Microbiota Alterations and Their Association with Oncogenomic Changes in Pancreatic Cancer Patients
Abstract
:1. Introduction
2. Genomic Characteristics of Pancreatic Cancer
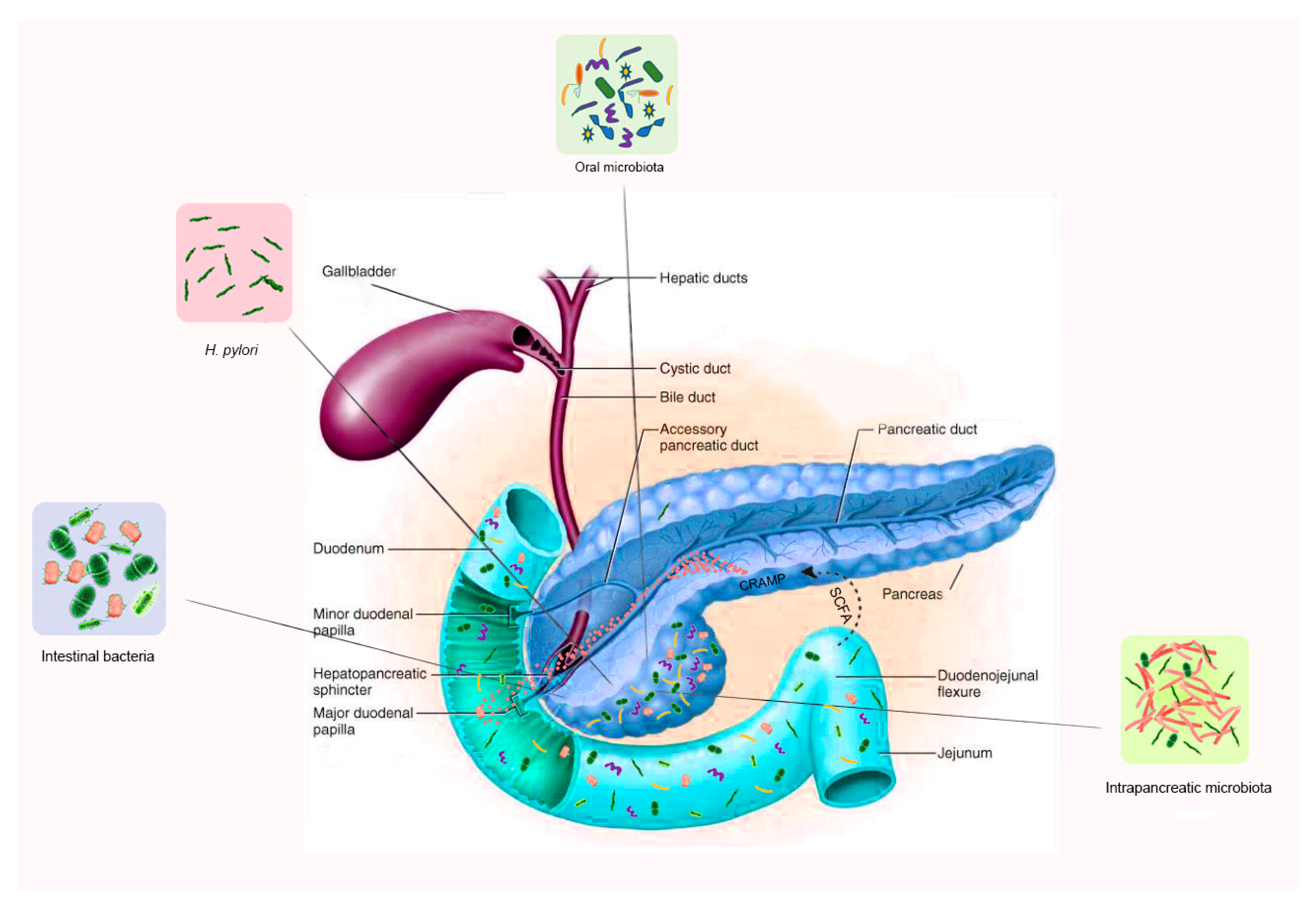
3. The Microbiome in Pancreatic Health
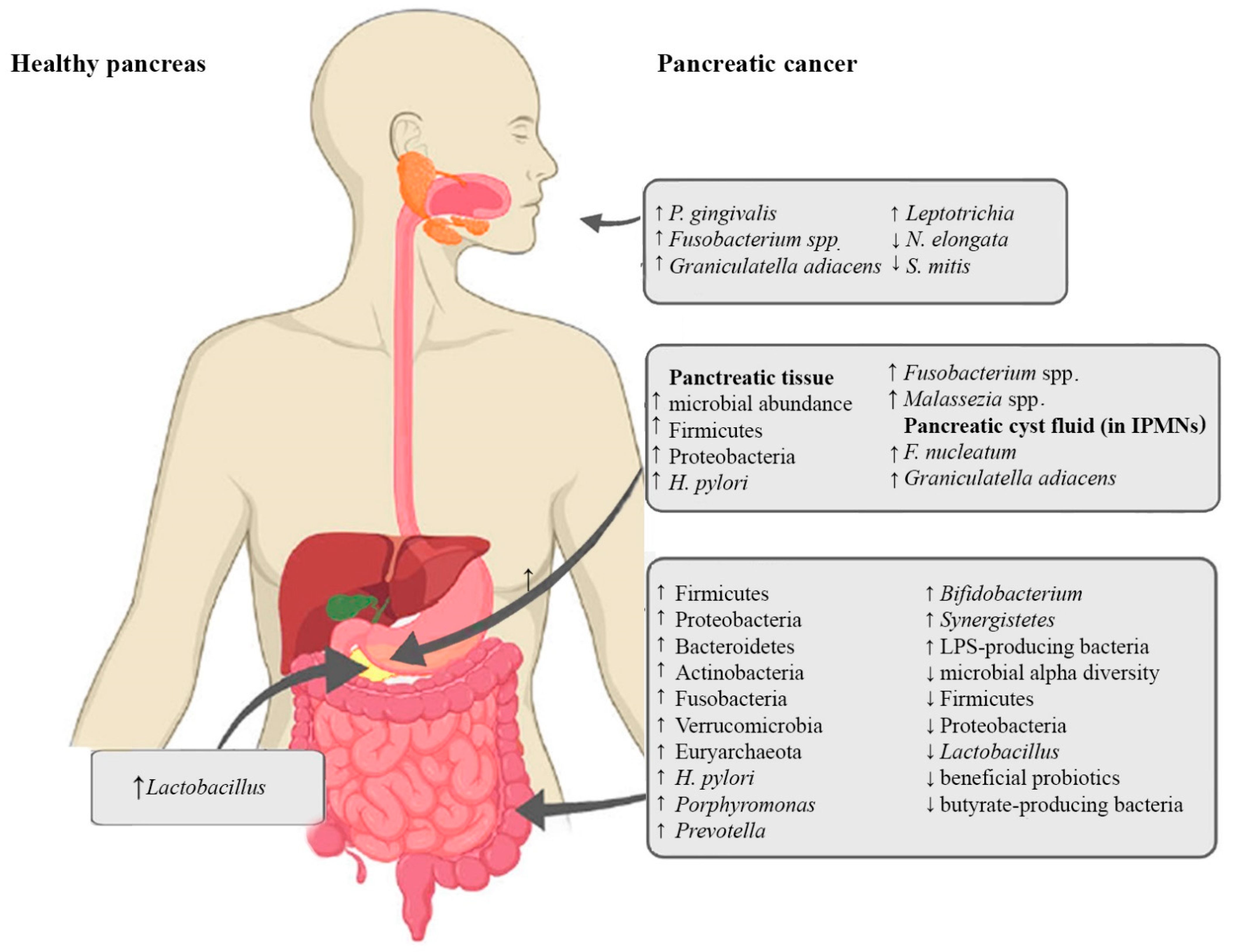
4. Microbiota Alterations in PC
4.1. Oral Microbiota and PC
4.2. Pancreatic Microbiota and PC
4.3. The Intestinal Microbiota and PC
5. Microbiota in Pancreatic Inflammation, Oncogenesis and Tumor Immunity
6. The Microbiota and Drug Response in PC
7. Associations of Bacterial Metabolites with Carcinogenic and Oncogenomic Changes in PC
7.1. Bacterial Metabolites and Their Effect on the Pancreas and PC
7.2. Bacterial Metabolites and Their Effect on Cancer Mutations
7.3. Dysbiosis and Oncogenomic Changes in PC
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef]
- European Cancer Information System. Available online: https://ecis.jrc.ec.europa.eu/explorer.php?$0-0$1-All$2-All$4-1,2$3-19$6-0,85$5-2008,2008$7-7$CEstByCountry$X0_8-3$X0_19-AE27$X0_20-No$CEstBySexByCountry$X1_8-3$X1_19-AE27$X1_-1-1$CEstByIndiByCountry$X2_8-3$X2_19-AE27$X2_20-No$CEstRelative$X3_8-3$X3_9-AE27$X3_19-AE27$CEstByCountryTable$X4_19-AE27 (accessed on 10 May 2021).
- Ahmadloo, N.; Bidouei, F.; Omidvari, S.H.; Ansari, M.; Mosalaei, A.; Mohammadianpanah, M. Pancreatic Cancer in Southern Iran. Iran. Red Crescent Med. J. 2010, 12, 624–630. [Google Scholar]
- Hadizadeh, M.; Padashi, M.; Alizadeh, A.H.M.; Zali, M.R. Clinical, Laboratory Biomarkers and Imaging Findings of Pancreatic Adenocarcinoma in Iran. Asian Pac. J. Cancer Prev. APJCP 2014, 15, 4349–4352. [Google Scholar] [CrossRef] [Green Version]
- Ansari, D.; Tingstedt, B.; Andersson, B.; Holmquist, F.; Sturesson, C.; Williamsson, C.; Sasor, A.; Borg, D.; Bauden, M.; Andersson, R. Pancreatic cancer: Yesterday, today and tomorrow. Future Oncol. 2016, 12, 1929–1946. [Google Scholar] [CrossRef] [Green Version]
- Howlader, N.; Noone, A.M.; Krapcho, M.; Miller, D.; Brest, A.; Yu, M.; Ruhl, J.; Tatalovich, Z.; Mariotto, A.; Lewis, D.R.; et al. SEER Cancer Statistics Review, 1975–2017; National Cancer Institute: Bethesda, MD, USA, 2020. [Google Scholar]
- Carioli, G.; Malvezzi, M.; Bertuccio, P.; Boffetta, P.; Levi, F.; La Vecchia, C.; Negri, E. European cancer mortality predictions for the year 2021 with focus on pancreatic and female lung cancer. Ann. Oncol. 2021, 32, 478–487. [Google Scholar] [CrossRef]
- Rahib, L.; Smith, B.D.; Aizenberg, R.; Rosenzweig, A.B.; Fleshman, J.M.; Matrisian, L.M. Projecting cancer incidence and deaths to 2030: The unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014, 74, 2913–2921. [Google Scholar] [CrossRef] [Green Version]
- Pishvaian, M.J.; Brody, J.R. Therapeutic Implications of Molecular Subtyping for Pancreatic Cancer. Oncology 2017, 31, 159–166. [Google Scholar]
- Mizrahi, J.D.; Surana, R.; Valle, J.W.; Shroff, R.T. Pancreatic cancer. Lancet 2020, 395, 2008–2020. [Google Scholar] [CrossRef]
- Klein, A.P. Pancreatic cancer epidemiology: Understanding the role of lifestyle and inherited risk factors. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Solomon, S.; Das, S.; Brand, R.; Whitcomb, D.C. Inherited Pancreatic Cancer Syndromes. Cancer J. 2012, 18, 485–491. [Google Scholar] [CrossRef] [Green Version]
- Zhou, B.; Xu, J.W.; Cheng, Y.G.; Gao, J.Y.; Hu, S.Y.; Wang, L.; Zhan, H.X. Early detection of pancreatic cancer: Where are we now and where are we going? Int. J. Cancer 2017, 141, 231–241. [Google Scholar] [CrossRef] [Green Version]
- Raphael, B.J.; Hruban, R.H.; Aguirre, A.J.; Gupta, M.; Getz, G.; Meyerson, M.; Fei, S.S.; Loher, P.; Rigoutsos, I.; Telonis, A.G.; et al. Integrated Genomic Characterization of Pancreatic Ductal Adenocarcinoma. Cancer Cell 2017, 32, 185–203.e13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- NIH Human Microbiome Portfolio Analysis Team. A review of 10 years of human microbiome research activities at the US National Institutes of Health, Fiscal Years 2007–2016. Microbiome 2019, 7, 31. [Google Scholar] [CrossRef] [PubMed]
- Pushalkar, S.; Hundeyin, M.; Daley, D.; Zambirinis, C.P.; Kurz, E.; Mishra, A.; Mohan, N.; Aykut, B.; Usyk, M.; Torres, L.E.; et al. The Pancreatic Cancer Microbiome Promotes Oncogenesis by Induction of Innate and Adaptive Immune Suppression. Cancer Discov. 2018, 8, 403–416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oh, J.; Weiderpass, E. Infection and Cancer: Global Distribution and Burden of Diseases. Ann. Glob. Health 2014, 80, 384–392. [Google Scholar] [CrossRef]
- Yu, Q.; Jobin, C.; Thomas, R.M. Implications of the microbiome in the development and treatment of pancreatic cancer: Thinking outside of the box by looking inside the gut. Neoplasia 2021, 23, 246–256. [Google Scholar] [CrossRef]
- Kalia, V.C.; Patel, S.K.S.; Cho, B.; Wood, T.K.; Lee, J. Emerging applications of bacteria as antitumor agents [published online ahead of print, 2021 May 11]. Semin. Cancer Biol. 2021, S1044-579X(21), 140. [Google Scholar] [CrossRef]
- Youssef, O.; Lahti, L.; Kokkola, A.; Karla, T.; Tikkanen, M.; Ehsan, H.; Carpelan-Holmström, M.; Koskensalo, S.; Böhling, T.; Rautelin, H.; et al. Stool Microbiota Composition Differs in Patients with Stomach, Colon, and Rectal Neoplasms. Dig. Dis. Sci. 2018, 63, 2950–2958. [Google Scholar] [CrossRef] [Green Version]
- Sarhadi, V.; Mathew, B.; Kokkola, A.; Karla, T.; Tikkanen, M.; Rautelin, H.; Lahti, L.; Puolakkainen, P.; Knuutila, S. Gut microbiota of patients with different subtypes of gastric cancer and gastrointestinal stromal tumors. Gut Pathog. 2021, 13, 11. [Google Scholar] [CrossRef]
- Johns, M.S.; Petrelli, N.J. Microbiome and colorectal cancer: A review of the past, present, and future. Surg. Oncol. 2021, 37, 101560. [Google Scholar] [CrossRef] [PubMed]
- Arsenijevic, T.; Nicolle, R.; Bouchart, C.; D’haene, N.; Demetter, P.; Puleo, F.; Van Laethem, J. Pancreatic Cancer Meets Human Microbiota: Close Encounters of the Third Kind. Cancers 2021, 13, 1231. [Google Scholar] [CrossRef]
- Heim, S.; Mitelman, F. Cancer Cytogenetics, 4th ed.; Wiley Blackwell: Chichester, UK, 2015; pp. 386–388. [Google Scholar]
- Corbo, V.; Mafficini, A.; Amato, E.; Scarpa, A. Pancreatic Cancer Genomics. In Cancer Genomics: Molecular Classification, Prognosis and Response Prediction; Pfeffer, U., Ed.; Springer: Dordrecht, The Netherlands, 2013; pp. 219–253. [Google Scholar]
- Chang, E.B.; Leung, P.S. Pancreatic Physiology. In The Gastrointestinal System; Springer: Dordrecht, The Netherlands, 2014; pp. 87–105. [Google Scholar]
- Adolph, T.E.; Mayr, L.; Grabherr, F.; Schwärzler, J.; Tilg, H. Pancreas-Microbiota Cross Talk in Health and Disease. Annu. Rev. Nutr. 2019, 39, 249–266. [Google Scholar] [CrossRef] [PubMed]
- Gallo, R.L.; Kim, K.J.; Bernfield, M.; Kozak, C.A.; Zanetti, M.; Merluzzi, L.; Gennaro, R. Identification of CRAMP, a Cathelin-related Antimicrobial Peptide Expressed in the Embryonic and Adult Mouse. J. Biol. Chem. 1997, 272, 13088–13093. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahuja, M.; Schwartz, D.M.; Tandon, M.; Son, A.; Zeng, M.; Swaim, W.; Eckhaus, M.; Hoffman, V.; Cui, Y.; Xiao, B.; et al. Orai1-Mediated Antimicrobial Secretion from Pancreatic Acini Shapes the Gut Microbiome and Regulates Gut Innate Immunity. Cell Metab. 2017, 25, 635–646. [Google Scholar] [CrossRef] [Green Version]
- Sun, J.; Furio, L.; Mecheri, R.; van der Does, A.; Lundeberg, E.; Saveanu, L.; Chen, Y.; van Endert, P.; Agerberth, B.; Diana, J. Pancreatic β-Cells Limit Autoimmune Diabetes via an Immunoregulatory Antimicrobial Peptide Expressed under the Influence of the Gut Microbiota. Immunity 2015, 43, 304–317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perry, R.J.; Peng, L.; Barry, N.A.; Cline, G.W.; Zhang, D.; Cardone, R.L.; Petersen, K.F.; Kibbey, R.G.; Goodman, A.L.; Shulman, G.I. Acetate mediates a microbiome-brain-β-cell axis to promote metabolic syndrome. Nature 2016, 534, 213–217. [Google Scholar] [CrossRef] [Green Version]
- Geller, L.T.; Barzily-Rokni, M.; Danino, T.; Jonas, O.H.; Shental, N.; Nejman, D.; Gavert, N.; Zwang, Y.; Cooper, Z.A.; Shee, K.; et al. Potential role of intratumor bacteria in mediating tumor resistance to the chemotherapeutic drug gemcitabine. Science 2017, 357, 1156–1160. [Google Scholar] [CrossRef] [Green Version]
- Del Castillo, E.; Meier, R.; Chung, M.; Koestler, D.C.; Chen, T.; Paster, B.J.; Charpentier, K.P.; Kelsey, K.T.; Izard, J.; Michaud, D.S. The Microbiomes of Pancreatic and Duodenum Tissue Overlap and Are Highly Subject Specific but Differ between Pancreatic Cancer and Noncancer Subjects. Cancer Epidemiol. Biomark. Prev. 2019, 28, 370–383. [Google Scholar] [CrossRef] [Green Version]
- Thomas, R.M.; Gharaibeh, R.Z.; Gauthier, J.; Beveridge, M.; Pope, J.L.; Guijarro, M.V.; Yu, Q.; He, Z.; Ohland, C.; Newsome, R.; et al. Intestinal microbiota enhances pancreatic carcinogenesis in preclinical models. Carcinogenesis (New York) 2018, 39, 1068–1078. [Google Scholar] [CrossRef]
- Thomas, R.M.; Jobin, C. Microbiota in pancreatic health and disease: The next frontier in microbiome research. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Kiss, B.; Mikó, E.; Sebő, É.; Toth, J.; Ujlaki, G.; Szabó, J.; Uray, K.; Bai, P.; Árkosy, P. Oncobiosis and Microbial Metabolite Signaling in Pancreatic Adenocarcinoma. Cancers 2020, 12, 1068. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [Green Version]
- Hanahan, D.; Weinberg, R.A. The hallmarks of cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef] [Green Version]
- Gopalakrishnan, V.; Helmink, B.A.; Spencer, C.N.; Reuben, A.; Wargo, J.A. The Influence of the Gut Microbiome on Cancer, Immunity, and Cancer Immunotherapy. Cancer Cell 2018, 33, 570–580. [Google Scholar] [CrossRef] [Green Version]
- Stolzenberg-Solomon, R.Z.; Dodd, K.W.; Blaser, M.J.; Virtamo, J.; Taylor, P.R.; Albanes, D. Tooth loss, pancreatic cancer, and Helicobacter pylori. Am. J. Clin. Nutr. 2003, 78, 176–181. [Google Scholar] [CrossRef]
- Mitsuhashi, K.; Nosho, K.; Sukawa, Y.; Matsunaga, Y.; Ito, M.; Kurihara, H.; Kanno, S.; Igarashi, H.; Naito, T.; Adachi, Y.; et al. Association of Fusobacterium species in pancreatic cancer tissues with molecular features and prognosis. Oncotarget 2015, 6, 7209–7220. [Google Scholar] [CrossRef] [Green Version]
- Ahn, J.; Segers, S.; Hayes, R.B. Periodontal disease, porphyromonas gingivalis serum antibody levels and orodigestive cancer mortality. Carcinogenesis 2012, 33, 1055–1058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michaud, D.S. Role of bacterial infections in pancreatic cancer. Carcinogenesis 2013, 34, 2193–2197. [Google Scholar] [CrossRef]
- Hayashi, C.; Gudino, C.V.; Gibson, F.C.; Genco, C.A. Pathogen-induced inflammation at sites distant from oral infection: Bacterial persistence and induction of cell-specific innate immune inflammatory pathways. Mol. Oral Microbiol. 2010, 25, 305–316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vogtmann, E.; Han, Y.; Caporaso, J.G.; Bokulich, N.; Mohamadkhani, A.; Moayyedkazemi, A.; Hua, X.; Kamangar, F.; Wan, Y.; Suman, S.; et al. Oral microbial community composition is associated with pancreatic cancer: A case-control study in Iran. Cancer Med. 2020, 9, 797–806. [Google Scholar] [CrossRef] [Green Version]
- Fan, X.; Alekseyenko, A.V.; Wu, J.; Peters, B.A.; Jacobs, E.J.; Gapstur, S.M.; Purdue, M.P.; Abnet, C.C.; Stolzenberg-Solomon, R.; Miller, G.; et al. Human oral microbiome and prospective risk for pancreatic cancer: A population-based nested case-control study. Gut 2018, 67, 120–127. [Google Scholar] [CrossRef] [Green Version]
- Torres, P.J.; Fletcher, E.M.; Gibbons, S.M.; Bouvet, M.; Doran, K.S.; Kelley, S.T. Characterization of the salivary microbiome in patients with pancreatic cancer. PeerJ 2015, 3, e1373. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Ren, Z.; Li, A.; Li, J.; Xu, S.; Zhang, H.; Jiang, J.; Yang, J.; Luo, Q.; Zhou, K.; et al. Tongue coating microbiome data distinguish patients with pancreatic head cancer from healthy controls. J. Oral Microbiol. 2019, 11, 1563409. [Google Scholar] [CrossRef] [PubMed]
- Farrell, J.J.; Zhang, L.; Zhou, H.; Chia, D.; Elashoff, D.; Akin, D.; Paster, B.J.; Joshipura, K.; Wong, D.T. Variations of oral microbiota are associated with pancreatic diseases including pancreatic cancer. Gut 2012, 61, 582–588. [Google Scholar] [CrossRef]
- Pandya, G.; Kirtonia, A.; Singh, A.; Goel, A.; Mohan, C.D.; Rangappa, K.S.; Pandey, A.K.; Kapoor, S.; Tandon, S.; Sethi, G.; et al. A comprehensive review of the multifaceted role of the microbiota in human pancreatic carcinoma [published online ahead of print, 2021 May 26]. Semin. Cancer Biol. 2021, S1044-579X, 157. [Google Scholar] [CrossRef]
- Aykut, B.; Pushalkar, S.; Chen, R.; Li, Q.; Abengozar, R.; Kim, J.I.; Shadaloey, S.A.; Wu, D.; Preiss, P.; Verma, N.; et al. The fungal mycobiome promotes pancreatic oncogenesis via activation of MBL. Nature 2019, 574, 264–267. [Google Scholar] [CrossRef] [PubMed]
- Riquelme, E.; Maitra, A.; McAllister, F. Immunotherapy for Pancreatic Cancer: More Than Just a Gut Feeling. Cancer Discov. 2018, 8, 386–388. [Google Scholar] [CrossRef] [Green Version]
- Chakladar, J.; Kuo, S.Z.; Castaneda, G.; Li, W.T.; Gnanasekar, A.; Yu, M.A.; Chang, E.Y.; Wang, X.Q.; Ongkeko, W.M. The Pancreatic Microbiome is Associated with Carcinogenesis and Worse Prognosis in Males and Smokers. Cancers 2020, 12, 2672. [Google Scholar] [CrossRef]
- Nejman, D.; Livyatan, I.; Fuks, G.; Gavert, N.; Zwang, Y.; Geller, L.T.; Rotter-Maskowitz, A.; Weiser, R.; Mallel, G.; Gigi, E.; et al. The human tumor microbiome is composed of tumor type-specific intracellular bacteria. Science 2020, 368, 973–980. [Google Scholar] [CrossRef]
- Bao, Y.; Spiegelman, D.; Li, R.; Giovannucci, E.; Fuchs, C.S.; Michaud, D.S. History of Peptic Ulcer Disease and Pancreatic Cancer Risk in Men. Gastroenterology 2010, 138, 541–549. [Google Scholar] [CrossRef] [Green Version]
- Nilsson, H.; Stenram, U.; Ihse, I.; Wadstrom, T. Helicobacter species ribosomal DNA in the pancreas, stomach and duodenum of pancreatic cancer patients. World J. Gastroenterol. WJG 2006, 12, 3038–3043. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Fuhler, G.M.; Bn, N.; Jose, T.; Bruno, M.J.; Peppelenbosch, M.P.; Konstantinov, S.R. Pancreatic cyst fluid harbors a unique microbiome. Microbiome 2017, 5, 147. [Google Scholar] [CrossRef] [PubMed]
- Gaiser, R.A.; Halimi, A.; Alkharaan, H.; Lu, L.; Davanian, H.; Healy, K.; Hugerth, L.W.; Ateeb, Z.; Valente, R.; Fernández Moro, C.; et al. Enrichment of oral microbiota in early cystic precursors to invasive pancreatic cancer. Gut 2019, 68, 2186–2194. [Google Scholar] [CrossRef] [Green Version]
- Half, E.; Keren, N.; Reshef, L.; Dorfman, T.; Lachter, I.; Kluger, Y.; Reshef, N.; Knobler, H.; Maor, Y.; Stein, A.; et al. Fecal microbiome signatures of pancreatic cancer patients. Sci. Rep. 2019, 9, 16801–16804. [Google Scholar] [CrossRef]
- Ren, Z.; Jiang, J.; Xie, H.; Li, A.; Lu, H.; Xu, S.; Zhou, L.; Zhang, H.; Cui, G.; Chen, X.; et al. Gut microbial profile analysis by MiSeq sequencing of pancreatic carcinoma patients in China. Oncotarget 2017, 8, 95176–95191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cougnoux, A.; Dalmasso, G.; Martinez, R.; Buc, E.; Delmas, J.; Gibold, L.; Sauvanet, P.; Darcha, C.; Déchelotte, P.; Bonnet, M.; et al. Bacterial genotoxin colibactin promotes colon tumour growth by inducing a senescence-associated secretory phenotype. Gut 2014, 63, 1932–1942. [Google Scholar] [CrossRef]
- Maekawa, T.; Fukaya, R.; Takamatsu, S.; Itoyama, S.; Fukuoka, T.; Yamada, M.; Hata, T.; Nagaoka, S.; Kawamoto, K.; Eguchi, H.; et al. Possible involvement of Enterococcus infection in the pathogenesis of chronic pancreatitis and cancer. Biochem. Biophys. Res. Commun. 2018, 506, 962–969. [Google Scholar] [CrossRef]
- Kohi, S.; Macgregor-Das, A.; Dbouk, M.; Yoshida, T.; Chuidian, M.; Abe, T.; Borges, M.; Lennon, A.M.; Shin, E.J.; Canto, M.I.; et al. Alterations in the Duodenal Fluid Microbiome of Patients With Pancreatic Cancer [published online ahead of print, 2020 Nov 5]. Clin. Gastroenterol. Hepatol. 2020. [Google Scholar] [CrossRef]
- Stolzenberg-Solomon, R.Z.; Blaser, M.J.; Limburg, P.J.; Perez-Perez, G.; Taylor, P.R.; Virtamo, J.; Albanes, D. Helicobacter pylori Seropositivity as a Risk Factor for Pancreatic Cancer. JNCI J. Natl. Cancer Inst. 2001, 93, 937–941. [Google Scholar] [CrossRef]
- Guo, Y.; Liu, W.; Wu, J. Helicobacter pylori infection and pancreatic cancer risk: A meta-analysis. J. Cancer Res. Ther. 2016, 12, C229–C232. [Google Scholar] [CrossRef]
- Kunovsky, L.; Dite, P.; Jabandziev, P.; Dolina, J.; Vaculova, J.; Blaho, M.; Bojkova, M.; Dvorackova, J.; Uvirova, M.; Kala, Z.; et al. Helicobacter pylori infection and other bacteria in pancreatic cancer and autoimmune pancreatitis. World J. Gastrointest. Oncol. 2021, 13, 835–844. [Google Scholar] [CrossRef]
- Mei, Q.X.; Huang, C.L.; Luo, S.Z.; Zhang, X.M.; Zeng, Y.; Lu, Y.Y. Characterization of the duodenal bacterial microbiota in patients with pancreatic head cancer vs. healthy controls. Pancreatology 2018, 18, 438–445. [Google Scholar] [CrossRef] [PubMed]
- Friedlander, S.Y.G.; Chu, G.C.; Snyder, E.L.; Girnius, N.; Dibelius, G.; Crowley, D.; Vasile, E.; DePinho, R.A.; Jacks, T. Context-Dependent Transformation of Adult Pancreatic Cells by Oncogenic K-Ras. Cancer Cell 2009, 16, 379–389. [Google Scholar] [CrossRef] [Green Version]
- Tijeras-Raballand, A.; Hilmi, M.; Astorgues-Xerri, L.; Nicolle, R.; Bièche, I.; Neuzillet, C. Microbiome and pancreatic ductal adenocarcinoma. Clin. Res. Hepatol. Gastroenterol. 2021, 45, 101589. [Google Scholar] [CrossRef]
- Huang, H.; Daniluk, J.; Liu, Y.; Chu, J.; Li, Z.; Ji, B.; Logsdon, C.D. Oncogenic K-Ras requires activation for enhanced activity. Oncogene 2014, 33, 532–535. [Google Scholar] [CrossRef] [Green Version]
- Torphy, R.J.; Fujiwara, Y.; Schulick, R.D. Pancreatic cancer treatment: Better, but a long way to go. Surg. Today 2020, 50, 1117–1125. [Google Scholar] [CrossRef]
- Zeng, S.; Pöttler, M.; Lan, B.; Grützmann, R.; Pilarsky, C.; Yang, H. Chemoresistance in Pancreatic Cancer. Int. J. Mol. Sci. 2019, 20, 4504. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Liu, Q.; Liao, Q.; Zhao, Y. Pancreatic Cancer, Gut Microbiota, and Therapeutic Efficacy. J. Cancer 2020, 11, 2749–2758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramos, A.; Hemann, M.T. Drugs, Bugs, and Cancer: Fusobacterium nucleatum Promotes Chemoresistance in Colorectal Cancer. Cell 2017, 170, 411–413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- An, J.; Ha, E. Combination therapy of Lactobacillus plantarum supernatant and 5-fluouracil increases chemosensitivity in colorectal cancer cells. J. Microbiol. Biotechnol. 2016, 26, 1490–1503. [Google Scholar] [CrossRef] [PubMed]
- Daillère, R.; Vétizou, M.; Waldschmitt, N.; Yamazaki, T.; Isnard, C.; Poirier-Colame, V.; Duong, C.M.; Flament, C.; Lepage, P.; Roberti, M.; et al. Enterococcus hirae and Barnesiella intestinihominis Facilitate Cyclophosphamide-Induced Therapeutic Immunomodulatory Effects. Immunity 2016, 45, 931–943. [Google Scholar] [CrossRef] [Green Version]
- Carpenter, E.; Nelson, S.; Bednar, F.; Cho, C.; Nathan, H.; Sahai, V.; Magliano, M.P.; Frankel, T.L. Immunotherapy for pancreatic ductal adenocarcinoma. J. Surg. Oncol. 2021, 123, 751–759. [Google Scholar] [CrossRef]
- Schizas, D.; Charalampakis, N.; Kole, C.; Economopoulou, P.; Koustas, E.; Gkotsis, E.; Ziogas, D.; Psyrri, A.; Karamouzis, M.V. Immunotherapy for pancreatic cancer: A 2020 update. Cancer Treat. Rev. 2020, 86, 102016. [Google Scholar] [CrossRef] [PubMed]
- Rezasoltani, S.; Yadegar, A.; Asadzadeh Aghdaei, H.; Reza Zali, M. Modulatory effects of gut microbiome in cancer immunotherapy: A novel paradigm for blockade of immune checkpoint inhibitors. Cancer Med. 2021, 10, 1141–1154. [Google Scholar] [CrossRef]
- Sethi, V.; Vitiello, G.A.; Saxena, D.; Miller, G.; Dudeja, V. The Role of the Microbiome in Immunologic Development and its Implication For Pancreatic Cancer Immunotherapy. Gastroenterology 2019, 156, 2097-2115.e2. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Wang, Y.; Yang, G.; Zhang, Q.; Meng, L.; Xin, Y.; Jiang, X. The role of short-chain fatty acids in intestinal barrier function, inflammation, oxidative stress, and colonic carcinogenesis. Pharmacol. Res. 2021, 165, 105420. [Google Scholar] [CrossRef]
- Mullins, T.D.; Kern, H.F.; Metzgar, R.S. Ultrastructural Differentiation of Sodium Butyrate-Treated Human Pancreatic Adenocarcinoma Cell Lines. Pancreas 1991, 6, 578–587. [Google Scholar] [CrossRef]
- Asai, Y.; Itoi, T.; Sugimoto, M.; Sofuni, A.; Tsuchiya, T.; Tanaka, R.; Tonozuka, R.; Honjo, M.; Mukai, S.; Fujita, M.; et al. Elevated Polyamines in Saliva of Pancreatic Cancer. Cancers 2018, 10, 43. [Google Scholar] [CrossRef] [Green Version]
- Arruabarrena-Aristorena, A.; Zabala-Letona, A.; Carracedo, A. Oil for the cancer engine: The cross-talk between oncogenic signaling and polyamine metabolism. Sci. Adv. 2018, 4, eaar2606. [Google Scholar] [CrossRef] [Green Version]
- Casero, J.; Robert, A.; Murray Stewart, T.; Pegg, A.E. Polyamine metabolism and cancer: Treatments, challenges and opportunities. Nat. Rev. Cancer 2018, 18, 681–695. [Google Scholar] [CrossRef]
- Tofalo, R.; Cocchi, S.; Suzzi, G. Polyamines and gut microbiota. Front. Nutr. 2019, 6, 16. [Google Scholar] [CrossRef] [Green Version]
- Mendez, R.; Kesh, K.; Arora, N.; Di Martino, L.; McAllister, F.; Merchant, N.; Banerjee, S.; Banerjee, S. Microbial dysbiosis and polyamine metabolism as predictive markers for early detection of pancreatic cancer. Carcinogenesis 2020, 41, 561–570. [Google Scholar] [CrossRef]
- Avila-Calderón, E.D.; Ruiz-Palma, M.D.S.; Aguilera-Arreola, M.G.; Velázquez-Guadarrama, N.; Ruiz, E.A.; Gomez-Lunar, Z.; Witonsky, S.; Contreras-Rodríguez, A. Outer Membrane Vesicles of Gram-Negative Bacteria: An Outlook on Biogenesis. Front. Microbiol. 2021, 12, 557902. [Google Scholar] [CrossRef]
- Kadosh, E.; Snir-Alkalay, I.; Venkatachalam, A.; May, S.; Lasry, A.; Elyada, E.; Zinger, A.; Shaham, M.; Vaalani, G.; Mernberger, M.; et al. The gut microbiome switches mutant p53 from tumour-suppressive to oncogenic. Nature 2020, 586, 133–138. [Google Scholar] [CrossRef]
- Dieterich, W.; Schink, M.; Zopf, Y. Microbiota in the Gastrointestinal Tract. Med. Sci. 2018, 6, 116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarhadi, V.; Lahti, L.; Saberi, F.; Youssef, O.; Kokkola, A.; Karla, T.; Tikkanen, M.; Rautelin, H.; Puolakkainen, P.; Salehi, R.; et al. Gut Microbiota and Host Gene Mutations in Colorectal Cancer Patients and Controls of Iranian and Finnish Origin. Anticancer Res. 2020, 40, 1325–1334. [Google Scholar] [CrossRef] [PubMed]
- Janney, A.; Powrie, F.; Mann, E.H. Host-microbiota maladaptation in colorectal cancer. Nature 2020, 585, 509–517. [Google Scholar] [CrossRef] [PubMed]
- Shirazi, M.S.R.; Al-Alo, K.Z.K.; Al-Yasiri, M.H.; Lateef, Z.M.; Ghasemian, A. Microbiome Dysbiosis and Predominant Bacterial Species as Human Cancer Biomarkers. J. Gastrointest. Cancer 2020, 51, 725–728. [Google Scholar] [CrossRef]
- Öğrendik, M. Periodontal Pathogens in the Etiology of Pancreatic Cancer. Gastrointest. Tumors 2017, 3, 125–127. [Google Scholar] [CrossRef] [Green Version]
- Gnanasekaran, J.; Binder Gallimidi, A.; Saba, E.; Pandi, K.; Eli Berchoer, L.; Hermano, E.; Angabo, S.; Makkawi, H.A.; Khashan, A.; Daoud, A.; et al. Intracellular Porphyromonas gingivalis Promotes the Tumorigenic Behavior of Pancreatic Carcinoma Cells. Cancers 2020, 12, 2331. [Google Scholar] [CrossRef]
- Guo, W.; Zhang, Y.; Guo, S.; Mei, Z.; Liao, H.; Dong, H.; Wu, K.; Ye., H.; Zhang., Y.; Zhu., Y.; et al. Tumor microbiome contributes to an aggressive phenotype in the basal-like subtype of pancreatic cancer. Commun. Biol. 2021, 4, 1019. [Google Scholar] [CrossRef]
| Reference | Study Population | Specimen | Analytical Methods | Main Findings | Microbial Changes | Oncogenomic Changes | Possible Associations between the Microbiota and Oncogenetics in PC |
|---|---|---|---|---|---|---|---|
| Mitsuhashi 2015 [42] | Human PDAC vs. HC | Pancreatic | TaqMan Gene Expression Assay | Fusobacterium spp. is present in 8.8% of PC tissue and independently associated with a worse prognosis; F. spp. could be used as a prognostic biomarker of PC. | ↑ F. spp. detected in 8.8% of PC tissue specimens. | Mutations in KRAS, NRAS, BRAF or PIK3CA, epigenetic changes or mi-R21, mi-R31 or mi-R143 expression levels. | No significant association was found between the Fusobacterium species status and molecular alterations of pancreatic cancers. |
| Michaud 2013 [44] | NA | NA | Review on bacterial infections linked to PC and their possible pathways | Bacteria may cause an inflammatory response of the host immune defense, promoting carcinogenesis. | H. pylori and P. gingivalis are positively associated with PC. | Aberrant miRNA expression | Microbes like P. gingivalis may regulate miRNA expression (even in a far-distance manner), which may influence important immunologic and cancer-related signaling pathways. |
| Shirazi et al. 2020 [94] | NA | NA | Review aiming to evaluate bacterial agents as cancer biomarkers | Bacteria can influence the cell cycle through inflammation, aberrant cell signaling, immune evasion, DNA damage and mutations, aberrant miRNA expression and epigenetic changes. | H. pylori and P. gingivalis are associated with PC. | DNA damage, mutations, expression of certain microRNAs, and epigenetic effects | Bacteria involved in carcinogenesis cause alterations in the cell cycle by the induction of DNA damage, mutations, expression of microRNA and epigenetic effects, amongst others. H. pylori and P. gingivalis cause inflammation and P.gingivalis may regulate miRNAs. |
| Ögrendik 2017 [95] | Human PC | Oral | Hypothesis based on earlier findings | P. gingivalis, Tannerella forsythia and Treponema denticola secrete peptidylarginine deaminase, which might cause p53 and KRAS point mutations. | P. gingivalis, T. forsythia and T. denticola are major pathogens of CPO. | Mutations in p53, KRAS | Bacterial peptidylarginine deaminases originating from P. gingivalis, T. forsythia and T. denticola might cause p53 and KRAS point mutations by the degradation of arginine. CPO has been associated with orodigestive cancer. |
| Gnanasekaran et al. 2020 [96] | Human (PC cell lines, xenograft model) | Pancreatic | Gene expression analysis by qRT-PCR, detection of P. gingivalis by RT PCR | Exposure to P. gingivalis increases tumorigenic behavior in PC cell lines. | P. gingivalis influences PC progression. | Mutant KRAS | P.gingivalis may synergize with mutant KRAS to promote tumorigenesis. |
| Pushalkar et al. 2018 [17] | Human PDAC, mouse (KPC or KRASG12D Trp53R172H PdxCre) | Pancreatic (mouse); pancreatic and fecal (human) | 16S rRNA gene sequencing | The PDAC microbiome promotes oncogenesis by immune suppression via TLR; this could be used as a therapeutic target. | ↑ Probacteria (Pseudomonas, Elizabethkingia) in human PC tissue, is associated with advanced disease; ↑↑ Proteobacteria, Synergistetes, Euryarchaeotain the feces of PC patients. | Mutated Kras(G12D) | The composition and diversity of the gut and pancreatic microbiota may be influenced by oncogenic Kras expression. |
| Thomas et al. 2018 [35] | Human PDAC vs. CP and HC; mouse (Kras(G12D)/PTENlox/+) | Pancreatic | 16S rRNA gene sequencing, RNAseq of human PDAC xenografts in mice | The pancreatic microbiota in PC accelerates carcinogenesis. No distinct microbiota profile is significantly associated with PC. Gut bacteria exert a long-distance effect on PC carcinogenesis. Bacterial colonization of the pancreas is not a physiological process. | 50% of PC mice harbored intrapancreatic bacteria. ↑ Acinetobacter, Afipia, Enterobacter, Pseudomonas in human PC tissue. | Mutated Kras (Kras(G12D)/ PTENlox/+ mouse model) | The gut microbiota accelerates pancreatic carcinogenesis in a mouse model of PC. Many genes involved in carcinogenesis are differently expressed depending on the gut microbiota state. The microbial effect seems to be independent of the Kras mutational status. The pancreatic microbiota is not correlated with carcinogenesis. |
| Riquelme et al. 2019 [53] | Human PDAC STS vs. PDAC LTS | Pancreatic | 16S rRNA gene sequencing | Higher α-diversity in the LTS tumor microbiome; predominant taxa could be used as biomarkers for the prediction of LTS; PDAC microbiome composition influences host immune response. | Enrichment of proteobacteria (Pseudoxanthomanas), Actinobacteria (Streptomyces, Saccharopolyspora), Bacillus clausii in LTS compared to STS. | No genomic differences in PDAC LTS vs. STS. | No genomic differences in stage matched PDAC LTS compared to STS. |
| Chakladar et al. 2020 [54] | Human PDAC | Pancreatic | Next generation RNA sequencing | The PC tumor microbiota is associated with gene expression dysregulation, metastasis and immune suppression. A worse prognosis in males and smokers is linked to the presence of cancer-promoting microbiota profiles. | A. ebreus (Betaproteobacteria) correlated with immune dysregulation and poor prognosis, Gammaproteobacteria correlated with increased metastasis. A. baumannii and M. hypneumoniae associated with smokers. | Oncogenic gene expression signatures, CNA; Deletions at the 9q13 locus. | An increased abundance of A. baumannii and M. hypneumoniae is associated with an increase of oncogenic and decrease of tumor suppressive and immune signatures in smokers; E. coli abundance is correlated with CNA; M. hyopneumoniae is significantly correlated with deletions at 9q13 (potential tumor suppressor) in smokers. |
| Guo et al. 2021 [97] | Human (different PDAC subtypes) | Pancreatic | Metagenomic sequencing, RNA-seq | Analysis of the tumor microbiome in different subtypes of PDAC: the microbial profile in basal-like PDAC was highly associated with carcinogenesis, possibly through the induction of pathogen-related inflammation. Host genetics influence the composition of the tumor microbiome. | ↑ Acinetobacter, Pseudomonas and Sphingopyxis in basal-like tumors | KRAS signaling | Acinetobacter, Pseudomonas and Sphingopyxis are associated with carcinogenic gene-expression, KRAS signalling, DNA replication and other PC-related pathways. Bacterial LPS can hyperstimulate KRAS and initiate carcinogenesis. A microbial procarcinogenic effect is caused by continuous inflammation rather than direct mutagenesis. Host genetics participate in shaping the tumor microbiome. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sammallahti, H.; Kokkola, A.; Rezasoltani, S.; Ghanbari, R.; Asadzadeh Aghdaei, H.; Knuutila, S.; Puolakkainen, P.; Sarhadi, V.K. Microbiota Alterations and Their Association with Oncogenomic Changes in Pancreatic Cancer Patients. Int. J. Mol. Sci. 2021, 22, 12978. https://doi.org/10.3390/ijms222312978
Sammallahti H, Kokkola A, Rezasoltani S, Ghanbari R, Asadzadeh Aghdaei H, Knuutila S, Puolakkainen P, Sarhadi VK. Microbiota Alterations and Their Association with Oncogenomic Changes in Pancreatic Cancer Patients. International Journal of Molecular Sciences. 2021; 22(23):12978. https://doi.org/10.3390/ijms222312978
Chicago/Turabian StyleSammallahti, Heidelinde, Arto Kokkola, Sama Rezasoltani, Reza Ghanbari, Hamid Asadzadeh Aghdaei, Sakari Knuutila, Pauli Puolakkainen, and Virinder Kaur Sarhadi. 2021. "Microbiota Alterations and Their Association with Oncogenomic Changes in Pancreatic Cancer Patients" International Journal of Molecular Sciences 22, no. 23: 12978. https://doi.org/10.3390/ijms222312978
APA StyleSammallahti, H., Kokkola, A., Rezasoltani, S., Ghanbari, R., Asadzadeh Aghdaei, H., Knuutila, S., Puolakkainen, P., & Sarhadi, V. K. (2021). Microbiota Alterations and Their Association with Oncogenomic Changes in Pancreatic Cancer Patients. International Journal of Molecular Sciences, 22(23), 12978. https://doi.org/10.3390/ijms222312978






