Certain, but Not All, Tetraether Lipids from the Thermoacidophilic Archaeon Sulfolobus acidocaldarius Can Form Black Lipid Membranes with Remarkable Stability and Exhibiting Mthk Channel Activity with Unusually High Ca2+ Sensitivity
Abstract
1. Introduction
2. Results and Discussions
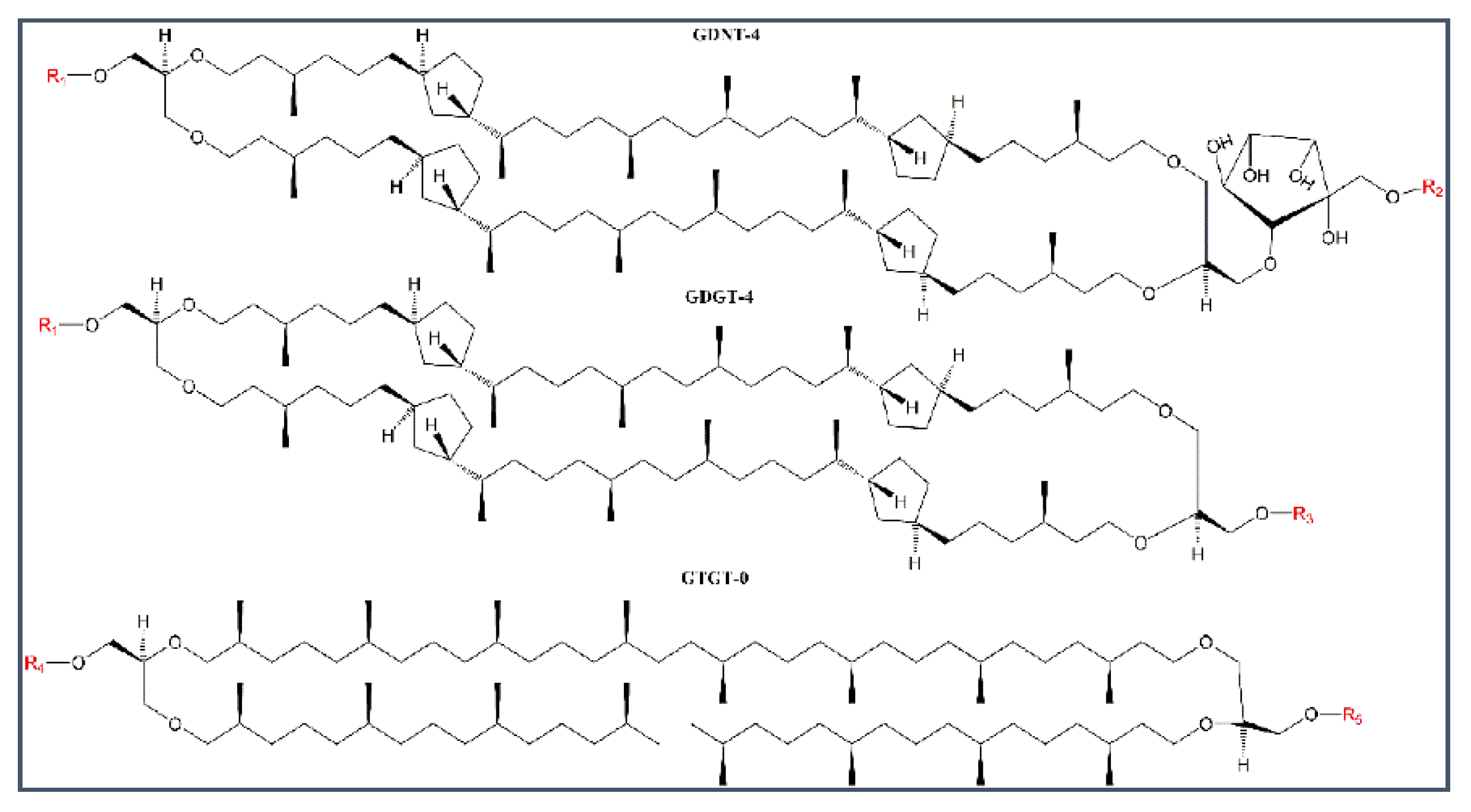
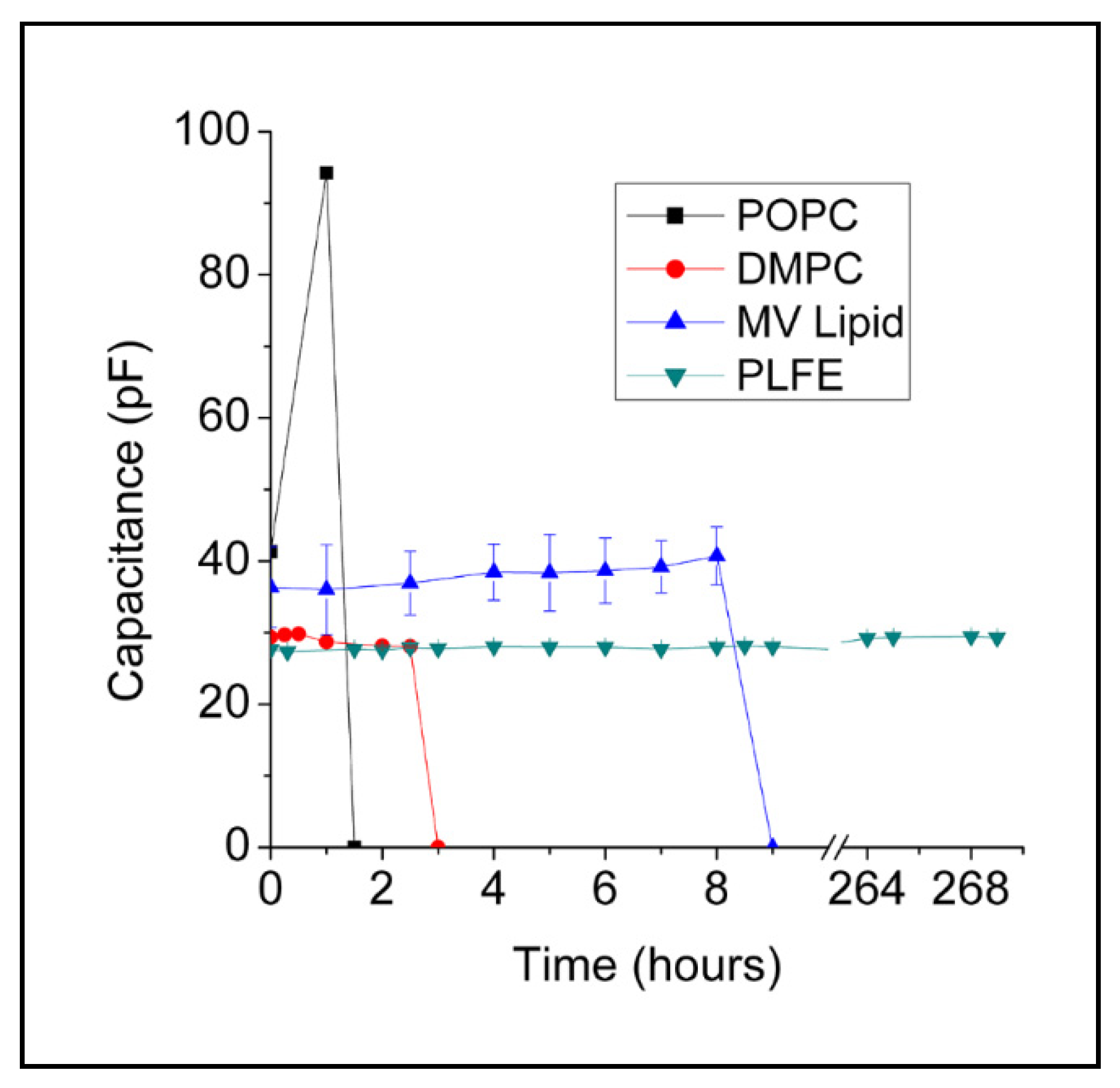
2.1. Stability of BLMPLFE Compared to Other BLMs
2.1.1. Explanations for the Differential BLM Stabilities
2.1.2. Future Directions and Implications
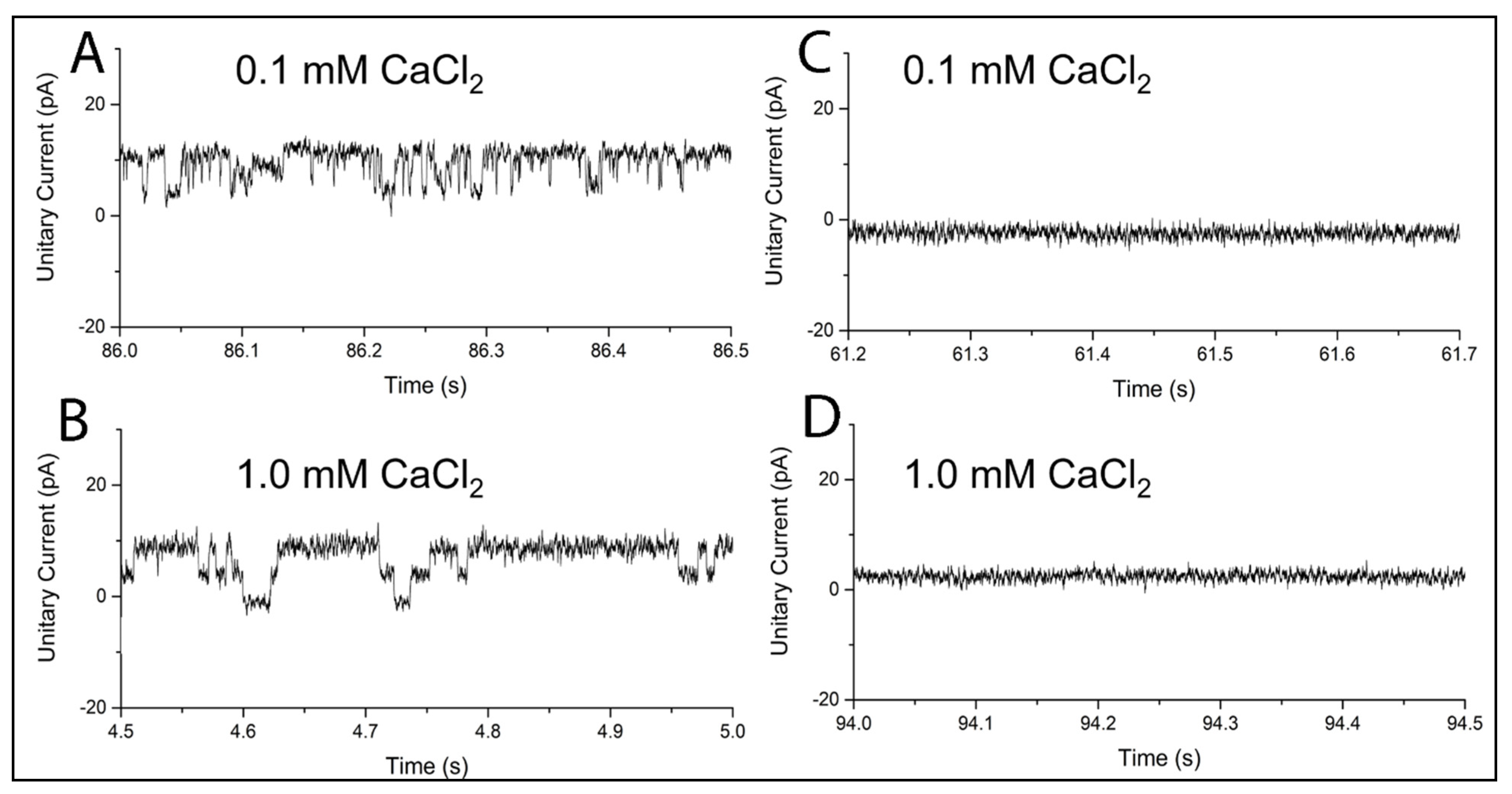
2.2. MthK in BLMPLFE
3. Materials and Methods
3.1. Isolation of PLFE and Sa-MV Lipids from the Archaeon S. acidocaldarius
3.2. Formation of BLM and Measurements of Membrane Stability
3.3. Incorporation of MthK into BLM
3.4. Measurements of MthK Ion Channel Activities
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siliakus, M.F.; van der Oost, J.; Kengen, S.W.M. Adaptations of Archaeal and Bacterial Membranes to Variations in Temperature, pH and Pressure. Extremophiles 2017, 21, 651–670. [Google Scholar] [CrossRef]
- Zeng, Z.; Liu, X.-L.; Wei, J.H.; Summons, R.E.; Welander, P.V. Calditol-linked Membrane Lipids Are Required for Acid Tolerance in Sulfolobus acidocaldarius. Proc. Natl. Acad. Sci. USA 2018, 115, 12932–12937. [Google Scholar] [CrossRef]
- Heitz, B.A.; Jones, I.W.; Hall, J.H.K.; Aspinwall, C.A.; Saavedra, S.S. Fractional Polymerization of a Suspended Planar Bilayer Creates a Fluid, Highly Stable Membrane for Ion Channel Recordings. J. Am. Chem. Soc. 2010, 132, 7086–7093. [Google Scholar] [CrossRef] [PubMed]
- van den Hurk, R.; Evoy, S. A Review of Membrane-Based Biosensors for Pathogen Detection. Sensors 2015, 15, 14045–14078. [Google Scholar] [CrossRef]
- Khan, M.S.; Dosoky, N.S.; Williams, J.D. Engineering Lipid Bilayer Membranes for Protein Studies. Int. J. Mol. Sci. 2013, 14, 21561–21597. [Google Scholar] [CrossRef]
- Freisleben, H.-J. The Main (Glyco) Phospholipid (MPL) of Thermoplasma acidophilum. Int. J. Mol. Sci. 2019, 20, 5217. [Google Scholar] [CrossRef]
- Kumar, M.; Habel, J.E.O.; Shen, Y.; Meier, W.P.; Walz, T. High-Density Reconstitution of Functional Water Channels into Vesicular and Planar Block Copolymer Membranes. J. Am. Chem. Soc. 2012, 134, 18631–18637. [Google Scholar] [CrossRef] [PubMed]
- Meier, W.; Nardin, C.; Winterhalter, M. Reconstitution of Channel Proteins in (Polymerized) ABA Triblock Copolymer Membranes. Angew. Chem. Int. Ed. 2000, 39, 4599–4602. [Google Scholar] [CrossRef]
- Lee, A.G. How Lipids Affect the Activities of Integral Membrane Proteins. Biochim. Biophys. Acta 2004, 1666, 62–87. [Google Scholar] [CrossRef]
- Zhang, X.; Fu, W.; Palivan, C.G.; Meier, W. Natural Channel Protein Inserts and Functions in a Completely Artificial, Solid-Supported Bilayer Membrane. Sci. Rep. 2013, 3, 2196. [Google Scholar] [CrossRef] [PubMed]
- Nardin, C.; Winterhalter, M.; Meier, W. Giant Free-Standing ABA Triblock Copolymer Membranes. Langmuir 2000, 16, 7708–7712. [Google Scholar] [CrossRef]
- Mitra, K.; Ubarretxena-Belandia, I.; Taguchi, T.; Warren, G.; Engelman, D.M. Modulation of the Bilayer Thickness of Exocytic Pathway Membranes by Membrane Proteins rather than Cholesterol. Proc. Natl. Acad. Sci. USA 2004, 101, 4083–4088. [Google Scholar] [CrossRef] [PubMed]
- Andersen, O.S.; Koeppe, I.R.E. Bilayer Thickness and Membrane Protein Function: An Energetic Perspective. Annu. Rev. Biophys. Biomol. Struct. 2007, 36, 107–130. [Google Scholar] [CrossRef] [PubMed]
- Chong, P.L.-G.; Fortes, P.A.; Jameson, D.M. Mechanisms of Inhibition of (Na,K)-ATPase by Hydrostatic Pressure Studied with Fluorescent Probes. J. Biol. Chem. 1985, 260, 14484–14490. [Google Scholar] [CrossRef]
- Avsar, S.Y.; Kyropoulou, M.; Di Leone, S.; Schoenenberger, C.; Meier, W.P.; Palivan, C.G. Biomolecules Turn Self-Assembling Amphiphilic Block Co-Polymer Platforms into Biomimetic Interfaces. Front. Chem. 2019, 6, 645. [Google Scholar] [CrossRef]
- Massana, R.; DeLong, E.F.; Pedros-Alio, C. A Few Cosmopolitan Phylotypes Dominate Planktonic Archaeal Assemblages in Widely Different Oceanic Provinces. Appl. Environ. Microbiol. 2000, 66, 1777–1787. [Google Scholar] [CrossRef]
- Powers, L.A.; Werne, J.P.; Johnson, T.C.; Hopmans, E.C.; Sinninghe Damste, J.S.; Schouten, S. Crenarchaeotal Membrane Lipids in Lake Sediments: A New Paleotemperature Proxy for Continental Paleoclimate Reconstruction? Geology 2004, 32, 613–616. [Google Scholar] [CrossRef]
- Yoshinaga, M.Y.; Gagen, E.J.; Wörmer, L.; Broda, N.K.; Meador, T.B.; Wendt, J.; Thomm, M.; Hinrichs, K.U. Methanothermobacter thermautotrophicus Modulates its Membrane Lipids in Response to Hydrogen and Nutrient Availability. Front. Microbiol. 2015, 6, 5. [Google Scholar] [CrossRef] [PubMed]
- Sprott, G.D.; Brisson, J.; Dicaire, C.J.; Pelletier, A.K.; Deschatelets, L.A.; Krishnan, L.; Patel, G.B. A Structural Comparison of the Total Polar Lipids from the Human Archaea Methanobrevibacter smithii and Methanosphaera stadtmanae and its Relevance to the Adjuvant Activities of their Liposomes. Biochim. Biophys. Acta 1999, 1440, 275–288. [Google Scholar] [CrossRef]
- Koga, Y.; Morii, H. Recent Advances in Structural Research on Ether Lipids from Archaea Including Comparative and Physiological Aspects. Biosci. Biotechnol. Biochem. 2005, 69, 2019–2034. [Google Scholar] [CrossRef]
- Ulrich, N.P.; Gmajner, D.; Raspor, P. Structural and Physicochemical Properties of Polar Lipids from Thermophilic Archaea. Appl. Microbiol. Biotechnol. 2009, 84, 249–260. [Google Scholar] [CrossRef]
- Schouten, S.; Hopmans, E.C.; Sinninghe Damste, J.S. The Organic Geochemistry of Glycerol Dialkyl Glycerol Tetraether Lipids: A Review. Org. Geochem. 2013, 54, 19–61. [Google Scholar] [CrossRef]
- Chong, P.L.-G. Archaebacterial Bipolar Tetraether Lipids: Physico-Chemical and Membrane Properties. Chem. Phys. Lipids 2010, 163, 253–265. [Google Scholar] [CrossRef] [PubMed]
- Gliozzi, A.; Rolandi, R.; De Rosa, M.; Gambacorta, A. Artificial Black Membranes from Bipolar Lipids of Thermophilic Archaebacteria. Biophys. J. 1982, 37, 563–566. [Google Scholar] [CrossRef][Green Version]
- Stern, J.; Freisleben, H.-J.; Janku, S.; Ring, K. Black Lipid Membranes of Tetraether Lipids from Thermoplasma Acidophilum. Biochim. Biophys. Acta 1992, 1128, 227–236. [Google Scholar] [CrossRef]
- Fittabile, L.; Robello, M.; Relini, A.; De Rosa, M.; Gliozzi, A. Organization of Monolayer-Formed Membranes Made from Archaeal Ether Lipids. Thin Solid Films 1996, 284–285, 735–738. [Google Scholar] [CrossRef]
- Ren, X.; Liu, K.; Zhang, Q.; Noh, H.M.; Kumbur, E.C.; Yuan, W.W.; Zhou, J.G.; Chong, P.L.-G. Design, Fabrication and Characterization of Archaeal Tetraether Free-Standing Planar Membranes in a PDMS- and PCB-Based Fluidic Platform. ACS Appl. Mater. Interfaces 2014, 6, 12618–12628. [Google Scholar] [CrossRef]
- Ren, X.; Kumbur, E.C.; Zhou, J.G.; Noh, H.M.; Chong, P.L.-G. Stability of Free-Standing Tetraether Planar Membranes in Microchips. J. Membr. Sci. 2017, 540, 27–34. [Google Scholar] [CrossRef]
- Lo, S.L.; Montague, C.E.; Chang, E.L. Purification of Glycerol Dialkyl Nonitol Tetraether from Sulfolobus acidocaldarius. J. Lipid Res. 1989, 30, 944–949. [Google Scholar] [CrossRef]
- Zadek, B.; Nimigean, C.M. Calcium-Dependent Gating of MthK, a Prokaryotic Potassium Channel. J. Gen. Physiol. 2006, 6, 673–685. [Google Scholar] [CrossRef]
- Pau, V.P.T.; Abarca-Heidemann, K.; Rothberg, B.S. Allosteric Mechanism of Ca2+ Activation and H+-Inhibited Gating of the MthK K+ Channel. J. Gen. Physiol 2010, 135, 509–526. [Google Scholar] [CrossRef]
- Ellen, A.F.; Albers, S.V.; Huibers, W.; Pitcher, A.; Hobel, C.F.; Schwarz, H.; Folea, M.; Schouten, S.; Boekema, E.J.; Poolman, B.; et al. Proteomic Analysis of Secreted Membrane Vesicles of Archaeal Sulfolobus Species Reveals the Presence of Endosome Sorting Complex Components. Extremophiles 2009, 13, 67–79. [Google Scholar] [CrossRef]
- De Rosa, M.; Gambacorta, A.; Nicolaus, B.; Chappe, B.; Albrecht, P. Isoprenoid Ethers: Backbone of Complex Lipids of the Archaebacterium Sulfolobus solfataricus. Biochim. Biophys. Acta 1983, 753, 249–256. [Google Scholar] [CrossRef]
- Bonanno, A.; Blake, R.C.I.; Chong, P.L.-G. Sulfolobus acidocaldarius Microvesicles Exhibit Unusually Tight Packing Properties as Revealed by Optical Spectroscopy. Int. J. Mol. Sci. 2019, 20, 5308. [Google Scholar] [CrossRef] [PubMed]
- Kang, X.F.; Cheley, S.; Rice-Ficht, A.C.; Bayley, H. A Storable Encapsulated Bilayer Chip Containing a Single Protein Nanopore. J. Am. Chem. Soc. 2007, 129, 4701–4705. [Google Scholar] [CrossRef] [PubMed]
- Heitz, B.A.; Xu, J.; Hall, J.H.K.; Aspinwall, C.A.; Saavedra, S.S. Enhanced Long-Term Stability for Single Ion Channel Recordings Using Suspended Poly(Lipid) Bilayers. J. Am. Chem. Soc. 2009, 131, 6662–6663. [Google Scholar] [CrossRef]
- Cheng, Y.; Bushby, R.J.; Evans, S.D.; Knowles, P.F.; Miles, R.E.; Ogier, S.D. Single Ion Channel Sensitivity in Suspended Bilayers on Micromachined Supports. Langmuir 2001, 17, 1240–1242. [Google Scholar] [CrossRef]
- Schmidt, C.; Mayer, M.; Vogel, H. A Chip-Based Biosensor for the Functional Analysis of Single Ion Channels. Angew. Chem. Int. 2000, 39, 3137–3140. [Google Scholar] [CrossRef]
- Bright, L.K.; Baker, C.A.; Branstrom, R.; Saavedra, S.S. Methacrylate Polymer Scaffolding Enhances the Stability of Suspended Lipid Bilayers for Ion Channel Recordings and Biosensor Development. ACS Biomater. Sci. Eng. 2015, 1, 955–963. [Google Scholar] [CrossRef]
- Gabriel, J.L.; Chong, P.L.-G. Molecular Modeling of Archaebacterial Bipolar Tetraether Lipid Membranes. Chem. Phys. Lipids 2000, 105, 193–200. [Google Scholar] [CrossRef]
- Shimada, H.; Nemoto, N.; Shida, Y.; Oshima, T.; Yamagishi, A. Effects of pH and Temperature on the Composition of Polar Lipids in Thermoplasma acidophilum HO-62. J. Bacteriol. 2008, 190, 5404–5411. [Google Scholar] [CrossRef]
- Zhai, Y.; Chong, P.L.-G.; Taylor, L.J.-A.; Erlkamp, M.; Grobelny, S.; Czeslik, C.; Watkins, E.; Winter, R. Physical Properties of Archaeal Tetraether Lipid Membranes as Revealed by Differential Scanning and Pressure Perturbation Calorimetry, Molecular Acoustics, and Neutron Reflectometry: Effects of Pressure and Cell Growth Temperature. Langmuir 2012, 28, 5211–5217. [Google Scholar] [CrossRef]
- Swain, M.; Brisson, J.R.; Sprott, G.D.; Cooper, F.P.; Patel, G.B. Identification of Beta-L-Gulose as the Sugar Moiety of the Main Polar Lipids of Thermoplasma acidophilum. Biochim. Biophys. Acta 1997, 1345, 56–64. [Google Scholar] [CrossRef]
- Han, X.; Studer, A.; Sehr, H.; Geissbuhler, I.; Di Berardino, M.; Winkler, F.K.; Tiefenauer, L.X. Nanopore Arrays for Stable and Functional Free-Standing Lipid Bilayers. Adv. Mater. 2007, 19, 4466–4470. [Google Scholar] [CrossRef]
- Romer, W.; Steinem, C. Impedance Analysis and Single-Channel Recordings on Nano-Black Lipid Membranes Based on Porous Alumina. Biophys. J. 2004, 86, 955–965. [Google Scholar] [CrossRef]
- Febo-Ayala, W.; Morera-Felix, S.L.; Hrycyna, C.A.; Thompson, D.H. Functional Reconstitution of the Integral Membrane Enzyme, Isoprenylcysteine Carboxyl Methyltransferase, in Synthetic Bolalipid Membrane Vesicles. Biochemistry 2006, 45, 14683–14694. [Google Scholar] [CrossRef][Green Version]
- Elferink, M.G.L.; Bosma, T.; Lolkema, J.S.; Gleiszner, M.; Driessen, A.J.M.; Konings, W.N. Thermostability of Respiratory Terminal Oxidases in the Lipid Environment. Biochim. Biophys. Acta 1995, 1230, 31–37. [Google Scholar] [CrossRef][Green Version]
- Elferink, M.G.; de Wit, J.G.; Demel, R.; Driessen, A.J.; Konings, W.N. Functional Reconstitution of Membrane Proteins in Monolayer Liposomes from Bipolar Lipids of Sulfolobus acidocaldarius. J. Biol. Chem. 1992, 267, 1375–1381. [Google Scholar] [CrossRef]
- Elferink, M.G.; de Wit, J.G.; Driessen, A.J.; Konings, W.N. Energy-Transducing Properties of Primary Proton Pumps Reconstituted into Archaeal Bipolar Lipid Vesicles. Eur. J. Biochem. 1993, 214, 917–925. [Google Scholar] [CrossRef]
- Freisleben, H.J.; Zwicker, K.; Jezek, P.; John, G.; Bettin-Bogutzki, A.; Ring, K.; Nawroth, T. Reconstitution of Bacteriorhodopsin and ATP Synthase from Micrococcus Luteus into Liposomes of the Purified Main Tetraether Lipid from Thermoplasma acidophilum: Proton Conductance and Light-Driven ATP Synthesis. Chem. Phys. Lipids 1995, 78, 137–147. [Google Scholar] [CrossRef]
- In’t Veld, G.; Elferink, M.G.; Driessen, A.J.; Konings, W.N. Reconstitution of the Leucine Transport System of Lactococcus Lactis into Liposomes Composed of Membrane-Spanning Lipids from Sulfolobus acidocaldarius. Biochemistry 1992, 31, 12493–12499. [Google Scholar] [CrossRef] [PubMed]
- Jacquemet, A.; Barbeau, J.; Lemiègre, L.; Benvegnu, T. Archaeal Tetraether Bipolar Lipids: Structures, Functions and Applications. Biochimie 2009, 91, 711–717. [Google Scholar] [CrossRef]
- Pau, V.P.; Smith, F.J.; Taylor, A.B.; Parfenova, L.V.; Samakai, E.; Callaghan, M.M.; Abarca-Heidemann, K.; Hart, P.J.; Rothberg, B.S. Structure and Function of Multiple Ca2+-Binding Sites in a K+ Channel Regulator of K+ Conductance (RCK) Domain. Proc. Natl. Acad. Sci. USA 2011, 108, 17684–17689. [Google Scholar] [CrossRef]
- Norimatsu, Y.; Hasegawa, K.; Shimizu, N.; Toyoshima, C. Protein–Phospholipid Interplay Revealed with Crystals of a Calcium Pump. Nature 2017, 545, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Iida, K.; Kiriyama, H.; Fukai, A.; Konings, W.N.; Nango, M. Two-Dimensional Self-Organization of the Light-Harvesting Polypeptides/BChl a Complex into a Thermostable Liposomal Membrane. Langmuir 2001, 17, 2821–2827. [Google Scholar] [CrossRef]
- Lo, S.L.; Chang, E.L. Purification and Characterization of a Liposomal-Forming Tetraether Lipid Fraction. Biochem. Biophys. Res. Commun. 1990, 167, 238–243. [Google Scholar] [CrossRef]
- Bonanno, A.; Chen, K.; Chong, P.L.-G. Sulfolobus acidocaldarius Microvesicles are Naturally Occurring Nanoparticles with Unusual Stability Against Various Environmental Stressors. Materialia 2019, 7, 100405. [Google Scholar] [CrossRef]
- Bagatolli, L.; Gratton, E.; Khan, T.K.; Chong, P.L.-G. Two-Photon Fluorescence Microscopy Studies of Bipolar Tetraether Giant Liposomes from Thermoacidophilic Archaebacteria Sulfolobus acidocaldarius. Biophys. J. 2000, 79, 416–425. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonanno, A.; Chong, P.L.-G. Certain, but Not All, Tetraether Lipids from the Thermoacidophilic Archaeon Sulfolobus acidocaldarius Can Form Black Lipid Membranes with Remarkable Stability and Exhibiting Mthk Channel Activity with Unusually High Ca2+ Sensitivity. Int. J. Mol. Sci. 2021, 22, 12941. https://doi.org/10.3390/ijms222312941
Bonanno A, Chong PL-G. Certain, but Not All, Tetraether Lipids from the Thermoacidophilic Archaeon Sulfolobus acidocaldarius Can Form Black Lipid Membranes with Remarkable Stability and Exhibiting Mthk Channel Activity with Unusually High Ca2+ Sensitivity. International Journal of Molecular Sciences. 2021; 22(23):12941. https://doi.org/10.3390/ijms222312941
Chicago/Turabian StyleBonanno, Alexander, and Parkson Lee-Gau Chong. 2021. "Certain, but Not All, Tetraether Lipids from the Thermoacidophilic Archaeon Sulfolobus acidocaldarius Can Form Black Lipid Membranes with Remarkable Stability and Exhibiting Mthk Channel Activity with Unusually High Ca2+ Sensitivity" International Journal of Molecular Sciences 22, no. 23: 12941. https://doi.org/10.3390/ijms222312941
APA StyleBonanno, A., & Chong, P. L.-G. (2021). Certain, but Not All, Tetraether Lipids from the Thermoacidophilic Archaeon Sulfolobus acidocaldarius Can Form Black Lipid Membranes with Remarkable Stability and Exhibiting Mthk Channel Activity with Unusually High Ca2+ Sensitivity. International Journal of Molecular Sciences, 22(23), 12941. https://doi.org/10.3390/ijms222312941





