Validation of SV2A-Targeted PET Imaging for Noninvasive Assessment of Neuroendocrine Differentiation in Prostate Cancer
Abstract
1. Introduction
2. Results
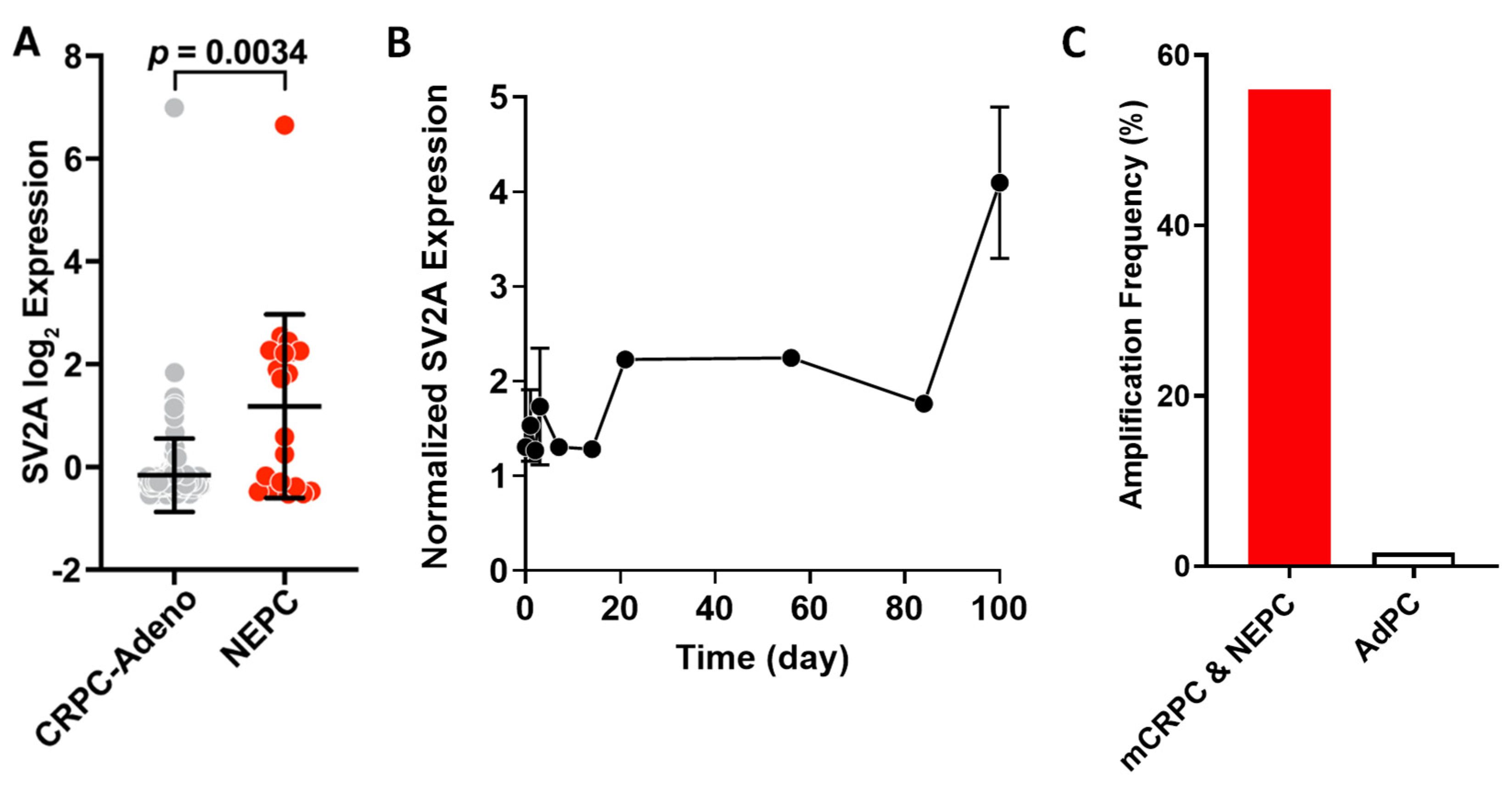
2.1. SV2A Gene Expression in Clinical Tumor Tissues: NEPC vs. CRPC-Adeno
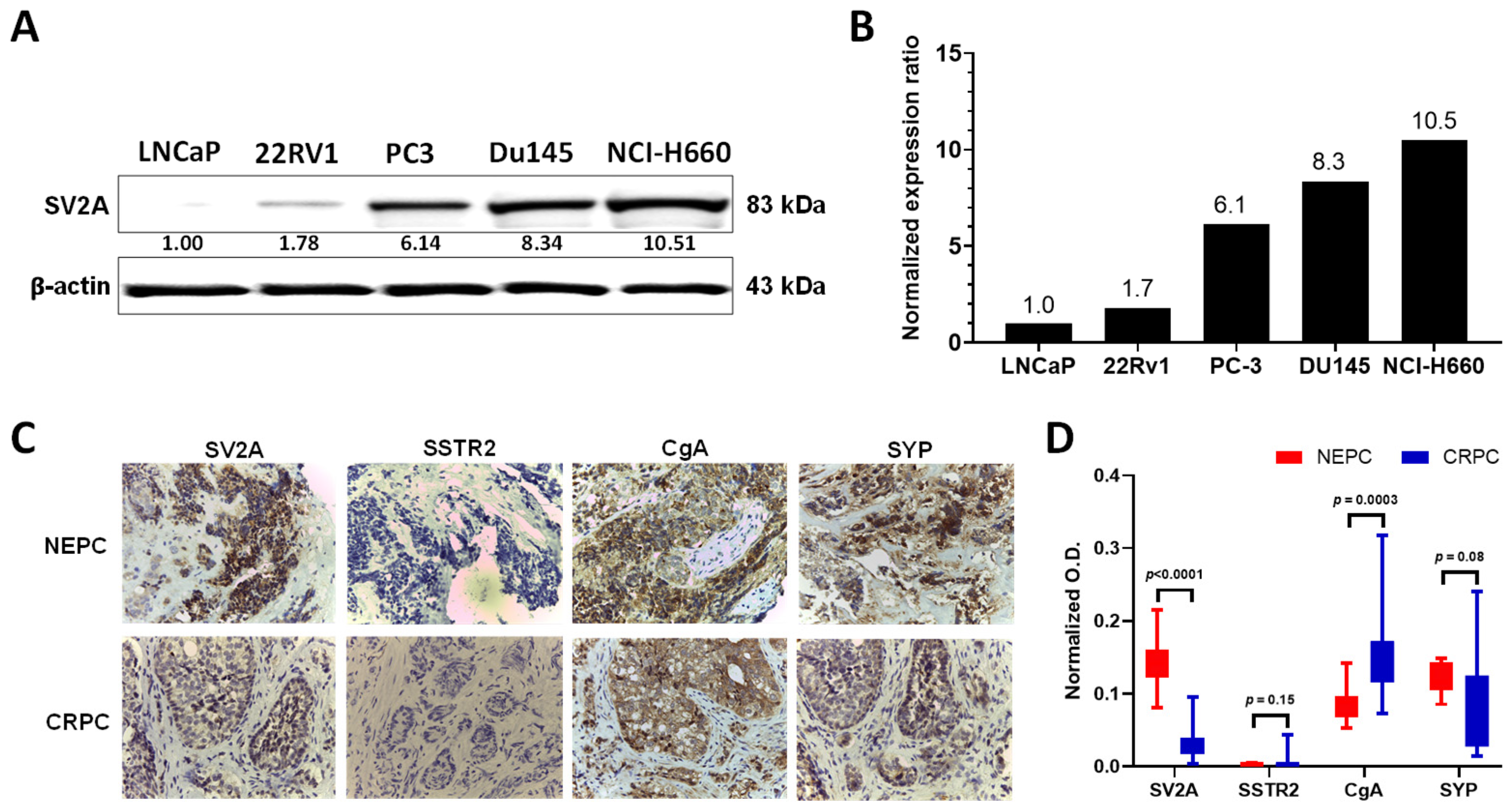
2.2. SV2A Protein Expression in PCa Cell Lines and Patient Tumor Specimens
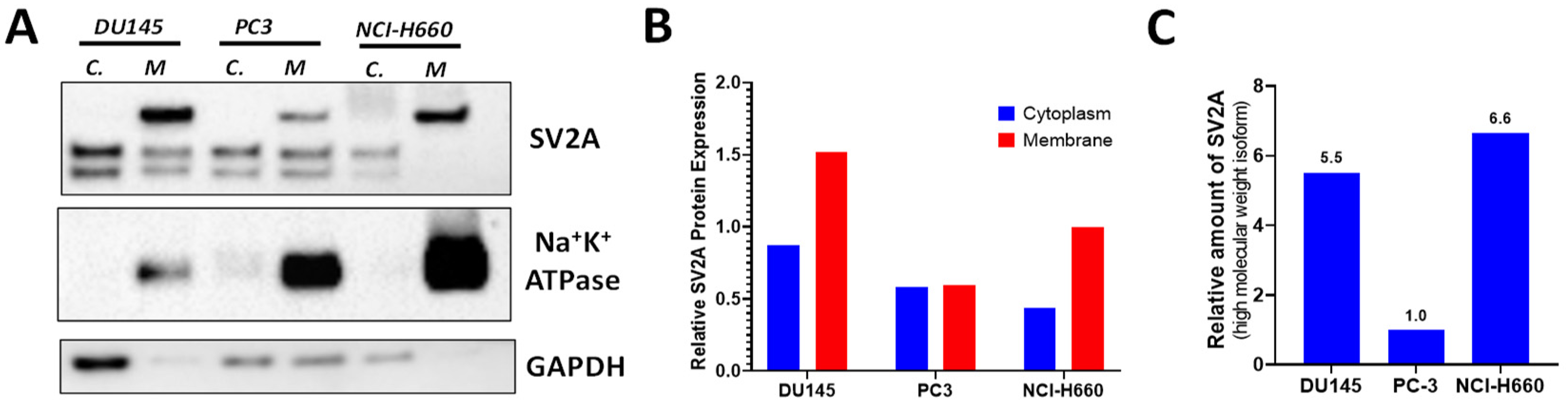
2.3. SV2A Localization in PCa Cells
2.4. Automated Radiosynthesis of 18F-SynVesT-1 on GE TRACERlab FX-N Synthesizer
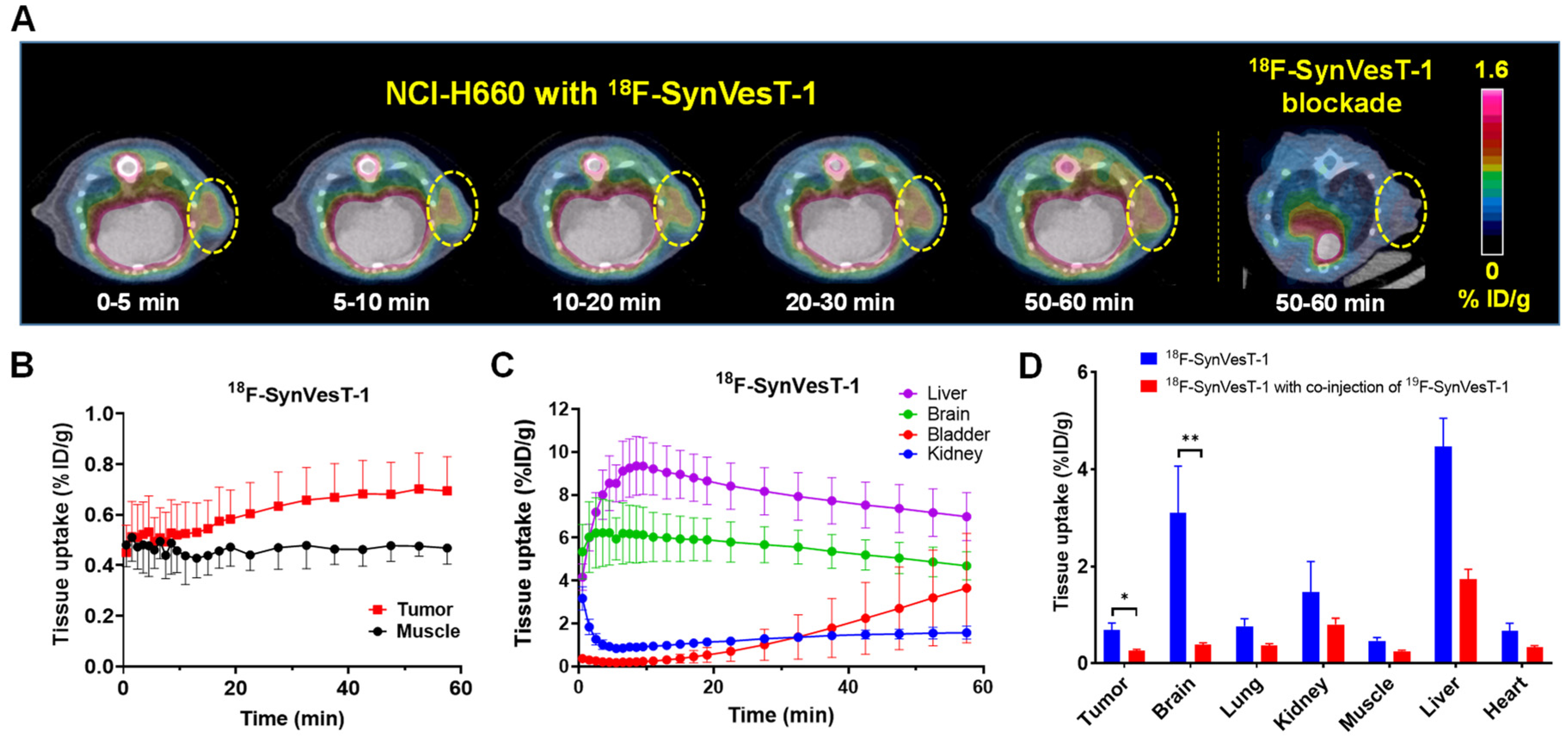
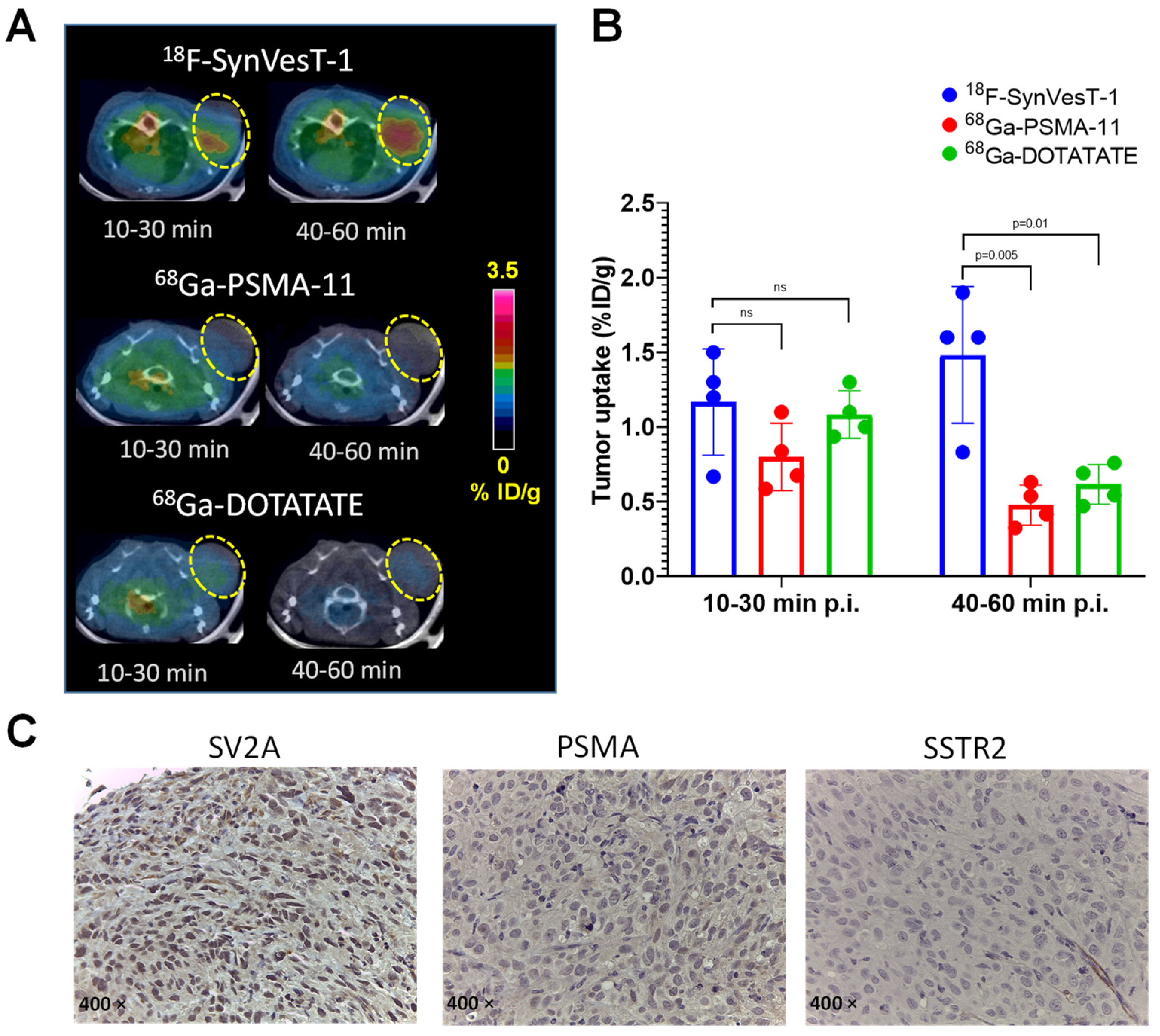
2.5. PET Imaging of SV2A with 18F-SynVesT-1 in NEPC Xenograft Model
3. Discussion
4. Materials and Methods
4.1. General
4.2. Bioinformatic Analyses Data Source
4.3. Cell Culture and Animal Models
4.4. Western Blotting
4.5. Determination of the SV2A Location in PCa Cells
4.6. Automated Radiosynthesis of 18F-SynVesT-1
4.7. Small Animal PET/CT Imaging
4.8. Immunohistochemistry (IHC)
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef]
- Survival Rates for Prostate Cancer. American Cancer Society, Inc. Available online: https://www.cancer.org/cancer/prostate-cancer/detection-diagnosis-staging/survival-rates.html (accessed on 27 August 2020).
- Beltran, H.; Prandi, D.; Mosquera, J.M.; Benelli, M.; Puca, L.; Cyrta, J.; Marotz, C.; Giannopoulou, E.; Chakravarthi, B.V.; Varambally, S.; et al. Divergent clonal evolution of castration-resistant neuroendocrine prostate cancer. Nat. Med. 2016, 22, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Conteduca, V.; Aieta, M.; Amadori, D.; De Giorgi, U. Neuroendocrine differentiation in prostate cancer: Current and emerging therapy strategies. Crit. Rev. Oncol. Hematol. 2014, 92, 11–24. [Google Scholar] [CrossRef]
- Grigore, A.D.; Ben-Jacob, E.; Farach-Carson, M.C. Prostate Cancer and Neuroendocrine Differentiation: More Neuronal, Less Endocrine? Front. Oncol. 2015, 5, 37. [Google Scholar] [CrossRef] [PubMed]
- Palmgren, J.S.; Karavadia, S.S.; Wakefield, M.R. Unusual and underappreciated: Small cell carcinoma of the prostate. Semin. Oncol. 2007, 34, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Davies, A.H.; Beltran, H.; Zoubeidi, A. Cellular plasticity and the neuroendocrine phenotype in prostate cancer. Nat. Rev. Urol. 2018, 15, 271–286. [Google Scholar] [CrossRef]
- Carlson, R.H. The Treatment Challenges of Neuroendocrine Prostate Cancer. Oncol. Times 2015, 37, 18–19. [Google Scholar] [CrossRef]
- Beltran, H.; Rickman, D.S.; Park, K.; Chae, S.S.; Sboner, A.; MacDonald, T.Y.; Wang, Y.; Sheikh, K.L.; Terry, S.; Tagawa, S.T.; et al. Molecular Characterization of Neuroendocrine Prostate Cancer and Identification of New Drug Targets. Cancer Discov. 2011, 1, 487–495. [Google Scholar] [CrossRef]
- di Sant’Agnese, P.A. Neuroendocrine differentiation in carcinoma of the prostate. Diagnostic, prognostic, and therapeutic implications. Cancer 1992, 70 (Suppl. S1), 254–268. [Google Scholar] [CrossRef]
- Zahalka, A.H.; Frenette, P.S. Nerves in cancer. Nat. Rev. Cancer 2020, 20, 143–157. [Google Scholar] [CrossRef]
- Venkatesh, H.S.; Morishita, W.; Geraghty, A.C.; Silverbush, D.; Gillespie, S.M.; Arzt, M.; Tam, L.T.; Espenel, C.; Ponnuswami, A.; Ni, L.; et al. Electrical and synaptic integration of glioma into neural circuits. Nature 2019, 573, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Venkataramani, V.; Tanev, D.I.; Strahle, C.; Studier-Fischer, A.; Fankhauser, L.; Kessler, T.; Korber, C.; Kardorff, M.; Ratliff, M.; Xie, R.; et al. Glutamatergic synaptic input to glioma cells drives brain tumour progression. Nature 2019, 573, 532–538. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Michael, I.P.; Zhang, P.; Saghafinia, S.; Knott, G.; Jiao, W.; McCabe, B.D.; Galvan, J.A.; Robinson, H.P.C.; Zlobec, I.; et al. Synaptic proximity enables NMDAR signalling to promote brain metastasis. Nature 2019, 573, 526–531. [Google Scholar] [CrossRef]
- Mauffrey, P.; Tchitchek, N.; Barroca, V.; Bemelmans, A.; Firlej, V.; Allory, Y.; Romeo, P.H.; Magnon, C. Progenitors from the central nervous system drive neurogenesis in cancer. Nature 2019, 569, 672–678. [Google Scholar] [CrossRef] [PubMed]
- Magnon, C.; Hall, S.J.; Lin, J.; Xue, X.; Gerber, L.; Freedland, S.J.; Frenette, P.S. Autonomic Nerve Development Contributes to Prostate Cancer Progression. Science 2013, 341, 1236361. [Google Scholar] [CrossRef]
- Ayala, G.E.; Dai, H.; Powell, M.; Li, R.; Ding, Y.; Wheeler, T.M.; Shine, D.; Kadmon, D.; Thompson, T.; Miles, B.J.; et al. Cancer-related axonogenesis and neurogenesis in prostate cancer. Clin. Cancer Res. 2008, 14, 7593–7603. [Google Scholar] [CrossRef]
- Ayala, G.E.; Dai, H.; Ittmann, M.; Li, R.; Powell, M.; Frolov, A.; Wheeler, T.M.; Thompson, T.C.; Rowley, D. Growth and Survival Mechanisms Associated with Perineural Invasion in Prostate Cancer. Cancer Res. 2004, 64, 6082–6090. [Google Scholar] [CrossRef]
- Ayala, G.E.; Wheeler, T.M.; Shine, H.D.; Schmelz, M.; Frolov, A.; Chakraborty, S.; Rowley, D. In vitro dorsal root ganglia and human prostate cell line interaction: Redefining perineural invasion in prostate cancer. Prostate 2001, 49, 213–223. [Google Scholar] [CrossRef]
- Zhu, Y.; Liu, C.; Cui, Y.; Nadiminty, N.; Lou, W.; Gao, A.C. Interleukin-6 induces neuroendocrine differentiation (NED) through suppression of RE-1 silencing transcription factor (REST). Prostate 2014, 74, 1086–1094. [Google Scholar] [CrossRef]
- Beltran, H.; Jendrisak, A.; Landers, M.; Mosquera, J.M.; Kossai, M.; Louw, J.; Krupa, R.; Graf, R.P.; Schreiber, N.A.; Nanus, D.M.; et al. The Initial Detection and Partial Characterization of Circulating Tumor Cells in Neuroendocrine Prostate Cancer. Clin. Cancer Res. 2016, 22, 1510–1519. [Google Scholar] [CrossRef]
- Carlucci, G.; Ippisch, R.; Slavik, R.; Mishoe, A.; Blecha, J.; Zhu, S. (68)Ga-PSMA-11 NDA Approval: A Novel and Successful Academic Partnership. J. Nucl. Med. 2021, 62, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.Y.C.; Ettinger, S.L.; Lin, D.; Xue, H.; Ci, X.; Nabavi, N.; Bell, R.H.; Mo, F.; Gout, P.W.; Fleshner, N.E.; et al. Targeting MCT4 to reduce lactic acid secretion and glycolysis for treatment of neuroendocrine prostate cancer. Cancer Med. 2018, 7, 3385–3392. [Google Scholar] [CrossRef] [PubMed]
- Terry, S.; Beltran, H. The many faces of neuroendocrine differentiation in prostate cancer progression. Front. Oncol. 2014, 4, 60. [Google Scholar] [CrossRef] [PubMed]
- Parida, G.K.; Tripathy, S.; Datta Gupta, S.; Singhal, A.; Kumar, R.; Bal, C.; Shamim, S.A. Adenocarcinoma Prostate With Neuroendocrine Differentiation: Potential Utility of 18F-FDG PET/CT and 68Ga-DOTANOC PET/CT Over 68Ga-PSMA PET/CT. Clin. Nucl. Med. 2018, 43, 248–249. [Google Scholar] [CrossRef]
- Liu, Y.Y. FDG PET-CT demonstration of metastatic neuroendocrine tumor of prostate. World J. Surg. Oncol. 2008, 6, 64. [Google Scholar] [CrossRef]
- Spratt, D.E.; Gavane, S.; Tarlinton, L.; Fareedy, S.B.; Doran, M.G.; Zelefsky, M.J.; Osborne, J.R. Utility of FDG-PET in clinical neuroendocrine prostate cancer. Prostate 2014, 74, 1153–1159. [Google Scholar] [CrossRef]
- Savelli, G.; Muni, A.; Falchi, R.; Zaniboni, A.; Barbieri, R.; Valmadre, G.; Minari, C.; Casi, C.; Rossini, P. Somatostatin receptors over-expression in castration resistant prostate cancer detected by PET/CT: Preliminary report of in six patients. Ann. Transl. Med. 2015, 3, 145. [Google Scholar] [CrossRef]
- Gofrit, O.N.; Frank, S.; Meirovitz, A.; Nechushtan, H.; Orevi, M. PET/CT With 68Ga-DOTA-TATE for Diagnosis of Neuroendocrine: Differentiation in Patients With Castrate-Resistant Prostate Cancer. Clin. Nucl. Med. 2017, 42, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Iravani, A.; Mitchell, C.; Akhurst, T.; Sandhu, S.; Hofman, M.S.; Hicks, R.J. Molecular Imaging of Neuroendocrine Differentiation of Prostate Cancer: A Case Series. Clin. Genitourin. Cancer 2021, 19, e200–e205. [Google Scholar] [CrossRef] [PubMed]
- Puranik, A.D.; Dromain, C.; Fleshner, N.; Sathekge, M.; Pavel, M.; Eberhardt, N.; Zengerling, F.; Marienfeld, R.; Grunert, M.; Prasad, V. Target Heterogeneity in Oncology: The Best Predictor for Differential Response to Radioligand Therapy in Neuroendocrine Tumors and Prostate Cancer. Cancers 2021, 13, 3607. [Google Scholar] [CrossRef]
- Bilen, M.A.; Akintayo, A.A.; Abiodun-Ojo, O.A.; Kucuk, O.; Carthon, B.C.; Schuster, D.M.; Parent, E.E. The role of 68Ga-DOTATATE for evaluation of patients with metastatic castration-resistant prostate cancer (mCRPC). J. Clin. Oncol. 2020, 38 (Suppl. S6), 217. [Google Scholar] [CrossRef]
- Schmitt, A.M.; Blank, A.; Marinoni, I.; Komminoth, P.; Perren, A. Histopathology of NET: Current concepts and new developments. Best Pract. Res. Clin. Endocrinol. Metab. 2016, 30, 33–43. [Google Scholar] [CrossRef]
- Tomita, T. Significance of chromogranin A and synaptophysin in pancreatic neuroendocrine tumors. Bosn. J. Basic Med. Sci. 2020, 20, 336–346. [Google Scholar] [CrossRef]
- Karsenty, G.; Rocha, J.; Chevalier, S.; Scarlata, E.; Andrieu, C.; Zouanat, F.Z.; Rocchi, P.; Giusiano, S.; Elzayat, E.A.; Corcos, J. Botulinum toxin type A inhibits the growth of LNCaP human prostate cancer cells in vitro and in vivo. Prostate 2009, 69, 1143–1150. [Google Scholar] [CrossRef]
- Bandala, C.; Cortes-Algara, A.L.; Mejia-Barradas, C.M.; Ilizaliturri-Flores, I.; Dominguez-Rubio, R.; Bazan-Mendez, C.I.; Floriano-Sanchez, E.; Luna-Arias, J.P.; Anaya-Ruiz, M.; Lara-Padilla, E. Botulinum neurotoxin type A inhibits synaptic vesicle 2 expression in breast cancer cell lines. Int. J. Clin. Exp. Pathol. 2015, 8, 8411–8418. [Google Scholar]
- Jiang, S.-H.; Hu, L.-P.; Wang, X.; Li, J.; Zhang, Z.-G. Neurotransmitters: Emerging targets in cancer. Oncogene 2020, 39, 503–515. [Google Scholar] [CrossRef]
- DeLellis, R.A.; Shin, S.J.; Treaba, D.O. Chapter 10—Immunohistology of Endocrine Tumors. In Diagnostic Immunohistochemistry, 3rd ed.; Dabbs, D.J., Ed.; W.B. Saunders: Philadelphia, PA, USA, 2011; pp. 291–339. [Google Scholar]
- Bhargava, R.; Dabbs, D.J. Chapter 8—Immunohistology of Metastatic Carcinomas of Unknown Primary. In Diagnostic Immunohistochemistry, 3rd ed.; Dabbs, D.J., Ed.; W.B. Saunders: Philadelphia, PA, USA, 2011; pp. 206–255. [Google Scholar]
- Jakobsen, A.-M.; Ahlman, H.; Wängberg, B.; Kölby, L.; Bengtsson, M.; Nilsson, O. Expression of synaptic vesicle protein 2 (SV2) in neuroendocrine tumours of the gastrointestinal tract and pancreas. J. Pathol. 2002, 196, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Georgantzi, K.; Tsolakis, A.V.; Jakobson, A.; Christofferson, R.; Janson, E.T.; Grimelius, L. Synaptic Vesicle Protein 2 and Vesicular Monoamine Transporter 1 and 2 Are Expressed in Neuroblastoma. Endocr. Pathol. 2019, 30, 173–179. [Google Scholar] [CrossRef]
- Lu, R.; Fan, C.; Shangguan, W.; Liu, Y.; Li, Y.; Shang, Y.; Yin, D.; Zhang, S.; Huang, Q.; Li, X.; et al. Neurons generated from carcinoma stem cells support cancer progression. Signal Transduct. Target. Ther. 2017, 2, e16036. [Google Scholar] [CrossRef]
- Cortes-Algara, A.; Cardenas-Rodriguez, N.; Lara-Padilla, E.; Floriano-Sanchez, E.; Martinez-Contreras, R.; Anaya-Ruiz, M.; Uribe-Escamilla, R.; Alfaro-Rodriguez, A.; Ilizaliturri-Flores, I.; Perez-Santos, M.; et al. Synaptic vesicle protein isoforms (SV2A, SV2B, SV2C): Expression in breast cancer and their association with risk factors and metastasis in Mexican women. Int. J. Clin. Exp. Pathol. 2017, 10, 1998–2004. [Google Scholar]
- Jaskula-Sztul, R.; Whitt, J.D.; Ou, J.; Liu, M.X.; Aburjania, Z.; Chen, H. Synaptic vesicle 2 receptors as a novel targets for neuroendocrine cancer therapy. J. Clin. Transl. Sci. 2018, 2 (Suppl. S1), 28–29. [Google Scholar] [CrossRef][Green Version]
- Stridsberg, M. The Use of Chromogranin, Synaptophysin and Islet Amyloid Polypeptide as Markers for Neuroendocrine Tumours. Upsala J. Med Sci. 1995, 100, 169–199. [Google Scholar] [CrossRef][Green Version]
- Estrada, S.; Lubberink, M.; Thibblin, A.; Sprycha, M.; Buchanan, T.; Mestdagh, N.; Kenda, B.; Mercier, J.; Provins, L.; Gillard, M.; et al. [11C]UCB-A, a novel PET tracer for synaptic vesicle protein 2A. Nucl. Med. Biol. 2016, 43, 325–332. [Google Scholar] [CrossRef]
- Nabulsi, N.B.; Mercier, J.; Holden, D.; Carré, S.; Najafzadeh, S.; Vandergeten, M.C.; Lin, S.F.; Deo, A.; Price, N.; Wood, M.; et al. Synthesis and Preclinical Evaluation of 11C-UCB-J as a PET Tracer for Imaging the Synaptic Vesicle Glycoprotein 2A in the Brain. J. Nucl. Med. 2016, 57, 777–784. [Google Scholar] [CrossRef]
- Becker, G.; Warnier, C.; Serrano, M.E.; Bahri, M.A.; Mercier, J.; Lemaire, C.; Salmon, E.; Luxen, A.; Plenevaux, A. Pharmacokinetic Characterization of [18F]UCB-H PET Radiopharmaceutical in the Rat Brain. Mol. Pharm. 2017, 14, 2719–2725. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Cai, Z.; Wu, X.; Holden, D.; Pracitto, R.; Kapinos, M.; Gao, H.; Labaree, D.; Nabulsi, N.; Carson, R.E.; et al. Synthesis and in Vivo Evaluation of a Novel PET Radiotracer for Imaging of Synaptic Vesicle Glycoprotein 2A (SV2A) in Nonhuman Primates. ACS Chem. Neurosci. 2019, 10, 1544–1554. [Google Scholar] [CrossRef]
- Cai, Z.; Li, S.; Zhang, W.; Pracitto, R.; Wu, X.; Baum, E.; Finnema, S.J.; Holden, D.; Toyonaga, T.; Lin, S.-F.; et al. Synthesis and Preclinical Evaluation of an 18F-Labeled Synaptic Vesicle Glycoprotein 2A PET Imaging Probe: [18F]SynVesT-2. ACS Chem. Neurosci. 2020, 11, 592–603. [Google Scholar] [CrossRef]
- Cai, Z.X.; Li, S.Y.; Matuskey, D.; Nabulsi, N.; Huang, Y.Y. PET imaging of synaptic density: A new tool for investigation of neuropsychiatric diseases. Neurosci. Lett. 2019, 691, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Becker, G.; Dammicco, S.; Bahri, M.A.; Salmon, E. The Rise of Synaptic Density PET Imaging. Molecules 2020, 25, 2303. [Google Scholar] [CrossRef]
- Chlenski, A.; Nakashiro, K.; Ketels, K.V.; Korovaitseva, G.I.; Oyasu, R. Androgen receptor expression in androgen-independent prostate cancer cell lines. Prostate 2001, 47, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Nakayama, K.; Cardiff, R.D.; Borowsky, A.D.; Kaul, K.; Williams, R.; Krajewski, S.; Mercola, D.; Carpenter, P.M.; Bowtell, D.; et al. Siah2-dependent concerted activity of HIF and FoxA2 regulates formation of neuroendocrine phenotype and neuroendocrine prostate tumors. Cancer Cell 2010, 18, 23–38. [Google Scholar] [CrossRef] [PubMed]
- Pauwels, E.; Cleeren, F.; Bormans, G.; Deroose, C.M. Somatostatin receptor PET ligands—The next generation for clinical practice. Am. J. Nucl. Med. Mol. Imaging 2018, 8, 311–331. [Google Scholar] [PubMed]
- Aggarwal, R.; Zhang, T.; Small, E.J.; Armstrong, A.J. Neuroendocrine prostate cancer: Subtypes, biology, and clinical outcomes. J. Natl. Compr. Cancer Netw. 2014, 12, 719–726. [Google Scholar] [CrossRef]
- Mathis, C.A.; Lopresti, B.J.; Ikonomovic, M.D.; Klunk, W.E. Small-molecule PET Tracers for Imaging Proteinopathies. Semin. Nucl. Med. 2017, 47, 553–575. [Google Scholar] [CrossRef]
- Rankovic, Z. CNS drug design: Balancing physicochemical properties for optimal brain exposure. J. Med. Chem. 2015, 58, 2584–2608. [Google Scholar] [CrossRef]
- Pike, V.W. PET radiotracers: Crossing the blood-brain barrier and surviving metabolism. Trends Pharmacol. Sci. 2009, 30, 431–440. [Google Scholar] [CrossRef]
- Mojtahedi, A.; Thamake, S.; Tworowska, I.; Ranganathan, D.; Delpassand, E.S. The value of (68)Ga-DOTATATE PET/CT in diagnosis and management of neuroendocrine tumors compared to current FDA approved imaging modalities: A review of literature. Am. J. Nucl. Med. Mol. Imaging 2014, 4, 426–434. [Google Scholar] [PubMed]
- Maxwell, J.E.; Howe, J.R. Imaging in neuroendocrine tumors: An update for the clinician. Int. J. Endocr. Oncol. 2015, 2, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Bodei, L.; Sundin, A.; Kidd, M.; Prasad, V.; Modlin, I.M. The status of neuroendocrine tumor imaging: From darkness to light? Neuroendocrinology 2015, 101, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Qian, Z.R.; Li, T.T.; Ter-Minassian, M.; Yang, J.H.; Chan, J.A.; Brais, L.K.; Masugi, Y.; Thiaglingam, A.; Brooks, N.; Nishihara, R.; et al. Association Between Somatostatin Receptor Expression and Clinical Outcomes in Neuroendocrine Tumors. Pancreas 2016, 45, 1386–1393. [Google Scholar] [CrossRef]
- Hofland, J.; Zandee, W.T.; de Herder, W.W. Role of biomarker tests for diagnosis of neuroendocrine tumours. Nat. Rev. Endocrinol. 2018, 14, 656–669. [Google Scholar] [CrossRef] [PubMed]
- de Groot, M.; Toering, S.T.; Boer, K.; Spliet, W.G.; Heimans, J.J.; Aronica, E.; Reijneveld, J.C. Expression of synaptic vesicle protein 2A in epilepsy-associated brain tumors and in the peritumoral cortex. Neuro. Oncol. 2010, 12, 265–273. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guan, B.; Zhou, N.; Wu, C.-Y.; Li, S.; Chen, Y.-A.; Debnath, S.; Hofstad, M.; Ma, S.; Raj, G.V.; He, D.; et al. Validation of SV2A-Targeted PET Imaging for Noninvasive Assessment of Neuroendocrine Differentiation in Prostate Cancer. Int. J. Mol. Sci. 2021, 22, 13085. https://doi.org/10.3390/ijms222313085
Guan B, Zhou N, Wu C-Y, Li S, Chen Y-A, Debnath S, Hofstad M, Ma S, Raj GV, He D, et al. Validation of SV2A-Targeted PET Imaging for Noninvasive Assessment of Neuroendocrine Differentiation in Prostate Cancer. International Journal of Molecular Sciences. 2021; 22(23):13085. https://doi.org/10.3390/ijms222313085
Chicago/Turabian StyleGuan, Bing, Ning Zhou, Cheng-Yang Wu, Songye Li, Yu-An Chen, Sashi Debnath, Mia Hofstad, Shihong Ma, Ganesh V. Raj, Dalin He, and et al. 2021. "Validation of SV2A-Targeted PET Imaging for Noninvasive Assessment of Neuroendocrine Differentiation in Prostate Cancer" International Journal of Molecular Sciences 22, no. 23: 13085. https://doi.org/10.3390/ijms222313085
APA StyleGuan, B., Zhou, N., Wu, C.-Y., Li, S., Chen, Y.-A., Debnath, S., Hofstad, M., Ma, S., Raj, G. V., He, D., Hsieh, J.-T., Huang, Y., Hao, G., & Sun, X. (2021). Validation of SV2A-Targeted PET Imaging for Noninvasive Assessment of Neuroendocrine Differentiation in Prostate Cancer. International Journal of Molecular Sciences, 22(23), 13085. https://doi.org/10.3390/ijms222313085





