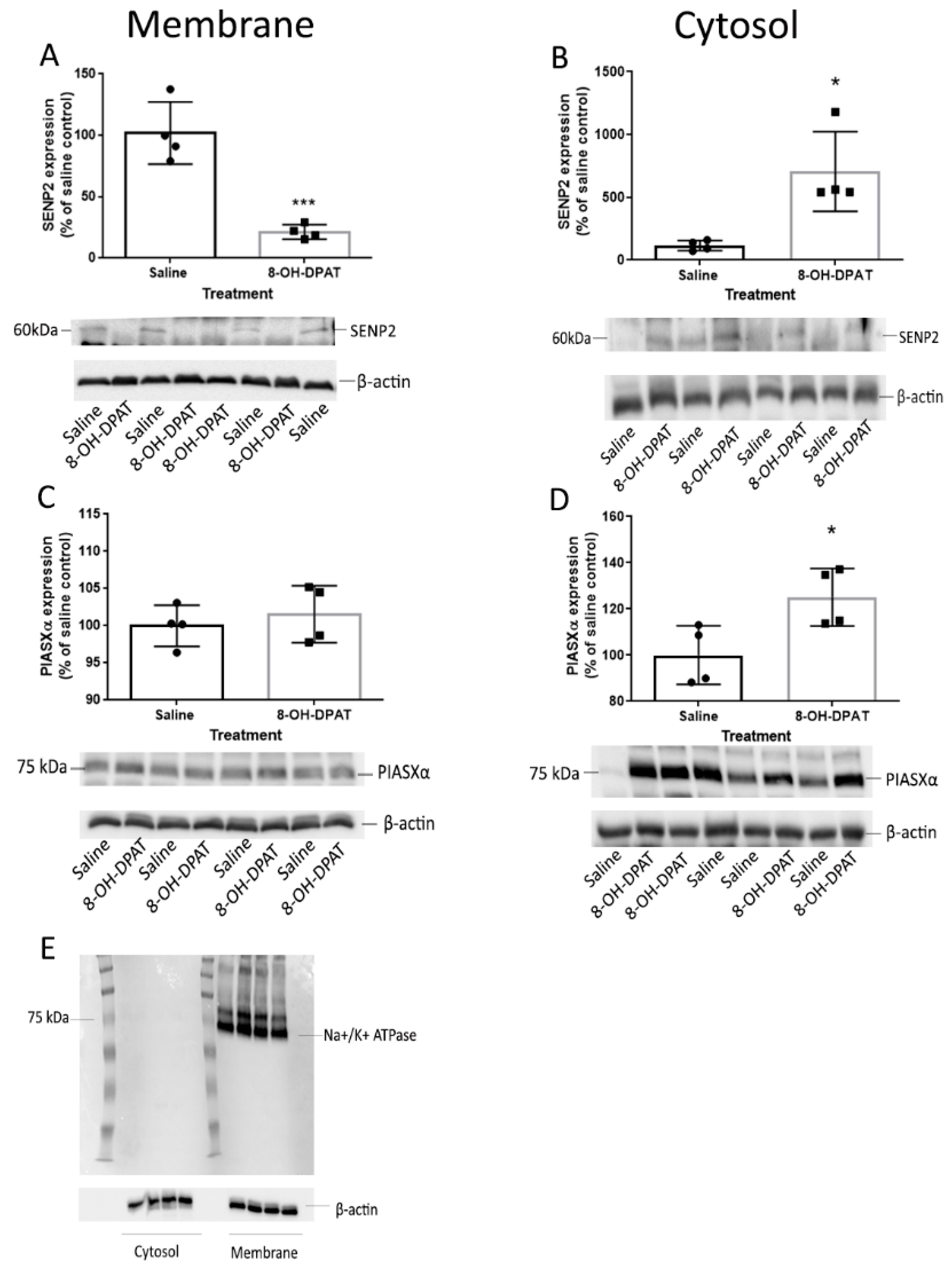
We found that agonist treatment alone increased the levels of SUMO1–5-HT1ARs in the membrane fraction of the rat frontal cortex, similar to that previously reported for the rat hypothalamic membrane and the cortical detergent resistant membrane fractions [
10]. After we identified SENP2 as capable of deSUMOylating 5-HT1ARs, we compared the levels of SENP2 in the 8-OH-DPAT-treated rat frontal cortex to saline-treated controls to determine if a decrease in SENP2 in the membrane could underlie the agonist-induced increase in the SUMOylation of 5-HT1ARs. Indeed, SENP2 decreased in the membrane fraction and increased in the cytosol fraction of the 8-OH-DPAT-treated rat frontal cortex, suggesting a translocation of SENP2 proteins following agonist treatment. These results further suggest that the translocation of SENP2 away from the membrane is responsible for the increase in the SUMOylation of 5-HT1ARs after agonist treatment. Similarly, a previous study demonstrated that SENP1 is also highly mobile and translocates to the synapse after the stimulation of metabotropic glutamate receptors (mGluR) [
24]. PIASxα does not appear to be involved in regulating the increase in 5-HT1AR SUMOylation after agonist treatment, as 8-OH-DPAT did not increase the expression of PIASxα in the membrane fraction of the PVN.
The mechanisms involved in translocation of PIASxα and SENP2 have not been investigated but might involve post-translation modifications. PIASxα is subject to both SUMOylation and phosphorylation, and SUMOylation is known to alter the cellular location of proteins [
14]. However, it is not known if these modifications play a role in regulating the translocation of PIASxα. Phosphorylation of SENP2 promotes its translocation from the nucleus to the cytosol [
29] but it is not known if phosphorylation alters the localization of SENP2 to the cell membrane.
To date, only four other GPCRs, cannabinoid receptor 1 (CB1), M1 muscarinic acetylcholine receptors (M1Rs) and mGluR7 and mGluR8 have been identified as SUMOylated in mammals [
30,
31,
32,
33,
34,
35]. In contrast to 5-HT1ARs, agonist treatment decreases the SUMOylation of M1Rs, CB1, and mGluR7, however, no data are available regarding the effects of SUMOylation on the function of mGluR8. The deSUMOylation of mGluR7 increases the internalization of the receptors [
30]. The SUMOylation of M1Rs increases ligand affinity and signaling of M1Rs, as well as endocytosis [
35]. In drosophila, the GPCR smoothened is SUMOylated, resulting in the stabilization of the receptor [
36] as well as promoting the translocation of the receptor to the cell surface [
37]. Clearly, the effects of SUMOylation on receptor signaling are receptor specific.
4. Materials and Methods
4.1. Cell Culture Experiments
N2a cells obtained from ATCC were maintained in 50% Dulbecco’s Modified Eagle Medium (1× DMEM, high glucose, pyruvate, ThermoFisher Scientific, Waltham, MA, USA) and 50% OptiMEM (ThermoFisher Scientific) supplemented with 10% Fetal Bovine Serum (FBS) (Atlanta Biologicals, Flowery Branch, GA, USA) and 1% penicillin-streptomycin solution (Sigma Aldrich, St. Louis, MO, USA). N2a cells were plated in 10 cm dishes at a density of 2.2 × 106 cells per dish. After 16–24 h, cells were transfected with the mammalian expression plasmid constructs using Lipofectamine 3000 (ThermoFisher Scientific). The cell culture media was changed 6 and 24 h after transfection. To allow for the maximal expression of 5-HT1ARs for the SENP transfection experiments, cells were transfected with 5-HT1ARs plasmids 16 h after transfecting SENP plasmids. Subsequently, cells were harvested at 48 h after SENP transfection and 32 h after 5-HT1AR transfection. In all other experiments, cells were harvested 48 h after transfection. Cells were washed with a phosphate-buffered saline and hypotonic buffer (0.25 M sucrose 50 mM Tris, pH 7.5, 5 mM EDTA, 100 mM NaCl, and 20 mM N-ethylmaleimide (NEM)). The cells were harvested with a hypotonic buffer containing 20 mM NEM, 1/100 dilution of phosphatase inhibitors (Sigma Aldrich), and protease inhibitors (Sigma Aldrich) and were centrifuged for 1 h. Then, the supernatant was removed as the cytosolic fraction and the pellet was resuspended in the solubilization buffer (20 mM Tris, pH 8, 1 mM EDTA, 100 mM NaCl, (1% sodium cholate, 20 mM NEM, 1/100 dilution of phosphatase inhibitors, and protease inhibitors were added immediately before use)). After sonication, the vials were shaken horizontally at 4 °C for 1 h. After this, vials were centrifuged at 25,000× g for 1 h. The supernatant was removed as the membrane fraction. The samples were aliquoted and stored in −80 °C. A BCA assay (ThermoFisher Scientific) was used to measure the protein concentration.
4.2. Plasmid Constructs
The flag-tagged PIAS and SENP plasmid constructs, his-tagged 5-HT1AR and his- and HA-tagged SUMO-1 constructs were from Addgene (Watertown, MA, USA). The (K)DYK tagged 5-HT1AR construct was from GenScript (Piscataway, NJ, USA). The QIAGEN (Germantown, MD, USA) plasmid MIDI prep kit was used to isolate the plasmids.
4.3. Treatment of Rats and Preparation of Tissue Fractions
The frontal cortex was used to examine the effects of acute treatment with the 5-HT1A/7R agonist, 8-OH-DPAT, since it was previously demonstrated that 8-OH-DPAT increases the SUMOylation of 5-HT1AR in this brain region [
10]. 8-OH-DPAT was purchased from Tocris (Ellisville, MO, USA), was dissolved in 0.85% NaCl at a concentration of 0.2 mg/mL and was administered at a dose of 0.2 mg/kg s.c. Solutions were made fresh before injection. Sixteen ovariectomized female rats were injected with either saline or 8-OH-DPAT (200 µg/kg) subcutaneously and then decapitated 5 min later. Brains were removed and the frontal cortex was dissected, frozen on dry ice, and stored at −80 °C until use. Tissue was homogenized and membrane and cytosol fractions were prepared as previously described [
10]. Four rats from each treatment group were examined on each Western blot and three to five Western blots were used for each antibody tested.
The paraventricular nucleus of the hypothalamus (PVN) of rats used in this study was from ovariectomized female rats used in a previously published study to determine the effects of EB on oxytocin and ACTH responses [
28]. Our previous study [
10] demonstrated that 8-OH-DPAT increased the SUMOylation of 5-HT1ARs in the hypothalamus and EB synergized this increase. EB from Sigma-Aldrich was first dissolved in 100% ethanol to a concentration of 25 μg/mL and then diluted to the final concentration with sesame oil. The EB solution and sesame oil were administered at 0.4 ml/kg (the EB dose was 10 μg/kg subcutaneous (s.c.)). 8-OH-DPAT was prepared and administered as described above. Both solutions were made fresh before the injection. Rats were given unilateral intra-PVN injections of GPR30-mis-Ads, a control vector that had no effect, as previously described [
28]. Five days after the injection, rats were treated with either EB (10 μg/kg, 0.4 mL/kg, s.c.) or sesame oil once daily for 2 days. Twenty hours after the last injection, rats were treated with 8-OH-DPAT (200 μ/kg, s.c.) or saline and decapitated 15 min after the treatment. Brains were removed and the PVN was isolated and stored at −80 °C until use; the PVN was then homogenized and membrane and cytosol fractions were prepared as previously described [
10]. Membrane fractions from three rats for each treatment group were used for immunoblots to examine the relative expression of PIAS proteins. All animal experiments were conducted according to the National Institutes of Health guide for the care and use of laboratory animals and was approved by the IACUC at the University of Kansas.
4.4. Immunoprecipitation
Immunoprecipitation was conducted using 250 µg to 450 µg of protein of the membrane fraction of N2a cells. The preparation of the membrane fraction is described above. The sample was added to 50 µL prewashed protein G agarose (Invitrogen ThermoFisher Scientific, Waltham, MA, USA) in a total volume of 250 µL IP buffer (50 mM Tris, pH 7.4, 10 mM EGTA, 100 mM NaCl, 0.5% Triton X-100, containing 20 mM NEM, 1× protease inhibitor cocktail, 1× phosphatase inhibitor cocktails I and II (Sigma Aldrich, St. Louis, MO, USA)) and rotated at 4 °C for 1 h. After centrifugation at 10,000 rpm at 4 °C for 10 min, the supernatant was incubated with a 2.5 μg anti-SUMO1 mouse antibody (Cat#sc-5308, Santa Cruz Biotechnology, Inc., Dallas, TX, USA) or mouse IgG control (Cat#sc-2025, Santa Cruz Biotechnology) on a rotator at 4 °C overnight. The solutions were added to 100 µL of pre-washed beads and rotated at 4 °C for 2 h. The protein G beads–immune complex was centrifuged at 1000 rpm for 3 min at 4 °C. The pellet was washed with a 0.5 ml ice-cold IP buffer 3 times. After washing, the proteins were eluted from the immune complexes using the 50 µL 2× sample buffer with β-mercaptoethanol and incubated at 95 °C for 5 min followed by centrifugation at 12,000 rpm for 5 min at room temperature. The eluate was stored in −80 °C or was loaded onto 10% SDS-PAGE gels.
4.5. Immunoblot Assays
Protein samples were resolved using 10% SDS-PAGE and then transferred to polyvinylidene fluoride (PVDF) membranes. After transferring, the PVDF membranes were incubated in 5% non-fat milk in Tris-buffered saline, pH 7.6, with 0.1% Tween-20. The membranes were incubated overnight with primary antibodies (PIAS antibodies (1:2000) from Dr. Yoshiaki Azuma, [
38], rabbit anti-5-HT1AR (1:1000, Thermo Fisher Scientific Cat#PA5-28090), rat-anti-flag (1:2000 Agilent Technologies, (Santa Clara, CA, US), Cat#200474-21), rabbit anti-SENP2 (1:500, Invitrogen, Thermo Fisher Scientific, Cat# PA5-86255), mouse anti-Na
+, K
+ ATPase (1:1000, Millipore Sigma, (Burlington, MA, USA) Cat#05-369), and mouse-anti-β actin (1:20,000, MP Biomedicals, LLC, (Irvine, CA, USA) Cat#691001)). Immunodetection was performed using an ECL kit (Millipore Sigma or Bio-Rad Laboratories, Hercules, CA, USA) and ImageLab 3.0 software (Bio-Rad Laboratories). The intensity of bands was normalized to the amount of protein loaded in each lane, the mean intensity of the bands of the empty vector transfected samples, and the beta-actin band.
4.6. Statistical Analysis
All statistical analyses were conducted using GraphPad Prism version 9.1 (San Diego, CA, USA) software. The Shapiro–Wilk test for normality and the Brown–Forsythe–Levene test for homogeneity of variance were used to determine if the data met the requirements for a parametric analysis of variance. Immunoblot data were analyzed by a one-way or two-way analysis of variance (ANOVA) followed by post-hoc tests; Dunnett’s was used to compare only to the control group, or Tukey’s multiple comparisons test was used to identify differences among the treatment groups where appropriate. All data are represented as mean ± SEM.