Target-Based Physiological Modulations and Chloroplast Proteome Reveals a Drought Resilient Rootstock in Okra (Abelmoschus esculentus) Genotypes
Abstract
1. Introduction
2. Materials and Methods
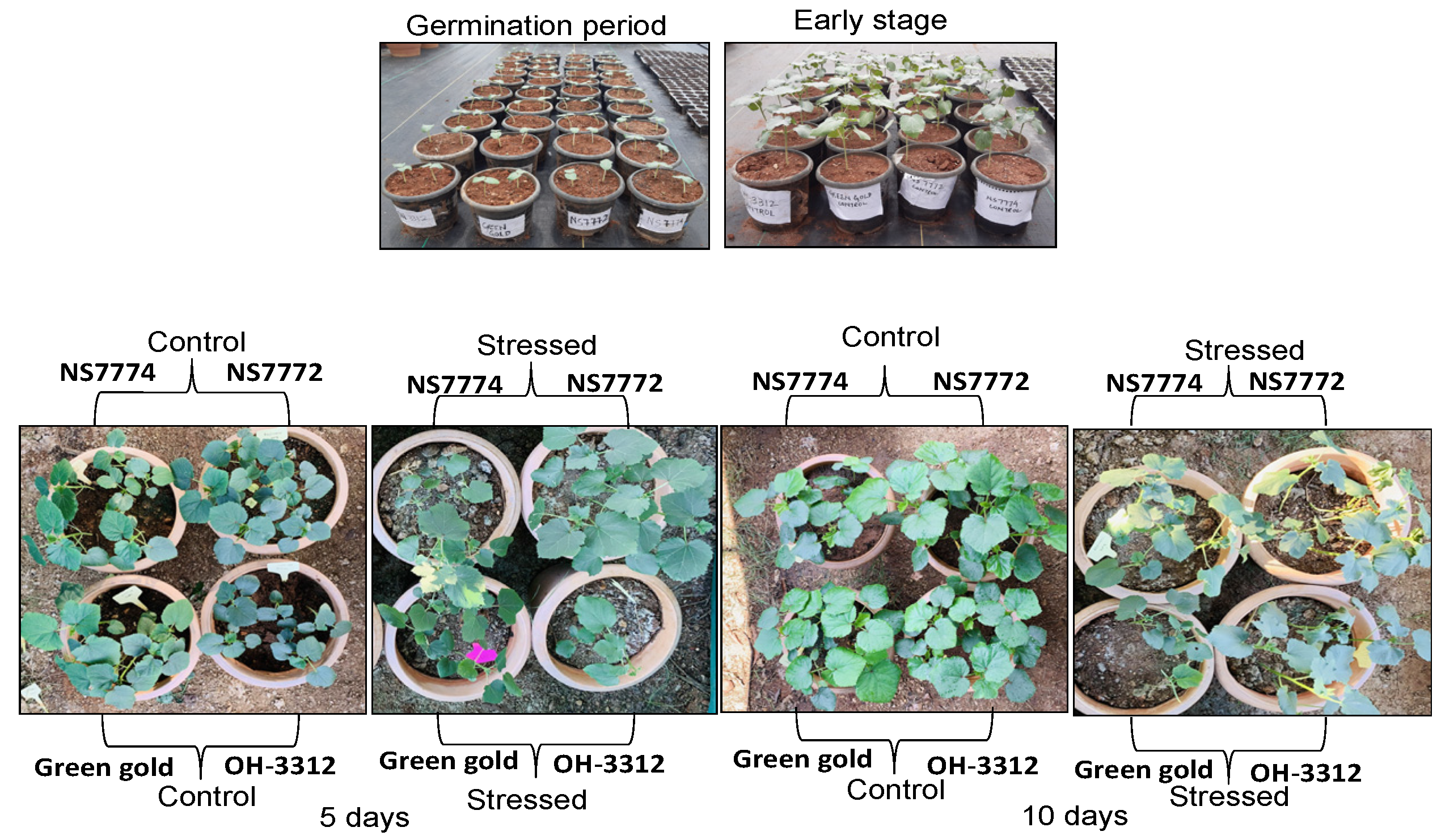
2.1. Plant Materials and Drought Treatments
2.2. Morphological Measurements
2.3. Measurement of Relative Water Content (RWC), MDA Content and Proline Content
2.4. H2O2 and O2−1 Localizations
2.5. Antioxidant Enzyme Assays
2.6. Water Transport Activity
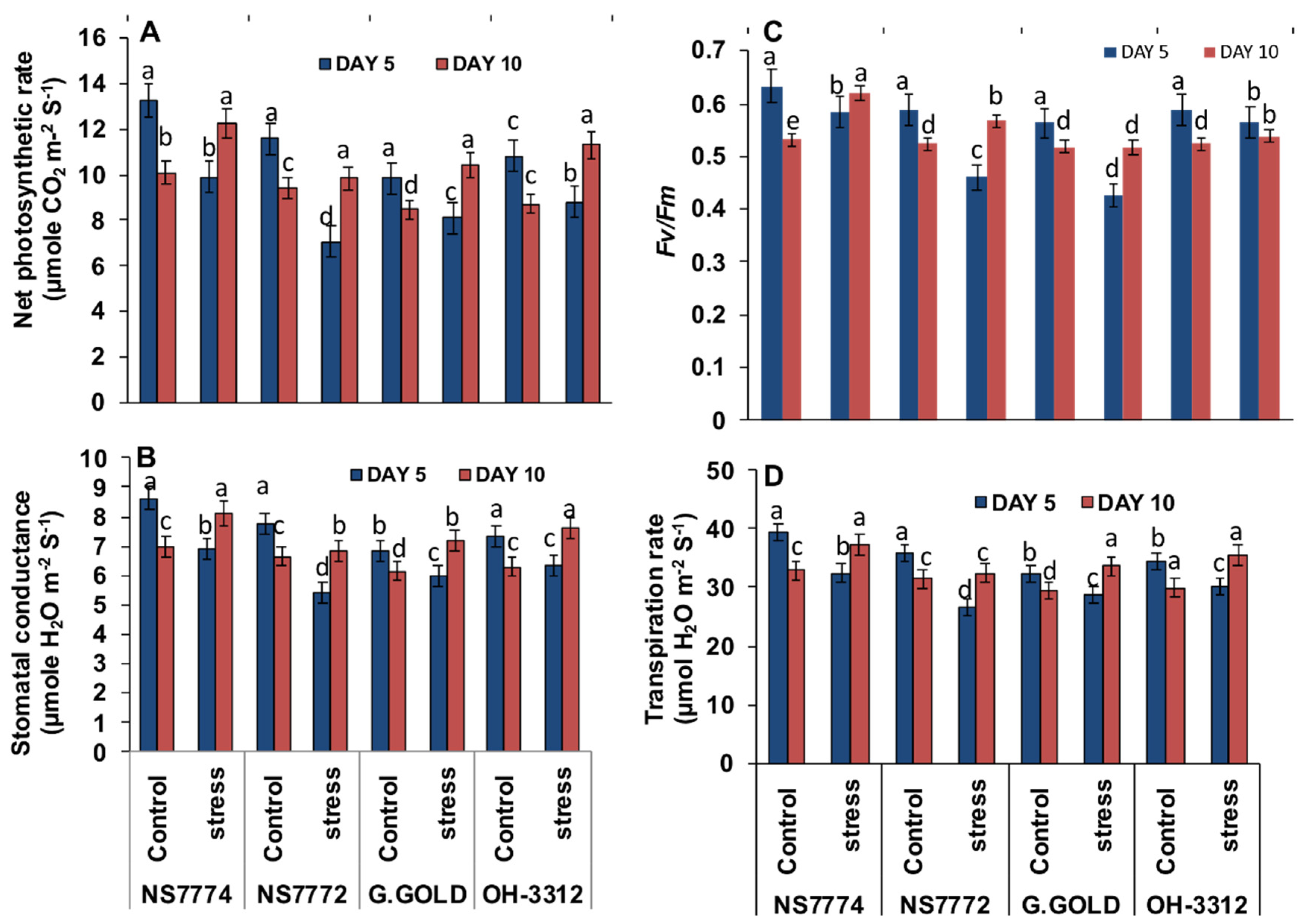
2.7. Photosynthetic Measurements
2.8. Pigment Analysis
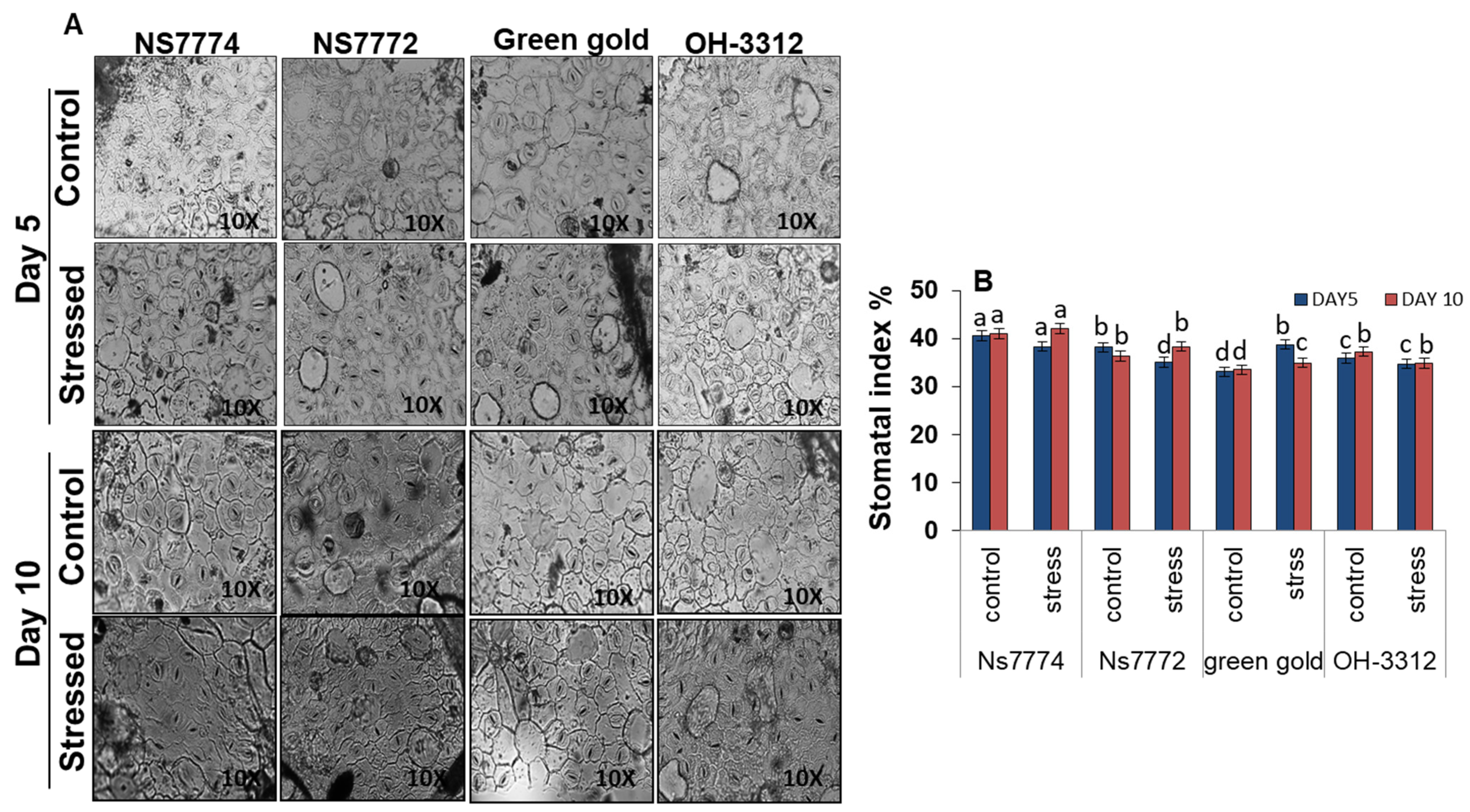
2.9. Determination of Stomatal Index
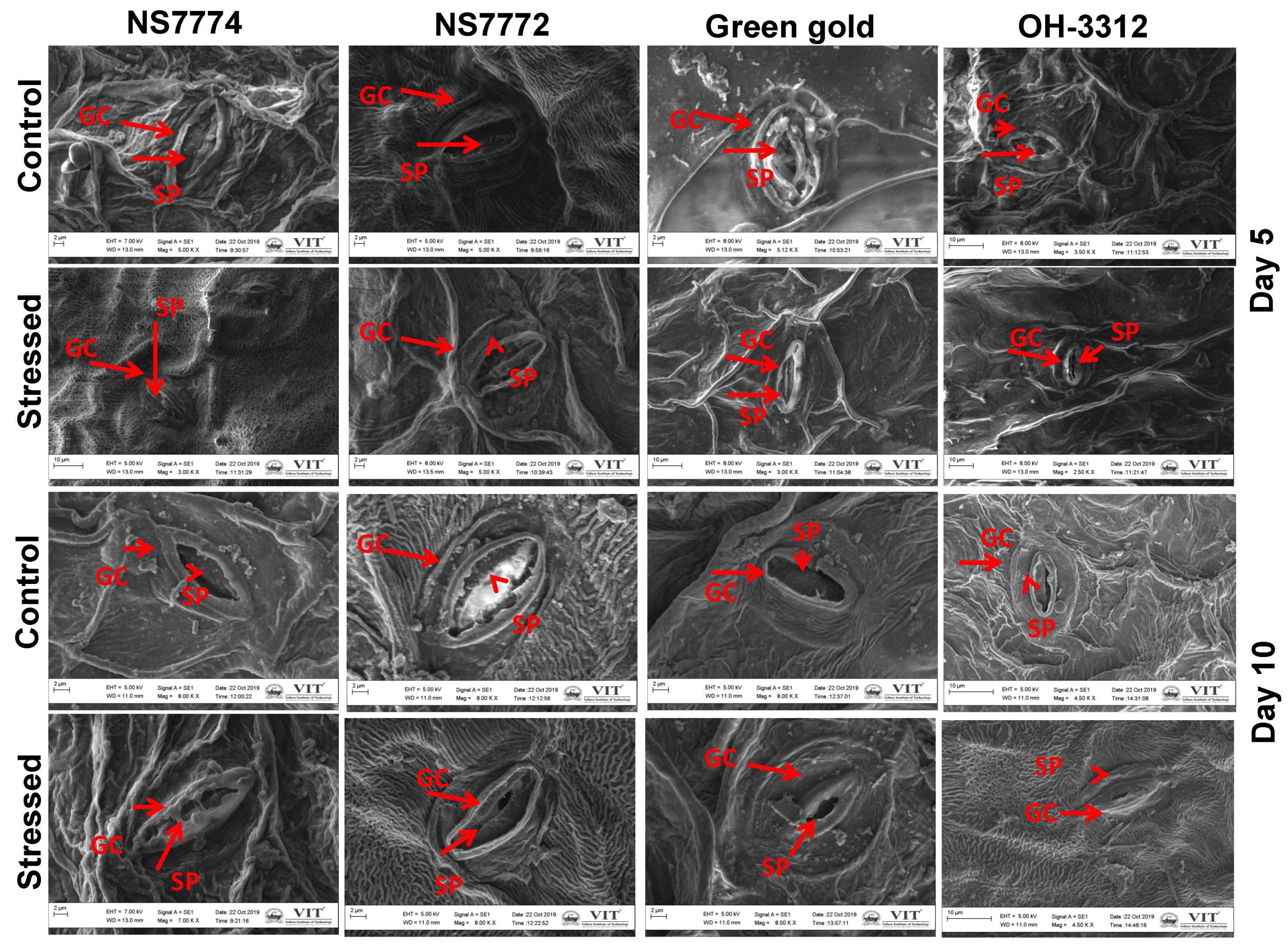
2.10. Scanning Electron Microscope (SEM) Analysis for the Structure of Stomata
2.11. Total Protein Profile by SDS-PAGE (Sodium Dodecyl Sulphate Polyacrylamide Gel Electrophoresis)
2.12. D BN-SDS-PAGE
2.13. Image Analysis
2.14. Protein In-Gel Digestion and Identification by Matrix Assisted Laser Desorption and Ionization Time of Flight Mass Spectrometry (MALDI-TOF-TOF-MS)
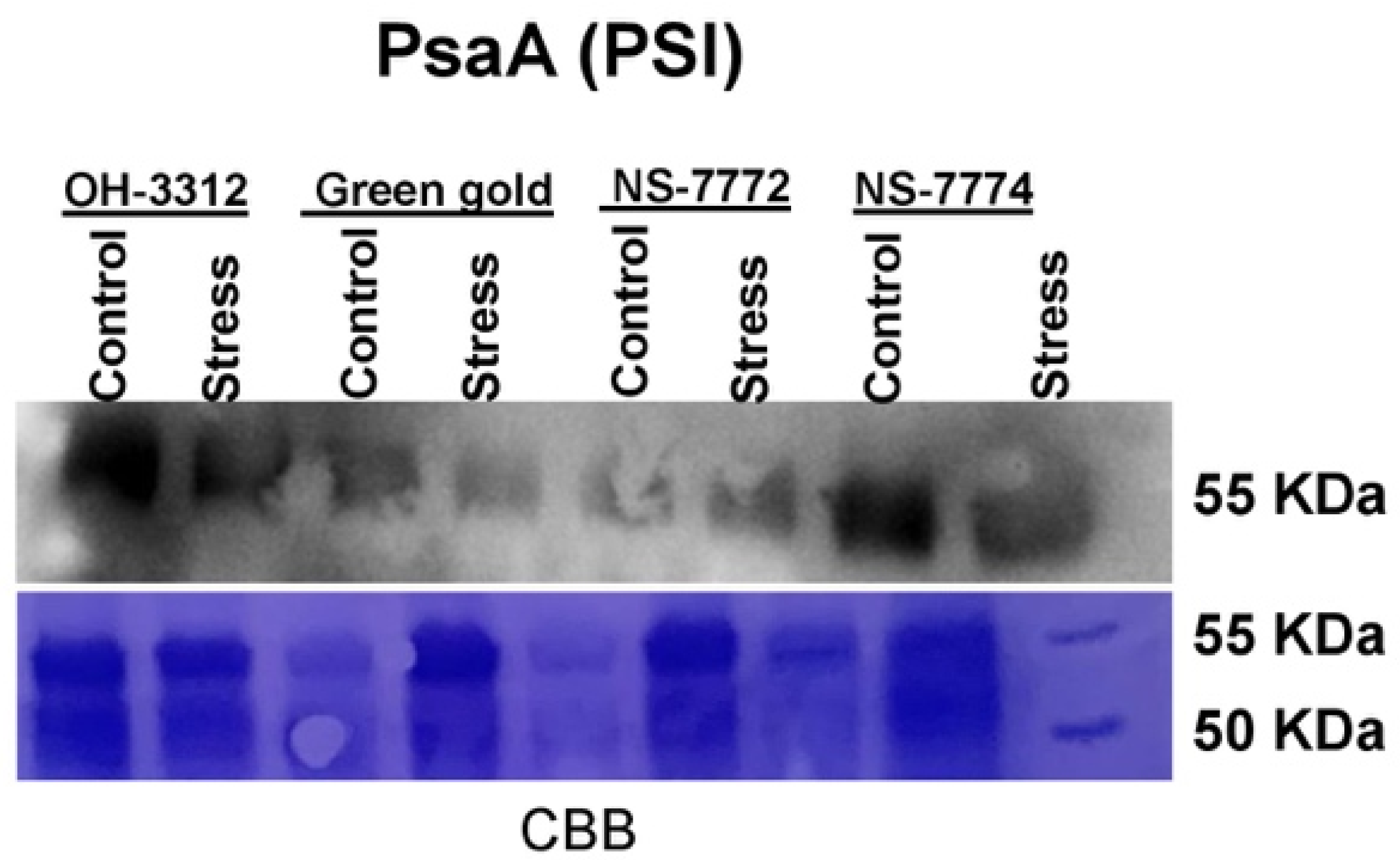
2.15. Western Blots (Immunoblot)
2.16. Statistical Analysis
3. Results
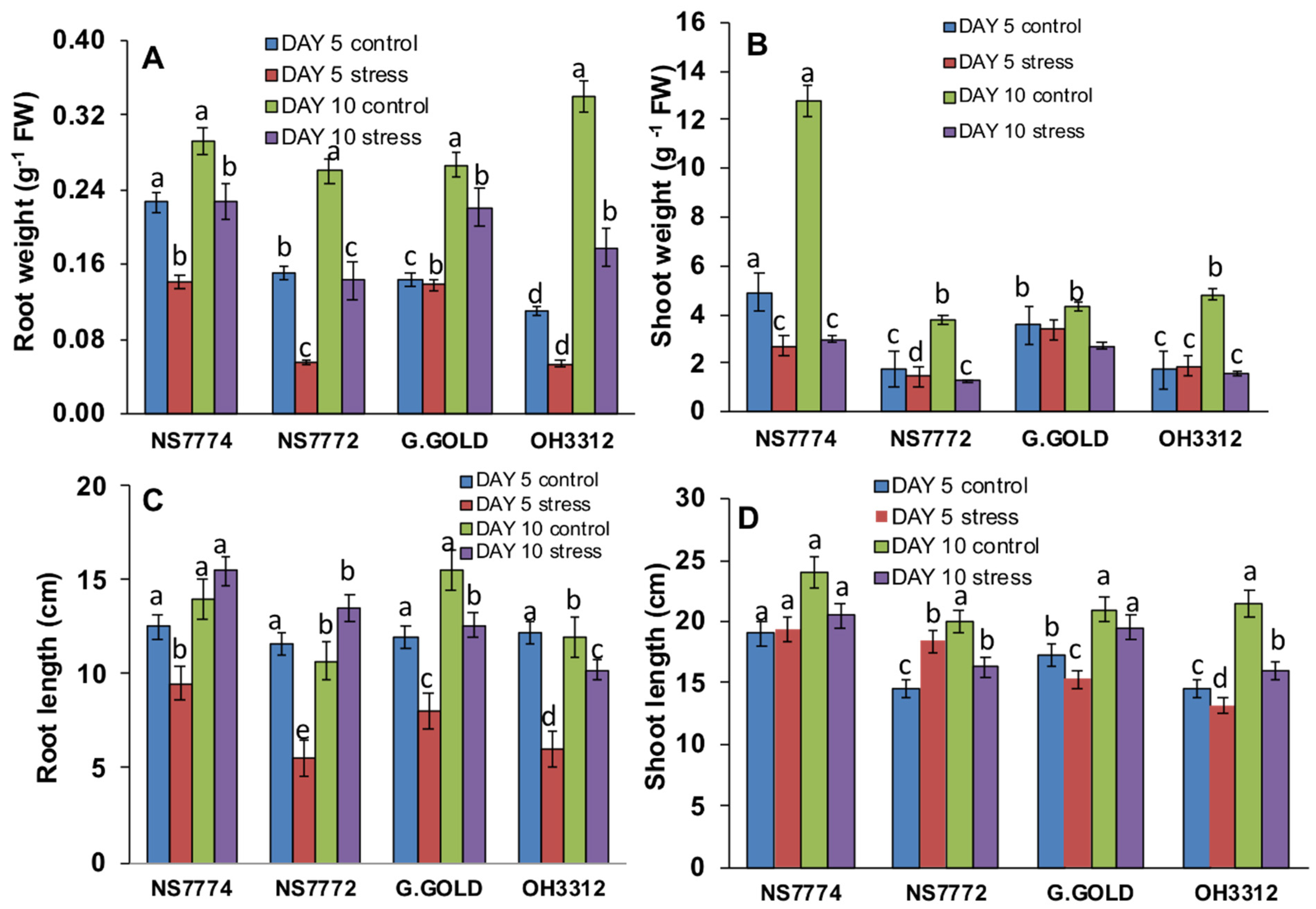
3.1. Genotypic Variation on Morphology of Drought-Stressed Okra
3.2. Genotypic Changes in Relative Water, MDA, and Proline Content in Drought-Stressed Okra
3.3. Oxidative Damage in Drought-Stressed Okra Genotypes
3.4. Proline Content Changes in Drought-Stressed Okra Genotypes
3.5. In situ H2O2 and O2−1 Localization in Drought-Stressed Okra Genotypes
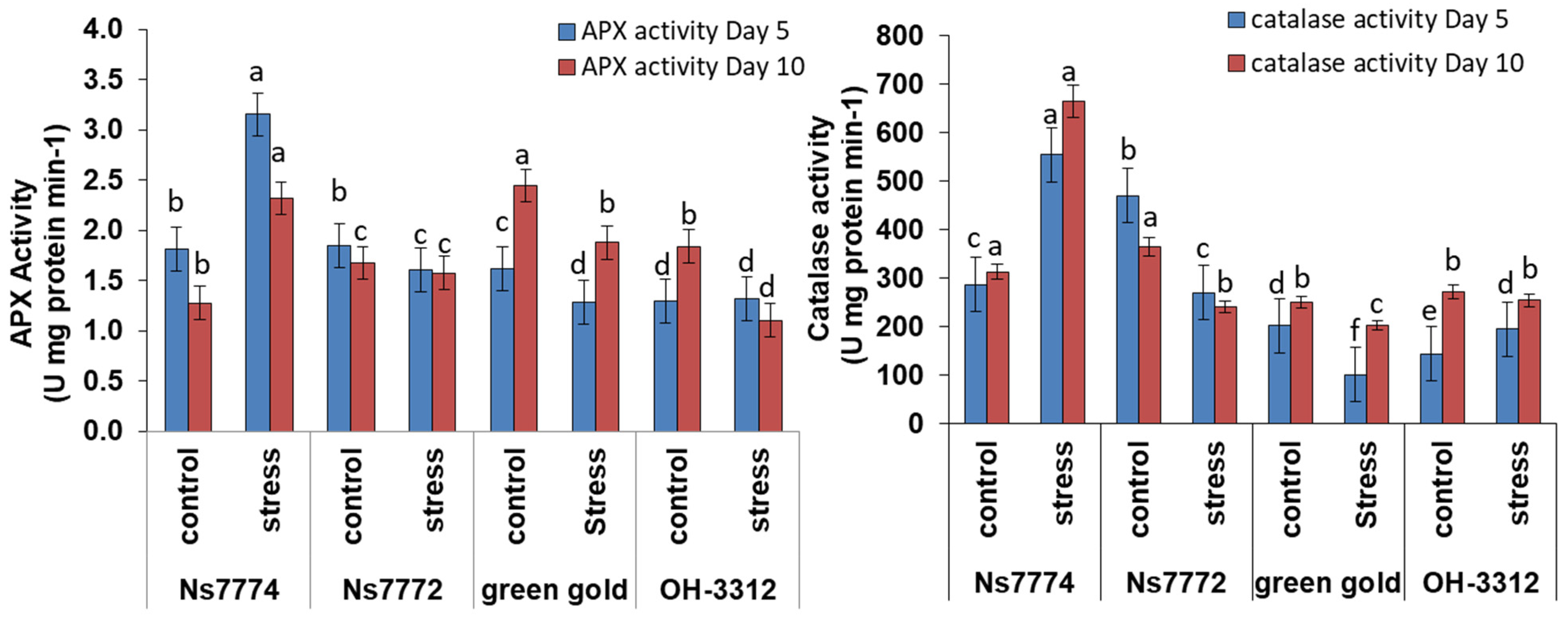
3.6. Enzyme Activities in Drought-Stressed Okra Genotypes
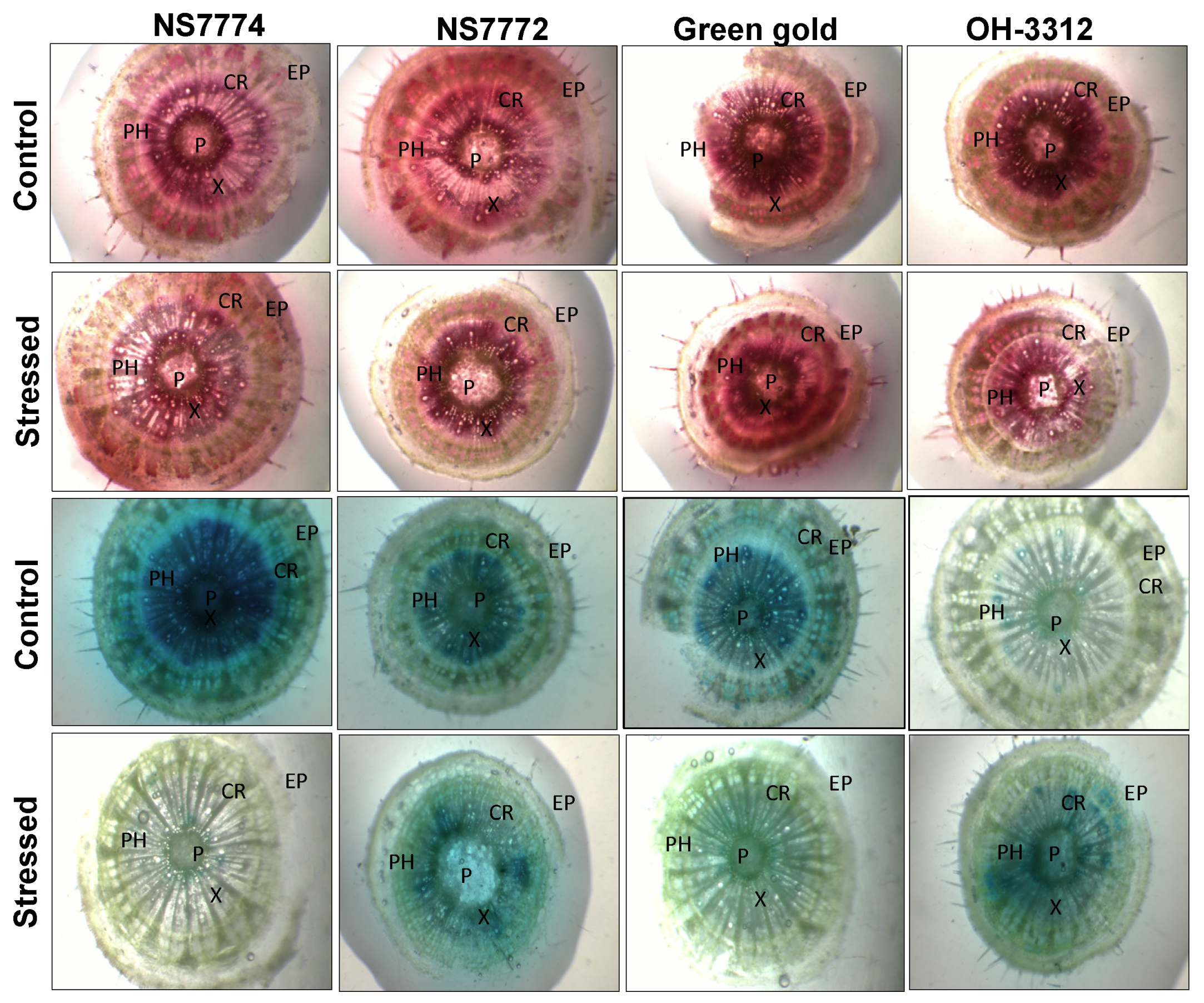
3.7. Vascular Activity in Drought-Stressed Okra Genotypes
3.8. Photosynthetic Changes in Drought-Stressed Okra Genotypes
3.9. Changes in Photosynthetic Pigments in Drought-Stressed Okra Genotypes
3.10. Stomatal Observations in Drought-Stressed Okra Genotypes
3.11. Proteomic Changes in Drought-Stressed Okra Genotypes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Todaka, D.; Zhao, Y.; Yoshida, T.; Kudo, M.; Kidokoro, S.; Mizoi, J.; Kodaira, K.-S.; Takebayashi, Y.; Kojima, M.; Sakakibara, H.; et al. Temporal and spatial changes in gene expression, metabolite accumulation and phytohormone content in rice seedlings grown under drought stress conditions. Plant J. 2016, 90, 61–78. [Google Scholar] [CrossRef]
- Pan, J.; Li, Z.; Wang, Q.; Garrell, A.K.; Liu, M.; Guan, Y.; Zhou, W.; Liu, W. Comparative proteomic investigation of drought responses in foxtail millet. BMC Plant Biol. 2018, 18, 315. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Chen, S.; Li, T.; Ma, X.; Liang, X.; Ding, X.; Liu, H.; Luo, L. OsGRAS23, a rice GRAS transcription factor gene, is involved in drought stress response through regulating expression of stress-responsive genes. BMC Plant Biol. 2015, 15, 141. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Wang, Y.; Wang, X.; Yang, Q.; Quan, X.; Zeng, J.; Dai, F.; Wu, F.; Zhang, G.; Chen, Z.H. Leaf Epidermis Transcriptome Reveals Drought-Induced Hormonal Signaling for Stomatal Regulation in Wild Barley. Plant Growth Regul. 2018, 87, 39–54. [Google Scholar] [CrossRef]
- Hou, Z.; Yin, J.; Lu, Y.; Song, J.; Wang, S.; Wei, S.; Liu, Z.; Zhang, Y.; Fang, Z. Transcriptomic Analysis Reveals the Temporal and Spatial Changes in Physiological Process and Gene Expression in Common Buckwheat (Fagopyrum esculentum Moench) Grown under Drought Stress. Agronomy 2019, 9, 569. [Google Scholar] [CrossRef]
- Kaya, M.D.; Okcub, G.; Ataka, M.; Cikilic, Y.; Kolsaricia, O. Seed Treatments to Overcome Salt and Drought Stress during Germination in Sunflower (Helianthus annuus L.). Eur. J. Agron. 2006, 24, 291–295. [Google Scholar] [CrossRef]
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S.M.A. Plant drought stress: Effects, mechanisms and management. Agron. Sustain. Dev. 2009, 29, 185–212. [Google Scholar] [CrossRef]
- Muneer, S.; Ko, C.H.; Wei, H.; Chen, Y.; Jeong, B.R. Physiological and Proteomic Investigations to Study the Response of Tomato Graft Unions under Temperature Stress. PLoS ONE 2016, 11, e0157439. [Google Scholar] [CrossRef]
- Taiz, L.; Zeiger, E. Plant Physiology, 4th ed.; Sinauer Associates Inc.: Sunderland, UK, 2016. [Google Scholar]
- Zhao, T.J.; Sun, S.; Liu, Y.; Liu, J.M.; Liu, Q.; Yan, Y.B.; Zhou, H.M. Regulating the Drought-Responsive Element (DRE)-Mediated Signaling Pathway by Synergic Functions of Trans-Active and Trans-Inactive DRE Binding Factors in Brassica napus. J. Biol. Chem. 2006, 281, 10752–10759. [Google Scholar] [CrossRef]
- Khan, M.B.; Hussain, M.; Raza, A.; Farooq, S.; Jabran, K. Seed priming with CaCl2 and ridge planting for improved drought resistance in maize. Turk. J. Agric. For. 2015, 39, 193–203. [Google Scholar] [CrossRef]
- Ladrera, R.; Marino, D.; Larrainzar, E.; Gonzalez, E.M.; ArreseIgor, C. Reduced Carbon Availability to Bacteroids and Elevated Ureides in Nodules, But Not in Shoots, are Involved in the Nitrogn Fixation Response to Early Drought in Soybean. Plant Physiol. 2007, 145, 539–546. [Google Scholar] [CrossRef] [PubMed]
- Wahid, A.; Gelani, S.; Ashraf, M.; Foolad, M. Heat tolerance in plants: An overview. Environ. Exp. Bot. 2007, 61, 199–223. [Google Scholar] [CrossRef]
- Razi, K.; Muneer, S. Drought stress-induced physiological mechanisms, signaling pathways and molecular response of chloroplasts in common vegetable crops. Crit. Rev. Biotechnol. 2021, 41, 669–691. [Google Scholar] [CrossRef]
- Liu, C.; Zhou, J.; Bracha-Drori, K.; Yalovsky, S.; Ito, T.; Yu, H. Specification of Arabidopsis floral Meristem Identity by Repression of Flowering Time Genes. Development 2007, 134, 1901–1910. [Google Scholar] [CrossRef]
- Yin, J.; Jia, J.; Lian, Z.; Hu, Y.; Guo, J.; Huo, H.; Zhu, Y.; Gong, H. Silicon enhances the salt tolerance of cucumber through increasing polyamine accumulation and decreasing oxidative damage. Ecotoxicol. Environ. Saf. 2018, 169, 8–17. [Google Scholar] [CrossRef]
- Luo, W.; Song, F.; Xie, Y. Trade-off between tolerance to drought and tolerance to flooding in three wetland plants. Wetlands 2008, 28, 866–873. [Google Scholar] [CrossRef]
- Biyani, K.; Tripathi, D.K.; Lee, J.H.; Muneer, S. Dynamic Role of Iron Supply in Amelioration of Cadmium Stress by Modulating Antioxidative Pathways and Peroxidase Enzymes in Mungbean. AOB Plants 2019, 11, plz005. [Google Scholar] [CrossRef]
- Zhang, Y.; Lou, H.; Guo, D.; Zhang, R.; Su, M.; Hou, Z.; Zhou, H.; Liang, R.; Xie, C.; You, M.; et al. Identifying changes in the wheat kernel proteome under heat stress using iTRAQ. Crop J. 2018, 6, 600–610. [Google Scholar] [CrossRef]
- Rocco, M.; Arena, S.; Renzone, G.; Scippa, G.S.; Lomaglio, T.; Verrillo, F.; Scaloni, A.; Marra, M. Proteomic Analysis of Drought Stress-Responsive Proteins in Arabidopsis thaliana Rosette Leaves. Mol. Biosyst. 2013, 9, 1257–1267. [Google Scholar] [CrossRef]
- Chaturvedi, A.K.; Surendran, U.; Gopinath, G.; Chandran, K.M.; Nk, A.; Ct, M.F. Elucidation of stage specific physiological sensitivity of okra to drought stress through leaf gas exchange, spectral indices, growth and yield parameters. Agric. Water Manag. 2019, 222, 92–104. [Google Scholar] [CrossRef]
- Dilruba, S.; Hasanuzzaman, M.; Karim, R.; Nahar, K. Yield Response of Okra to Different Sowing Time and Application of Growth Hormones. J. Hort. Sci. Ornam. Plants 2009, 1, 10–14. [Google Scholar]
- Sindhu, R.K.; Vishal, P. Phytochemical, Nutritional and Pharmacological evidences for Abelmoschus esculentus (L.). J. Phytopharm. 2016, 5, 238–241. [Google Scholar] [CrossRef]
- Al-Harbi, A.R.; Al-Omran, A.M.; El-Adgham, F.I. Effect of Drip Irrigation Levels and Emitters Depth on Okra (Abelmoschus esculentus) Growth. J. Appl. Sci. 2008, 8, 2764–2769. [Google Scholar] [CrossRef][Green Version]
- Muneer, S.; Park, Y.G.; Manivannan, A.; Soundararajan, P.; Jeong, B.R. Physiological and Proteomic Analysis in Chloroplasts of Solanum lycopersicum L. under Silicon Efficiency and Salinity Stress. Int. J. Mol. Sci. 2014, 15, 21803–21824. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in Isolated Chloroplast I. Kinetics and Stoichiometry of Fatty Acid Peroxidation. Arch. Biochem. Biophy. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Muneer, S.; Lee, B.R.; Bae, D.W.; Kim, T.H. Changes in expression of proteins involved in alleviation of Fedeficiency by sulfur nutrition in Brassica napus L. Acta Physiol. Plant. 2013, 35, 3037–3045. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen Peroxide is Scavenged by Ascorbate-specific Peroxidase in Spinach Chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar] [CrossRef]
- Cakmak, I.; Marschner, H. Magnesium Deficiency and High Light Intensity Enhance Activities of Superoxide Dismutase, Ascorbate Peroxidase, and Glutathione Reductase in Bean Leaves. Plant Physiol. 1992, 98, 1222–1227. [Google Scholar] [CrossRef]
- Muneer, S.; Ko, C.H.; Soundararajan, P.; Manivnnan, A.; Park, Y.G.; Jeong, B.R. Proteomic Study Related to Vascular Connections in Watermelon Scions Grafted onto Bottle-Gourd Rootstock under Different Light Intensities. PLoS ONE 2015, 10, e0120899. [Google Scholar] [CrossRef]
- Yang, L.; Tian, D.; Todd, C.D.; Luo, Y.; Hu, X. Comparative Proteome Analyses Reveal that Nitric Oxide is an Important Signal Molecule in the Response of Rice to Aluminium Toxicity. J. Proteome Res. 2013, 12, 1316–1330. [Google Scholar] [CrossRef]
- Hiscox, J.D.; Israelstam, G.F. A method for the extraction of chlorophyll from leaf tissue without maceration. Can. J. Bot. 1979, 57, 1332–1334. [Google Scholar] [CrossRef]
- Arnon, J.D.; Israelstam, G.F. Copper Enzymes in Isolated Chloroplast Oxidase in Beta vulgaris. Plant Physiol. 1949, 42, 287–292. [Google Scholar]
- Muneer, S.; Lee, J.H. Hazardous gases (CO, NOx, CH4 and C3H8) released from CO2 fertilizer unit lead to oxidative damage and degrades photosynthesis in strawberry plants. Sci. Rep. 2018, 8, 12291. [Google Scholar] [CrossRef] [PubMed]
- Bi, H.; Luang, S.; Li, Y.; Bazanova, N.; Morran, S.; Song, Z.; Perera, M.A.; Hrmova, M.; Borisjuk, N.; Lopato, S. Identification and Characterization of Wheat Drought Responsive MYB Transcription Factors Involved in the Regulation of Cuticle Biosynthesis. J. Exp. Bot. 2016, 67, 5363–5380. [Google Scholar] [CrossRef] [PubMed]
- Usuda, H. The Activation State of Ribulose 1,5-bisphosphate Carboxylase in Maize Leaves in Dark and Light. Plant Cell Physiol. 1985, 26, 1455–1463. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for The Quantitation of Microgram Quantities of Protein Utilizing the Principal of Protein-Dye Binding. Ann. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Muneer, S.; Park, Y.G.; Jeong, B.R. Red and Blue Light Emitting Diodes (LEDs) Participate in Mitigation of Hyperhydricity in In Vitro-Grown Carnation Genotypes (Dianthus caryophyllus). J. Plant Growth Regul. 2017, 37, 370–379. [Google Scholar] [CrossRef]
- Hossain, A.; Sarkar, M.A.Z.; Saifuzzaman, M.; Teixeira da silva, J.A.; Lozovskaya, M.V.; Akhtar, M.M. Evaluation of Growth, Yield, Relative Performance and Heat Susceptibility of Eight Wheat (Triticum aestivum L.) Genotypes Grown Under Heat Stress. Int. J. Plant Prod. 2013, 7, 615–636. [Google Scholar]
- Hassan, H.M.; Arafat, E.F.A.; Sabagh, A.E. Genetic Studies on Agro-Morphological Traits in Rice (Oryza sativa L.) under Water Stress Conditions. J. Agri. Biotechnol. 2016, 1, 76–84. [Google Scholar]
- Islam, M.S.; Akhter, M.M.; El Sabagh, A.; Liu, L.Y.; Nguyen, N.T.; Ueda, A.; Masaoka, Y.; Saneoka, H. Comparative Studies on Growth and Physiological Responses to Saline and Alkaline Stresses of Foxtail Millet (Setaria italica L.) and Proso Millet (Panicum miliaceum L.). Aust. J. Crop Sci. 2011, 5, 1269–1277. [Google Scholar]
- Thakur, M.; Bhattacharya, S.; Khosla, P.K.; Puri, S. Improving production of plant secondary metabolites through biotic and abiotic elicitation. J. Appl. Res. Med. Aromat. Plants 2018, 12, 1–12. [Google Scholar] [CrossRef]
- Li, J.; Leisner, S.M.; Frantz, J. Alleviation of Copper Toxicity in Arabidopsis thaliana by Silicon Addition to Hydroponic Solutions. J. Am. Soc. Hortic. Sci. 2008, 133, 670–677. [Google Scholar] [CrossRef]
- Greenway, H.; Munns, R. Mechanisms of Salt Tolerance in Nonhalophytes. Annu. Rev. Plant Physiol. 1980, 31, 149–190. [Google Scholar] [CrossRef]
- Haq, I.-U.; Khan, A.A.; Khan, I.A.; Azmat, M.A. Comprehensive screening and selection of okra (Abelmoschus esculentus) germplasm for salinity tolerance at the seedling stage and during plant ontogeny. J. Zhejiang Univ. Sci. B 2012, 13, 533–544. [Google Scholar] [CrossRef] [PubMed]
- Ray, I.M.; Segovia-Lerma, A.; Murray, L.W. Diallel Analysis of Carbon Isotope Discrimination and its Association with Forage Yield Among Nine Historically Recognized Alfalfa Germplasms. Crop Sci. 2004, 44, 1970–1975. [Google Scholar] [CrossRef]
- Cao, H.-X.; Zhang, Z.-B.; Xu, P.; Chu, L.-Y.; Shao, H.-B.; Lu, Z.-H.; Liu, J.-H. Mutual physiological genetic mechanism of plant high water use efficiency and nutrition use efficiency. Colloids Surf. B Biointerfaces 2007, 57, 1–7. [Google Scholar] [CrossRef]
- Parida, A.; Das, A.B. Salt tolerance and salinity effects on plants: A review. Ecotoxicol. Environ. Saf. 2005, 60, 324–349. [Google Scholar] [CrossRef]
- Guo, Y.Y.; Yu, H.Y.; Yang, M.M.; Kong, D.S.; Zhang, Y.J. Effect of Drought Stress on Lipid Peroxidation, Osmotic Adjustment and Antioxidant Enzyme Activity of Leaves and Roots of Lycium ruthenicum Murr. Seedling. Russ. J. Plant Physiol. 2018, 65, 244–250. [Google Scholar] [CrossRef]
- Tanveer, M.; Shahzad, B.; Sharma, A.; Khan, E.A. 24-Epibrassinolide application in plants: An implication for improving drought stress tolerance in plants. Plant Physiol. Biochem. 2018, 135, 295–303. [Google Scholar] [CrossRef]
- Sharma, S.; Lin, W.; Villamor, J.G.; Verslues, P.E. Divergent Low Water Potential Response in Arabidopsis thaliana Accessions Landsberg erecta and Shahdara. Plant Cell Environ. 2013, 36, 994–1008. [Google Scholar] [CrossRef]
- Xing, X.-H.; Fang, C.-W.; Li, L.; Jiang, H.-Q.; Zhou, Q.; Jiang, H.-D.; Wang, S.-H. Improved drought tolerance by α-naphthaleneacetic acid-induced ROS accumulation in two soybean cultivars. J. Integr. Agric. 2016, 15, 1770–1784. [Google Scholar] [CrossRef]
- Yi, X.; Burgess, P.; Zhang, X.; Huang, B. Enhancing Cytokinin Synthesis by Overexpressing IPT Alleviated Drought Inhibition of Root Growth through Activating ROS-Scavenging Systems in Agrostis stolonifera. J. Exp. Bot. 2016, 67, 1979–1992. [Google Scholar]
- Zhan, Y.; Wu, Q.; Chen, Y.; Tang, M.; Sun, C.; Sun, J.; Yu, C. Comparative proteomic analysis of okra (Abelmoschus esculentus L.) seedlings under salt stress. BMC Genom. 2019, 20, 381. [Google Scholar] [CrossRef] [PubMed]
- Hasanzadehi, Z.; Ghahremani, Z.; Barzegar, T. The Effect of Naphthalene Acetic Acid on Growth, Yield and Fruit Quality of Okra cv. Kano Dwarf. J. Plant Prod. Res. 2017, 24, 33–45. [Google Scholar]
- Ayub, Q.; Khan, S.M.; Hussain, I.; Gurmani, A.R.; Naveed, K.; Mehmood, A.; Ali, S.; Ahmad, T.; Haq, N.U.; Hussain, A. Mitigating the adverse effects of NaCl salinity on pod yield and ionic attributes of okra plants by silicon and gibberellic acid application. Italus Hortus 2021, 28, 59. [Google Scholar] [CrossRef]
- Ezeh, O.S.; Adejumo, S.A. Ameliorative roles of compost on okra (Abelmoschus esculentus L.) exposed to drought stress at vegetative and reproductive growth stages. Not. Sci. Biol. 2020, 12, 884–900. [Google Scholar] [CrossRef]
- Rosales, M.A.; Ocampo, E.; Rodriguez-Valentin, R.; Olvera-Carrillo, Y.; Acosta-Gallegos, J.; Covarrubias, A.A. hysiological Analysis of Common Bean (Phaseolus vulgaris L.) Cultivars Uncovers Characteristics Related to Terminal Drought Resistance. Plant Physiol. Biochem. 2012, 56, 24–34. [Google Scholar] [CrossRef]
- Li, Z.; Peng, Y.; Ma, X. Different response on drought tolerance and post-drought recovery between the small-leafed and the large-leafed white clover (Trifolium repens L.) associated with antioxidative enzyme protection and lignin metabolism. Acta Physiol. Plant. 2012, 35, 213–222. [Google Scholar] [CrossRef]
- Mansori, M.; Chernane, H.; Latique, S.; Benaliat, A.; Hsissou, D.; El Kaoua, M. Seaweed extract effect on water deficit and antioxidative mechanisms in bean plants (Phaseolus vulgaris L.). Environ. Boil. Fishes 2014, 27, 1689–1698. [Google Scholar] [CrossRef]
- Singh, A.; Kumar, A.; Yadav, S.; Singh, I.K. Reactive oxygen species-mediated signaling during abiotic stress. Plant Gene 2019, 18, 100173. [Google Scholar] [CrossRef]
- Jia, X.; Sun, C.; Li, G.; Li, G.; Chen, G. Effects of progressive drought stress on the physiology, antioxidative enzymes and secondary metabolites of Radix Astragali. Acta Physiol. Plant. 2015, 37, 262. [Google Scholar] [CrossRef]
- Kusvuran, S.; Kiran, S.; Ellialtioglu, S.S. Antioxidant Enzyme Activities and Abiotic Stress Tolerance Relationship in Vegetable Crops. In Abiotic and Biotic Stress in Plants—Recent Advances and Future Perspectives; InTech: London, UK, 2016; pp. 481–506. [Google Scholar]
- Slabbert, M.; Krüger, G. Antioxidant enzyme activity, proline accumulation, leaf area and cell membrane stability in water stressed Amaranthus leaves. S. Afr. J. Bot. 2014, 95, 123–128. [Google Scholar] [CrossRef]
- Hong-Bo, S.; Xiao-Yan, C.; Li-Ye, C.; Xi-Ning, Z.; Gang, W.; Yong-Bing, Y.; Chang-Xing, Z.; Zan-Min, H. Investigation on the relationship of proline with wheat anti-drought under soil water deficits. Colloids Surf. B Biointerfaces 2006, 53, 113–119. [Google Scholar] [CrossRef]
- Sánchez-Rodríguez, E.; Rubio-Wilhelmi, M.; Cervilla, L.M.; Blasco, B.; Rios, J.J.; Rosales, M.A.; Romero, L.; Ruiz, J.M. Genotypic differences in some physiological parameters symptomatic for oxidative stress under moderate drought in tomato plants. Plant Sci. 2010, 178, 30–40. [Google Scholar] [CrossRef]
- Sorkheh, K.; Shiran, B.; Khodambashi, M.; Moradi, H.; Gradziel, T.; Martinez-Gomez, P. Correlations between quantitative tree and fruit almond traits and their implications for breeding. Sci. Hortic. 2010, 125, 323–331. [Google Scholar] [CrossRef]
- Lin, K.H.; Chao, P.Y.; Yang, C.M.; Cheing, W.C.; Lo, H.F.; Chang, T.R. The Effects of Flooding and Drought Stresses on the Antioxidant Constituents in Sweet Potato Leaves. Bot. Stud. 2006, 47, 417–426. [Google Scholar]
- Zlatev, Z.; Lidon, F.C. An Overview on Drought Induced Changes in Plant Growth, Water Relations and Photosynthesis. Emir. J. Food Agri. 2012, 24, 57–72. [Google Scholar]
- Van Iersel, M.W.; Weaver, G.; Martin, M.T.; Ferrarezi, R.S.; Mattos, E.; Haidekker, M. A Chlorophyll Fluorescence-Based Biofeedback System to Control Photosynthetic Lighting in Controlled Environment Agriculture. J. Am. Soc. Hortic. Sci. 2016, 141, 169–176. [Google Scholar] [CrossRef]
- Mathobo, R.; Marais, D.; Steyn, J.M. The Effect of Drought Stress on Yield, Leaf Gaseous Exchange and Chlorophyll Fluorescence of Dry Beans (Phaseolus vulgaris L.). Agric. Water Manag. 2017, 180, 118–125. [Google Scholar] [CrossRef]
- Ahmed, C.; Rouina, B.; Sensoy, S.; Boukhris, M.; Abdallah, F. Changes in gas exchange, proline accumulation and antioxidative enzyme activities in three olive cultivars under contrasting water availability regimes. Environ. Exp. Bot. 2009, 67, 345–352. [Google Scholar] [CrossRef]
- Dias, M.C.; Correia, S.; Serôdio, J.; Silva, A.M.S.; Freitas, H.; Santos, C. Chlorophyll fluorescence and oxidative stress endpoints to discriminate olive cultivars tolerance to drought and heat episodes. Sci. Hortic. 2018, 231, 31–35. [Google Scholar] [CrossRef]
- Chen, Y.-E.; Cui, J.-M.; Su, Y.-Q.; Zhang, C.-M.; Ma, J.; Zhang, Z.-W.; Yuan, M.; Liu, W.-J.; Zhang, H.-Y.; Yuan, S. Comparison of phosphorylation and assembly of photosystem complexes and redox homeostasis in two wheat cultivars with different drought resistance. Sci. Rep. 2017, 7, 12718. [Google Scholar] [CrossRef]
- Zhang, Y.P.; Wang, Z.M.; Wu, Y.C.; Zhang, X. Stomatal Characteristics of Different Green Organs in Wheat under Different Irrigation Regimes. Acta Agron. Sin. 2006, 32, 70–75. [Google Scholar]
- Xu, Z.; Zhou, G. Responses of leaf stomatal density to water status and its relationship with photosynthesis in a grass. J. Exp. Bot. 2008, 59, 3317–3325. [Google Scholar] [CrossRef]
- Khan, M.A.; Rab, A. Plant Spacing Affects the Growth and Seed Production of Okra Varieties. Sarhad J. Agric. 2019, 35. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, B.; Jiang, D.; Chen, G. Silicon improves photosynthetic performance by optimizing thylakoid membrane protein components in rice under drought stress. Environ. Exp. Bot. 2018, 158, 117–124. [Google Scholar] [CrossRef]
- Chen, Y.-E.; Liu, W.-J.; Su, Y.-Q.; Cui, J.-M.; Zhang, Z.-W.; Yuan, M.; Zhang, H.-Y.; Yuan, S. Different response of photosystem II to short and long-term drought stress in Arabidopsis thaliana. Physiol. Plant. 2016, 158, 225–235. [Google Scholar] [CrossRef]
- Wang, S.; Zhuang, K.; Zhang, S.; Yang, M.; Kong, F.; Meng, Q. Overexpression of a tomato carotenoid ε-hydroxylase gene (SlLUT1) improved the drought tolerance of transgenic tobacco. J. Plant Physiol. 2018, 222, 103–112. [Google Scholar] [CrossRef]
- Zhu, Y.; Gong, H. Beneficial effects of silicon on salt and drought tolerance in plants. Agron. Sustain. Dev. 2013, 34, 455–472. [Google Scholar] [CrossRef]
- Abed, M.Y.; Ibrahim, E.A.; El-Shoura, A.M. Development of Okra (Abelmoschus esculentus L. Moench) Hybrids Derived from Selected Inbreds under Drought Stress. J. Plant Prod. 2020, 11, 61–69. [Google Scholar] [CrossRef]
- Gunawardhana, M.; De Silva, C. Impact of Temperature and Water Stress on Growth Yield and Related Biochemical Parameters of Okra. Trop. Agric. Res. 2012, 23, 77. [Google Scholar] [CrossRef]
- Adejumo, S.A.; Ezeh, O.S.; Mur, L.A. Okra growth and drought tolerance when exposed to water regimes at different growth stages. Int. J. Veg. Sci. 2018, 25, 226–258. [Google Scholar] [CrossRef]
- Kusvuran, S. Influence of Drought Stress on Growth, Ion Accumulation and Antioxidative Enzymes in Okra Genotypes. Int. J. Agric. Biol. 2012, 14, 401–406. [Google Scholar]




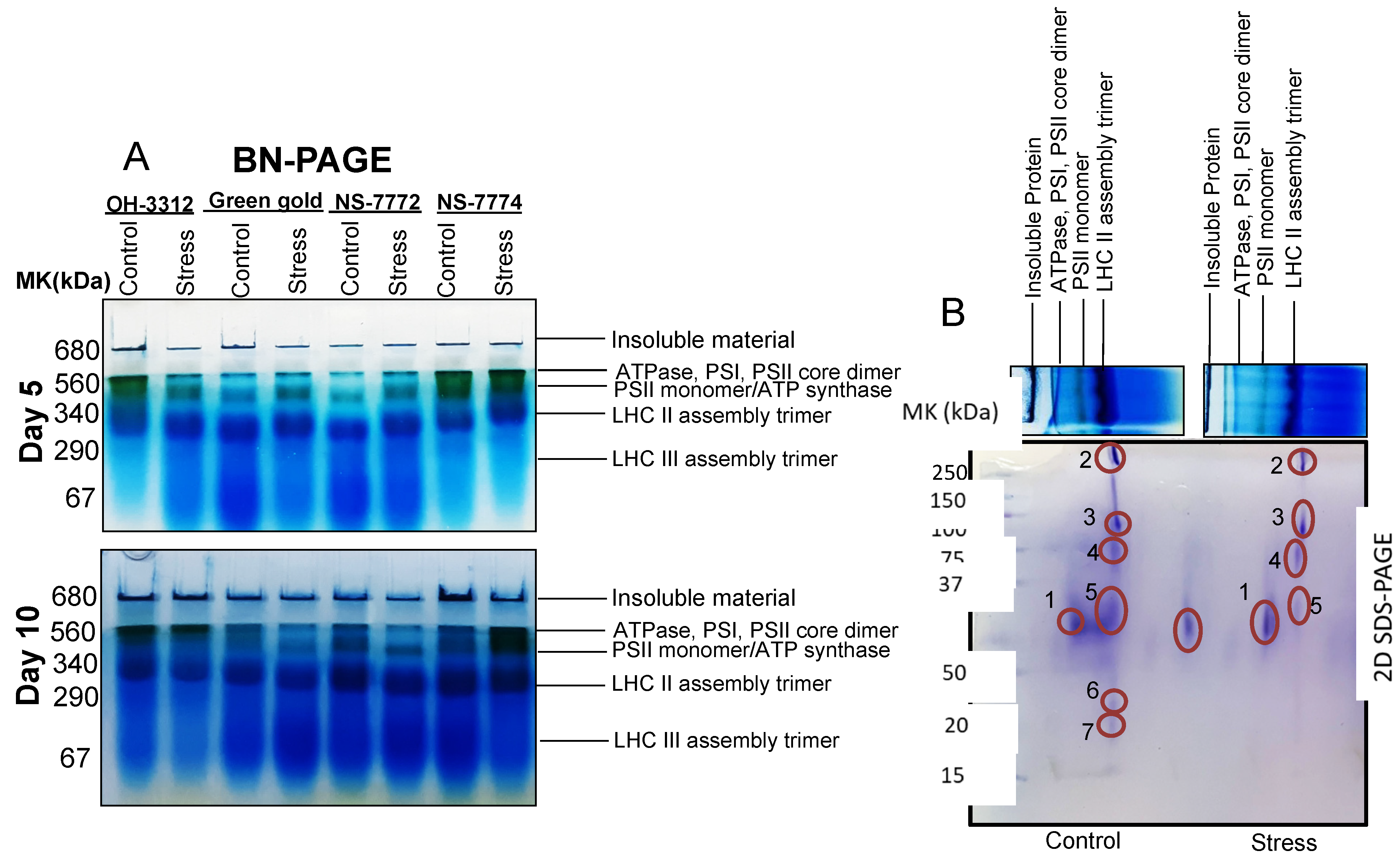
| Spot No. | Protein Name | Plant Species | Accession Number | Protein Score | Biological Function | Mr Value | Calcu. pI/Exp. pI | Sequence Coverage |
| 1 | Chlorophyll a-b binding protein | Capsicum baccatum | A0A2G2XDV1 | 78 | Photosynthesis | 28,213 | 5.1/4.0 | 36 |
| 2 | Photosystem I P700 chlorophyll a apoprotein | Lupinus angustifolius | A0A394D0H9 | 166 | Photosynthesis | 163,602 | 6.7/5.0 | 10 |
| 3 | Photosystem I P700 chlorophyll a apoprotein | Lupinus angustifolius | A0A394D0H9 | 130 | Photosynthesis | 163,602 | 6.7/5.0 | 13 |
| 4 | ATP synthase gamma chain | Desulforudis audaxviator | ATPG_DESAP | 30 | Photosynthesis | 33,245 | 9.6/5.0 | 38 |
| 5 | CDP-4-dehydro-6-deoxyglucose reductase | Pseudomonas | A0A560PPL8 | 59 | Starch and Sucrose Metabolism | 36,242 | 5.57/5.0 | 13 |
| 6 | Photosystem Q(B) protein | Glycine tomentella | R9ZRU2 | 40 | Photosynthesis | 38,866 | 5.1/5.0 | 23 |
| 7 | Cytochrome b6 | Citrus sinensis | Q09ME7 | 170 | Photosynthesis | 24,277 | 8.89/5.0 | 33 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Razi, K.; Bae, D.-W.; Muneer, S. Target-Based Physiological Modulations and Chloroplast Proteome Reveals a Drought Resilient Rootstock in Okra (Abelmoschus esculentus) Genotypes. Int. J. Mol. Sci. 2021, 22, 12996. https://doi.org/10.3390/ijms222312996
Razi K, Bae D-W, Muneer S. Target-Based Physiological Modulations and Chloroplast Proteome Reveals a Drought Resilient Rootstock in Okra (Abelmoschus esculentus) Genotypes. International Journal of Molecular Sciences. 2021; 22(23):12996. https://doi.org/10.3390/ijms222312996
Chicago/Turabian StyleRazi, Kaukab, Dong-Won Bae, and Sowbiya Muneer. 2021. "Target-Based Physiological Modulations and Chloroplast Proteome Reveals a Drought Resilient Rootstock in Okra (Abelmoschus esculentus) Genotypes" International Journal of Molecular Sciences 22, no. 23: 12996. https://doi.org/10.3390/ijms222312996
APA StyleRazi, K., Bae, D.-W., & Muneer, S. (2021). Target-Based Physiological Modulations and Chloroplast Proteome Reveals a Drought Resilient Rootstock in Okra (Abelmoschus esculentus) Genotypes. International Journal of Molecular Sciences, 22(23), 12996. https://doi.org/10.3390/ijms222312996






