Dickkopf-1 Inhibition Reactivates Wnt/β-Catenin Signaling in Rhabdomyosarcoma, Induces Myogenic Markers In Vitro and Impairs Tumor Cell Survival In Vivo
Abstract
1. Introduction
2. Results
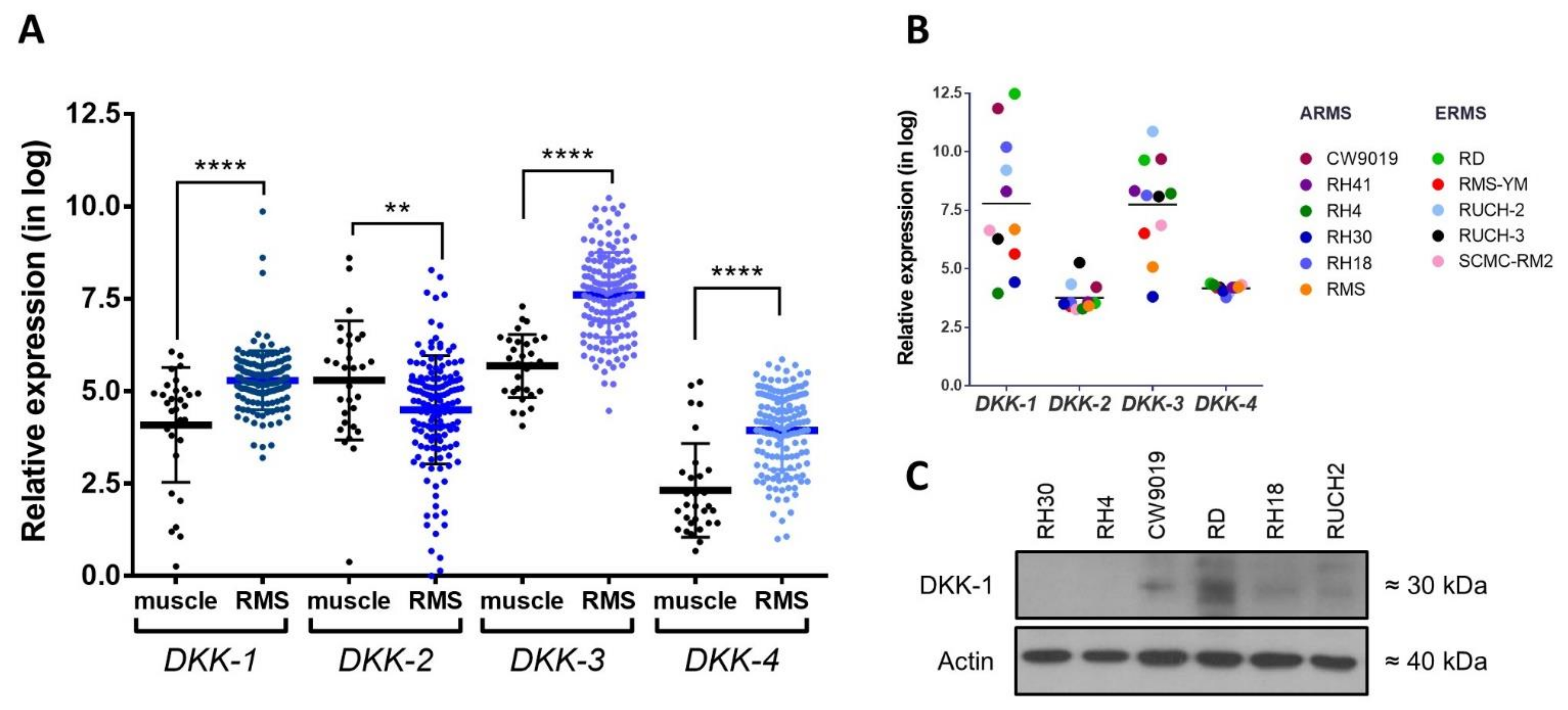
2.1. Analysis of DKK Expression in RMS
2.2. Genetic DKK-1 Inhibition Led to Reduced Proliferation in RMS Cells
2.3. DKK-1 Knockdown Activated Wnt Signaling and Induced Expression of Myogenic Markers
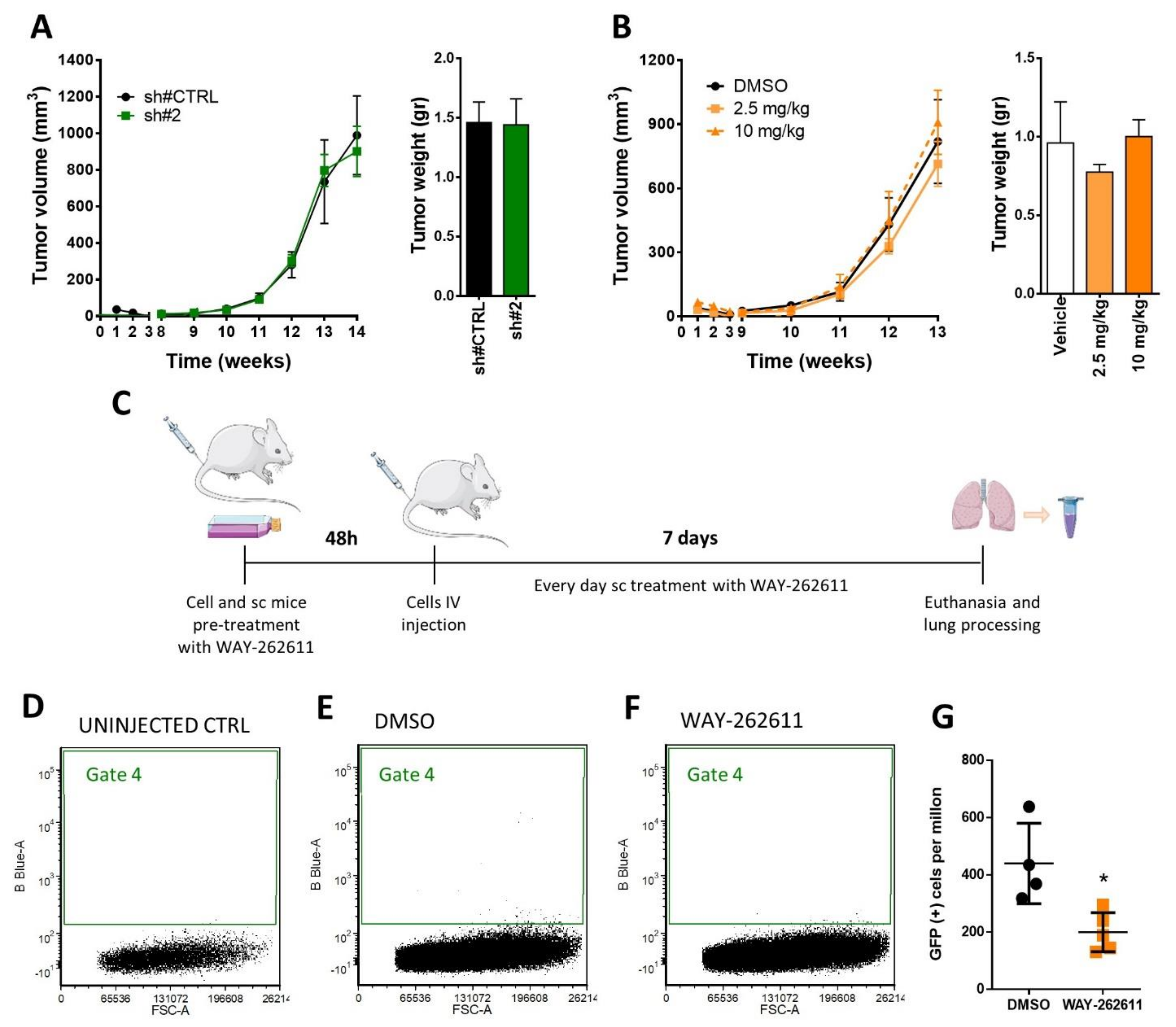
2.4. DKK-1 Pharmacological Inhibition Decreased Cell Proliferation and Invasion Rates
2.5. WAY-262611 Treatment Produced an Increase in β-Catenin Levels, an Induction of Myogenic Markers and a Decrease in FAK Levels
2.6. WAY-262611 Treatment Decreased the Survival of Intravenously Injected Tumor Cells
3. Discussion
4. Materials and Methods
4.1. Database and mRNA Expression Analyses
4.2. Cell Culture
4.3. Lentiviral Transduction
4.4. Cell Proliferation Assay and IC50 Determination
4.5. Transwell Cell Invasion Assay
4.6. Western Blot
4.7. Luciferase Assay of β-Catenin Transcriptional Activity
4.8. Primary Tumor and Metastasis Models in Mice
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| RMS | rhabdomyosarcoma |
| DKK | Dickkopf |
| LRP | low-density lipoprotein receptor-related protein |
| Wnt | Wingless-INT |
| Fz | Frizzled |
| FAK | focal adhesion kinase |
| MYOD1 | myogenic differentiation 1 |
| WB | Western blot |
References
- Haseeb, M.; Pirzada, R.H.; Ain, Q.U.; Choi, S. Wnt Signaling in the Regulation of Immune Cell and Cancer Therapeutics. Cells 2019, 8, 1380. [Google Scholar] [CrossRef]
- Hartung, N.; Benary, U.; Wolf, J.; Kofahl, B. Paracrine and autocrine regulation of gene expression by Wnt-inhibitor Dickkopf in wild-type and mutant hepatocytes. BMC Syst. Biol. 2017, 11, 98. [Google Scholar] [CrossRef] [PubMed]
- Niehrs, C. Function and biological roles of the Dickkopf family of Wnt modulators. Oncogene 2006, 25, 7469–7481. [Google Scholar] [CrossRef]
- Choe, J.-Y.Y.; Kim, J.H.; Park, K.-Y.Y.; Choi, C.-H.H.; Kim, S.-K.K.; Kim, J.H.; Park, K.-Y.Y.; Choi, C.-H.H.; Kim, S.-K.K. Activation of dickkopf-1 and focal adhesion kinase pathway by tumour necrosis factor a induces enhanced migration of fibroblast-like synoviocytes in rheumatoid arthritis. Rheumatology 2016, 55, 928–938. [Google Scholar] [CrossRef]
- Pelletier, J.C.; Lundquist, J.T., IV; Gilbert, A.M.; Alon, N.; Bex, F.J.; Bhat, B.M.; Bursavich, M.G.; Coleburn, V.E.; Felix, L.A.; Green, D.M.; et al. (1-(4-(Naphthalen-2-yl)pyrimidin-2-yl)piperidin-4-yl)methanamine: A wingless β-catenin agonist that increases bone formation rate. J. Med. Chem. 2009, 52, 6962–6965. [Google Scholar] [CrossRef] [PubMed]
- Vibhakar, R.; Foltz, G.; Yoon, J.G.; Field, L.; Lee, H.; Ryu, G.Y.; Pierson, J.; Davidson, B.; Madan, A. Dickkopf-1 is an epigenetically silenced candidate tumor suppressor gene in medulloblastoma 1. Neuro-Oncol. 2007, 9, 135–144. [Google Scholar] [CrossRef]
- Koppen, A.; Ait-Aissa, R.; Hopman, S.; Koster, J.; Haneveld, F.; Versteeg, R.; Valentijn, L.J. Dickkopf-1 is down-regulated by MYCN and inhibits neuroblastoma cell proliferation. Cancer Lett. 2007, 256, 218–228. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, S.D.; Trucco, M.; Guzman, W.B.; Hayashi, M.; Loeb, D.M. A monoclonal antibody against the Wnt signaling inhibitor dickkopf-1 inhibits osteosarcoma metastasis in a preclinical model. Oncotarget 2016, 7, 21114–21123. [Google Scholar] [CrossRef]
- Pal, A.; Leung, J.Y.; Ang, G.C.K.; Rao, V.K.; Pignata, L.; Lim, H.J.; Hebrard, M.; Chang, K.T.; Lee, V.K.M.; Guccione, E.; et al. Ehmt2 epigenetically suppresses wnt signaling and is a potential target in embryonal rhabdomyosarcoma. Elife 2020, 9, e57683. [Google Scholar] [CrossRef]
- Ward, E.; DeSantis, C.; Robbins, A.; Kohler, B.; Jemal, A. Childhood and adolescent cancer statistics, 2014. CA Cancer J. Clin. 2014, 64, 83–103. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.H.; Barr, F.G. Therapeutic approaches targeting PAX3-FOXO1 and its regulatory and transcriptional pathways in rhabdomyosarcoma. Molecules 2018, 23, 2798. [Google Scholar] [CrossRef]
- Bhakta, N.; Force, L.M.; Allemani, C.; Atun, R.; Bray, F.; Coleman, M.P.; Steliarova-Foucher, E.; Frazier, A.L.; Robison, L.L.; Rodriguez-Galindo, C.; et al. Childhood cancer burden: A review of global estimates. Lancet Oncol. 2019, 20, e42–e53. [Google Scholar] [CrossRef]
- Melguizo, C.; Prados, J.; Rama, A.R.; Ortiz, R.; Álvarez, P.J.; Fernández, J.E.; Aranega, A. Multidrug resistance and rhabdomyosarcoma (review). Oncol. Rep. 2011, 26, 755–761. [Google Scholar] [CrossRef] [PubMed]
- Missiaglia, E.; Selfe, J.; Hamdi, M.; Williamson, D.; Schaaf, G.; Fang, C.; Koster, J.; Summersgill, B.; Messahel, B.; Versteeg, R.; et al. Genomic imbalances in rhabdomyosarcoma cell lines affect expression of genes frequently altered in primary tumors: An approach to identify candidate genes involved in tumor development. Genes Chromosom. Cancer 2009, 48, 455–467. [Google Scholar] [CrossRef]
- Charrasse, S.; Comunale, F.; Gilbert, E.; Delattre, O.; Gauthier-Rouvière, C. Variation in cadherins and catenins expression is linked to both proliferation and transformation of Rhabdomyosarcoma. Oncogene 2004, 23, 2420–2430. [Google Scholar] [CrossRef][Green Version]
- Soglio, D.B.D.; Rougemont, A.L.; Absi, R.; Giroux, L.M.; Sanchez, R.; Barrette, S.; Fournet, J.C. Beta-catenin mutation does not seem to have an effect on the tumorigenesis of pediatric rhabdomyosarcomas. Pediatr. Dev. Pathol. 2009, 12, 371–373. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.S.; Huang, G.; Yu, B.; Wen, X.Q. Clinical significance and prognostic value of serum Dickkopf-1 concentrations in patients with lung cancer. Clin. Chem. 2009, 55, 1656–1664. [Google Scholar] [CrossRef]
- Qiao, R.; Zhong, R.; Chang, Q.; Teng, J.; Pei, J.; Han, B.; Chu, T. Serum dickkopf-1 as a clinical and prognostic factor in nonsmall cell lung cancer patients with bone metastases. Oncotarget 2017, 8, 79469–79479. [Google Scholar] [CrossRef] [PubMed]
- Zarea, M.; Mohammadian Bajgiran, A.; Sedaghati, F.; Hatami, N.; Taheriazam, A.; Yahaghi, E.; Shakeri, M. Diagnostic investigations of DKK-1 and PDCD5 expression levels as independent prognostic markers of human chondrosarcoma. IUBMB Life 2016, 68, 597–601. [Google Scholar] [CrossRef] [PubMed]
- Chen, E.Y.; DeRan, M.T.; Ignatius, M.S.; Grandinetti, K.B.; Clagg, R.; McCarthy, K.M.; Lobbardi, R.M.; Brockmann, J.; Keller, C.; Wu, X.; et al. Glycogen synthase kinase 3 inhibitors induce the canonical WNT/β-catenin pathway to suppress growth and self-renewal in embryonal rhabdomyosarcoma. Proc. Natl. Acad. Sci. USA 2014, 111, 5349–5354. [Google Scholar] [CrossRef] [PubMed]
- Chien, A.J.; Moore, E.C.; Lonsdorf, A.S.; Kulikauskas, R.M.; Rothberg, B.G.; Berger, A.J.; Major, M.B.; Hwang, S.T.; Rimm, D.L.; Moon, R.T. Activated Wnt/β-catenin signaling in melanoma is associated with decreased proliferation in patient tumors and a murine melanoma model. Proc. Natl. Acad. Sci. USA 2009, 106, 1193–1198. [Google Scholar] [CrossRef] [PubMed]
- Geyer, F.C.; Lacroix-Triki, M.; Savage, K.; Arnedos, M.; Lambros, M.B.; MacKay, A.; Natrajan, R.; Reis-Filho, J.S. Β-Catenin pathway activation in breast cancer is associated with triple-negative phenotype but not with CTNNB1 mutation. Mod. Pathol. 2011, 24, 209–231. [Google Scholar] [CrossRef]
- Ragab, N.; Viehweger, F.; Bauer, J.; Geyer, N.; Yang, M.; Seils, A.; Belharazem, D.; Brembeck, F.H.; Schildhaus, H.-U.; Marx, A.; et al. Canonical WNT/β-Catenin Signaling Plays a Subordinate Role in Rhabdomyosarcomas. Front. Pediatr. 2018, 6, 378. [Google Scholar] [CrossRef] [PubMed]
- Annavarapu, S.R.; Cialfi, S.; Dominici, C.; Kokai, G.K.; Uccini, S.; Ceccarelli, S.; McDowell, H.P.; Hellxxiwell, T.R. Characterization of Wnt/β-catenin signaling in rhabdomyosarcoma. Lab. Investig. 2013, 93, 1090–1099. [Google Scholar] [CrossRef]
- Kim, C.-H.; Neiswender, H.; Baik, E.J.; Xiong, W.C.; Mei, L. β-Catenin Interacts with MyoD and Regulates Its Transcription Activity. Mol. Cell. Biol. 2008, 28, 2941–2951. [Google Scholar] [CrossRef]
- Maucort, C.; Di Giorgio, A.; Azoulay, S.; Duca, M. Differentiation of Cancer Stem Cells by Using Synthetic Small Molecules: Toward New Therapeutic Strategies against Therapy Resistance. ChemMedChem 2021, 16, 14–29. [Google Scholar] [CrossRef] [PubMed]
- Di Martino, O.; Welch, J.S. Retinoic acid receptors in acute myeloid leukemia therapy. Cancers 2019, 11, 1915. [Google Scholar] [CrossRef]
- Zhou, J.; Yi, Q.; Tang, L. The roles of nuclear focal adhesion kinase (FAK) on Cancer: A focused review. J. Exp. Clin. Cancer Res. 2019, 38, 250. [Google Scholar] [CrossRef]
- Wörthmüller, J.; Rüegg, C. The crosstalk between FAK and Wnt signaling pathways in cancer and its therapeutic implication. Int. J. Mol. Sci. 2020, 21, 9107. [Google Scholar] [CrossRef] [PubMed]
- Wörthmüller, J.; Salicio, V.; Oberson, A.; Blum, W.; Schwaller, B. Modulation of Calretinin Expression in Human Mesothelioma Cells Reveals the Implication of the FAK and Wnt Signaling Pathways in Conferring Chemoresistance towards Cisplatin. Int. J. Mol. Sci. 2019, 20, 5391. [Google Scholar] [CrossRef]
- Nguyen, N.; Yi, J.S.; Park, H.; Lee, J.S.; Ko, Y.G. Mitsugumin 53 (MG53) Ligase ubiquitinates Focal Adhesion Kinase during Skeletal Myogenesis. J. Biol. Chem. 2014, 289, 3209–3216. [Google Scholar] [CrossRef] [PubMed]
- Sastry, S.K.; Lakonishok, M.; Wu, S.; Truong, T.Q.; Huttenlocher, A.; Turner, C.E.; Horwitz, A.F. Quantitative changes in integrin and focal adhesion signaling regulate myoblast cell cycle withdrawal. J. Cell Biol. 1999, 144, 1295–1309. [Google Scholar] [CrossRef]
- Al Shareef, Z.; Kardooni, H.; Murillo-Garzón, V.; Domenici, G.; Stylianakis, E.; Steel, J.H.; Rabano, M.; Gorroño-Etxebarria, I.; Zabalza, I.; Vivanco, M.M.; et al. Protective effect of stromal Dickkopf-3 in prostate cancer: Opposing roles for TGFBI and ECM-1. Oncogene 2018, 37, 5305–5324. [Google Scholar] [CrossRef]
- Cruciat, C.-M.; Niehrs, C. Secreted and transmembrane wnt inhibitors and activators. Cold Spring Harb. Perspect. Biol. 2013, 5, a015081. [Google Scholar] [CrossRef] [PubMed]
- Semënov, M.V.; Tamai, K.; Brott, B.K.; Kühl, M.; Sokol, S.; He, X. Head inducer dickkopf-1 is a ligand for Wnt coreceptor LRP6. Curr. Biol. 2001, 11, 951–961. [Google Scholar] [CrossRef]
- Malladi, S.; MacAlinao, D.G.; Jin, X.; He, L.; Basnet, H.; Zou, Y.; De Stanchina, E.; Massagué, J. Metastatic Latency and Immune Evasion through Autocrine Inhibition of WNT. Cell 2016, 165, 45–60. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, N.; Fukushima, T.; Yorita, K.; Tanaka, H.; Chijiiwa, K.; Kataoka, H. Dickkopf-1 is overexpressed in human pancreatic ductal adenocarcinoma cells and is involved in invasive growth. Int. J. Cancer 2010, 126, 1611–1620. [Google Scholar] [CrossRef]
- Zhuang, X.; Zhang, H.; Li, X.; Li, X.; Cong, M.; Peng, F.; Yu, J.; Zhang, X.; Yang, Q.; Hu, G. Differential effects on lung and bone metastasis of breast cancer by Wnt signalling inhibitor DKK1. Nat. Cell Biol. 2017, 19, 1274–1285. [Google Scholar] [CrossRef] [PubMed]
- Almazán-Moga, A.; Zarzosa, P.; Vidal, I.; Molist, C.; Giralt, I.; Navarro, N.; Soriano, A.; Segura, M.F.; Alfranca, A.; Garcia-Castro, J.; et al. Hedgehog Pathway Inhibition Hampers Sphere and Holoclone Formation in Rhabdomyosarcoma. Stem Cells Int. 2017, 2017, 7507380. [Google Scholar] [CrossRef]
- Molist, C.; Navarro, N.; Giralt, I.; Zarzosa, P.; Gallo-Oller, G.; Pons, G.; Magdaleno, A.; Moreno, L.; Guillén, G.; Hladun, R.; et al. miRNA-7 and miRNA-324-5p regulate alpha9-Integrin expression and exert anti-oncogenic effects in rhabdomyosarcoma. Cancer Lett. 2020, 477, 49–59. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giralt, I.; Gallo-Oller, G.; Navarro, N.; Zarzosa, P.; Pons, G.; Magdaleno, A.; Segura, M.F.; Sábado, C.; Hladun, R.; Arango, D.; et al. Dickkopf-1 Inhibition Reactivates Wnt/β-Catenin Signaling in Rhabdomyosarcoma, Induces Myogenic Markers In Vitro and Impairs Tumor Cell Survival In Vivo. Int. J. Mol. Sci. 2021, 22, 12921. https://doi.org/10.3390/ijms222312921
Giralt I, Gallo-Oller G, Navarro N, Zarzosa P, Pons G, Magdaleno A, Segura MF, Sábado C, Hladun R, Arango D, et al. Dickkopf-1 Inhibition Reactivates Wnt/β-Catenin Signaling in Rhabdomyosarcoma, Induces Myogenic Markers In Vitro and Impairs Tumor Cell Survival In Vivo. International Journal of Molecular Sciences. 2021; 22(23):12921. https://doi.org/10.3390/ijms222312921
Chicago/Turabian StyleGiralt, Irina, Gabriel Gallo-Oller, Natalia Navarro, Patricia Zarzosa, Guillem Pons, Ainara Magdaleno, Miguel F. Segura, Constantino Sábado, Raquel Hladun, Diego Arango, and et al. 2021. "Dickkopf-1 Inhibition Reactivates Wnt/β-Catenin Signaling in Rhabdomyosarcoma, Induces Myogenic Markers In Vitro and Impairs Tumor Cell Survival In Vivo" International Journal of Molecular Sciences 22, no. 23: 12921. https://doi.org/10.3390/ijms222312921
APA StyleGiralt, I., Gallo-Oller, G., Navarro, N., Zarzosa, P., Pons, G., Magdaleno, A., Segura, M. F., Sábado, C., Hladun, R., Arango, D., Sánchez de Toledo, J., Moreno, L., Gallego, S., & Roma, J. (2021). Dickkopf-1 Inhibition Reactivates Wnt/β-Catenin Signaling in Rhabdomyosarcoma, Induces Myogenic Markers In Vitro and Impairs Tumor Cell Survival In Vivo. International Journal of Molecular Sciences, 22(23), 12921. https://doi.org/10.3390/ijms222312921






