Mutants of the white ABCG Transporter in Drosophila melanogaster Have Deficient Olfactory Learning and Cholesterol Homeostasis
Abstract
1. Introduction
2. Results
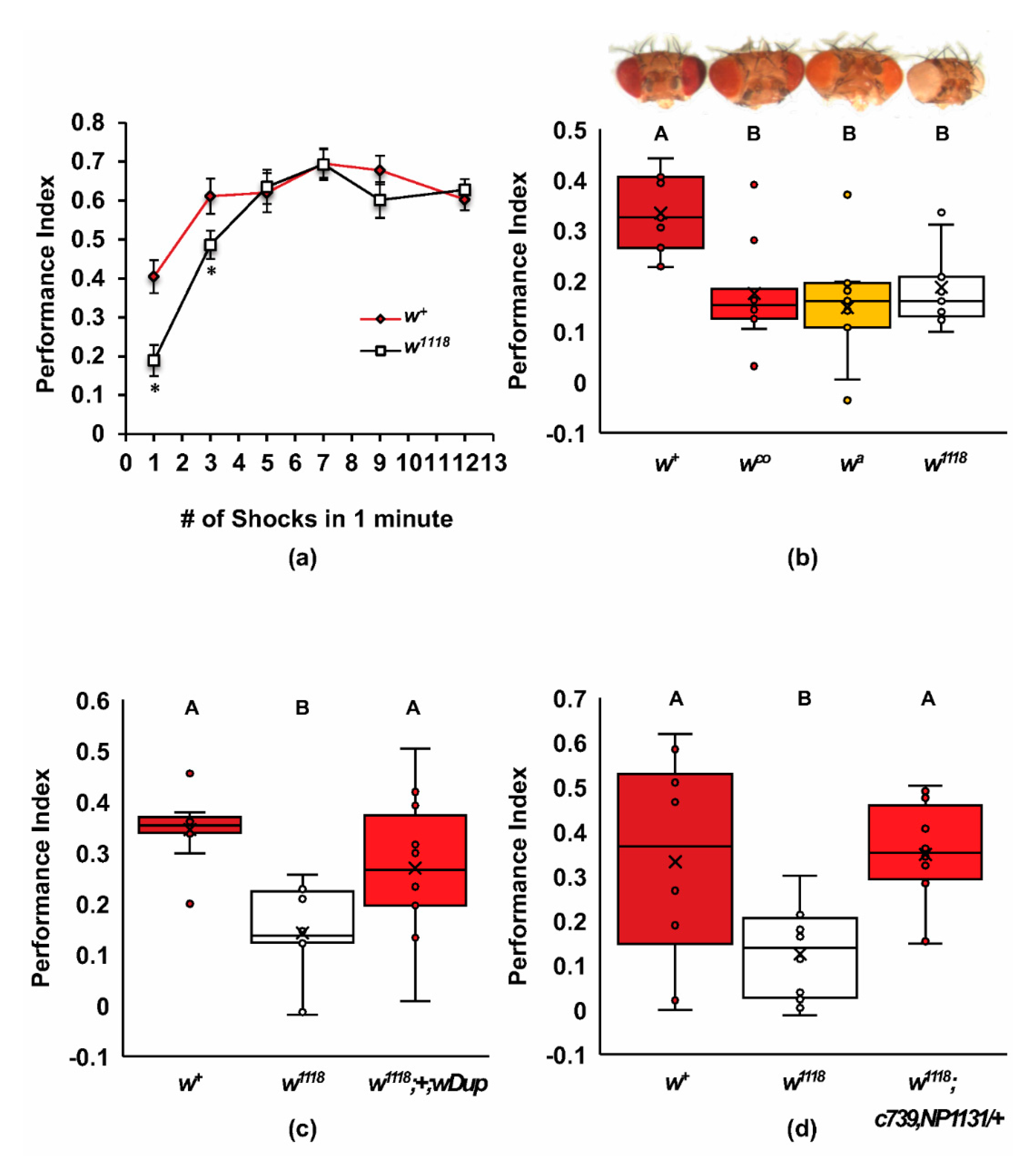
2.1. Mutants of white Have Poor Learning Acquisition
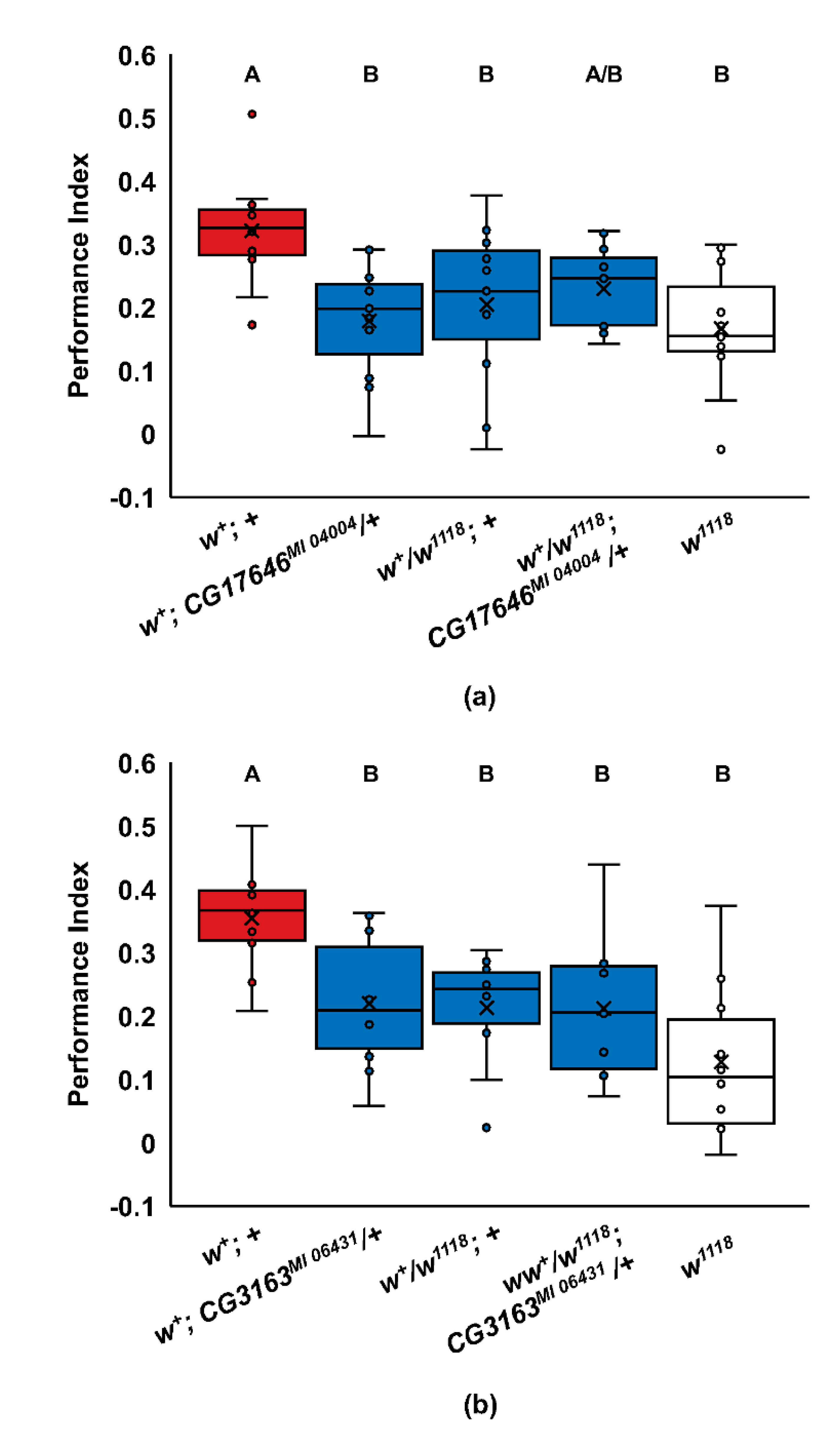
2.2. Screen for Additional ABCG Learning Mutants
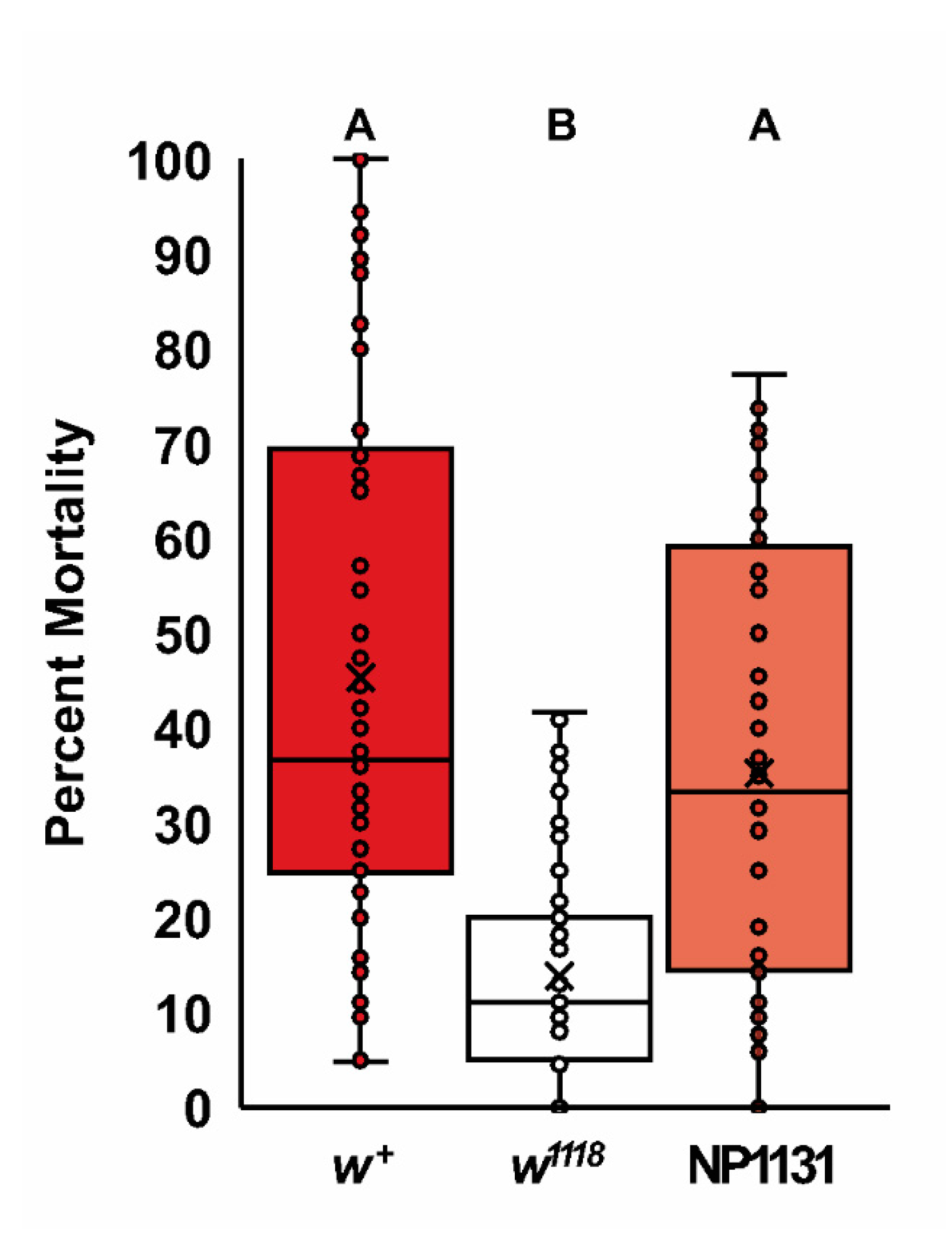
2.3. A Role for white in Cholesterol Homeostasis
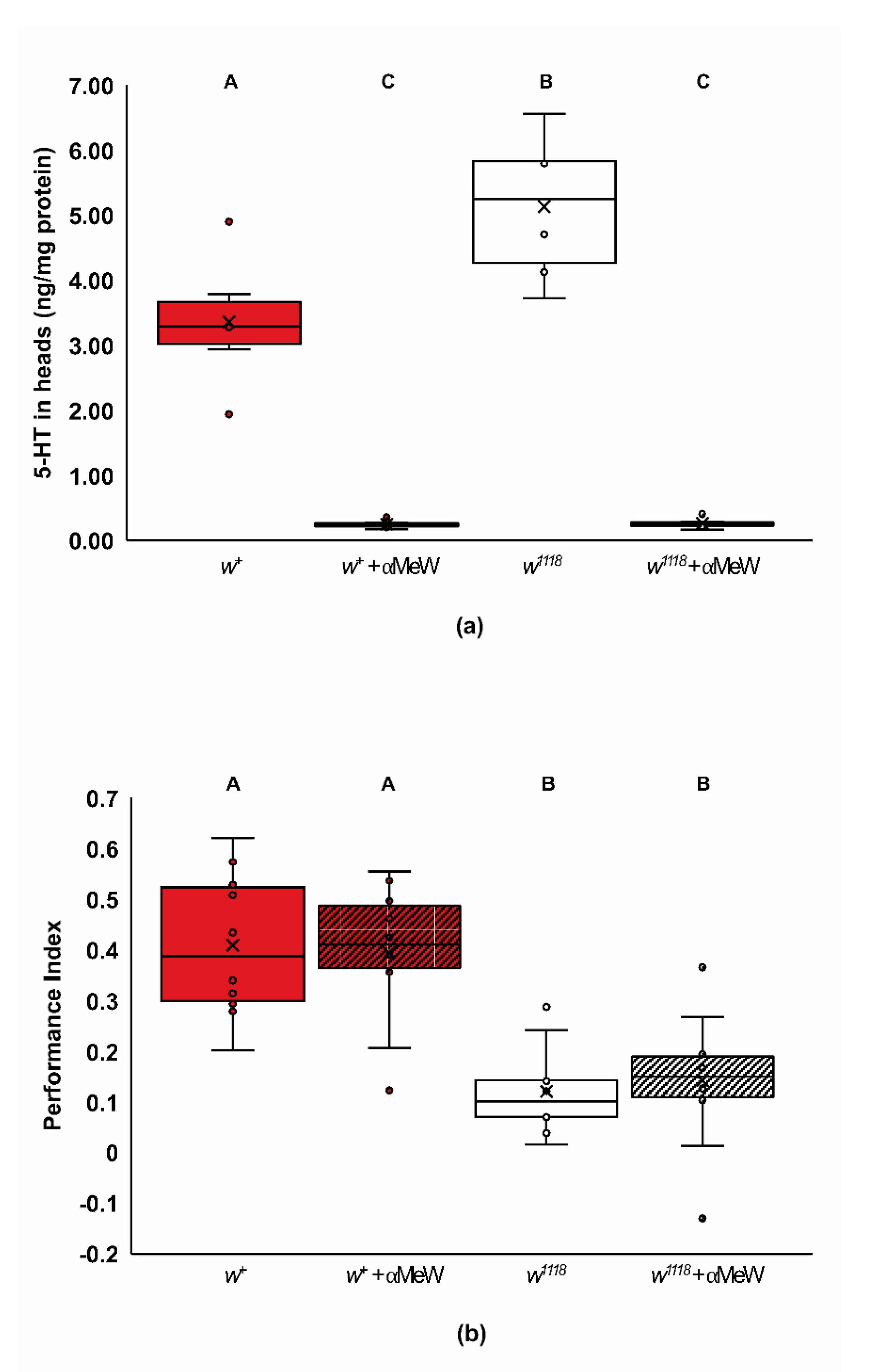
2.4. Biogenic Amine Signaling and the w1118 Learning Phenotype
3. Discussion
3.1. Known Biochemical Activities of white and Cellular Locations
3.2. The Role of white in Behavior
3.3. A Role for white in Olfactory Learning
3.4. Dominance and Gene Dose Sensitivity
3.5. ABCGs with Cholesterol and Other Lipid Functions
3.6. Cholesterol Regulation in Drosophila
3.7. Differences in the Cholesterol and Cholesterol Ester Levels of w1118
3.8. Roles of Cholesterol in Synaptic Signaling
3.9. white and Biogenic Amine Signaling in Olfactory Learning
3.10. Possible Mechanisms for L-Dopa and 5-HTP Rescue of Wildtype Learning Phenotype
4. Materials and Methods
4.1. Drosophila Stocks and Genetics
4.2. Pharmacology
4.3. Olfactory Classical Conditioning Assay
4.4. Cholesterol Treatments
4.5. Cholesterol and Cholesterol Ester Quantification
4.6. Freeze Tolerance
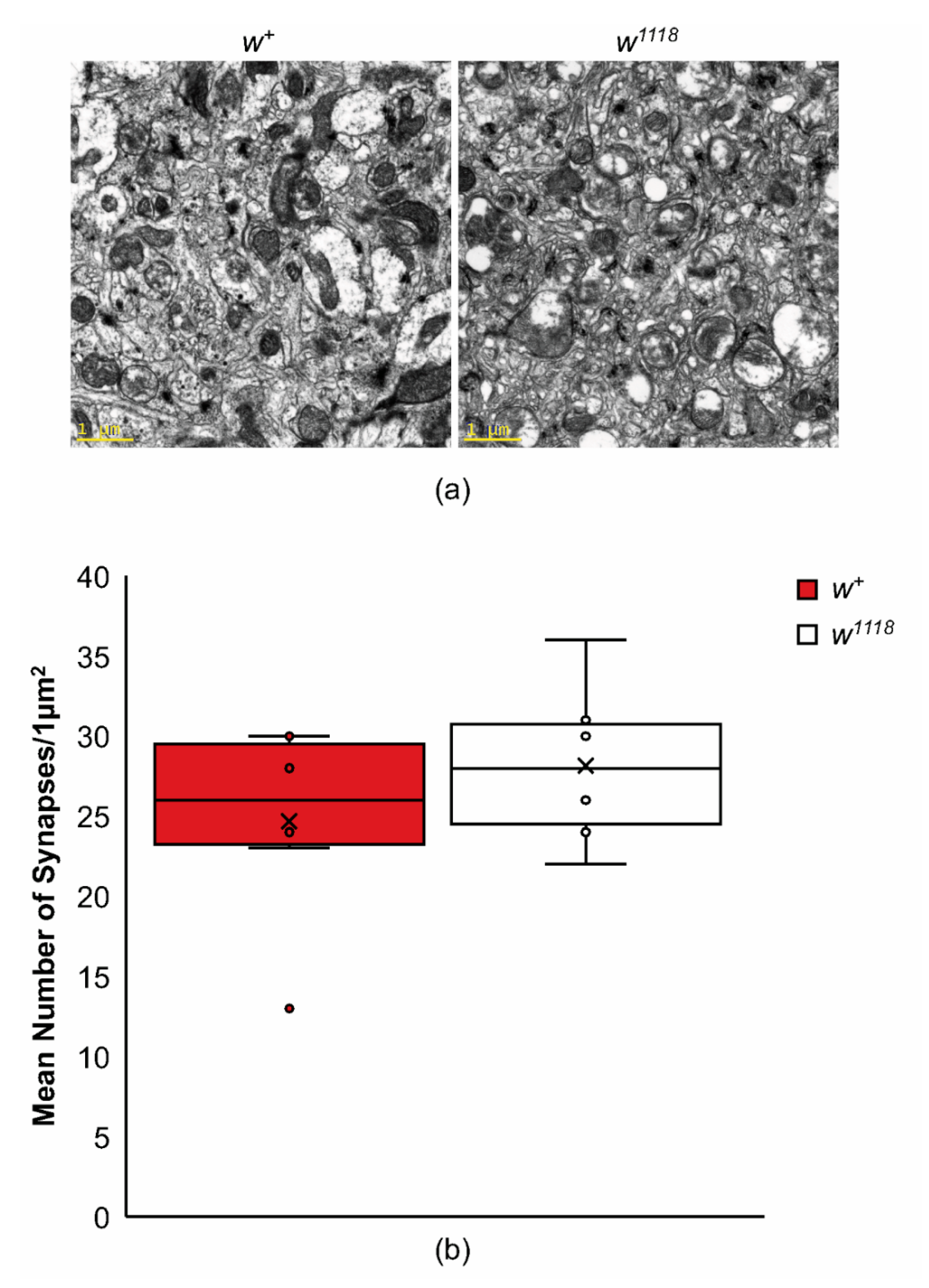
4.7. Quantifying Synapse Numbers
4.8. HPLC for 5-HT
4.9. Data Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Morgan, T.H. Sex Limited Inheritance in Drosophila. Science 1910, 32, 120–122. [Google Scholar] [CrossRef]
- Bridges, C.B. Non-disjunction as proof of the chromosome theory of heredity. Genetics 1916, 1, 1–52. [Google Scholar] [CrossRef]
- Muller, H.J. Artificial Transmutation of the Gene. Science 1927, 66, 84–87. [Google Scholar] [CrossRef]
- Rubin, G.; Spradling, A.C. Vectors for P element-mediated gene transfer in Drosophila. Nucleic Acids Res. 1983, 11, 6341–6351. [Google Scholar] [CrossRef][Green Version]
- Brand, A.H.; Perrimon, N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 1993, 118, 401–415. [Google Scholar] [CrossRef]
- Kalmus, H. The optomotor responses of some eye mutants of Drosophila. J. Genet. 1943, 45, 206–213. [Google Scholar] [CrossRef]
- Hengstenberg, R.; Gotz, K.G. Effect of facet-separating pigments on perception of light and contrast in eye mutants of Dro-sophila. Kybernetik 1967, 3, 276–285. [Google Scholar]
- Liu, L.; Davis, R.; Roman, G. Exploratory Activity in Drosophila Requires the kurtz Nonvisual Arrestin. Genetics 2007, 175, 1197–1212. [Google Scholar] [CrossRef] [PubMed]
- Soibam, B.; Mann, M.; Liu, L.; Tran, J.; Lobaina, M.; Kang, Y.Y.; Gunaratne, G.H.; Pletcher, S.; Roman, G. Open-field arena boundary is a primary object of exploration for Drosophila. Brain Behav. 2012, 2, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Campbell, J.L.; Nash, H.A. Volatile general anesthetics reveal a neurobiological role for the white and brown genes of Drosophila melanogaster. J. Neurobiol. 2001, 49, 339–349. [Google Scholar] [CrossRef] [PubMed]
- Diegelmann, S.; Zars, M.; Zars, T. Genetic dissociation of acquisition and memory strength in the heat-box spatial learning paradigm in Drosophila. Learn. Mem. 2006, 13, 72–83. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sitaraman, D.; Zars, M.; LaFerriere, H.; Chen, Y.-C.; Sable-Smith, A.; Kitamoto, T.; Rottinghaus, G.E.; Zars, T. Serotonin is necessary for place memory in Drosophila. Proc. Natl. Acad. Sci. USA 2008, 105, 5579–5584. [Google Scholar] [CrossRef] [PubMed]
- Chan, R.F.; Lewellyn, L.; Deloyht, J.M.; Sennett, k.; Coffman, S.; Hewitt, M. Contrasting influences of Drosophila white/miniwhite on ethanol sensitivity in two different behavioral assays. Alcoholism: Clin. Exp. Res. 2014, 38, 1582–1593. [Google Scholar] [CrossRef]
- Krstic, D.; Boll, W.; Noll, M. Influence of the White Locus on the Courtship Behavior of Drosophila Males. PLoS ONE 2013, 8, e77904. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.D.; Odenwald, W.F. Misexpression of the white (w) gene triggers male-male courtship in Drosophila. Proc. Natl. Acad. Sci. USA 1995, 92, 5525–5529. [Google Scholar] [CrossRef] [PubMed]
- Au-van der Linde, K. The FlyBar: Administering alcohol to flies. J. Vis. Experiments 2014, 87, e50442. [Google Scholar] [CrossRef]
- Vasiliou, V.; Vasiliou, K.; Nebert, D.W. Human ATP-binding cassette (ABC) transporter family. Hum. Genom. 2008, 3, 281–290. [Google Scholar] [CrossRef]
- Dermauw, W.; Van Leeuwen, T. The ABC gene family in arthropods: Comparative genomics and role in insecticide transport and resistance. Insect Biochem. Mol. Biol. 2014, 45, 89–110. [Google Scholar] [CrossRef]
- Hersh, B.M. More than Meets the Eye: A Primer for Timing of Locomotor Recovery from Anoxia Modulated by the white Gene in Drosophila melanogaster. Genetics 2016, 204, 1369–1375. [Google Scholar] [CrossRef]
- Ford, R.C.; Beis, K. Learning the ABCs one at a time: Structure and mechanism of ABC transporters. Biochem. Soc. Trans. 2019, 47, 23–36. [Google Scholar] [CrossRef]
- Wilkens, S. Structure and mechanism of ABC transporters. F1000Prime Rep. 2015, 7, 14. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, D.T.; Sullivan, M.C. Transport defects as the physiological basis for eye color mutants of Drosophila melanogaster. Biochem. Genet. 1975, 13, 603–613. [Google Scholar] [CrossRef] [PubMed]
- Dean, M.; Rzhetsky, A.; Allikmets, R. The Human ATP-Binding Cassette (ABC) Transporter Superfamily. Genome Res. 2001, 11, 1156–1166. [Google Scholar] [CrossRef] [PubMed]
- Linton, K.J. Structure and Function of ABC Transporters. Physiology 2007, 22, 122–130. [Google Scholar] [CrossRef]
- Dong, J.; Yang, G.; Mchaourab, H.S. Structural Basis of Energy Transduction in the Transport Cycle of MsbA. Science 2005, 308, 1023–1028. [Google Scholar] [CrossRef]
- Klein, I.; Sarkadi, B.; Váradi, A. An inventory of the human ABC proteins. Biochim. Biophys. Acta (BBA)—Biomembr. 1999, 1461, 237–262. [Google Scholar] [CrossRef]
- Schmitz, G.; Langmann, T.; Heimerl, S. Role of ABCG1 and other ABCG family members in lipid metabolism. J. Lipid Res. 2001, 42, 1513–1520. [Google Scholar] [CrossRef]
- Tarling, E.J.; Edwards, P.A. ATP binding cassette transporter G1 (ABCG1) is an intracellular sterol transporter. Proc. Natl. Acad. Sci. USA 2011, 108, 19719–19724. [Google Scholar] [CrossRef] [PubMed]
- Tarr, P.T.; Tarling, E.J.; Bojanic, D.D.; Edwards, P.A.; Baldán, Á. Emerging new paradigms for ABCG transporters. Biochim. Biophys. Acta (BBA)—Mol. Cell Biol. Lipids 2009, 1791, 584–593. [Google Scholar] [CrossRef]
- Bojanic, D.D.; Tarr, P.T.; Gale, G.D.; Smith, D.J.; Bok, D.; Chen, B.; Nusinowitz, S.; Lövgren-Sandblom, A.; Björkhem, I.; A Edwards, P. Differential expression and function of ABCG1 and ABCG4 during development and aging. J. Lipid Res. 2010, 51, 169–181. [Google Scholar] [CrossRef]
- Qiu, S.; Xiao, C.; Robertson, R.M. Different age-dependent performance in Drosophila wild-type Canton-S and the white mutant w1118 flies. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2017, 206, 17–23. [Google Scholar] [CrossRef]
- Berge, K.E.; Tian, H.; Graf, G.A.; Yu, L.; Grishin, N.V.; Schultz, J.; Kwiterovich, P.; Shan, B.; Barnes, R.; Hobbs, H.H. Accumulation of Dietary Cholesterol in Sitosterolemia Caused by Mutations in Adjacent ABC Transporters. Science 2000, 290, 1771–1775. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Sun, F.; Zhang, D.; Ma, Y.M.; Xu, F.; Belani, J.D.; Cohen, J.C.; Hobbs, H.H.; Xie, X.S. Sterol transfer by ABCG5 and ABCG8: In vitro assay and reconstitution. J. Biol. Chem. 2006, 281, 27894–27904. [Google Scholar] [CrossRef]
- Yamanaka, N.; Marqués, G.; O’Connor, M.B. Vesicle-Mediated Steroid Hormone Secretion in Drosophila melanogaster. Cell 2015, 163, 907–919. [Google Scholar] [CrossRef]
- Horner, M.A.; Pardee, K.; Liu, S.; King-Jones, K.; Lajoie, G.; Edwards, A.; Krause, H.M.; Thummel, C.S. The Drosophila DHR96 nuclear receptor binds cholesterol and regulates cholesterol homeostasis. Genes Dev. 2009, 23, 2711–2716. [Google Scholar] [CrossRef]
- Borycz, J.; Borycz, J.A.; Kubow, A.; Lloyd, V.; Meinertzhagen, I.A. Drosophila ABC transporter mutants white, brown and scarlet have altered contents and distribution of biogenic amines in the brain. J. Exp. Biol. 2008, 211 Pt 21, 3454–3466. [Google Scholar] [CrossRef] [PubMed]
- McClung, C.; Hirsh, J. The trace amine tyramine is essential for sensitization to cocaine in Drosophila. Curr. Biol. 1999, 9, 853–860. [Google Scholar] [CrossRef]
- Hardie, S.L.; Hirsh, J. An improved method for the separation and detection of biogenic amines in adult Drosophila brain extracts by high performance liquid chromatography. J. Neurosci. Methods 2006, 153, 243–249. [Google Scholar] [CrossRef]
- Zhang, Y.Q.; Friedman, D.B.; Wang, Z.; Woodruff, E.; Pan, L.; O’donnell, J.; Broadie, K. Protein expression profiling of the Drosophila fragile X mutant brain reveals up-regulation of monoamine synthesis. Mol. Cell. Proteom. 2005, 4, 278–290. [Google Scholar] [CrossRef] [PubMed]
- Yarali, A.; Krischke, M.; Michels, B.; Saumweber, T.; Mueller, M.J.; Gerber, B. Genetic Distortion of the Balance between Punishment and Relief Learning in Drosophila. J. Neurogenet. 2009, 23, 235–247. [Google Scholar] [CrossRef]
- Tully, T.; Quinn, W.G. Classical conditioning and retention in normal and mutant Drosophila melanogaster. J. Comp. Physiol. A 1985, 157, 263–277. [Google Scholar] [CrossRef]
- Roman, G.; Davis, R.L. Molecular biology and anatomy of Drosophila olfactory associative learning. BioEssays 2001, 23, 571–581. [Google Scholar] [CrossRef] [PubMed]
- Beck, C.; Schroeder, B.; Davis, R.L. Learning performance of normal and mutant Drosophila after repeated conditioning trials with discrete stimuli. J. Neurosci. 2000, 20, 2944–2953. [Google Scholar] [CrossRef]
- Venken, K.; Popodi, E.; Holtzman, S.L.; Schulze, K.L.; Park, S.; Carlson, J.W.; Hoskins, R.A.; Bellen, H.; Kaufman, T.C. A Molecularly Defined Duplication Set for the X Chromosome of Drosophila melanogaster. Genetics 2010, 186, 1111–1125. [Google Scholar] [CrossRef] [PubMed]
- Golic, K.G.; Lindquist, S. The FLP recombinase of yeast catalyzes site-specific recombination in the drosophila genome. Cell 1989, 59, 499–509. [Google Scholar] [CrossRef]
- Sullivan, D.T.; Bell, L.A.; Paton, D.R.; Sullivan, M.C. Purine transport by Malpighian tubules of pteridine-deficient eye color mutants of Drosophila melanogaster. Biochem. Genet. 1979, 17, 565–573. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.M.; Day, J.P.; Cabrero, P.; Dow, J.A.T.; Davies, S.-A. A new role for a classical gene: White transports cyclic GMP. J. Exp. Biol. 2008, 211, 890–899. [Google Scholar] [CrossRef]
- Dreesen, T.D.; Johnson, D.H.; Henikoff, S. The brown protein of Drosophila melanogaster is similar to the white protein and to components of active transport complexes. Mol. Cell. Biol. 1988, 8, 5206–5215. [Google Scholar] [CrossRef]
- Tearle, R.G.; Belote, J.M.; McKeown, M.; Baker, B.S.; Howells, A.J. Cloning and characterization of the scarlet gene of Drosophila melanogaster. Genetics 1989, 122, 595–606. [Google Scholar] [CrossRef]
- Berkeley Drosophila Genome Project. Patterns of Gene Expression in Drosophila Embryogenesis. 2010. Available online: https://www.fruitfly.org/ (accessed on 25 November 2021).
- Nagarkar-Jaiswal, S.; Lee, P.-T.; E Campbell, M.; Chen, K.; Anguiano-Zarate, S.; Gutierrez, M.C.; Busby, T.; Lin, W.-W.; Theodore, B.; Schulze, K.L.; et al. A library of MiMICs allows tagging of genes and reversible, spatial and temporal knockdown of proteins in Drosophila. eLife 2015, 4, e5338. [Google Scholar] [CrossRef]
- Nagao, K.; Juni, N.; Umeda, M. Membrane Lipid Transporters in Drosophila melanogaster in Bioactive Lipid Mediators; Yokomizo, T., Murakami, M., Eds.; Springer: Tokyo, Japan, 2015; pp. 165–180. [Google Scholar]
- Liu, Z.; Huang, X. Lipid metabolism in Drosophila: Development and disease. Acta Biochim. Biophys. Sin. 2013, 45, 44–50. [Google Scholar] [CrossRef]
- Sjögren, B.; Csöregh, L.; Svenningsson, P. Cholesterol reduction attenuates 5-HT1A receptor-mediated signaling in human primary neuronal cultures. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2008, 378, 441–446. [Google Scholar] [CrossRef]
- Dason, J.; Smith, A.; Marin, L.; Charlton, M.P. Cholesterol and F-actin are required for clustering of recycling synaptic vesicle proteins in the presynaptic plasma membrane. J. Physiol. 2014, 592, 621–633. [Google Scholar] [CrossRef] [PubMed]
- Doktorova, M.; Symons, J.L.; Levental, I. Structural and functional consequences of reversible lipid asymmetry in living membranes. Nat. Chem. Biol. 2020, 16, 1321–1330. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.S.; Ho, Y.K.; Goldstein, J.L. The cholesteryl ester cycle in macrophage foam cells. Continual hydrolysis and re-esterification of cytoplasmic cholesteryl esters. J. Biol. Chem. 1980, 255, 9344–9352. [Google Scholar] [CrossRef]
- Steponkus, P.L. Role of the plasma membrane in freezing injury and cold acclimation. Annu. Rev. Plant Physiol. 1984, 35, 543–584. [Google Scholar] [CrossRef]
- Shreve, S.M.; Yi, S.-X.; E Lee, R. Increased dietary cholesterol enhances cold tolerance in Drosophila melanogaster. Cryo Lett. 2007, 28, 33–37. [Google Scholar]
- Teets, N.M.; Denlinger, D.L. Physiological mechanisms of seasonal and rapid cold-hardening in insects. Physiol. Èntomol. 2013, 38, 105–116. [Google Scholar] [CrossRef]
- Tempel, B.L.; Bonini, N.; Dawson, D.R.; Quinn, W.G. Reward learning in normal and mutant Drosophila. Proc. Natl. Acad. Sci. USA 1983, 80, 1482–1486. [Google Scholar] [CrossRef]
- Schwaerzel, M.; Monastirioti, M.; Scholz, H.; Friggi-Grelin, F.; Birman, S.; Heisenberg, M. Dopamine and Octopamine Differentiate between Aversive and Appetitive Olfactory Memories in Drosophila. J. Neurosci. 2003, 23, 10495–10502. [Google Scholar] [CrossRef]
- Johnson, O.; Becnel, J.; Nichols, C. Serotonin receptor activity is necessary for olfactory learning and memory in Drosophila melanogaster. Neuroscience 2011, 192, 372–381. [Google Scholar] [CrossRef]
- A Dierick, H.; Greenspan, R.J. Serotonin and neuropeptide F have opposite modulatory effects on fly aggression. Nat. Genet. 2007, 39, 678–682. [Google Scholar] [CrossRef] [PubMed]
- Bainton, R.J.; Tsai, L.; Singh, C.M.; Moore, M.S.; Neckameyer, W.S.; Heberlein, U. Dopamine modulates acute responses to cocaine, nicotine and ethanol in Drosophila. Curr. Biol. 2000, 10, 187–194. [Google Scholar] [CrossRef]
- Kain, J.S.; Stokes, C.; de Bivort, B.L. Phototactic personality in fruit flies and its suppression by serotonin and white. Proc. Natl. Acad. Sci. USA 2012, 109, 19834–19839. [Google Scholar] [CrossRef] [PubMed]
- Gazi, M.; Shyamala, B.V.; Bhat, K.M. A neurodegenerative disease affecting synaptic connections in Drosophila mutant for the tumor suppressor morphogen Patched. Dev. Biol. 2009, 334, 311–323. [Google Scholar] [CrossRef][Green Version]
- Green, M.M. 2010: A century of Drosophila genetics through the prism of the white gene. Genetics 2010, 184, 3–7. [Google Scholar] [CrossRef]
- Bellen, H.J.; Tong, C.; Tsuda, H. 100 years of Drosophila research and its impact on vertebrate neuroscience: A history lesson for the future. Nat. Rev. Neurosci. 2010, 11, 514–522. [Google Scholar] [CrossRef]
- Anaka, M.; MacDonald, C.D.; Barkova, E.; Simon, K.; Rostom, R.; Godoy, R.A.; Haigh, A.J.; Meinertzhagen, I.A.; Lloyd, V. The white gene of Drosophila melanogaster encodes a protein with a role in courtship behavior. J. Neurogenet. 2008, 22, 243–276. [Google Scholar] [CrossRef]
- Buchmann, J.; Meyer, C.; Neschen, S.; Schmolz, K.; Augustin, R.; Kluge, R.; Eulenberg, K.; Wehr, R.; Dohrmann, C.; Joost, H.G.; et al. Ablation of cholesterol transporter ABCG1 in mice reduces adipose cell size and corrects diet-induced insulin resistance. Aktuelle Ernährungsmedizin 2006, 31, V5. [Google Scholar] [CrossRef]
- MacKenzie, S.M.; Howells, A.J.; Cox, G.B.; Ewart, G.D. Sub-cellular Localisation of the White/Scarlet ABC Transporter to Pigment Granule Membranes within the Compound Eye of Drosophila Melanogaster. Genetics 2000, 108, 239–252. [Google Scholar] [CrossRef]
- Ewart, G.D.; Howells, A.J. [15] ABC transporters involved in transport of eye pigment precursors in Drosophila melanogaster. Methods Enzymol. 1998, 292, 213–224. [Google Scholar] [CrossRef]
- Sullivan, D.T.; Bell, L.A.; Paton, D.R.; Sullivan, M.C. Genetic and functional analysis of tryptophan transport in Malpighian tubules of Drosophila. Biochem. Genet. 1980, 18, 1109–1130. [Google Scholar] [CrossRef]
- Tejeda-Guzmán, C.; Rosas-Arellano, A.; Kroll, T.; Webb, S.M.; Barajas-Aceves, M.; Osorio, B.; Missirlis, F. Biogenesis of zinc storage granules in Drosophila melanogaster. J. Exp. Biol. 2018, 221, jeb.168419. [Google Scholar] [CrossRef]
- Sasaki, A.; Nishimura, T.; Takano, T.; Naito, S.; Yoo, S.K. white regulates proliferative homeostasis of intestinal stem cells during ageing in Drosophila. Nat. Metab. 2021, 3, 546–557. [Google Scholar] [CrossRef]
- Joh, T.H.; Shikimi, T.; Pickel, V.M.; Reis, D.J. Brain tryptophan hydroxylase: Purification of, production of antibodies to, and cellular and ultrastructural localization in serotonergic neurons of rat midbrain. Proc. Natl. Acad. Sci. USA 1975, 72, 3575–3579. [Google Scholar] [CrossRef] [PubMed]
- Howells, A.J.; Summers, K.M.; Ryall, R.L. Developmental patterns of 3-hydroxykynurenine accumulation in white and various other eye color mutants of Drosophila melanogaster. Biochem. Genet. 1977, 15, 1049–1059. [Google Scholar] [CrossRef]
- Chintapalli, V.R.; Wang, J.; Dow, J. Using FlyAtlas to identify better Drosophila melanogaster models of human disease. Nat. Genet. 2007, 39, 715–720. [Google Scholar] [CrossRef]
- Xiao, C.; Robertson, R.M. Timing of Locomotor Recovery from Anoxia Modulated by the white Gene in Drosophila. Genetics 2016, 203, 787–797. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.; Qiu, S.; Robertson, R.M. The white gene controls copulation success in Drosophila melanogaster. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef]
- Buchmann, J.; Meyer, C.; Neschen, S.; Augustin, R.; Schmolz, K.; Kluge, R.; Al-Hasani, H.; Jürgens, H.; Eulenberg, K.; Wehr, R.; et al. Ablation of the Cholesterol Transporter Adenosine Triphosphate-Binding Cassette Transporter G1 Reduces Adipose Cell Size and Protects against Diet-Induced Obesity. Endocrinology 2007, 148, 1561–1573. [Google Scholar] [CrossRef]
- Tarr, P.T.; Edwards, P.A. ABCG1 and ABCG4 are coexpressed in neurons and astrocytes of the CNS and regulate cholesterol homeostasis through SREBP-2. J. Lipid Res. 2008, 49, 169–182. [Google Scholar] [CrossRef]
- Lee, M.-H.; Lu, K.; Hazard, S.; Yu, H.; Shulenin, S.; Hidaka, H.; Kojima, H.; Allikmets, R.; Sakuma, N.; Pegoraro, R.; et al. Identification of a gene, ABCG5, important in the regulation of dietary cholesterol absorption. Nat. Genet. 2001, 27, 79–83. [Google Scholar] [CrossRef]
- Lee, M.-H.; Lu, K.; Patel, S.B. Genetic basis of sitosterolemia. Curr. Opin. Lipidol. 2001, 12, 141–149. [Google Scholar] [CrossRef]
- Chintapalli, V.; Wang, J.; Dow, J. FlyAtlas: The Drosophila Gene Expression Atlas; flyatlas.org; University of Glasgow: Glasgow, UK, 2007. [Google Scholar]
- Tomancak, P.; Berman, B.P.; Beaton, A.; Weiszmann, R.; Kwan, E.; Hartenstein, V.; E Celniker, S.; Rubin, G.M. Global analysis of patterns of gene expression during Drosophila embryogenesis. Genome Biol. 2007, 8, R145. [Google Scholar] [CrossRef]
- Kopczynski, C.C.; Noordermeer, J.N.; Serano, T.L.; Chen, W.-Y.; Pendleton, J.D.; Lewis, S.; Goodman, C.S.; Rubin, G. A high throughput screen to identify secreted and transmembrane proteins involved in Drosophila embryogenesis. Proc. Natl. Acad. Sci. USA 1998, 95, 9973–9978. [Google Scholar] [CrossRef]
- Parkin, C.; Burnet, B. Growth arrest of Drosophila melanogaster on erg-2 and erg-6 sterol mutant strains of Saccharomyces cerevisiae. J. Insect Physiol. 1986, 32, 463–471. [Google Scholar] [CrossRef]
- Bujold, M.; Gopalakrishnan, A.; Nally, E.; King-Jones, K. Nuclear Receptor DHR96 Acts as a Sentinel for Low Cholesterol Concentrations in Drosophila melanogaster. Mol. Cell. Biol. 2010, 30, 793–805. [Google Scholar] [CrossRef]
- Hazel, J.R. Thermal adaptation in biological membranes: Is homeoviscous adaptation the explanation? Annu. Rev. Physiol. 1995, 57, 19–42. [Google Scholar] [CrossRef]
- Goritz, C.; Mauch, D.H.; Pfrieger, F.W. Multiple mechanisms mediate cholesterol-induced synaptogenesis in a CNS neuron. Mol. Cell. Neurosci. 2005, 29, 190–201. [Google Scholar] [CrossRef]
- Renner, M.; Choquet, D.; Triller, A. Control of the Postsynaptic Membrane Viscosity. J. Neurosci. 2009, 29, 2926–2937. [Google Scholar] [CrossRef]
- Puchkov, D.; Haucke, V. Greasing the synaptic vesicle cycle by membrane lipids. Trends Cell Biol. 2013, 23, 493–503. [Google Scholar] [CrossRef]
- Korinek, M.; Gonzalez-Gonzalez, M.I.; Smejkalova, T.; Hajdukovic, D.; Skrenkova, K.; Krusek, J.; Horak, M.; Vyklicky, L. Cholesterol modulates presynaptic and postsynaptic properties of excitatory synaptic transmission. Sci. Rep. 2020, 10, 1–18. [Google Scholar] [CrossRef]
- Smith, A.J.; Sugita, S.; Charlton, M.P. Cholesterol-Dependent Kinase Activity Regulates Transmitter Release from Cerebellar Synapses. J. Neurosci. 2010, 30, 6116–6121. [Google Scholar] [CrossRef] [PubMed]
- Zamir, O.; Charlton, M.P. Cholesterol and synaptic transmitter release at crayfish neuromuscular junctions. J. Physiol. 2006, 571, 83–99. [Google Scholar] [CrossRef]
- Takamori, S.; Holt, M.; Stenius, K.; Lemke, E.A.; Grønborg, M.; Riedel, D.; Urlaub, H.; Schenck, S.; Brügger, B.; Ringler, P.; et al. Molecular Anatomy of a Trafficking Organelle. Cell 2006, 127, 831–846. [Google Scholar] [CrossRef]
- Dason, J.S.; Smith, A.J.; Marin, L.; Charlton, M.P. Vesicular Sterols Are Essential for Synaptic Vesicle Cycling. J. Neurosci. 2010, 30, 15856–15865. [Google Scholar] [CrossRef] [PubMed]
- Magnani, F.; Tate, C.; Wynne, S.; Williams, C.; Haase, J. Partitioning of the Serotonin Transporter into Lipid Microdomains Modulates Transport of Serotonin. J. Biol. Chem. 2004, 279, 38770–38778. [Google Scholar] [CrossRef]
- Gabriel, L.R.; Wu, S.; Kearney, P.; Bellvé, K.D.; Standley, C.; Fogarty, K.E.; Melikian, H.E. Dopamine transporter endocytic trafficking in striatal dopaminergic neurons: Differential dependence on dynamin and the actin cytoskeleton. J. Neurosci. 2013, 33, 17836–17846. [Google Scholar] [CrossRef] [PubMed]
- Samuvel, D.J.; Jayanthi, L.D.; Bhat, N.R.; Ramamoorthy, S. A Role for p38 Mitogen-Activated Protein Kinase in the Regulation of the Serotonin Transporter: Evidence for Distinct Cellular Mechanisms Involved in Transporter Surface Expression. J. Neurosci. 2005, 25, 29–41. [Google Scholar] [CrossRef]
- Foster, J.D.; Adkins, S.D.; Lever, J.R.; Vaughan, R.A. Phorbol ester induced trafficking-independent regulation and enhanced phosphorylation of the dopamine transporter associated with membrane rafts and cholesterol. J. Neurochem. 2008, 105, 1683–1699. [Google Scholar] [CrossRef] [PubMed]
- Zeppelin, T.; Ladefoged, L.K.; Sinning, S.; Periole, X.; Schiøtt, B. A direct interaction of cholesterol with the dopamine transporter prevents its out-to-inward transition. PLoS Comput. Biol. 2018, 14, e1005907. [Google Scholar] [CrossRef] [PubMed]
- Manna, M.; Niemelä, M.; Tynkkynen, J.; Javanainen, M.; Kulig, W.; Muller, D.J.; Rog, T.; Vattulainen, I. Mechanism of allosteric regulation of β2-adrenergic receptor by cholesterol. eLife 2016, 5, e18432. [Google Scholar] [CrossRef]
- Guixà-González, R.; Albasanz, J.L.; Rodriguez-Espigares, I.; Pastor, M.; Sanz, F.; Marti-Solano, M.; Manna, M.; Martinez-Seara, H.; Hildebrand, P.W.; Martín, M.; et al. Membrane cholesterol access into a G-protein-coupled receptor. Nat. Commun. 2017, 8, 14505. [Google Scholar] [CrossRef]
- Marino, K.A.; Prada-Gracia, D.; Provasi, D.; Filizola, M. Impact of Lipid Composition and Receptor Conformation on the Spatio-temporal Organization of μ-Opioid Receptors in a Multi-component Plasma Membrane Model. PLoS Comput. Biol. 2016, 12, e1005240. [Google Scholar] [CrossRef]
- Riemensperger, T.; Völler, T.; Stock, P.; Buchner, E.; Fiala, A. Punishment Prediction by Dopaminergic Neurons in Drosophila. Curr. Biol. 2005, 15, 1953–1960. [Google Scholar] [CrossRef] [PubMed]
- McCurdy, L.Y.; Sareen, P.; Davoudian, P.A.; Nitabach, M.N. Dopaminergic mechanism underlying reward-encoding of punishment omission during reversal learning in Drosophila. Nat. Commun. 2021, 12, 1–17. [Google Scholar] [CrossRef]
- Berry, J.A.; Cervantes-Sandoval, I.; Nicholas, E.P.; Davis, R.L. Dopamine Is Required for Learning and Forgetting in Drosophila. Neuron 2012, 74, 530–542. [Google Scholar] [CrossRef] [PubMed]
- Busto, G.U.; Cervantes-Sandoval, I.; Davis, R.L. Olfactory Learning in Drosophila. Physiology 2010, 25, 338–346. [Google Scholar] [CrossRef]
- Krashes, M.J.; DasGupta, S.; Vreede, A.; White, B.; Armstrong, D.; Waddell, S. A Neural Circuit Mechanism Integrating Motivational State with Memory Expression in Drosophila. Cell 2009, 139, 416–427. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Keene, A.C.; Srivatsan, A.; Waddell, S.; Davis, R.L. Drosophila DPM Neurons Form a Delayed and Branch-Specific Memory Trace after Olfactory Classical Conditioning. Cell 2005, 123, 945–957. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.-T.; Lin, H.-W.; Chang, Y.-H.; Fu, T.-F.; Dubnau, J.; Hirsh, J.; Lee, T.; Chiang, A.-S. Serotonin-mushroom body circuit modulating the formation of anesthesia-resistant memory in Drosophila. Proc. Natl. Acad. Sci. USA 2011, 108, 13794–13799. [Google Scholar] [CrossRef]
- Yuan, Q.; Lin, F.; Zheng, X.; Sehgal, A. Serotonin Modulates Circadian Entrainment in Drosophila. Neuron 2005, 47, 115–127. [Google Scholar] [CrossRef]
- Yuan, Q.; Joiner, W.J.; Sehgal, A. A Sleep-Promoting Role for the Drosophila Serotonin Receptor 1A. Curr. Biol. 2006, 16, 1051–1062. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, M.; Schwudke, D.; Sampaio, J.; Palm, W.; Riezman, I.; Dey, G.; Gupta, G.D.; Mayor, S.; Riezman, H.; Shevchenko, A.; et al. Survival strategies of a sterol auxotroph. Development 2010, 137, 3675–3685. [Google Scholar] [CrossRef] [PubMed]
- Caragata, E.P.; Rancès, E.; Hedges, L.M.; Gofton, A.; Johnson, K.; O’Neill, S.; McGraw, E.A. Dietary Cholesterol Modulates Pathogen Blocking by Wolbachia. PLoS Pathog. 2013, 9, e1003459. [Google Scholar] [CrossRef] [PubMed]
- Venken, K.J.T.; Schulze, K.L.; A Haelterman, N.; Pan, H.; He, Y.; Evans-Holm, M.; Carlson, J.W.; Levis, R.; Spradling, A.C.; A Hoskins, R.; et al. MiMIC: A highly versatile transposon insertion resource for engineering Drosophila melanogaster genes. Nat. Methods 2011, 8, 737–743. [Google Scholar] [CrossRef]
- Ferris, J.; Ge, H.; Liu, L.; Roman, G. G(o) signaling is required for Drosophila associative learning. Nat. Neurosci. 2006, 9, 1036–1040. [Google Scholar] [CrossRef]
- Niwa, R.; Niwa, Y.S. The Fruit Fly Drosophila melanogaster as a Model System to Study Cholesterol Metabolism and Homeostasis. Cholesterol 2011, 2011, 176802. [Google Scholar] [CrossRef]
- Bodennec, J.; Koul, O.; Aguado, I.; Brichon, G.; Zwingelstein, G.; Portoukalian, J. A procedure for fractionation of sphingolipid classes by solid-phase extraction on aminopropyl cartridges. J. Lipid Res. 2000, 41, 1524–1531. [Google Scholar] [CrossRef]
- Hutchins, P.M.; Barkley, R.M.; Murphy, R.C. Separation of cellular nonpolar neutral lipids by normal-phase chromatography and analysis by electrospray ionization mass spectrometry. J. Lipid Res. 2008, 49, 804–813. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, S.S.; Wilk, M.B. An analysis of variance test for normality (complete samples). Biometrika 1965, 52, 591–611. [Google Scholar] [CrossRef]
- Kruskal, W.H.; Wallis, W.A. Use of ranks in one-criterion variance analysis. J. Am. Stat. Assoc. 1952, 47, 583–621. [Google Scholar] [CrossRef]



| Fly Stock Used | Annotation in Text | Identifiers |
|---|---|---|
| w[1118];Dp(1;3)DC050,PBac[y[+mDint2] w[+mC] = DC050]VK00033 | w1118;+,wDup | BDSC 30234 |
| bw1; st1 | bw1; st1 | RRID:BDSC_686 |
| wa | wa | RRID:BDSC_48 |
| wco, sn2 | wco, sn2 | RRID:BDSC_153 |
| w[1118]; P[w[+mW.hs] = GawB]Hr39[c739], P[GawB]NP1131 | w1118;c739, NP1131 | RRID:BDSC_7362, RRID:FBti0034208 |
| MiMIC lines and y[1] w[*]; Mi(y[+mDint2] = MIC)CG3164[MI06431] | CG3164MI 06431 | RRID:BDSC_42392 |
| y[1] w[*]; Mi(y[+mDint2] = MIC)ND-15[MI10825] CG3164[MI10825]/SM6a | CG3164MI 10825 | RRID:BDSC_55545 |
| y[1] w[*]; Mi(y[+mDint2] = MIC)ND-15[MI13074] CG4822[MI13074] | CG4822MI 13074 | RRID:BDSC_58020 |
| y[1] w[*]; Mi(y[+mDint2] = MIC)CG17646[MI04004] | CG17646MI 04004 | RRID:BDSC_42302 |
| y[1] w[*]; Mi(y[+mDint2] = MIC)CG9663[MI11447] | CG9663MI 11447 | RRID:BDSC_56321 |
| y[1] w[*]; Mi(y[+mDint2] = MIC)Atet[MI01881] | AtetMI 01881 | RRID:BDSC_37314 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Myers, J.L.; Porter, M.; Narwold, N.; Bhat, K.; Dauwalder, B.; Roman, G. Mutants of the white ABCG Transporter in Drosophila melanogaster Have Deficient Olfactory Learning and Cholesterol Homeostasis. Int. J. Mol. Sci. 2021, 22, 12967. https://doi.org/10.3390/ijms222312967
Myers JL, Porter M, Narwold N, Bhat K, Dauwalder B, Roman G. Mutants of the white ABCG Transporter in Drosophila melanogaster Have Deficient Olfactory Learning and Cholesterol Homeostasis. International Journal of Molecular Sciences. 2021; 22(23):12967. https://doi.org/10.3390/ijms222312967
Chicago/Turabian StyleMyers, Jennifer L., Maria Porter, Nicholas Narwold, Krishna Bhat, Brigitte Dauwalder, and Gregg Roman. 2021. "Mutants of the white ABCG Transporter in Drosophila melanogaster Have Deficient Olfactory Learning and Cholesterol Homeostasis" International Journal of Molecular Sciences 22, no. 23: 12967. https://doi.org/10.3390/ijms222312967
APA StyleMyers, J. L., Porter, M., Narwold, N., Bhat, K., Dauwalder, B., & Roman, G. (2021). Mutants of the white ABCG Transporter in Drosophila melanogaster Have Deficient Olfactory Learning and Cholesterol Homeostasis. International Journal of Molecular Sciences, 22(23), 12967. https://doi.org/10.3390/ijms222312967






