Sanguiins—Promising Molecules with Broad Biological Potential
Abstract
1. Introduction
2. Methodology
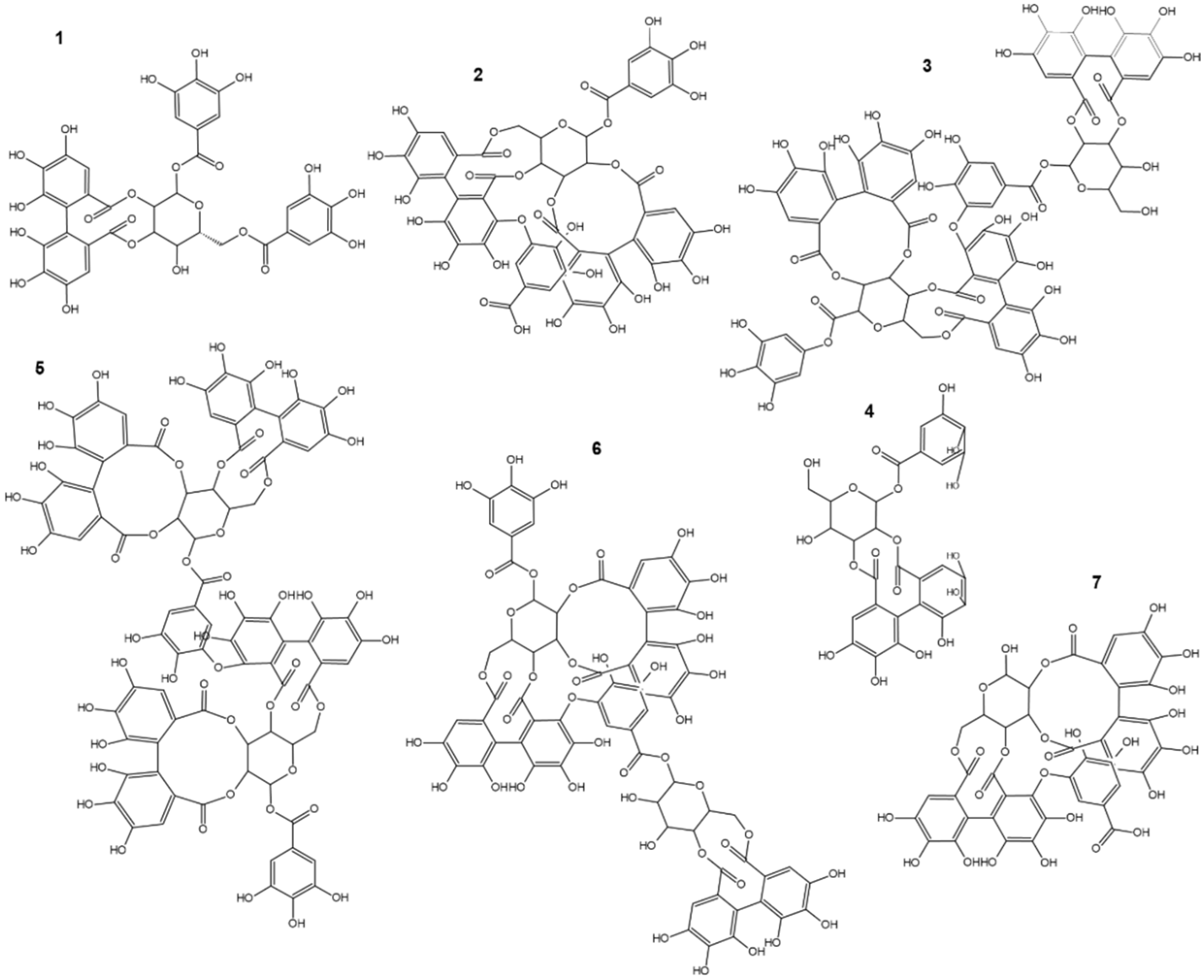
3. Natural Occurrence of Sanguiins
4. Chromatographic Techniques for the Analysis of Sanguiins
5. Biological Potential of Sanguiins
5.1. Antioxidant and Anti-Inflammatory Activities
5.2. Osteoclastogenesis Inhibitory Activity
5.3. Antibacterial Activity
5.4. Antifungal Activity
5.5. Antiviral Activity (Including SARS-CoV-2)
5.6. Anticancer Activity
5.7. Estrogenic Activity
5.8. Neuroprotective Activity
5.9. Clinical Trials
6. Pharmacokinetics of Sanguiins
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SH2 | sanguiin H-2 |
| SH3 | sanguiin H-3 |
| SH5 | sanguiin H-5 |
| SH6 | sanguiin H-6 |
| SH10 | sanguiin H-10 |
| SH11 | sanguiin H-11 |
| ADMET | absorption, distribution, metabolism, excretion, toxicity |
| CINC-1 | cytokine-induced neutrophil chemoattractant |
| TNFα | tumor necrosis factor α |
| IL-1β | interleukin-1β |
| d.w. | dry weight |
| ONOO- | peroxynitrite |
| LPS | lipopolysaccharide |
| NFATc1 | nuclear factor of activated T cells 1 |
| NF-κB | nuclear factor-κB |
| c-Src | proto-oncogene tyrosine-protein kinase Src |
| TNF-α | tumor necrosis factor |
| PARP | poly (ADP-ribose) polymerase |
| EMT | epithelial-mesenchymal transition |
| TGF-β1 | transforming growth factor beta 1 |
| VEGF | vascular endothelial growth factor |
| iNOS | inducible NO synthase |
| ETs | ellagitannins |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| ROS | reactive oxygen species |
| ABTS | 2,29-azinobis-(3-ethylbenzo-thiazoline-6-sulfonic acid) |
| FRAP | ferric reducing ability of plasma |
| MRSA | methicillin-resistant Staphylococcus aureus |
| MSSA | methicillin-sensitive Staphylococcus aureus |
| MIC | minimum inhibitory concentration; |
| MBC | minimum bactericidal concentration |
| GSH | glutathione |
| GSSG | glutathione disulfide |
| BMMs | bone marrow macrophages |
| tBID | truncated BID |
| HUVECs | human umbilical vein endothelial cells |
| MCF-7/wt | MCF-7 human breast cancer cell (wild type) |
| FA | formic acid |
| TBA | thiobarbituric acid |
| ACN | acetonitrile |
| AMC | acceptable means of compliance |
| iNOS | inducible nitric oxide synthase |
| TRAP | tartrate-resistant acid phosphatase |
| ES/BS | eroded surface/bone surface |
| BMM | bone marrow-derived macrophages |
| NFATC1 Tmax | nuclear factor of activated T cells 1 time take to reach maximum concentration |
References
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J. Nat. Prod. 2012, 75, 311–335. [Google Scholar] [CrossRef] [PubMed]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Orhan, I.E.; Banach, M.; Rollinger, J.M.; Barreca, D.; Weckwerth, W.; Bauer, R.; Bayer, E.A.; et al. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef] [PubMed]
- Kiss, A.K.; Piwowarski, J.P. Ellagitannins, gallotannins and their metabolites—The contribution to the anti-inflammatory effect of food products and medicinal plants. Curr. Med. Chem. 2016, 25, 4946–4967. [Google Scholar] [CrossRef]
- Riaz, A.; Rasul, A.; Hussain, G.; Zahoor, M.K.; Jabeen, F.; Subhani, Z.; Younis, T.; Ali, M.; Sarfraz, I.; Selamoglu, Z. Astragalin: A bioactive phytochemical with potential therapeutic activities. Adv. Pharmacol. Sci. 2018, 2018, 9794625. [Google Scholar] [CrossRef]
- Clifford, M.N.; Scalbert, A. Ellagitannins—Nature, occurrence and dietary burden. J. Sci. Food Agric. 2000, 80, 1118–1125. [Google Scholar] [CrossRef]
- Prothmann, J.; Sun, M.; Spégel, P.; Sandahl, M.; Turner, C.; Scheuba, J.; Wronski, V.K.; Rollinger, J.M.; Grienke, U.; Santos-Buelga, C.; et al. Relationship between phenolic compounds, anthocyanins content and antioxidant activity in colored barley germplasm. J. Agric. Food Chem. 2017, 53, 1713. [Google Scholar]
- Su, X.; Surry, D.S.; Spandl, R.J.; Spring, D.R. Total synthesis of sanguiin H-5. Org. Lett. 2008, 10, 2593–2596. [Google Scholar] [CrossRef]
- Niemetz, R.; Gross, G.G. Enzymology of gallotannin and ellagitannin biosynthesis. Phytochemistry 2005, 66, 2001–2011. [Google Scholar] [CrossRef]
- Feldman, K.S.; Sambandam, A. Ellagitannin chemistry. the first total chemical synthesis of an O(2),O(3)-Galloyl-Coupled ellagitannin, sanguiin H-5. J. Org. Chem. 1995, 60, 8171–8178. [Google Scholar] [CrossRef]
- Bakkalbasi, E.; Mentes, O.; Artik, N. Food ellagitannins-occurrence, effects of processing and storage. Crit. Rev. Food Sci. Nutr. 2009, 49, 283–298. [Google Scholar] [CrossRef]
- Koponen, J.M.; Happonen, A.M.; Mattila, P.H.; Törrönen, A.R. Contents of anthocyanins and ellagitannins in selected foods consumed in Finland. J. Agric. Food Chem. 2007, 55, 1612–1619. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Surry, D.; Spandl, R.; Spring, D. Synthesis of Sanguiin H-5. Synfacts 2008, 2008, 1130. [Google Scholar] [CrossRef]
- Nonaka, G.; Tanaka, T.; Nita, M.; Nishioka, I. A dimeric hydrolyzable tannin, sanguiin H-6 from Sanguisorba officinalis L. Chem. Pharm. Bull. 1982, 30, 2255–2257. [Google Scholar] [CrossRef]
- Jakimiuk, K.; Wink, M.; Tomczyk, M. Flavonoids of the Caryophyllaceae. Phytochem. Rev. 2021, 20, 1–40. [Google Scholar] [CrossRef]
- Landete, J.M. Ellagitannins, ellagic acid and their derived metabolites: A review about source, metabolism, functions and health. Food Res. Int. 2011, 44, 1150–1160. [Google Scholar] [CrossRef]
- Patel, A.; Rojas-Vera, J.; Dacke, C.G. Therapeutic constituents and actions of Rubus species. Curr. Med. Chem. 2004, 11, 1501–1512. [Google Scholar] [CrossRef]
- Moreno-Medina, B.L.; Casierra-Posada, F.; Cutler, J. Phytochemical composition and potential use of Rubus species. Gesunde Pflanz. 2018, 70, 65–74. [Google Scholar] [CrossRef]
- Okuda, T.; Yoshida, T.; Hatano, T.; Iwasaki, M.; Kubo, M.; Orime, T.; Yoshizaki, M.; Naruhashi, N. Hydrolysable tannins as chemotaxonomic markers in the Rosaceae. Phytochemistry 1992, 31, 3091–3096. [Google Scholar] [CrossRef]
- Duckstein, S.M.; Lotter, E.M.; Meyer, U.; Lindequist, U. Phenolic constituents from Alchemilla vulgaris L. and Alchemilla mollis (Buser) Rothm. at different dates of harvest. Z. Naturforsch. C 2012, 67, 529–540. [Google Scholar] [CrossRef]
- Luo, S.; Guo, L.; Sheng, C.; Zhao, Y.; Chen, L.; Li, C.; Jiang, Z.; Tian, H. Rapid identification and isolation of neuraminidase inhibitors from mockstrawberry (Duchesnea indica Andr.) based on ligand fishing combined with HR-ESI-Q-TOF-MS. Acta Pharm. Sin. B 2020, 10, 1846–1855. [Google Scholar] [CrossRef]
- Lee, S.-H.; Tanaka, T.; Nonaka, G.; Nishioka, I.; Zhang, B. Allose gallates from Euphorbia fischeriana. Phytochemistry 1991, 30, 1251–1253. [Google Scholar] [CrossRef]
- Vrhovsek, U.; Guella, G.; Gasperotti, M.; Pojer, E.; Zancato, M.; Mattivi, F. Clarifying the identity of the main ellagitannin in the fruit of the strawberry, Fragaria vesca and Fragaria ananassa Duch. J. Agric. Food Chem. 2012, 60, 2507–2516. [Google Scholar] [CrossRef] [PubMed]
- Mena, P.; Calani, L.; Dall’Asta, C.; Galaverna, G.; Garcia-Viguera, C.; Bruni, R.; Crozier, A.; Del Rio, D. Rapid and comprehensive evaluation of polyphenolic compounds in pomegranate (Punica granatum L.) juice by UHPLC-MS. Molecules 2012, 17, 14821–14840. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.G.; Huang, W.T.; Lee, L.T.; Wang, C.C. Ellagitannins from Terminalia calamansanai induced apoptosis in HL-60 cells. Toxicol. Vitr. 2009, 23, 603–609. [Google Scholar] [CrossRef]
- Tanaka, T.; Tachibana, H.; Nonaka, G.; Nishioka, I.; Hsu, F.-L.; Kohda, H.; Tanaka, O. Tannins and related compounds. CXXII. New dimeric, trimeric and tetrameric ellagitannins, lambertianins A-D, from Rubus lambertianus SERINGE. Chem. Pharm. Bull. 1993, 41, 1214–1220. [Google Scholar] [CrossRef]
- Verma, R.; Gangrade, T.; Punasiya, R.; Ghulaxe, X. Rubus fruticosus (blackberry) use as an herbal medicine. Pharmacogn. Rev. 2014, 8, 101–104. [Google Scholar] [CrossRef]
- Krauze-Baranowska, M.; Majdan, M.; Hałasa, R.; Głód, D.; Kula, M.; Fecka, I.; Orzeł, A. The antimicrobial activity of fruits from some cultivar varieties of Rubus idaeus and Rubus occidentalis. Food Funct. 2014, 5, 2536–2541. [Google Scholar] [CrossRef]
- Yoshida, T.; Amakura, Y.; Yoshimura, M. Structural features and biological properties of ellagitannins in some plant families of the order myrtales. Int. J. Mol. Sci. 2010, 11, 79–106. [Google Scholar] [CrossRef]
- Aires, A. (Ed.) Tannins: Structural Properties, Biological Properties and Current Knowledge, 1st ed.; IntechOpen: London, UK, 2020; pp. 21–53. [Google Scholar]
- Zhu, M.; Dong, X.; Guo, M.; Ferreira, I.; McPhee, D.J. Phenolic profiling of Duchesnea indica combining macroporous resin chromatography (MRC) with HPLC-ESI-MS/MS and ESI-IT-MS. Molecules 2015, 20, 22463–22475. [Google Scholar] [CrossRef]
- Liberal, J.; Francisco, V.; Costa, G.; Figueirinha, A.; Amaral, M.T.; Marques, C.; Girão, H.; Lopes, M.; Cruz, M.T.; Teresa, B.M. Bioactivity of Fragaria vesca leaves through inflammation, proteasome and autophagy modulation. J. Ethnopharmacol. 2014, 158, 113–122. [Google Scholar] [CrossRef]
- Yoshida, T.; Tanaka, K.; Chen, X.-M.; Okuda, T. Dimeric ellagitannins, laevigatins E, F and G, from Rosa laevigata. Phytochemistry 1989, 28, 2451–2454. [Google Scholar] [CrossRef]
- Cui, C.; Zhao, Q.-C.; Cai, B.; Yao, X.-S.; Osadsa, H. Two new and four known polyphenolics obtained as new cell-cycle inhibitors from Rubus aleaefolius poir. J. Asian Nat. Prod. Res. 2002, 4, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Zhao, J.; Chen, W.; Lin, S.; Zhang, J.; Hong, Z. Hepatoprotection of 1β-hydroxyeuscaphic acid—The major constituent from Rubus aleaefolius against CCl4-induced injury in hepatocytes cells. Pharm. Biol. 2013, 51, 686–690. [Google Scholar] [CrossRef]
- Mertz, C.; Cheynier, V.; Günata, Z.; Brat, P. Analysis of phenolic compounds in two blackberry species (Rubus glaucus and Rubus adenotrichus) by high-performance liquid chromatography with diode array detection and electrospray ion trap mass spectrometry. J. Agric. Food Chem. 2007, 55, 8616–8624. [Google Scholar] [CrossRef] [PubMed]
- Gancel, A.; Feneuil, A.; Acosta, O.; Mercedes, A.; Vaillant, F. Impact of industrial processing and storage on major polyphenols and the antioxidant capacity of tropical highland blackberry (Rubus adenotrichus). Food Res. Int. J. 2011, 44, 2243–2251. [Google Scholar] [CrossRef]
- Hukkanen, A.; Kostamo, K.; Karenlampi, S.; Kokko, H. Impact of agrochemicals on Peronospora sparsa and phenolic profiles in three Rubus arcticus cultivars. J. Agric. Food Chem. 2008, 56, 1008–1016. [Google Scholar] [CrossRef]
- Martins, A.; Barros, L.; Carvalho, A.M.; Santos-Buelga, C.; Fernandes, I.P.; Barreiro, F.; Ferreira, I.C.F.R. Phenolic extracts of Rubus ulmifolius Schott flowers: Characterization, microencapsulation and incorporation into yogurts as nutraceutical sources. Food Funct. 2014, 5, 1091–1100. [Google Scholar] [CrossRef]
- Puupponen-Pimiä, R.; Nohynek, L.; Suvanto, J.; Salminen Juha-Pekka Seppänen-Laakso, T.; Tähtiharju, J.; Honkapää, K.; Oksman-Caldentey, K.-M. Natural antimicrobials from cloudberry (Rubus chamaemorus) seeds by sanding and hydrothermal extraction. ACS Foods Sci. Technol. 2001, 1, 917–927. [Google Scholar] [CrossRef]
- Wang, B.; Koivumäki, T.; Kylli, P.; Heinonen, M.; Poutanen, M. Protein-phenolic interaction of tryptic digests of β-lactoglobulin and cloudberry ellagitannin. J. Agric. Food Chem. 2014, 62, 5028–5037. [Google Scholar] [CrossRef]
- Thiem, B. Rubus chamaemorus L.—A boreal plant rich in biologically active metabolites: A review. Biol. Lett. 2003, 40, 3–13. [Google Scholar]
- Grochowski, D.M.; Paduch, R.; Wiater, A.; Dudek, A.; Pleszczynska, M.; Tomczykowa, M.; Granica, S.; Polak, P.; Tomczyk, M. In vitro antiproliferative and antioxidant effects of extracts from Rubus caesius leaves and their quality evaluation. Evid. Based Complement. Altern. Med. 2016, 2016, 5698685. [Google Scholar] [CrossRef]
- Oszmiański, J.; Wojdyło, A.; Nowicka, P.; Teleszko, M.; Cebulak, T.; Wolanin, M. Determination of phenolic compounds and antioxidant activity in leaves from wild Rubus L. species. Molecules 2015, 20, 4951–4966. [Google Scholar] [CrossRef]
- Gu, J.; Ahn-Jarvis, J.H.; Riedl, K.M.; Schwartz, S.J.; Clinton, S.K.; Vodovotz, Y. Characterization of black raspberry functional food products for cancer prevention human clinical trials. J. Agric. Food Chem. 2014, 62, 3997–4006. [Google Scholar] [CrossRef] [PubMed]
- Lim, T.K. Edible Medicinal and Non-Medicinal Plants: Fruits, 1st ed.; Springer: Dordrecht, The Netherlands, 2012; pp. 570–580. [Google Scholar]
- Krauze-Baranowska, M.; Głód, D.; Kula, M.; Majdan, M.; Hałasa, R.; Matkowski, A.; Kozłowska, W.; Kawiak, A. Chemical composition and biological activity of Rubus idaeus shoots—A traditional herbal remedy of Eastern Europe. BMC Complement. Altern. Med. 2014, 14, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Sakai, E.; Aoki, Y.; Yoshimatsu, M.; Nishishita, K.; Iwatake, M.; Fukuma, Y.; Okamoto, K.; Tanaka, T.; Tsukuba, T. Sanguiin H-6, a constituent of Rubus parvifolius L., inhibits receptor activator of nuclear factor-κB ligand-induced osteoclastogenesis and bone resorption in vitro and prevents tumor necrosis factor-α-induced osteoclast formation in vivo. Phytomedicine 2016, 23, 828–837. [Google Scholar] [CrossRef]
- Jong-Won, C.; Yeong-Min, Y.; Min-Young, K.; Jung-Hwan, N.; Agung, N.; Hee-Juhn, P. Anti-hyperglycemic and anti-hyperlipidemic effects of the triterpenoid-rich fractions from Rubus coreanus and Rubus crataegifolius and their main component, niga-ichigoside f1, in streptozotocin-induced diabetic rats. Nat. Prod. Sci. 2008, 14, 260–264. [Google Scholar]
- Li, X.; Sun, J.; Chen, Z.; Jiang, J.; Jackson, A. Characterization of carotenoids and phenolics during fruit ripening of Chinese raspberry (Rubus chingii Hu). RSC Adv. 2021, 11, 10804–10813. [Google Scholar] [CrossRef]
- Jia-Yun, S.; Si-Qi, W.; Kao-Hua, L.; Bo, Z.; Qiao-Yan, Z.; Lu-Ping, Q.; Jian-Jun, W. Rubus chingii Hu: An overview of botany, traditional uses, phytochemistry, and pharmacology. Chin. J. Nat. Med. 2020, 18, 401–4016. [Google Scholar]
- Sun, Z.-L.; Zhang, Y.; Wan, A.-H.; Zhang, X.-L.; Feng, J. A new active compound against kidney deficiency from the fruits of Rubus corchorifolius. J. Asian Nat. Prod. Res. 2011, 13, 68–74. [Google Scholar] [CrossRef]
- Gasperotti, M.; Masuero, D.; Vrhovsek, U.; Guella, G.; Mattivi, F. Profiling and accurate quantification of Rubus ellagitannins and ellagic acid conjugates using direct UPLC-Q-TOF hdms and HPLC-DAD analysis. J. Agric. Food Chem. 2010, 58, 4602–4616. [Google Scholar] [CrossRef]
- Piwowarski, J.P.; Granica, S.; Zwierzyńska, M.; Stefańska, J.; Schopohl, P.; Melzig, M.F.; Kiss, A.K. Role of human gut microbiota metabolism in the anti-inflammatory effect of traditionally used ellagitannin-rich plant materials. J. Ethnopharmacol. 2014, 155, 801–809. [Google Scholar] [CrossRef]
- Zhang, G.; Liu, Y.; Hai, P. The complete chloroplast genome of tibetan medicinal plant Rubus phoenicolasius Maxim. Mitochondrial DNA Part B 2021, 6, 886–887. [Google Scholar] [CrossRef]
- Kool, M.M.; Comeskey, D.J.; Cooney, J.M.; McGhie, T.K. Structural identification of the main ellagitannins of a boysenberry (Rubus loganbaccus × baileyanus Britt.) extract by LC-ESI-MS/MS, MALDI-TOF-MS and NMR spectroscopy. Food Chem. 2010, 119, 1535–1543. [Google Scholar] [CrossRef]
- Lee, Y.A.; Lee, M.W. Tannins from Rubus coreanum. Korean J. Pharmacogn. 1995, 26, 27–30. [Google Scholar]
- Pang, K.C.; Kim, M.S.; Lee, M.W. Hydrolyzable tannins from the fruits of Rubus coreanum. Korean J. Pharmacogn. 1996, 27, 366–370. [Google Scholar]
- Kim, M.S.; Pang, K.C.; Lee, S.M. Tannins from the leaves of Rubus coreanum. Korea Sci. 1996, 40, 666–669. [Google Scholar]
- Kim, L.S.; Youn, S.H.; Kim, J.Y. Comparative study on antioxidant effects of extracts from Rubus coreanus and Rubus occidentalis Lee. J. Korean Soc. Food Sci. Nutr. 2014, 43, 1357–1362. [Google Scholar] [CrossRef]
- Kosmala, M.; Jurgoński, A.; Juśkiewicz, J.; Karlińska, E.; Macierzuński, J.; Rój, E.; Zduńczyk, Z. Chemical composition of blackberry press cake, polyphenolic extract, and defatted seeds, and their effects on cecal fermentation, bacterial metabolites, and blood lipid profile in rats. J. Agric. Food Chem. 2017, 65, 5470–5479. [Google Scholar] [CrossRef]
- Sparzak, B.; Merino-Arevalo, M.; Vander Heyden, Y.; Krauze-Baranowska, M.; Majdan, M.; Fecka, I.; Głód, D.; Ba̧czek, T. HPLC analysis of polyphenols in the fruits of Rubus idaeus L. (Rosaceae). Nat. Prod. Res. 2010, 24, 1811–1822. [Google Scholar] [CrossRef]
- Tavares, L.; Figueira, I.; McDougall, G.; Vieira, H.; Stewart, D.; Alves, P.; Ferreira, R.; Santos, C. Neuroprotective effects of digested polyphenols from wild blackberry species. Eur. J. Nutr. 2013, 52, 225–236. [Google Scholar] [CrossRef]
- Nonaka, G.I.; Tanaka, T.; Nishioka, I. Tannins and related compounds. Part 3. A new phenolic acid, sanguisorbic acid dilactone, and three new ellagitannins, sanguiins H-1, H-2, and H-3, from Sanguisorba officinalis. J. Chem. Soc. Perkin Trans. 1 1982, 1067–1073. [Google Scholar] [CrossRef]
- Zhao, Z.; He, X.; Zhang, Q.; Wei, X.; Huang, L.; Fang, J.; Wang, X.; Zhao, M.; Bai, Y.; Zheng, X. Traditional uses, chemical constituents and biological activities of plants from the genus Sanguisorba L. Am. J. Chin. Med. 2017, 45, 199–224. [Google Scholar] [CrossRef] [PubMed]
- Arun, N.; Road, R.; Pradesh, U. Punica granatum: A review on pharmacological and therapeutic properties. Int. J. Pharm. Sci. Res. 2012, 3, 1240–1245. [Google Scholar]
- Lia, Y.-N.; He, J.; Zhang, J.; Shi, Y.-X.; Guo, L.-B.; Peng, Z.-C.; Yang, T.; Ding, K.; Zhang, W.-K.; Xu, J.-K. Existing knowledge on Euphorbia fischeriana Steud. (Euphorbiaceae): Traditional uses, clinical applications, phytochemistry, pharmacology and toxicology. J. Ethnopharmacol. 2021, 275, 114095. [Google Scholar] [CrossRef] [PubMed]
- Juszczak, A.M.; Zovko-Končić, M.; Tomczyk, M. Recent trends in the application of chromatographic techniques in the analysis of luteolin and its derivatives. Biomolecules 2019, 9, 731. [Google Scholar] [CrossRef] [PubMed]
- Kashiwada, Y.; Nonaka, G.I.; Nishioka, I.; Chang, J.J.; Lee, K.H. Tannins and related compounds as selective cytotoxic agents. J. Nat. Prod. 1992, 55, 1033–1043. [Google Scholar] [CrossRef]
- Jang, E.; Inn, K.S.; Jang, Y.P.; Lee, K.T.; Lee, J.H. Phytotherapeutic activities of Sanguisorba officinalis and its chemical constituents: A review. Am. J. Chin. Med. 2018, 46, 299–318. [Google Scholar] [CrossRef]
- Konishi, K.; Urada, M.; Adachi, I.; Tanaka, T. Inhibitory effect of sanguiin H-11 on chemotaxis of neutrophil. Biol. Pharm. Bull. 2000, 23, 213–218. [Google Scholar] [CrossRef]
- Sangiovanni, E.; Vrhovsek, U.; Rossoni, G.; Colombo, E.; Brunelli, C.; Brembati, L.; Trivulzio, S.; Gasperotti, M.; Mattivi, F.; Bosisio, E.; et al. Ellagitannins from Rubus berries for the control of gastric inflammation: In vitro and in vivo studies. PLoS ONE 2013, 8, e71762. [Google Scholar] [CrossRef]
- Yokozawa, T.; Chen, C.P.; Rhyu, D.Y.; Tanaka, T.; Park, J.C.; Kitani, K. Potential of sanguiin H-6 against oxidative damage in renal mitochondria and apoptosis mediated by peroxynitrite in vivo. Nephron 2002, 92, 133–141. [Google Scholar] [CrossRef]
- Mullen, W.; McGinn, J.; Lean, M.E.J.; MacLean, M.R.; Gardner, P.; Duthie, G.G.; Yokota, T.; Crozier, A. Ellagitannins, flavonoids, and other phenolics in red raspberries and their contribution to antioxidant capacity and vasorelaxation properties. J. Agric. Food Chem. 2002, 50, 5191–5196. [Google Scholar] [CrossRef]
- Song, J.H.; Kim, S.Y.; Hwang, G.S.; Kim, Y.S.; Kim, H.Y.; Kang, K.S. Sanguiin H-11 from Sanguisorbae radix protects HT22 murine hippocampal cells against glutamate-induced death. Bioorgan. Med. Chem. Lett. 2019, 29, 252–256. [Google Scholar] [CrossRef] [PubMed]
- Kähkönen, M.; Kylli, P.; Ollilainen, V.; Salminen, J.P.; Heinonen, M. Antioxidant activity of isolated ellagitannins from red raspberries and cloudberries. J. Agric. Food Chem. 2012, 60, 1167–1174. [Google Scholar] [CrossRef] [PubMed]
- Yokozawa, T.; Chen, C.P.; Tanaka, T.; Kitani, K. Effects of sanguiin H-6, a component of Sanguisorbae radix, on lipopolysaccharide-stimulated nitric oxide production. Biochem. Pharmacol. 2002, 63, 853–858. [Google Scholar] [CrossRef]
- Puljula, E.; Walton, G.; Woodward, M.J.; Karonen, M. Antimicrobial activities of ellagitannins against Clostridiales perfringens, Escherichia coli, Lactobacillus plantarum and Staphylococcus aureus. Molecules 2020, 25, 3714. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, L.P.; Pereira, E.; Pires, T.C.S.P.; Alves, M.J.; Pereira, O.R.; Barros, L.; Ferreira, I.C.F.R. Rubus ulmifolius Schott fruits: A detailed study of its nutritional, chemical and bioactive properties. Food Res. Int. 2019, 119, 34–43. [Google Scholar] [CrossRef]
- Park, E.J.; Lee, D.; Baek, S.E.; Kim, K.H.; Kang, K.S.; Jang, T.S.; Lee, H.L.; Song, J.H.; Yoo, J.E. Cytotoxic effect of sanguiin H-6 on MCF-7 and MDA-MB-231 human breast carcinoma cells. Bioorgan. Med. Chem. Lett. 2017, 27, 4389–4392. [Google Scholar] [CrossRef]
- Bhatia, S.; Giri, S.; Singh, S.; Lal, A.F. Identification of potential inhibitors of dietary polyphenols for SARS-CoV-2 M protease: An in silico study. One Health Bull. 2020, 1, 21–29. [Google Scholar]
- Trinh, T.A.; Park, E.-J.; Lee, D.; Song, J.H.; Lee, H.L.; Kim, K.H.; Kim, Y.; Jung, K.; Kang, K.S.; Yoo, J.-E. Estrogenic activity of sanguiin H-6 through activation of estrogen receptor α coactivator-binding site. Nat. Prod. Sci. 2019, 25, 28. [Google Scholar] [CrossRef][Green Version]
- Bastow, K.F.; Bori, I.D.; Fukushima, Y.; Kashiwada, Y.; Tanaka, T.; Nonaka, G.; Nishioka, I.; Lee, K.H. Inhibition of DNA topoisomerases by sanguiin H-6, a cytotoxic dimeric ellagitannin from Sanguisorba officinalis. Planta Med. 1993, 59, 240–245. [Google Scholar] [CrossRef]
- Lee, S.J.; Lee, H.K. Sanguiin H-6 blocks endothelial cell growth through inhibition of VEGF binding to VEGF receptor. Arch. Pharm. Res. 2005, 28, 1270–1274. [Google Scholar] [CrossRef] [PubMed]
- Ko, H.; Jeon, H.; Lee, D.; Choi, H.K.; Kang, K.S.; Choi, K.C. Sanguiin H6 suppresses TGF-β induction of the epithelial-mesenchymal transition and inhibits migration and invasion in A549 lung cancer. Bioorgan. Med. Chem. Lett. 2015, 25, 5508–5513. [Google Scholar] [CrossRef]
- Berdowska, I.; Zieliński, B.; Saczko, J.; Sopel, M.; Gamian, A.; Fecka, I. Modulatory impact of selected ellagitannins on the viability of human breast cancer cells. J. Funct. Foods 2018, 42, 122–128. [Google Scholar] [CrossRef]
- Park, E.-H.H.; Park, J.Y.; Yoo, H.-S.S.; Yoo, J.-E.E.; Lee, H.L. Assessment of the anti-metastatic properties of sanguiin H-6 in HUVECs and MDA-MB-231 human breast cancer cells. Bioorgan. Med. Chem. Lett. 2016, 26, 3291–3294. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Ko, H.; Kim, Y.-J.; Kim, S.-N.; Choi, K.-C.; Yamabe, N.; Kim, K.H.; Kang, K.S.; Kim, H.Y.; Shibamoto, T. Inhibition of A2780 human ovarian carcinoma cell proliferation by a Rubus component, sanguiin H-6. J. Agric. Food Chem. 2016, 64, 801–880. [Google Scholar] [CrossRef]
- Yokozawa, T.; Chen, C.P.; Tanaka, T. Direct scavenging of nitric oxide by traditional crude drugs. Phytomedicine 2000, 6, 453–463. [Google Scholar] [CrossRef]
- Frieri, M.; Kumar, K.; Boutin, A. Antibiotic resistance. J. Infect. Public Health 2017, 10, 369–378. [Google Scholar] [CrossRef]
- Oh, D.Y.; Hurt, A.C. A review of the antiviral susceptibility of human and avian influenza viruses over the last decade. Scientifica 2014, 2014, 430629. [Google Scholar] [CrossRef] [PubMed]
- Tu, Y.-F.; Chien, C.-S.; Yarmishyn, A.A.; Lin, Y.-Y.; Luo, Y.-H.; Lin, Y.-T.; Lai, W.-Y.; Yang, D.-M.; Chou, S.-J.; Yang, Y.-P.; et al. A review of SARS-CoV-2 and the ongoing clinical trials. Int. J. Mol. Sci. 2020, 21, 2657. [Google Scholar] [CrossRef]
- Lim, Y.X.; Ng, Y.L.; Tam, J.P.; Liu, D.X. Human coronaviruses a review of virus-host interactions. Diseases 2016, 4, 26. [Google Scholar] [CrossRef]
- Arnica, F.; Sabeena, G.; Sonam, B.; Shaminder, S.; Sonia, M. Identification of ellagitannins as natural inhibitors of spike proteins of COVID19 virus: An in silico- based study for drug development. Afr. J. Health Sci. 2020, 33, 78–97. [Google Scholar]
- Stoner, G.D.; Sardo, C.; Apseloff, G.; Mullet, D.; Wargo, W.; Pound, V.; Singh, A.; Sanders, J.; Aziz, R.; Casto, B.; et al. Pharmacokinetics of anthocyanins and ellagic acid in healthy volunteers fed freeze-dried black raspberries daily for 7 days. J. Clin. Pharmacol. 2005, 45, 1153–1164. [Google Scholar] [CrossRef]
- Cho, J.M.; Chae, J.; Jeong, S.R.; Moon, M.J.; Ha, K.C.; Kim, S.; Lee, J.H. The cholesterol-lowering effect of unripe Rubus coreanus is associated with decreased oxidized LDL and apolipoprotein B levels in subjects with borderline-high cholesterol levels: A randomized controlled trial. Lipids Health Dis. 2020, 19, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Lipińska, L.; Klewicka, E.; Sójka, M. The structure, occurrence and biological activity of ellagitannins: A general review. Acta Sci. Pol. Technol. Aliment. 2014, 13, 289–299. [Google Scholar] [CrossRef]
- Espín, J.C.; Larrosa, M.; García-Conesa, M.T.; Tomás-Barberán, F. Biological significance of urolithins, the gut microbial ellagic acid-derived metabolites: The evidence so far. Evid.-Based Complement. Altern. Med. 2013, 2013, 270418. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, I.A.; Mena, P.; Calani, L.; Borges, G.; Pereira-Caro, G.; Bresciani, L.; Del Rio, D.; Lean, M.E.J.; Crozier, A. New insights into the bioavailability of red raspberry anthocyanins and ellagitannins. Free Radic. Biol. Med. 2015, 89, 758–769. [Google Scholar] [CrossRef]
| Plant | Family | Geographical Location | Type of SH | Amount of SH | Traditional Medicine Uses | References |
|---|---|---|---|---|---|---|
| Alchemilla vulgaris | Rosaceae | Germany | SH6 SH10 isomers | not given | wounds, eczema, and inflamed mucosa | [19] |
| Alchemilla mollis | Rosaceae | Germany | SH6 SH10 isomers | not given | wounds, eczema, and inflamed mucosa | [19] |
| Duchesnea indica | Rosaceae | China | SH4 | 0.0046 mg/g of dried fruits | fever, inflammation, cancer | [20,30] |
| Fragaria vesca | Rosaceae | Italy | SH6 | not given | inflammation-related diseases | [22,31] |
| Fragaria ananassa | Rosaceae | Italy | SH6 | not given | not found | [22] |
| Rosa laevigata | Rosaceae | China | SH4 | 0.03 mg/g of dried pecarps | urinary incontinence, diarrhea, pain, burns, toothache | [32] |
| Rubus aleaefolius | Rosaceae | China | SH2 ethyl ester | 0.028 mg/g of dried roots | various types of hepatitis | [33,34] |
| Rubus adenotrichus | Rosaceae | Costa Rica, Trinidad Ecuador | SH6 | 4.2 mg/g of dried berries | not found | [35,36] |
| Rubus arcticus | Rosaceae | propagated vegetatively | SH5 SH6 SH10 | not given | not found | [37] |
| Rubus ulmifolius | Rosaceae | Portugal | SH10 isomer | not given | diarrhea, menstrual pain, menopause disorders, liver diseases, aphtha, gingivitis | [38] |
| Rubus chamaemorus | Rosaceae | Finland | SH6 SH10 isomers | not given | scurvy and diarrhea | [39,40,41] |
| Rubus caesius | Rosaceae | Poland | SH6 | 5.79 mg/g of dried leaves | uterine relaxant, stimulant during confinement, diarrhea and similar enteric disorders, an astringent | [16,42,43] |
| Rubus hirsutus | Rosaceae | Japan | SH6 SH11 | 73.92 mg/g of dried leaves | not found | [18] |
| not given | ||||||
| Rubus occidentalis | Rosaceae | Poland | SH6 | 10.78–50.45 mg/g of plant extract from shoots | common cold, fever and flu-like infections, management of impotence, spermatorrhea, enuresis, asthma, allergic diseases | [27,44,45,46] |
| Rubus lambertianus | Rosaceae | Taiwan, Japan | SH2 SH6 SH11 | not given | not found | [18,25] |
| Rubus parvifolius | Rosaceae | Japan | SH2 SH6 SH11 | not given | fever, angina, enteritis, hepatitis, concretion, eczema, rheumatism | [18,25,47] |
| Rubus crataegifolius | Rosaceae | Japan | SH2 SH6 SH11 | not given | diabetes mellitus | [18,25,48] |
| Rubus pedatus | Rosaceae | Japan | SH6 SH11 | not given | not found | [18] |
| Rubus palmatus | Rosaceae | Japan | SH2 SH6 SH11 | not given | not found | [18,25] |
| Rubus chingii | Rosaceae | Japan | SH2 SH6 SH11 | not given | invigorating Qi, losing weight, blackening hair, tonifying kidney, enriching essence, impotence | [18,25,49,50] |
| Rubus sieboldii | Rosaceae | Japan | SH2 | not given | not found | [25] |
| Rubus corchorifolius | Rosaceae | Japan | SH2 | not given | impotence, seminal emission | [25,51] |
| Rubus palmatus var. coptophyllus | Rosaceae | Japan | SH2 | not given | not found | [25] |
| Rubus idaeus | Rosaceae | Japan Poland Italy | SH2 | not given | enterocolitis, bronchitis, prostate disorders, analgesic, cold, cough, fever | [25,27,52,53] |
| SH6 | 1.7–6.33 mg/g of plant extract from shoots | |||||
| Rubus mesogeanus | Rosaceae | Japan | SH2 | not given | not found | [25] |
| Rubus calycinoides | Rosaceae | Taiwan | SH2 | not given | not found | [25] |
| Rubus phoenicolasius | Rosaceae | Japan | SH2 SH6 SH11 | not given | rheumatism, irregular menstruation, kidney ailments | [18,25,54] |
| Rubus loganbaccus x Rubus baileyanus | Rosaceae | New Zealand | SH2 SH6 SH10 | not given | not found | [55] |
| Rubus glaucus | Rosaceae | Trinidad, Costa Rica, Ecuador | SH6 | 2.45 mg/g of dried berries | diarrhea, wounds, burns | [17,35] |
| Rubus coreanus | Rosaceae | Korea, Japan | SH2 SH5 SH4 SH6 SH11 | not given | impotence, pollution, premature ejaculation, frequency of urination | [18,56,57,58,59,60] |
| Rubus fruticosus | Rosaceae | Poland, Japan | SH6 | 1.35–5.47 mg/g of dried berries | dysentery, diarrhea, whooping cough, colitis, toothache, pain | [18,26,61] |
| SH11 | not given | |||||
| SH2 isomer | not given | |||||
| Rubus irirasem | Rosaceae | Japan | SH6 SH11 | not given | not found | [18] |
| Rubus hiraseanus | Rosaceae | Japan | SH6 SH11 | not given | not found | [18] |
| Rubus vagabundus | Rosaceae | Portugal | SH2 SH6 SH10 | not given | not found | [62] |
| Rubus brigantinus | Rosaceae | Portugal | SH2 SH6 SH10 | not given | not found | [62] |
| Rubus radula | Rosaceae | Poland | SH6 | 16.66 mg/g of dried leaves | not found | [43] |
| Rubus montanus | Rosaceae | Poland | SH6 | 16.95 mg/g of dried leaves | not found | [43] |
| Rubus gracilis | Rosaceae | Poland | SH6 | 18.07 mg/g of dried leaves | not found | [43] |
| Rubus macrophyllus | Rosaceae | Poland | SH6 | 14.48 mg/g of dried leaves | not found | [43] |
| Rubus pericrispatus | Rosaceae | Poland | SH6 | 14.49 mg/g of dried leaves | not found | [43] |
| Rubus subcatus | Rosaceae | Poland | SH6 | 59.79 mg/g of dried leaves | not found | [43] |
| Rubus ambrosius | Rosaceae | Poland | SH6 | 21.11 mg/g of dried leaves | not found | [43] |
| Rubus fasciculatus | Rosaceae | Poland | SH6 | 23.24 mg/g of dried leaves | not found | [43] |
| Rubus nessensis | Rosaceae | Poland | SH6 | 12.22 mg/g of dried leaves | not found | [43] |
| Rubus glivicensis | Rosaceae | Poland | SH6 | 48.46 mg/g of dried leaves | not found | [43] |
| Rubus bifronus | Rosaceae | Poland | SH6 | 39.48 mg/g of dried leaves | not found | [43] |
| Rubus praecox | Rosaceae | Poland | SH6 | 18.49 mg/g of dried leaves | not found | [43] |
| Rubus perrobustus | Rosaceae | Poland | SH6 | 53.02 mg/g of dried leaves | not found | [43] |
| Rubus parthenocissus | Rosaceae | Poland | SH6 | 11.41 mg/g of dried leaves | not found | [43] |
| Rubus pseudidaeus | Rosaceae | Poland | SH6 | 15.07 mg/g of dried leaves | not found | [43] |
| Rubus constrictus | Rosaceae | Poland | SH6 | 24.38 mg/g of dried leaves | not found | [43] |
| Rubus wimmerianus | Rosaceae | Poland | SH6 | 64.44 mg/g of dried leaves | not found | [43] |
| Rubus orthostachys | Rosaceae | Poland | SH6 | 45.60 mg/g of dried leaves | not found | [43] |
| Rubus plicatus | Rosaceae | Poland | SH6 | 58.48 mg/g of dried leaves | not found | [43] |
| Rubus pedemontanus | Rosaceae | Poland | SH6 | 63.51 mg/g of dried leaves | not found | [43] |
| Rubus grabowski | Rosaceae | Poland | SH6 | 49.77 mg/g of dried leaves | not found | [43] |
| Sanguisorba tenuifolia var. parviflora | Rosaceae | Japan | SH2 SH11 | not given | not found | [25] |
| Sanguisorba officinalis | Rosaceae | Japan | SH1 | not given | leukopenia, hemorrhaging, burns | [13,25,63,64] |
| SH2 | not given | |||||
| SH3 | not given | |||||
| SH6 | 1.6 mg/g of dried leaves | |||||
| SH11 | not given | |||||
| Sanguisorba tenuijolia var. alba | Rosaceae | Japan | SH6 SH11 | not given | not found | [18] |
| Punica granatum | Lythraceae | Spain | SH10 isomers | not given | inflammation, rheumatism, pain, snakebites, diabetes, burns, leprosy, vermifugal and taenicidal agent | [23,65] |
| Euphorbia fischeriana | Euphorbiaceae | China | SH5 | 0.072 mg/g of dried roots | dyspepsia, abdominal distension, abdominal pain, cough, external applications as a cure for scabies and tuberculosis of lymph nodes | [21,66] |
| Terminalia calamansanai | Combretaceae | Taiwan | SH4 | 0.098 mg/g of dried leaves | lithotriptic | [24] |
| Compound | Stationary Phase/Column | Mobile Phase | Conditions (Flow Rate, Injection Volume) | Detection | References |
|---|---|---|---|---|---|
| SH6, SH10 isomers | SunFire C18 RP | 1% FA and ACN/H2O (9:1, v/v) | 0.21 mL/min; 5 μL | 280 nm | [19] |
| SH4 | Phenomenex Gemini C18; Waters Symmetry C18; Phenomenex Kinetex C18; Phenomenex Luna C18 | 1% FA and MeOH | 1–15 mL/min | 310 nm | [20] |
| Toyopearl HW-40F | 70% MeOH | - | - | [32] | |
| LiChroprep RP C18 | 0.05% TFA and CH3CN (95:5) | 1 mL/min | 280 nm | [24] | |
| SH2, ethyl ester | ODS | MeOH–H2O (35:65) | - | - | [33] |
| SH6 | Lichrospher ODS-2 RP | 2% FA and ACN/H2O/FA (80:18:2, v/v/v) | 0.5 mL/min; 10 μL | 200–600 nm | [35] |
| Discovery HS C18 | 0.1% TFA and 0.1% TFA in a mixture of H2O:ACN (50:50 v/v) | 0.3 mL/min; 1 μL | 520 nm | [27] | |
| Fuji-gel ODS-G3 | MeOH–H2O (7:3) | - | - | [25] | |
| UPLC BEH C18 | 4.5% FA and ACN | 0.45 mL/min; 10 μL | 240 nm | [43] | |
| SH5, SH6, SH10 | ODS Hypersil | ACN and 1% FA | 2 mL/min; 15 μL | 280 nm | [37] |
| SH10 isomer | Spherisorb S3 ODS-2 C18 | 1% FA and ACN | 0.5 mL/min; | 280 nm | [38] |
| BlueOrchid C18; Hypersil Gold C18; Kinetex PFP | ACN + 1% FA and H2O | 0.2 mL/min; 5 μL | - | [23] | |
| SH2 | MCI-gel CHP 20P | mixture of MeOH and H2O | - | - | [25] |
| SH5 | Sephadex LH-20 | mixture of MeOH and H2O | - | - | [21] |
| SH6, SH11 | Superspher Si 60 | hexane-MeOH-THF-HCO2H + (COH)2O | 1.5 mL/min; | 280 nm | [18] |
| SH2, SH6, SH10 | Synergy Hydro RP C18 | ACN:H2O | 10 mL/min; 50–200 μL | 280 nm | [55,62] |
| Activity | Experimental Model | Exposure | Concentration | Efficacy | References |
|---|---|---|---|---|---|
| Anti-inflammatory | Rat neutrophils | 60 min chemotaxis and 2 h toxicity in in vitro assays | 0, 1, 2.5, 5, and 10 μM SH11, SH6, and SH2 |
| [70] |
| Human AGS gastric epithelial cells | 1 h for NF-κB nuclear translocation, 6 h for NF-κB-driven transcription, and 6 h for IL-8 release in in vitro assays | 0.25–10 μM SH6 |
| [71] | |
| Antioxidant | Male LWH Wistar rats | In vivo, rats were fed orally with SH6 for 30 days | 10 mg/kg body weight/day |
| [72] |
| Fremy’s salt | 20 min electron spin resonance spectroscopy in situ assay | extracts diluted to 5% (v/v) with ethanol and water (12:88, v/v); 1.0 mL portion |
| [73] | |
| HT22 murine hippocampal cells | 8 h in vitro assay | 0, 10, and 20 μM SH11 |
| [74] | |
| DPPH, methyl linoleate and diene hydroperoxide | 15 min, 72 h, and 2 h in situ assays | 2, 5, 10, 50, and 250 μM of raspberry ET dimers and trimers |
| [75] | |
| ABTS and FRAP assays | 6 min in situ ABTS assay, 8 min in situ FRAP assay | not given |
| [43] | |
| mice macrophage and sodium nitroprusside | 24 in vitro macrophage incubation, 150 min in situ sodium nitroprusside assay | 0, 12.5, 25, and 50 μM of SH6 in macrophage assay, 0, 2.5, 5, 12.5, 25, 50, and 100 μM of SH6 in sodium nitroprusside assay |
| [76] | |
| Osteoclastogenesis inhibitory | 8-week-old male C57BL/6J mice | intraperitoneal injections for 5 days | 10 μg/body weight(g)/day of SH6 |
| [47] |
| bone marrow macrophages (BMMs) | 72 h in vitro assay | 0, 1, 5, 10, and 25 μM of SH6 |
| ||
| RAW-D cells | 72 h in vitro assay | 5 μM of SH6 |
| ||
| BMMs and RAW-D cells | 72 h in vitro assay | 0–50 μM of SH6 in BMM and RAW-D cells assays |
| ||
| Antibacterial | Streptococcus group A, B, C S. pneumoniae E. faecalis C. diphtheriae B. subtilis C. sporogenes S. aureus S. epidermidis N. meningitidis M. catarrhalis H. influenzae H. pylori K. pneumoniae | 48 h in vitro assay | SH6 concentrations: geometric progression from 0.015 to 1 mg/mL |
| [27] |
| C. perfringens E. coli L. plantarum S. aureus | 24 h in vitro incubation | 0.5 mM of SH6 |
| [77] | |
| E. coli, E. faecalis K. pneumoniae, M. morganii, P. mirabilis, P. aeruginosa, L. monocytogenes, MRSA, MSSA | not given | 100 mg/mL (stock solution) R. ulmifolius extract; SH10: 9.6 ± 0.1 mg/g |
| [78] | |
| Antifungal | C. albicans | not given | 100 mg/mL (stock solution) R. ulmifolius extract; SH10: 9.6 ± 0.1 mg/g |
| |
| Antiviral | NA from C. perfringens | 30 min in situ assay | SH4 solution |
| [20] |
| spike glycoprotein of SARS-CoV-2 virus | in silico molecular docking assay | SH6 and SH2 molecular structures |
| [79] | |
| Mpro protease and spike glycoprotein of SARS-CoV-2 virus | in silico molecular docking assay | SH6 molecular structure |
| [80] | |
| Estrogenic | MCF-7 human breast adenocarcinoma cell | 144 h in vitro proliferation assay | SH6 at 0, 25, 50 and 100 μM, Rubus coreanus: 0, 5, 10, 25, 50, and 100 μg/mL |
| [81] |
| Estrogen Receptor α | in silico molecular docking assay | SH6 molecular structure |
| ||
| Neuroprotective | SK-N-MC neuroblastoma cells | 2 and 24 h in vitro assay | commercial blackberry and R. vagabundus: 0, 0.25, 0.5, and 1 µg GAE/mL, R. brigantinus: 0, 0.1, 0.2, and 0.4 µg GAE/mL |
| [62] |
| 24 h in vitro assay | Commercial blackberry and R. vagabundus: 0, 0.25, 0.5, and 1 µg GAE/mL, R. brigantinus: 0, 0.1, 0.2, and 0.4 µg GAE/mL |
| |||
| Anticancer | HeLa cells | 72 h in vitro assay | Cytotoxicity: 0–25 µM of SH6 DNA cleavage: 10, 15, and 25 µM |
| [82] |
| Topoisomerase I and II | 30 min in situ assay | Topoisomerase I: 0, 19, 38, and 75 nM of SH6 Topoisomerase II: 0, 0.05, 0.1 0.2, 0.4, and 0.8 µM of SH6 |
| ||
| Topoisomerase I and II | 30 min in situ assay | 0, 0.1, 0.2, 0.4, 0.6, 1.2, and 2.4 µM of SH6 |
| ||
| HUVECs and HT1080 cells | 72 h in vitro XTT incorporation assay | SH6: concentrations up to 20 µg/mL |
| [83] | |
| PRMI-7951 melanoma cells | in vitro cytotoxicity assay | SH2, SH6, and SH11 solutions |
| [68] | |
| HL-60 and PBMCs | 12 h in vitro treatment | HL-60: 100 µM, PBMCs: 400 µM of SH4 |
| [24] | |
| AGS, HeLa, Hep G2, HT 29, and T 24 cell lines | 24 h in vitro treatment | 100 µM of SH4 |
| ||
| HL-60 cells | 12 h in vitro assay | serial dilution concentrations from 0 to 400 µM of SH4 |
| ||
| 25, 50, and 100 µM of SH4 |
| ||||
| 100 µM of SH4 |
| ||||
| 50 and 100 µM of SH4 |
| ||||
| A549 lung cancer cells | 48 h in vitro assay | 5 and 10 µM of SH6 |
| [84] | |
| 48 h in vitro assay | 5 and 10 µM of SH6 |
| |||
| 2 h in vitro pretreatment | 5 and 10 µM of SH6 |
| |||
| 5 and 10 µM of SH6 |
| ||||
| 5 and 10 µM of SH6 |
| ||||
| 48 h in vitro assay | 1, 2.5, 5, 10, 25, 50, 75, and 100 µM of SH6 |
| |||
| MCF-7/Adr and MCF-7/wt cells | 48 h in vitro incubation; MTT assay | 10, 20, 40, 79, 157, and 313 µM of SH6 |
| [85] | |
| MDA-MB-231 human breast cancer cells | 24 h in vitro assay | 0 and 6.25 µM of SH6 |
| ||
| 0, 6.25, 12.5, 25, 50, 100, and 200 µM of SH6 |
| ||||
| HUVECs | 0 and 6.25 µM of SH6 |
| [86] | ||
| 0, 6.25, 12.5, 25, 50, 100, and 200 µM of SH6 |
| ||||
| MCF-7 and MDA-MB-231 cells | 24 h in vitro assay | 0, 50, and 100 µM of SH6 for MCF-7 and MDA-MB-231 cells |
| [79] | |
| 50 and 100 µM of SH6 |
| ||||
| 0, 5, 10, 25, 50, and 100 µM of SH6 |
| ||||
| A2780 human ovarian carcinoma cells | 24 h in vitro assay | 0, 10, 20, and 40 µM of SH6 |
| [87] | |
| 20 and 40 µM of SH6 |
| ||||
| 0, 10, 20, and 40 µM of SH6 |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gesek, J.; Jakimiuk, K.; Atanasov, A.G.; Tomczyk, M. Sanguiins—Promising Molecules with Broad Biological Potential. Int. J. Mol. Sci. 2021, 22, 12972. https://doi.org/10.3390/ijms222312972
Gesek J, Jakimiuk K, Atanasov AG, Tomczyk M. Sanguiins—Promising Molecules with Broad Biological Potential. International Journal of Molecular Sciences. 2021; 22(23):12972. https://doi.org/10.3390/ijms222312972
Chicago/Turabian StyleGesek, Jakub, Katarzyna Jakimiuk, Atanas G. Atanasov, and Michał Tomczyk. 2021. "Sanguiins—Promising Molecules with Broad Biological Potential" International Journal of Molecular Sciences 22, no. 23: 12972. https://doi.org/10.3390/ijms222312972
APA StyleGesek, J., Jakimiuk, K., Atanasov, A. G., & Tomczyk, M. (2021). Sanguiins—Promising Molecules with Broad Biological Potential. International Journal of Molecular Sciences, 22(23), 12972. https://doi.org/10.3390/ijms222312972









