Suppression of Bone Necrosis around Tooth Extraction Socket in a MRONJ-like Mouse Model by E-rhBMP-2 Containing Artificial Bone Graft Administration
Abstract
:1. Introduction
2. Results
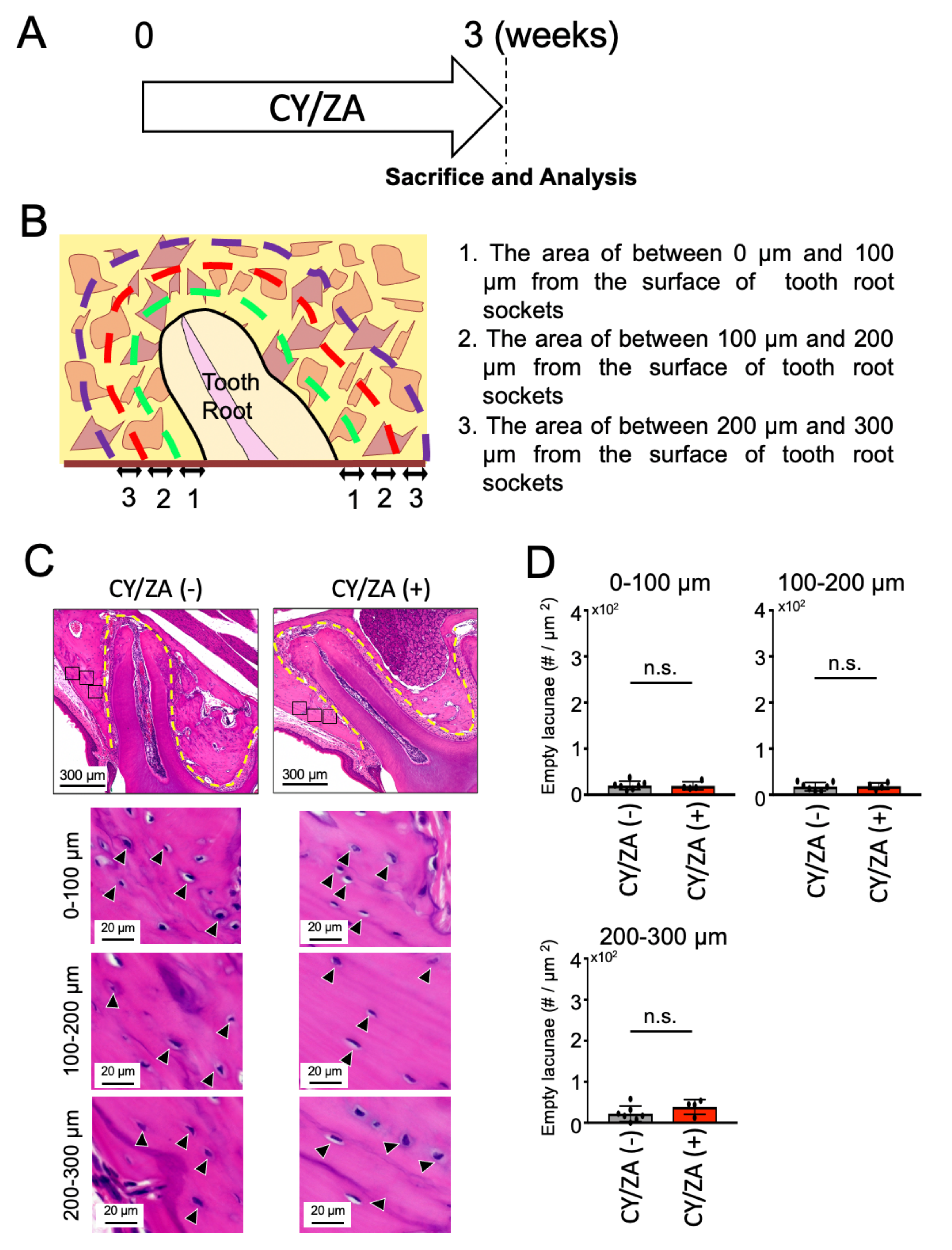
2.1. Effect of CY/ZA Administration before Tooth Extraction
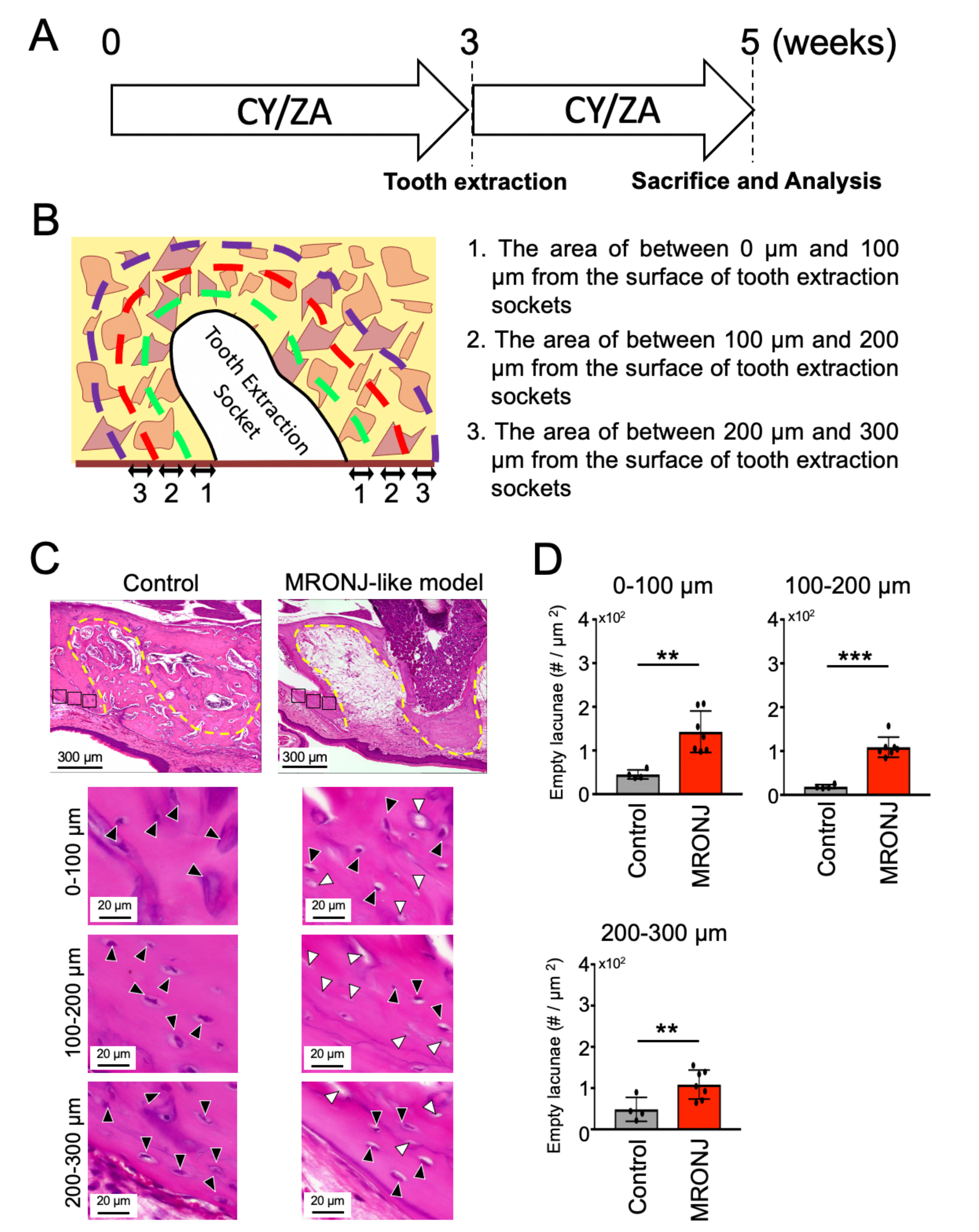
2.2. Effect of CY/ZA Administration 2 Weeks after Tooth Extraction
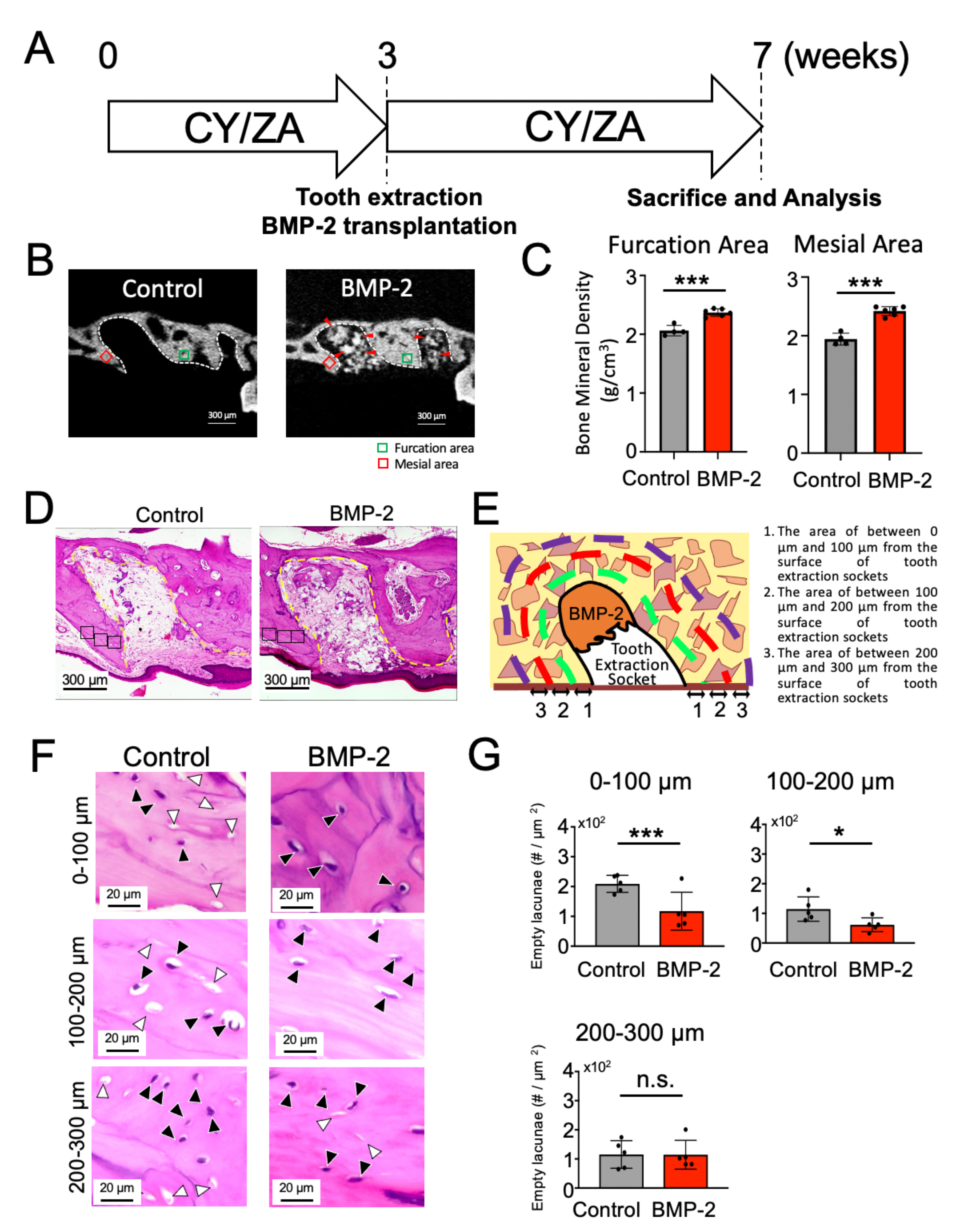
2.3. Effect of BMP-2/β-TCP Transplantation on the Alveolar Bone Surrounding the Tooth Extraction Socket in the Prevention of the Onset of MRONJ-like Symptoms
2.4. Effect of BMP-2/β-TCP Transplantation on the Alveolar Bone Surrounding the Tooth Extraction Socket in the Treatment of MRONJ-like Symptoms
3. Discussion
4. Materials and Methods
4.1. Preparation of E-rhBMP2/β-TCP Complex
4.2. Animal Model
4.3. Micro-CT Analysis
4.4. Histological Analysis
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marx, R.E. Pamidronate (Aredia) and zoledronate (Zometa) induced avascular necrosis of the jaws: A growing epidemic. J. Oral Maxillofac. Surg. 2003, 61, 1115–1117. [Google Scholar] [CrossRef]
- Hillner, B.E.; Ingle, J.N.; Chlebowski, R.T.; Gralow, J.; Yee, G.C.; Janjan, N.A.; Cauley, J.A.; Blumenstein, B.A.; Albain, K.S.; Lipton, A.; et al. American Society of Clinical Oncology 2003 update on the role of bisphosphonates and bone health issues in women with breast cancer. J. Clin. Oncol. 2003, 21, 4042–4057. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Huang, W.; Zhou, R.; Jia, S.; Tang, W.; Luo, Y.; Zhang, J. Bisphosphonates in the Treatment of Patients with Metastatic Breast, Lung, and Prostate Cancer: A Meta-Analysis. Medicine 2015, 94, e2014. [Google Scholar] [CrossRef] [PubMed]
- Russell, R.G. Bisphosphonates: The first 40 years. Bone 2011, 49, 2–19. [Google Scholar] [CrossRef] [PubMed]
- Hortobagyi, G.N.; Theriault, R.L.; Porter, L.; Blayney, D.; Lipton, A.; Sinoff, C.; Wheeler, H.; Simeone, J.F.; Seaman, J.; Knight, R.D. Efficacy of pamidronate in reducing skeletal complications in patients with breast cancer and lytic bone metastases. Protocol 19 Aredia Breast Cancer Study Group. N. Engl. J. Med. 1996, 335, 1785–1791. [Google Scholar] [CrossRef]
- Ruggiero, S.L.; Woo, S.B. Biophosphonate-related osteonecrosis of the jaws. Dent. Clin. N. Am. 2008, 52, 111–128. [Google Scholar] [CrossRef]
- Berenson, J.R.; Lichtenstein, A.; Porter, L.; Dimopoulos, M.A.; Bordoni, R.; George, S.; Lipton, A.; Keller, A.; Ballester, O.; Kovacs, M.J.; et al. Efficacy of pamidronate in reducing skeletal events in patients with advanced multiple myeloma. Myeloma Aredia Study Group. N. Engl. J. Med. 1996, 334, 488–493. [Google Scholar] [CrossRef]
- Haviv, Y.; Geller, Z.; Mazor, S.; Sharav, Y.; Keshet, N.; Zadik, Y. Pain characteristics in medication-related osteonecrosis of the jaws. Support Care Cancer 2021, 29, 1073–1080. [Google Scholar] [CrossRef]
- Ruggiero, S.L. Diagnosis and Staging of Medication-Related Osteonecrosis of the Jaw. Oral Maxillofac. Surg. Clin. N. Am. 2015, 27, 479–487. [Google Scholar] [CrossRef]
- Khan, A.A.; Morrison, A.; Hanley, D.A.; Felsenberg, D.; McCauley, L.K.; O’Ryan, F.; Reid, I.R.; Ruggiero, S.L.; Taguchi, A.; Tetradis, S.; et al. Diagnosis and management of osteonecrosis of the jaw: A systematic review and international consensus. J. Bone Miner. Res. 2015, 30, 3–23. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Morrison, A.; Cheung, A.; Hashem, W.; Compston, J. Osteonecrosis of the jaw (ONJ): Diagnosis and management in 2015. Osteoporos. Int. 2016, 27, 853–859. [Google Scholar] [CrossRef]
- Kuroshima, S.; Sasaki, M.; Sawase, T. Medication-related osteonecrosis of the jaw: A literature review. J. Oral Biosci. 2019, 61, 99–104. [Google Scholar] [CrossRef]
- Voss, P.J.; Poxleitner, P.; Schmelzeisen, R.; Stricker, A.; Semper-Hogg, W. Update MRONJ and perspectives of its treatment. J. Stomatol. Oral Maxillofac. Surg. 2017, 118, 232–235. [Google Scholar] [CrossRef] [PubMed]
- Miksad, R.A.; Lai, K.C.; Dodson, T.B.; Woo, S.B.; Treister, N.S.; Akinyemi, O.; Bihrle, M.; Maytal, G.; August, M.; Gazelle, G.S.; et al. Quality of life implications of bisphosphonate-associated osteonecrosis of the jaw. Oncologist 2011, 16, 121–132. [Google Scholar] [CrossRef] [Green Version]
- Nicolatou-Galitis, O.; Schiodt, M.; Mendes, R.A.; Ripamonti, C.; Hope, S.; Drudge-Coates, L.; Niepel, D.; Van den Wyngaert, T. Medication-related osteonecrosis of the jaw: Definition and best practice for prevention, diagnosis, and treatment. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2019, 127, 117–135. [Google Scholar] [CrossRef] [Green Version]
- Coleman, R.; Body, J.J.; Aapro, M.; Hadji, P.; Herrstedt, J.; Group, E.G.W. Bone health in cancer patients: ESMO Clinical Practice Guidelines. Ann. Oncol. 2014, 25 (Suppl. 3), iii124–iii137. [Google Scholar] [CrossRef] [PubMed]
- Ruggiero, S.L.; Dodson, T.B.; Fantasia, J.; Goodday, R.; Aghaloo, T.; Mehrotra, B.; O’Ryan, F. American Association of Oral and Maxillofacial Surgeons position paper on medication-related osteonecrosis of the jaw—2014 update. J. Oral Maxillofac. Surg. 2014, 72, 1938–1956. [Google Scholar] [CrossRef] [PubMed]
- Yoneda, T.; Hagino, H.; Sugimoto, T.; Ohta, H.; Takahashi, S.; Soen, S.; Taguchi, A.; Toyosawa, S.; Nagata, T.; Urade, M. Bisphosphonate-related osteonecrosis of the jaw: Position paper from the Allied Task Force Committee of Japanese Society for Bone and Mineral Research, Japan Osteoporosis Society, Japanese Society of Periodontology, Japanese Society for Oral and Maxillofacial Radiology, and Japanese Society of Oral and Maxillofacial Surgeons. J. Bone Miner. Metab. 2010, 28, 365–383. [Google Scholar] [PubMed]
- He, L.; Sun, X.; Liu, Z.; Qiu, Y.; Niu, Y. Pathogenesis and multidisciplinary management of medication-related osteonecrosis of the jaw. Int. J. Oral Sci. 2020, 12, 30. [Google Scholar] [CrossRef]
- Lorenzo-Pouso, A.I.; Bagan, J.; Bagan, L.; Gandara-Vila, P.; Chamorro-Petronacci, C.M.; Castelo-Baz, P.; Blanco-Carrion, A.; Blanco-Fernandez, M.A.; Alvarez-Calderon, O.; Carballo, J.; et al. Medication-Related Osteonecrosis of the Jaw: A Critical Narrative Review. J. Clin. Med. 2021, 10, 4367. [Google Scholar] [CrossRef] [PubMed]
- Bacci, C.; Cerrato, A.; Bardhi, E.; Frigo, A.C.; Djaballah, S.A.; Sivolella, S. A retrospective study on the incidence of medication-related osteonecrosis of the jaws (MRONJ) associated with different preventive dental care modalities. Support Care Cancer 2021. [Google Scholar] [CrossRef]
- Jung, J.; Yoo, H.Y.; Kim, G.T.; Lee, J.W.; Lee, Y.A.; Kim, D.Y.; Kwon, Y.D. Short-Term Teriparatide and Recombinant Human Bone Morphogenetic Protein-2 for Regenerative Approach to Medication-Related Osteonecrosis of the Jaw: A Preliminary Study. J. Bone Miner. Res. 2017, 32, 2445–2452. [Google Scholar] [CrossRef] [PubMed]
- On, S.W.; Cho, S.W.; Byun, S.H.; Yang, B.E. Various Therapeutic Methods for the Treatment of Medication-Related Osteonecrosis of the Jaw (MRONJ) and Their Limitations: A Narrative Review on New Molecular and Cellular Therapeutic Approaches. Antioxidants 2021, 10, 680. [Google Scholar] [CrossRef] [PubMed]
- Weiss, A.; Attisano, L. The TGFbeta superfamily signaling pathway. Wiley Interdiscip. Rev. Dev. Biol. 2013, 2, 47–63. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.S.; Akhtar, N.; Jamil, H.M.; Banik, R.S.; Asaduzzaman, S.M. TGF-beta/BMP signaling and other molecular events: Regulation of osteoblastogenesis and bone formation. Bone Res. 2015, 3, 15005. [Google Scholar] [CrossRef] [Green Version]
- Burkus, J.K.; Gornet, M.F.; Dickman, C.A.; Zdeblick, T.A. Anterior lumbar interbody fusion using rhBMP-2 with tapered interbody cages. J. Spinal Disord Tech. 2002, 15, 337–349. [Google Scholar] [CrossRef] [PubMed]
- Boyne, P.J. Application of bone morphogenetic proteins in the treatment of clinical oral and maxillofacial osseous defects. J. Bone Joint Surg. Am. 2001, 83 (Suppl. 1), S146–S150. [Google Scholar] [CrossRef]
- Cochran, D.L.; Schenk, R.; Buser, D.; Wozney, J.M.; Jones, A.A. Recombinant human bone morphogenetic protein-2 stimulation of bone formation around endosseous dental implants. J. Periodontol. 1999, 70, 139–150. [Google Scholar] [CrossRef]
- Wikesjo, U.M.; Sorensen, R.G.; Wozney, J.M. Augmentation of alveolar bone and dental implant osseointegration: Clinical implications of studies with rhBMP-2. J. Bone Jt. Surg. Am. 2001, 83 (Suppl. 1), S136–S145. [Google Scholar] [CrossRef]
- Yano, K.; Hoshino, M.; Ohta, Y.; Manaka, T.; Naka, Y.; Imai, Y.; Sebald, W.; Takaoka, K. Osteoinductive capacity and heat stability of recombinant human bone morphogenetic protein-2 produced by Escherichia coli and dimerized by biochemical processing. J. Bone Miner. Metab. 2009, 27, 355–363. [Google Scholar] [CrossRef]
- Uckan, S.; Deniz, K.; Dayangac, E.; Araz, K.; Ozdemir, B.H. Early implant survival in posterior maxilla with or without beta-tricalcium phosphate sinus floor graft. J. Oral Maxillofac. Surg. 2010, 68, 1642–1645. [Google Scholar] [CrossRef]
- Zhang, X.; Guo, W.G.; Cui, H.; Liu, H.Y.; Zhang, Y.; Muller, W.E.; Cui, F.Z. In vitro and in vivo enhancement of osteogenic capacity in a synthetic BMP-2 derived peptide-coated mineralized collagen composite. J. Tissue Eng. Regen. Med. 2016, 10, 99–107. [Google Scholar] [CrossRef]
- Ono, M.; Sonoyama, W.; Yamamoto, K.; Oida, Y.; Akiyama, K.; Shinkawa, S.; Nakajima, R.; Pham, H.T.; Hara, E.S.; Kuboki, T. Efficient bone formation in a swine socket lift model using Escherichia coli-derived recombinant human bone morphogenetic protein-2 adsorbed in beta-tricalcium phosphate. Cells Tissues Organs 2014, 199, 249–255. [Google Scholar] [CrossRef]
- Mikai, A.; Ono, M.; Tosa, I.; Nguyen, H.T.T.; Hara, E.S.; Nosho, S.; Kimura-Ono, A.; Nawachi, K.; Takarada, T.; Kuboki, T.; et al. BMP-2/β-TCP Local Delivery for Bone Regeneration in MRONJ-Like Mouse Model. Int. J. Mol. Sci. 2020, 21, 7028. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, H.; Arakawa, T.; Mano, H.; Kaneda, T.; Ogasawara, A.; Nakagawa, M.; Toyama, Y.; Yabe, Y.; Kumegawa, M.; Hakeda, Y. Direct stimulation of osteoclastic bone resorption by bone morphogenetic protein (BMP)-2 and expression of BMP receptors in mature osteoclasts. Bone 2000, 27, 479–486. [Google Scholar] [CrossRef]
- Itoh, K.; Udagawa, N.; Katagiri, T.; Iemura, S.; Ueno, N.; Yasuda, H.; Higashio, K.; Quinn, J.M.; Gillespie, M.T.; Martin, T.J.; et al. Bone morphogenetic protein 2 stimulates osteoclast differentiation and survival supported by receptor activator of nuclear factor-kappaB ligand. Endocrinology 2001, 142, 3656–3662. [Google Scholar] [CrossRef]
- Pearson, H.B.; Mason, D.E.; Kegelman, C.D.; Zhao, L.; Dawahare, J.H.; Kacena, M.A.; Boerckel, J.D. Effects of Bone Morphogenetic Protein-2 on Neovascularization During Large Bone Defect Regeneration. Tissue Eng. Part A 2019, 25, 1623–1634. [Google Scholar] [CrossRef]
- Chaudhry, A.N.; Ruggiero, S.L. Osteonecrosis and bisphosphonates in oral and maxillofacial surgery. Oral Maxillofac. Surg. Clin. N. Am. 2007, 19, 199–206. [Google Scholar] [CrossRef]
- Tannoury, C.A.; An, H.S. Complications with the use of bone morphogenetic protein 2 (BMP-2) in spine surgery. Spine J. 2014, 14, 552–559. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.B.; Sui, C.; Wu, T.T.; Wu, L.Z.; Zhu, Y.Y.; Ren, Z.H. Association of Bone Morphogenetic Protein (BMP)/Smad Signaling Pathway with Fracture Healing and Osteogenic Ability in Senile Osteoporotic Fracture in Humans and Rats. Med. Sci. Monit. 2018, 24, 4363–4371. [Google Scholar] [CrossRef] [PubMed]
- Hayashida, S.; Soutome, S.; Yanamoto, S.; Fujita, S.; Hasegawa, T.; Komori, T.; Kojima, Y.; Miyamoto, H.; Shibuya, Y.; Ueda, N.; et al. Evaluation of the Treatment Strategies for Medication-Related Osteonecrosis of the Jaws (MRONJ) and the Factors Affecting Treatment Outcome: A Multicenter Retrospective Study with Propensity Score Matching Analysis. J. Bone Miner. Res. 2017, 32, 2022–2029. [Google Scholar] [CrossRef] [Green Version]
- Fortunato, L.; Bennardo, F.; Buffone, C.; Giudice, A. Is the application of platelet concentrates effective in the prevention and treatment of medication-related osteonecrosis of the jaw? A systematic review. J. Craniomaxillofac. Surg. 2020, 48, 268–285. [Google Scholar] [CrossRef]
- Mauceri, R.; Panzarella, V.; Pizzo, G.; Oteri, G.; Cervino, G.; Mazzola, G.; Di Fede, O.; Campisi, G. Platelet-Rich Plasma (PRP) in Dental Extraction of Patients at Risk of Bisphosphonate-Related Osteonecrosis of the Jaws: A Two-Year Longitudinal Study. Appl. Dentistry Oral Sci. 2020, 10, 4487. [Google Scholar] [CrossRef]
- Gawai, K.T.; Sobhana, C.R. Clinical evaluation of use of platelet rich plasma in bone healing. J. Maxillofac. Oral Surg. 2015, 14, 67–80. [Google Scholar] [CrossRef] [Green Version]
- Aghaloo, T.; Hazboun, R.; Tetradis, S. Pathophysiology of Osteonecrosis of the Jaws. Oral Maxillofac. Surg. Clin. N. Am. 2015, 27, 489–496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wozney, J.M. Overview of bone morphogenetic proteins. Spine 2002, 27 (Suppl. 1), S2–S8. [Google Scholar] [CrossRef] [PubMed]
- Hughes-Fulford, M.; Li, C.F. The role of FGF-2 and BMP-2 in regulation of gene induction, cell proliferation and mineralization. J. Orthop. Surg. Res. 2011, 6, 8. [Google Scholar] [CrossRef] [Green Version]
- Brierly, G.I.; Ren, J.; Baldwin, J.; Saifzadeh, S.; Theodoropoulos, C.; Tsurkan, M.V.; Lynham, A.; Hsu, E.; Nikolarakos, D.; Werner, C.; et al. Investigation of Sustained BMP Delivery in the Prevention of Medication-Related Osteonecrosis of the Jaw (MRONJ) in a Rat Model. Macromol. Biosci. 2019, 19, e1900226. [Google Scholar] [CrossRef]
- Burkus, J.K.; Heim, S.E.; Gornet, M.F.; Zdeblick, T.A. Is INFUSE bone graft superior to autograft bone? An integrated analysis of clinical trials using the LT-CAGE lumbar tapered fusion device. J. Spinal Disord. Tech. 2003, 16, 113–122. [Google Scholar] [CrossRef]
- Misch, C.; Wang, H.L. Clinical Applications of Recombinant Human Bone Morphogenetic Protein-2 for Bone Augmentation Before Dental Implant Placement. Clin. Adv. Periodontics 2011, 1, 118–131. [Google Scholar] [CrossRef] [Green Version]
- Halloran, D.; Durbano, H.W.; Nohe, A. Bone Morphogenetic Protein-2 in Development and Bone Homeostasis. J. Dev. Biol. 2020, 8, 19. [Google Scholar] [CrossRef] [PubMed]
- Jung, R.E.; Glauser, R.; Scharer, P.; Hammerle, C.H.; Sailer, H.F.; Weber, F.E. Effect of rhBMP-2 on guided bone regeneration in humans. Clin. Oral Implant. Res. 2003, 14, 556–568. [Google Scholar] [CrossRef] [PubMed]
- Kuroshima, S.; Sasaki, M.; Nakajima, K.; Tamaki, S.; Hayano, H.; Sawase, T. Prevalence of bisphosphonate-related osteonecrosis of the jaw-like lesions is increased in a chemotherapeutic dose-dependent manner in mice. Bone 2018, 112, 177–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanaka, Y.; Aung, K.T.; Ono, M.; Mikai, A.; Dang, A.T.; Hara, E.S.; Tosa, I.; Ishibashi, K.; Ono-Kimura, A.; Nawachi, K.; et al. Suppression of Bone Necrosis around Tooth Extraction Socket in a MRONJ-like Mouse Model by E-rhBMP-2 Containing Artificial Bone Graft Administration. Int. J. Mol. Sci. 2021, 22, 12823. https://doi.org/10.3390/ijms222312823
Tanaka Y, Aung KT, Ono M, Mikai A, Dang AT, Hara ES, Tosa I, Ishibashi K, Ono-Kimura A, Nawachi K, et al. Suppression of Bone Necrosis around Tooth Extraction Socket in a MRONJ-like Mouse Model by E-rhBMP-2 Containing Artificial Bone Graft Administration. International Journal of Molecular Sciences. 2021; 22(23):12823. https://doi.org/10.3390/ijms222312823
Chicago/Turabian StyleTanaka, Yukie, Kyaw Thu Aung, Mitsuaki Ono, Akihiro Mikai, Anh Tuan Dang, Emilio Satoshi Hara, Ikue Tosa, Kei Ishibashi, Aya Ono-Kimura, Kumiko Nawachi, and et al. 2021. "Suppression of Bone Necrosis around Tooth Extraction Socket in a MRONJ-like Mouse Model by E-rhBMP-2 Containing Artificial Bone Graft Administration" International Journal of Molecular Sciences 22, no. 23: 12823. https://doi.org/10.3390/ijms222312823
APA StyleTanaka, Y., Aung, K. T., Ono, M., Mikai, A., Dang, A. T., Hara, E. S., Tosa, I., Ishibashi, K., Ono-Kimura, A., Nawachi, K., Kuboki, T., & Oohashi, T. (2021). Suppression of Bone Necrosis around Tooth Extraction Socket in a MRONJ-like Mouse Model by E-rhBMP-2 Containing Artificial Bone Graft Administration. International Journal of Molecular Sciences, 22(23), 12823. https://doi.org/10.3390/ijms222312823





