Application of Dynamic and Static Light Scattering for Size and Shape Characterization of Small Extracellular Nanoparticles in Plasma and Ascites of Ovarian Cancer Patients
Abstract
1. Introduction
2. Results
2.1. Preliminary DLS Measurements at θ = 90°
2.2. Analysis of LS Data at θ = 90°
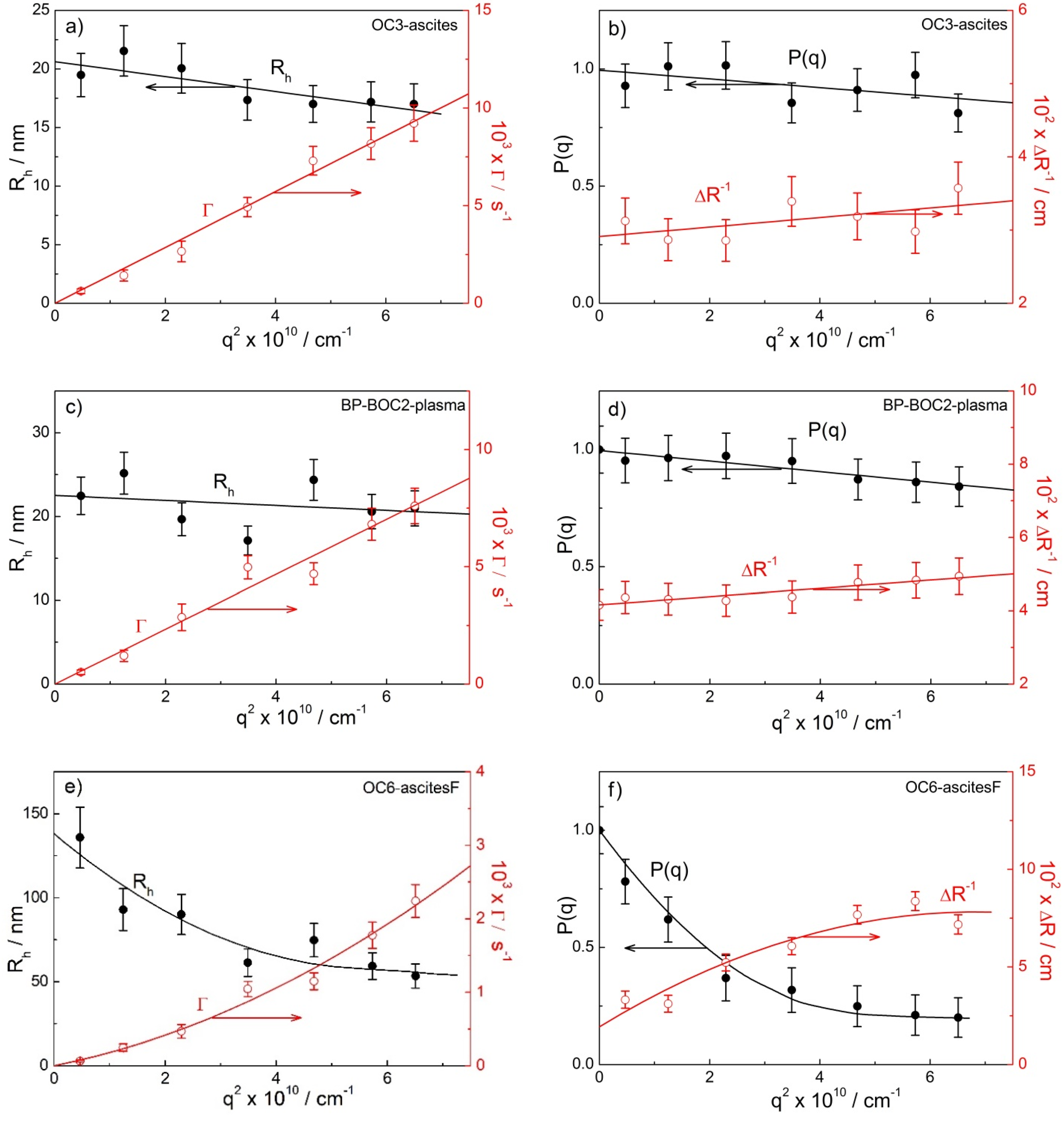
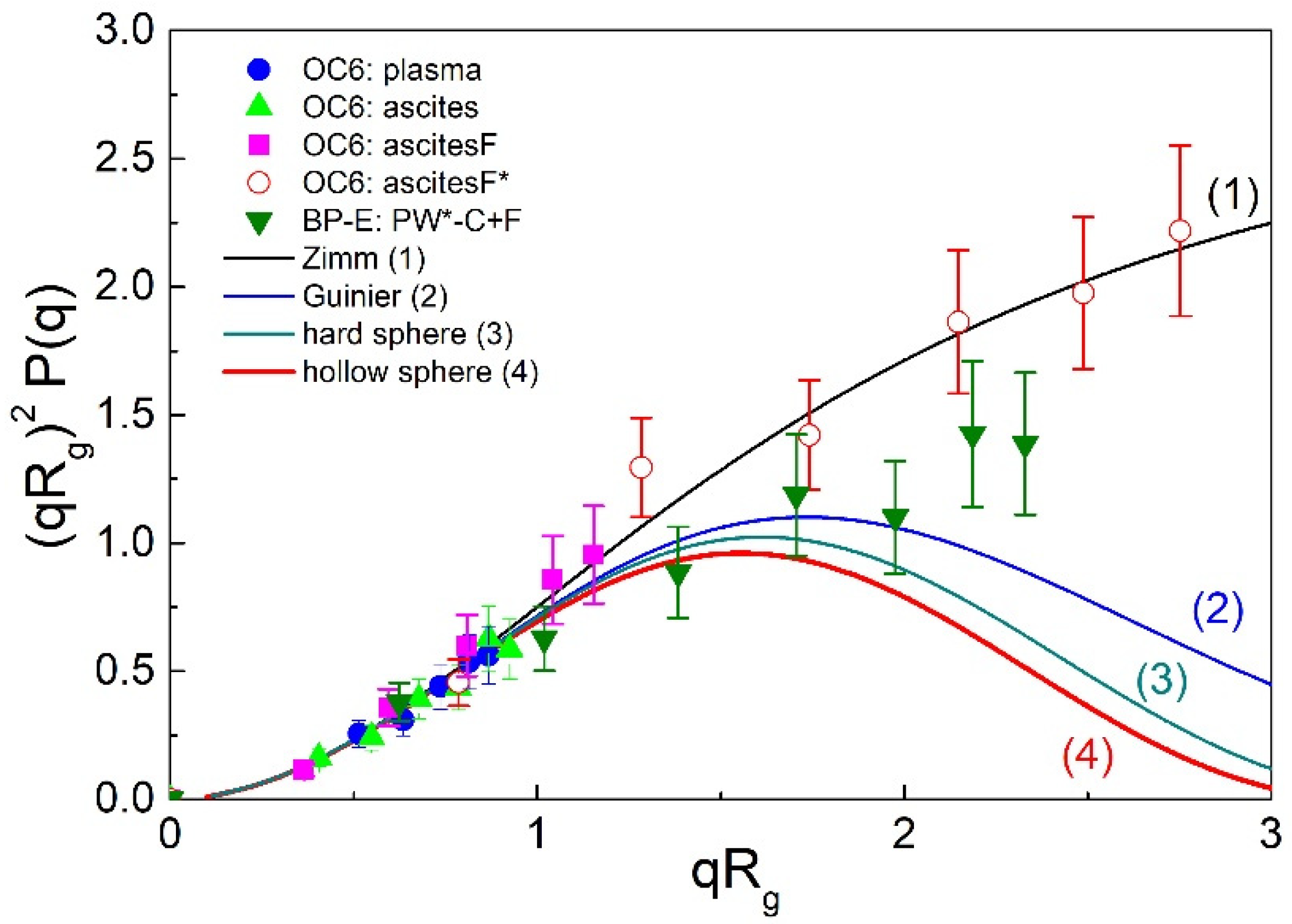
2.3. Angular Dependency and the Determination of Rg and ρ
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Collection of Samples
4.3. Preparation and Storage of Samples
Isolation/Concentration of Exosomes by Differential Ultracentrifugation
4.4. Static and Dynamic Light Scattering Measurements
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.; Naishadham, D.; Jemal, A. Cancer statistics, 2013. CA Cancer J. Clin. 2013, 63, 11–30. [Google Scholar] [CrossRef] [PubMed]
- Falzone, L.; Scandurra, G.; Lombardo, V.; Gattuso, G.; Lavoro, A.; Distefano, A.B.; Scibilia, G.; Scollo, P. A multidisciplinary approach remains the best strategy to improve and strengthen the management of ovarian cancer (Review). Int. J. Oncol. 2021, 59, 53. [Google Scholar] [CrossRef] [PubMed]
- Kurnit, K.C.; Fleming, G.F.; Lengyel, E. Updates and New Options in Advanced Epithelial Ovarian Cancer Treatment. Obstet. Gynecol. 2021, 137, 108–121. [Google Scholar] [CrossRef] [PubMed]
- Lheureux, S.; Msc, M.B.; Oza, A.M. Epithelial ovarian cancer: Evolution of management in the era of precision medicine. CA Cancer J. Clin. 2019, 69, 280–304. [Google Scholar] [CrossRef] [PubMed]
- Chen, V.W.; Ruiz, B.; Killeen, J.L.; Coté, T.R.; Wu, X.C.; Correa, C.N.; Howe, H.L. Pathology and classification of ovarian tumors. Cancer 2003, 97, 2631–2642. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.; Cardenas-Goicoechea, S.J.; Gordon, P.; Curtin, C.; Momeni, M.; Chuang, L.; Fishman, D. Biomarkers for Early Detection of Ovarian Cancer. Women’s Health 2013, 9, 171–187. [Google Scholar] [CrossRef]
- Hu, L.; McArthur, C.; Jaffe, R.B. Ovarian cancer stem-like side-population cells are tumourigenic and chemoresistant. Br. J. Cancer 2010, 102, 1276–1283. [Google Scholar] [CrossRef]
- Tang, M.K.; Wong, A.S. Exosomes: Emerging biomarkers and targets for ovarian cancer. Cancer Lett. 2015, 367, 26–33. [Google Scholar] [CrossRef]
- Xu, R.; Rai, A.; Chen, M.; Suwakulsiri, W.; Greening, D.; Simpson, R.J. Extracellular vesicles in cancer—Implications for future improvements in cancer care. Nat. Rev. Clin. Oncol. 2018, 15, 617–638. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, H.; Gu, J.; Zhang, J.; Shi, H.; Qian, H.; Wang, D.; Xu, W.; Pan, J.; Santos, H.A. Engineered Extracellular Vesicles for Cancer Therapy. Adv. Mater. 2021, 33, 2005709. [Google Scholar] [CrossRef]
- Kobayashi, M.; Tapia, J.; Mitchell, M.D.; Rice, G.E.; Salomon, C. Exosomes are fingerprints of originating cells: Potential biomarkers for ovarian cancer. Res. Rep. Biochem. 2015, 5, 101–109. [Google Scholar] [CrossRef][Green Version]
- Li, J.; Sherman-Baust, C.A.; Tsai-Turton, M.; Bristow, R.E.; Roden, R.B.; Morin, P.J. Claudin-containing exosomes in the peripheral circulation of women with ovarian cancer. BMC Cancer 2009, 9, 244. [Google Scholar] [CrossRef]
- Szajnik, M.; Derbis, M.; Lach, M.; Patalas, P.; Michalak, M.; Drzewiecka, H.; Szpurek, D.; Nowakowski, A.; Spaczynski, M.; Baranowski, W.; et al. Exosomes in Plasma of Patients with Ovarian Carcinoma: Potential Biomarkers of Tumor Progression and Response to Therapy. Gynecol. Obstet. 2012, 8, 40. [Google Scholar] [CrossRef]
- Keller, S.; König, A.-K.; Marmé, F.; Runz, S.; Wolterink, S.; Koensgen, D.; Mustea, A.; Sehouli, J.; Altevogt, P. Systemic presence and tumor-growth promoting effect of ovarian carcinoma released exosomes. Cancer Lett. 2009, 278, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Černe, K.; Kobal, B. Chapter Eight—Implications of Microvesicle and Cell Surface Protein Shedding for Biomarker Studies, Cancerogenesis, and Therapeutic Target Discovery in Ovarian Cancer. In Advances in Planar Lipid Bilayers and Liposomes; Aleš, I., Ed.; Academic Press: Cambridge, MA, USA, 2012; Volume 16, pp. 239–274. [Google Scholar]
- Dolo, V.; D’Ascenzo, S.; Violini, S.; Pompucci, L.; Festuccia, C.; Ginestra, A.; Vittorelli, M.L.; Canevari, S.; Pavan, A. Matrix-degrading proteinases are shed in membrane vesicles by ovarian cancer cells in vivo and in vitro. Clin. Exp. Metastasis 1999, 17, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Gercel-Taylor, C.; Atay, S.; Tullis, R.H.; Kesimer, M.; Taylor, D.D. Nanoparticle analysis of circulating cell-derived vesicles in ovarian cancer patients. Anal. Biochem. 2012, 428, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Ryuzaki, S.; Yasui, T.; Tsutsui, M.; Yokota, K.; Komoto, Y.; Paisrisarn, P.; Kaji, N.; Ito, D.; Tamada, K.; Ochiya, T.; et al. Rapid Discrimination of Extracellular Vesicles by Shape Distribution Analysis. Anal. Chem. 2021, 93, 7037–7044. [Google Scholar] [CrossRef]
- Lin, Q.; Liu, W.; Xu, S.; Sun, L. Associations of preoperative serum high-density lipoprotein cholesterol and low-density lipoprotein cholesterol levels with the prognosis of ovarian cancer. Arch. Gynecol. Obstet. 2021, 95, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Xi, Y.; Feng, Y. Ovarian cancer risk in relation to blood lipid levels and hyperlipidemia: A systematic review and meta-analysis of observational epidemiologic studies. Eur. J. Cancer Prev. 2021, 30, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Buas, M.F.; Drescher, C.W.; Urban, N.; Li, C.I.; Bettcher, L.; Hait, N.C.; Moysich, K.B.; Odunsi, K.; Raftery, D.; Yan, L. Quantitative global lipidomics analysis of patients with ovarian cancer versus benign adnexal mass. Sci. Rep. 2021, 11, 18156. [Google Scholar] [CrossRef]
- Niemi, R.J.; Braicu, E.I.; Kulbe, H.; Koistinen, K.M.; Sehouli, J.; Puistola, U.; Mäenpää, J.U.; Hilvo, M. Ovarian tumours of different histologic type and clinical stage induce similar changes in lipid metabolism. Br. J. Cancer 2018, 119, 847–854. [Google Scholar] [CrossRef]
- Théry, C. Exosomes: Secreted vesicles and intercellular communications. F1000 Biol. Rep. 2011, 3, 15. [Google Scholar] [CrossRef]
- Conde-Vancells, J.; Rodriguez-Suarez, E.; Embade, N.; Gil, D.; Matthiesen, R.; Valle, M.; Elortza, F.; Lu, S.C.; Mato, J.M.; Falcon-Perez, J.M. Characterization and Comprehensive Proteome Profiling of Exosomes Secreted by Hepatocytes. J. Proteome Res. 2008, 7, 5157–5166. [Google Scholar] [CrossRef] [PubMed]
- German, J.B.; Smilowitz, J.T.; Zivkovic, A.M. Lipoproteins: When size really matters. Curr. Opin. Colloid Interface Sci. 2006, 11, 171–183. [Google Scholar] [CrossRef] [PubMed]
- Božič, D.; Sitar, S.; Junkar, I.; Štukelj, R.; Pajnič, M.; Žagar, E.; Kralj-Iglič, V.; Kogej, K.; Iglič, K. Viscosity of Plasma as a Key Factor in Assessment of Extracellular Vesicles by Light Scattering. Cells 2019, 8, 1046. [Google Scholar] [CrossRef]
- Chaikov, L.L.; Kirichenko, M.N.; Krivokhizha, S.V.; Zaritskiy, A.R. Dynamics of statistically confident particle sizes and concentrations in blood plasma obtained by the dynamic light scattering method. J. Biomed. Opt. 2015, 20, 057003. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Gillespie, B.M.; Palanisamy, V.; Gimzewski, J.K. Quantitative Nanostructural and Single-Molecule Force Spectroscopy Biomolecular Analysis of Human-Saliva-Derived Exosomes. Langmuir 2011, 27, 14394–14400. [Google Scholar] [CrossRef]
- Peng, P.; Yan, Y.; Keng, S. Exosomes in the ascites of ovarian cancer patients: Origin and effects on anti-tumor immunity. Oncol. Rep. 2011, 25, 749–762. [Google Scholar] [CrossRef] [PubMed]
- Andre, F.; Schartz, N.E.; Movassagh, M.; Flament, C.; Pautier, P.; Morice, P.; Pomel, C.; Lhomme, C.; Escudier, B.; Le Chevalier, T.; et al. Malignant effusions and immunogenic tumour-derived exosomes. Lancet 2002, 360, 295–305. [Google Scholar] [CrossRef]
- Taylor, D.D.; Gerceltaylor, C. Tumour-derived exosomes and their role in cancer-associated T-cell signalling defects. Br. J. Cancer 2005, 92, 305–311. [Google Scholar] [CrossRef]
- Zhang, W.; Peng, P.; Kuang, Y.; Yang, J.; Cao, D.; You, Y.; Shen, K. Characterization of exosomes derived from ovarian cancer cells and normal ovarian epithelial cells by nanoparticle tracking analysis. Tumor Biol. 2016, 37, 4213–4221. [Google Scholar] [CrossRef]
- Erdbrügger, U.; Lannigan, J. Analytical challenges of extracellular vesicle detection: A comparison of different techniques. Cytom. Part A 2015, 89, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Van Der Pol, E.; Coumans, F.; Varga, Z.; Krumrey, M.; Nieuwland, M. Innovation in detection of microparticles and exosomes. J. Thromb. Haemost. 2013, 11 (Suppl. S1), 36–45. [Google Scholar] [CrossRef]
- Vogel, R.; Savage, J.; Muzard, J.; Della Camera, G.; Vella, G.; Law, A.; Marchioni, M.; Mehn, D.; Geiss, O.; Peacock, B.; et al. Measuring particle concentration of multimodal synthetic reference materials and extracellular vesicles with orthogonal techniques: Who is up to the challenge? J. Extracell. Vesicles 2021, 10, e12052. [Google Scholar] [CrossRef] [PubMed]
- Hartjes, T.A.; Mytnyk, S.; Jenster, G.W.; Van Steijn, V.; Van Royen, M.E. Extracellular Vesicle Quantification and Characterization: Common Methods and Emerging Approaches. Bioengineering 2019, 6, 7. [Google Scholar] [CrossRef]
- Sitar, S.; Kejžar, A.; Pahovnik, D.; Kogej, K.; Tušek-Žnidarič, M.; Lenassi, M.; Žagar, E. Size Characterization and Quantification of Exosomes by Asymmetrical-Flow Field-Flow Fractionation. Anal. Chem. 2015, 87, 9225–9233. [Google Scholar] [CrossRef] [PubMed]
- Schärtl, W. Light Scattering from Polymer Solutions and Nanoparticle Dispersions; Springer: Berlin, Germany, 2007. [Google Scholar]
- Brown, W. (Ed.) Dynamic Light Scattering: The Method and Some Applications; Clarendon Press: Oxford, UK, 1993. [Google Scholar]
- Tarassova, E.; Aseyev, V.; Filippov, A.; Tenhu, H. Structure of poly(vinyl pyrrolidone)—C70 complexes in aqueous solutions. Polymer 2007, 48, 4503–4510. [Google Scholar] [CrossRef]
- Sitar, S.; Aseyev, V.; Kogej, K. Differences in association behavior of isotactic and atactic poly(methacrylic acid). Polymer 2014, 55, 848–854. [Google Scholar] [CrossRef]
- Hriberšek, P.; Kogej, K. Temperature Dependent Behavior of Isotactic and Atactic Poly(Methacrylic Acid) in the Presence of MgCl2 and CaCl2. Acta Chim. Slov. 2019, 66, 1023–1037. [Google Scholar] [CrossRef]
- Hriberšek, P.; Kogej, K. Tacticity and Counterion Modulated Temperature Response of Weak Polyelectrolytes: The Case of Poly(methacrylic acid) Stereoisomers in Aqueous Solutions. Macromolecules 2019, 52, 7028–7041. [Google Scholar] [CrossRef]
- De Spirito, M.; Brunelli, R.; Mei, G.; Bertani, F.R.; Ciasca, G.; Greco, G.; Papi, M.; Arcovito, G.; Ursini, F.; Parasassi, T. Low Density Lipoprotein Aged in Plasma Forms Clusters Resembling Subendothelial Droplets: Aggregation via Surface Sites. Biophys. J. 2006, 90, 4239–4247. [Google Scholar] [CrossRef]
- Chandra, R.; Mellis, B.; Garza, K.; Hameed, S.A.; Jurica, J.M.; Hernandez, A.V.; Nguyen, M.N.; Mittal, C.K. Remnant lipoprotein size distribution profiling via dynamic light scattering analysis. Clin. Chim. Acta 2016, 462, 6–14. [Google Scholar] [CrossRef]
- Stauch, O.; Schubert, R.; Savin, G.; Burchard, W. Structure of Artificial Cytoskeleton Containing Liposomes in Aqueous Solution Studied by Static and Dynamic Light Scattering. Biomacromolecules 2002, 3, 565–578. [Google Scholar] [CrossRef]
- Božič, D.; Hočevar, M.; Kononenko, V.; Jeran, M.; Štibler, U.; Fiume, I.; Pajnič, M.; Pađen, L.; Kogej, K.; Drobne, D.; et al. Chapter Five—Pursuing mechanisms of extracellular vesicle formation. Effects of sample processing. In Advances in Biomembranes and Lipid Self-Assembly; Bongiovanni, A., Pocsfalvi, G., Manno, M., Kralj-Iglič, V., Eds.; Elsevier: London, UK; Academic Press: London, UK, 2020; Volume 32, pp. 113–155. [Google Scholar]
- Sitar, S.; Aseyev, V.; Kogej, K. Microgel-like aggregates of isotactic and atactic poly(methacrylic acid) chains in aqueous alkali chloride solutions as evidenced by light scattering. Soft Matter 2014, 10, 7712–7722. [Google Scholar] [CrossRef]
- Dong, L.; Zieren, R.C.; Horie, K.; Kim, C.; Mallick, E.; Jing, Y.; Feng, M.; Kuczler, M.D.; Green, J.; Amend, S.R.; et al. Comprehensive evaluation of methods for small extracellular vesicles separation from human plasma, urine and cell culture medium. J. Extracell. Vesicles 2020, 10, 12044. [Google Scholar] [CrossRef]
- Veerman, R.E.; Teeuwen, L.; Czarnewski, P.; Akpinar, G.G.; Sandberg, A.; Cao, X.; Pernemalm, M.; Orre, L.M.; Gabrielsson, S.; Eldh, M. Molecular evaluation of five different isolation methods for extracellular vesicles reveals different clinical applicability and subcellular origin. J. Extracell. Vesicles 2021, 10, e12128. [Google Scholar] [CrossRef]
- Tzaridis, T.; Bachurski, D.; Liu, S.; Surmann, K.; Babatz, F.; Salazar, M.G.; Völker, U.; Hallek, M.; Herrlinger, U.; Vorberg, I.; et al. Extracellular Vesicle Separation Techniques Impact Results from Human Blood Samples: Considerations for Diagnostic Applications. Int. J. Mol. Sci. 2021, 22, 9211. [Google Scholar] [CrossRef] [PubMed]
- Šuštar, V.; Bedina-Zavec, A.; Štukelj, R.; Frank, M.; Bobojevic, G.; Janša, R.; Ogorevc, E.; Kruljc, P.; Mam, K.; Simunič, B.; et al. Nanoparticles isolated from blood: A reflection of vesiculability of blood cells during the isolation process. Int. J. Nanomed. 2011, 6, 2737–2748. [Google Scholar] [CrossRef][Green Version]
- Alberro, A.; Iparraguirre, L.; Fernandes, A.; Otaegui, D. Extracellular Vesicles in Blood: Sources, Effects, and Applications. Int. J. Mol. Sci. 2021, 22, 8163. [Google Scholar] [CrossRef] [PubMed]
- Yáñez-Mó, M.; Siljander, P.R.-M.; Andreu, Z.; Zavec, A.B.; Borràs, F.E.; Buzas, E.I.; Buzas, K.; Casal, E.; Cappello, F.; Carvalho, J.; et al. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles 2015, 4, 27066. [Google Scholar] [CrossRef] [PubMed]
- Plochberger, B.; Sych, T.; Weber, F.; Novacek, J.; Axmann, M.; Stangl, H.; Sezgin, E. Lipoprotein Particles Interact with Membranes and Transfer Their Cargo without Receptors. Biochemistry 2020, 59, 4421–4428. [Google Scholar] [CrossRef]
- Boon, R.A.; Vickers, K.C. Intercellular Transport of MicroRNAs. Arter. Thromb. Vasc. Biol. 2013, 33, 186–192. [Google Scholar] [CrossRef]
- Chiva-Blanch, G.; Badimon, L. Cross-Talk between Lipoproteins and Inflammation: The Role of Microvesicles. J. Clin. Med. 2019, 8, 2059. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, K.; Saji, M.; Lazaroff, S.M.; Palmer, A.F.; Ringel, M.D.; Paulaitis, M.E. Analysis of Exosome Release as a Cellular Response to MAPK Pathway Inhibition. Langmuir 2015, 31, 5440–5448. [Google Scholar] [CrossRef]
- Kesimer, M.; Gupta, R. Physical characterization and profiling of airway epithelial derived exosomes using light scattering. Methods 2015, 87, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Palmieri, V.; Lucchetti, D.; Gatto, I.; Maiorana, A.; Marcantoni, M.; Maulucci, G.; Papi, M.; Pola, R.; De Spirito, M.; Sgambato, A. Dynamic light scattering for the characterization and counting of extracellular vesicles: A powerful noninvasive tool. J. Nanopart. Res. 2014, 16, 2583. [Google Scholar] [CrossRef]
- Colombo, M.; Moita, C.F.; van Niel, G.; Kowal, J.; Vigneron, J.; Benaroch, P.; Manel, N.; Moita, L.F.; Théry, C.; Raposo, G. Analysis of ESCRT functions in exosome biogenesis, composition and secretion highlights the heterogeneity of extracellular vesicles. J. Cell Sci. 2013, 126, 5553–5565. [Google Scholar] [CrossRef]
- Pencer, J.; Hallett, F.R. Effects of Vesicle Size and Shape on Static and Dynamic Light Scattering Measurements. Langmuir 2003, 19, 7488–7497. [Google Scholar] [CrossRef]
- Kratochvil, P. Particle scattering functions. In Light Scattering from Polymer Solutions; Huglin, M.B., Ed.; Academic Press: New York, NY, USA, 1972; pp. 333–384. [Google Scholar]
- Barrett, K.E.; Boitano, S.; Barman, S.M.; Brooks, H.L. Ganong’s Review of Medical Physiology Twenty, 23th ed.; McGraw-Hill Professional Publishing: New York, NY, USA, 2010. [Google Scholar]
- Bjørge, L.; Hakulinen, J.; Vintermyr, O.K.; Jarva, H.; Jensen, T.S.; Iversen, O.; Meri, S. Ascitic complement system in ovarian cancer. Br. J. Cancer 2005, 92, 895–905. [Google Scholar] [CrossRef]
- Gokturk, H.S.; Demir, M.; Ozturk, N.A.; Unler, G.K.; Kulaksizoglu, S.; Kozanoglu, I.; Serin, E.; Yilmaz, U. The Role of Ascitic Fluid Viscosity in the Differential Diagnosis of Ascites. Can. J. Gastroenterol. 2010, 24, 255–259. [Google Scholar] [CrossRef] [PubMed]
- Multia, E.; Tear, C.J.Y.; Palviainen, M.; Siljander, P.; Riekkola, M.-L. Fast isolation of highly specific population of platelet-derived extracellular vesicles from blood plasma by affinity monolithic column, immobilized with anti-human CD61 antibody. Anal. Chim. Acta 2019, 1091, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Johnsen, K.B.; Gudbergsson, J.M.; Andresen, T.L.; Simonsen, J.B. What is the blood concentration of extracellular vesicles? Implications for the use of extracellular vesicles as blood-borne biomarkers of cancer. Biochim. Biophys. Acta (BBA)—Bioenerg. 2019, 1871, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Rizi, E.P.; Baig, S.; Loh, T.P.; Toh, S.-A.; Khoo, C.M.; Tai, E.S. Two-Hour Postprandial Lipoprotein Particle Concentration Differs Between Lean and Obese Individuals. Front. Physiol. 2019, 10, 856. [Google Scholar] [CrossRef] [PubMed]
- Anderson, W.; Lane, R.; Korbie, D.; Trau, M. Observations of Tunable Resistive Pulse Sensing for Exosome Analysis: Improving System Sensitivity and Stability. Langmuir 2015, 31, 6577–6587. [Google Scholar] [CrossRef] [PubMed]
- Arraud, N.; Linares, R.; Tan, S.; Gounou, C.; Pasquet, J.-M.; Mornet, S.; Brisson, A.R. Extracellular vesicles from blood plasma: Determination of their morphology, size, phenotype and concentration. J. Thromb. Haemost. 2014, 12, 614–627. [Google Scholar] [CrossRef]
- Linares, R.; Tan, S.; Gounou, C.; Arraud, N.; Brisson, A.R. High-speed centrifugation induces aggregation of extracellular vesicles. J. Extracell. Vesicles 2015, 4, 29509. [Google Scholar] [CrossRef]
- Forstner, R. CT and MRI in Ovarian Carcinoma. In MRI and CT of the Female Pelvis; Hamm, B., Forstner, R., Eds.; Springer: New York, NY, USA, 2007; pp. 233–265. [Google Scholar]
- Milburn, J.A.; Ford, I.; Cassar, K.; Fluck, N.; Brittenden, J. Platelet activation, coagulation activation and C-reactive protein in simultaneous samples from the vascular access and peripheral veins of haemodialysis patients. Int. J. Lab. Hematol. 2012, 34, 52–58. [Google Scholar] [CrossRef]
- Jerman, K.G.; Kobal, B.; Jakimovska, M.; Verdenik, I.; Černe, K. Control values of ovarian cancer tumor markers and standardisation of a protocol for sampling peritoneal fluid and performing washing during laparoscopy. World J. Surg. Oncol. 2014, 12, 278. [Google Scholar] [CrossRef][Green Version]
- Théry, C.; Amigorena, S.; Raposo, G.; Clayton, A. Isolation and Characterization of Exosomes from Cell Culture Supernatants and Biological Fluids. Curr. Protoc. Cell Biol. 2006, 30, 3–22. [Google Scholar] [CrossRef]

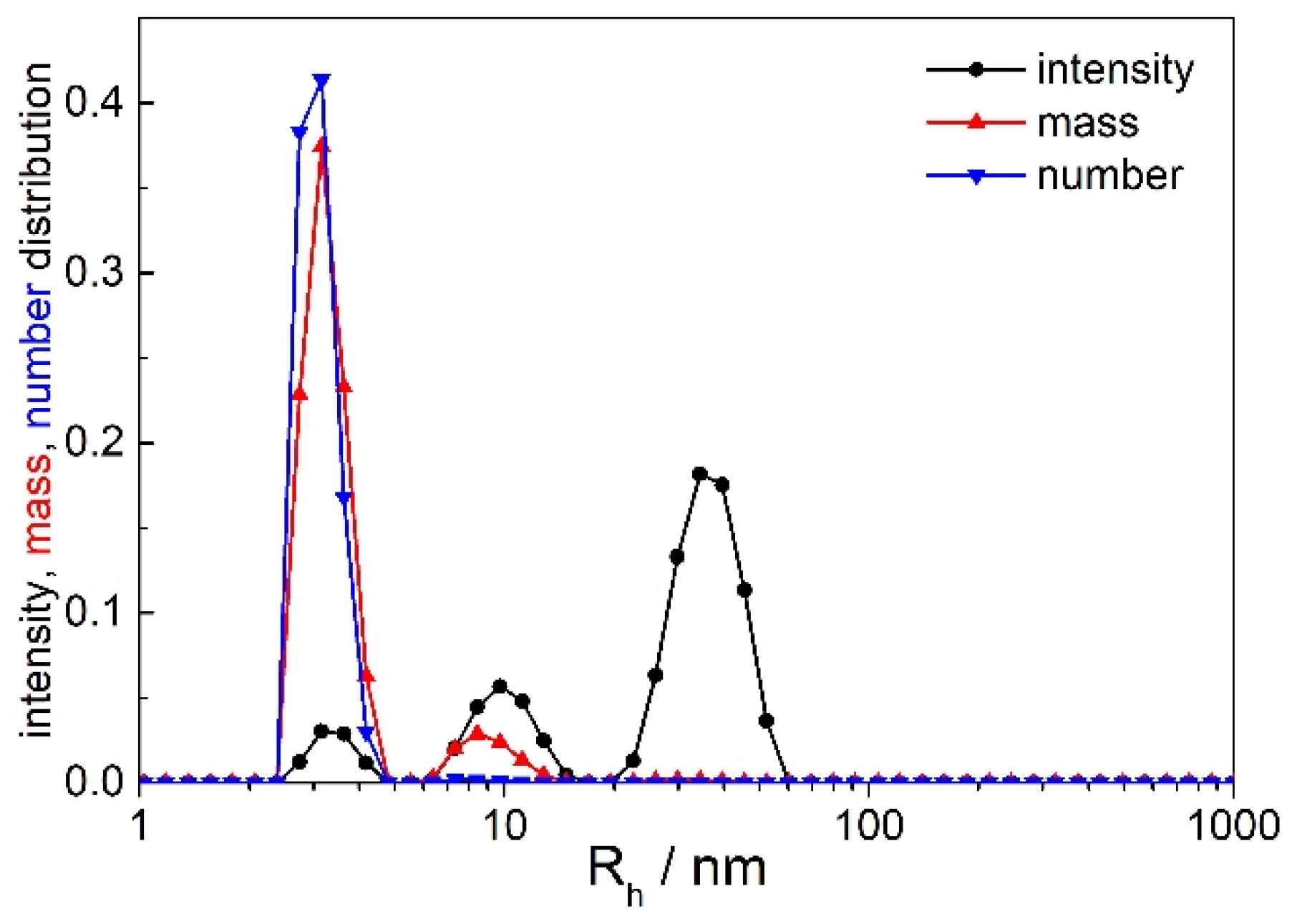
| Rh/nm | Origin | |
|---|---|---|
| Peak 1 | <10 | various proteins |
| Peak 2 | 15–35 | smaller ENPs |
| Peak 3 | >50 | larger ENPs |
| Peak 4 | several 100–1000 | larger aggregates |
| Rh,90/nm | Angular Dependency (Peak 2 or 3) | Peak 2 or 3 | ||||||
|---|---|---|---|---|---|---|---|---|
| Patient | Sample | Peak 1 | Peak 2 | Peak 3 | Rh,0/nm | Rg/nm | ρ | % Itot,90 (Peak 2 or 3) |
| OC1 # | plasma | 3.7 | 24 | 70 | ||||
| ascites | 7.0 | 27 | 80 | |||||
| ascitesF | / | 29 | / | |||||
| OC2 | plasma | 5.4 | 24 | / | 24.3 | 24.8 | 1.02 | 88 |
| ascites | 4.2 | 21 | / | 22.9 | 23.7 | 1.04 | 77 | |
| ascitesF | 4.0 | 20 | / | 20.2 | 20.4 | 1.01 | 76 | |
| OC3 | plasma | 4.4 | 17 | 46 & | 25.4 | 26.2 | 1.03 | 63 |
| ascites | 7.2 | 17 | / | 20.6 | 21.1 | 1.03 | 61 | |
| ascitesF | 4.6 | 20 | / | 21.9 | 25.5 | 1.16 | 77 | |
| OC4 | plasma | 2.7 | 22 | / | 24.7 | 25.5 | 1.03 | 64 |
| ascites | 2.9 | 17 | 102 & | 21.9 | 21.2 | 0.97 | 51 | |
| ascitesF | 4.1 | 24 | / | 21.2 | 21.8 | 1.03 | 56 | |
| OC5 | plasma | 4.7 | 23 | / | 34.0 | 34.0 | 1.00 | 50 |
| ascites | 3.7 | 20 | / | 34.2 | 36.3 | 1.06 | 46 | |
| ascitesF | 4.1 | 24 | / | 46.8 | 53.4 | 1.14 | 43 | |
| OC6 | plasma | 5.1 | 26 | / | 24.6 | 24.6 | 1.00 | 74 |
| ascites | 4.7 | 21 | / | 28.0 | 29.2 | 1.04 | 49 | |
| ascitesF | 4.0 | 18 | 22.9 | 22.7 | 0.99 | 63 | ||
| 68 | 150 | 160 | 1.07 | 13 | ||||
| ascites * | 5.6 | 27 | 28.7 | 29.0 | 1.01 | 18 | ||
| 150 | 143 | 165 | 1.10 | 15 | ||||
| ascitesF * | 3.8 | 14 | 17.0 | 36.0 | (~2.1) | 56 | ||
| 62 | 101 | 115 | 1.14 | 34 | ||||
| OC7 | plasma * | 4.1 | 20 | ~21 | ~32 | (~1.5) | 76 | |
| 173 | 188 | 185 | 0.99 | 11 | ||||
| ascites * | 3.2 | 16 | 26.9 | 28.4 | 1.05 | 71 | ||
| 83 | 203 | 230 | 1.13 | 13 | ||||
| ascitesF * | 4.5 | 20 | 22.3 | 33.4 | (~1.5) | 75 | ||
| 90 | 105 | 115 | 1.09 | 13 | ||||
| Rh,90/nm | Angular Dependency (Peak 2) | |||||||
|---|---|---|---|---|---|---|---|---|
| Patient | Sample | Peak 1 | Peak 2 | Peak 3 | Rh,0/nm | Rg/nm | ρ | % Itot,90 |
| BP-BOC1 | plasma | 6.1 | 31 | / | ~29 | / # | / # | 89 |
| PF | 4.7 | 19 & | 123 | / & | / # | / # | 25 | |
| BP-BOC2 | plasma | 3.3 | 17 | 107 | 24 | 26 | 1.1 | 76 |
| PF | 2.9, 7.7 | 31 | / | 51 | 67 | 1.3 | 18 | |
| BP-BOC3 | plasma | 4.3 | 18 | 102 | ~20 | / # | / # | 56 |
| PF | 3.7, 13.5 | 42 | 390 | 36 | 68 | 1.9 | 24 | |
| BP-BOC4 | plasma | 2.8, 7.2 | 36 | / | 28 | / # | / # | 69 |
| PF | 3.3 | 13 | 63.0 | 29 | 55 | 1.9 | 23 | |
| BP-BOC5 | plasma | 3.7 | 24 | / | 23 | 28 | 1.2 | 63 |
| PF | 3.1, 10 | 34 | / | 40 | 51 | 1.3 | 22 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kogej, K.; Božič, D.; Kobal, B.; Herzog, M.; Černe, K. Application of Dynamic and Static Light Scattering for Size and Shape Characterization of Small Extracellular Nanoparticles in Plasma and Ascites of Ovarian Cancer Patients. Int. J. Mol. Sci. 2021, 22, 12946. https://doi.org/10.3390/ijms222312946
Kogej K, Božič D, Kobal B, Herzog M, Černe K. Application of Dynamic and Static Light Scattering for Size and Shape Characterization of Small Extracellular Nanoparticles in Plasma and Ascites of Ovarian Cancer Patients. International Journal of Molecular Sciences. 2021; 22(23):12946. https://doi.org/10.3390/ijms222312946
Chicago/Turabian StyleKogej, Ksenija, Darja Božič, Borut Kobal, Maruša Herzog, and Katarina Černe. 2021. "Application of Dynamic and Static Light Scattering for Size and Shape Characterization of Small Extracellular Nanoparticles in Plasma and Ascites of Ovarian Cancer Patients" International Journal of Molecular Sciences 22, no. 23: 12946. https://doi.org/10.3390/ijms222312946
APA StyleKogej, K., Božič, D., Kobal, B., Herzog, M., & Černe, K. (2021). Application of Dynamic and Static Light Scattering for Size and Shape Characterization of Small Extracellular Nanoparticles in Plasma and Ascites of Ovarian Cancer Patients. International Journal of Molecular Sciences, 22(23), 12946. https://doi.org/10.3390/ijms222312946






