Topoisomerase IIIβ Deficiency Induces Neuro-Behavioral Changes and Brain Connectivity Alterations in Mice
Abstract
1. Introduction
2. Results
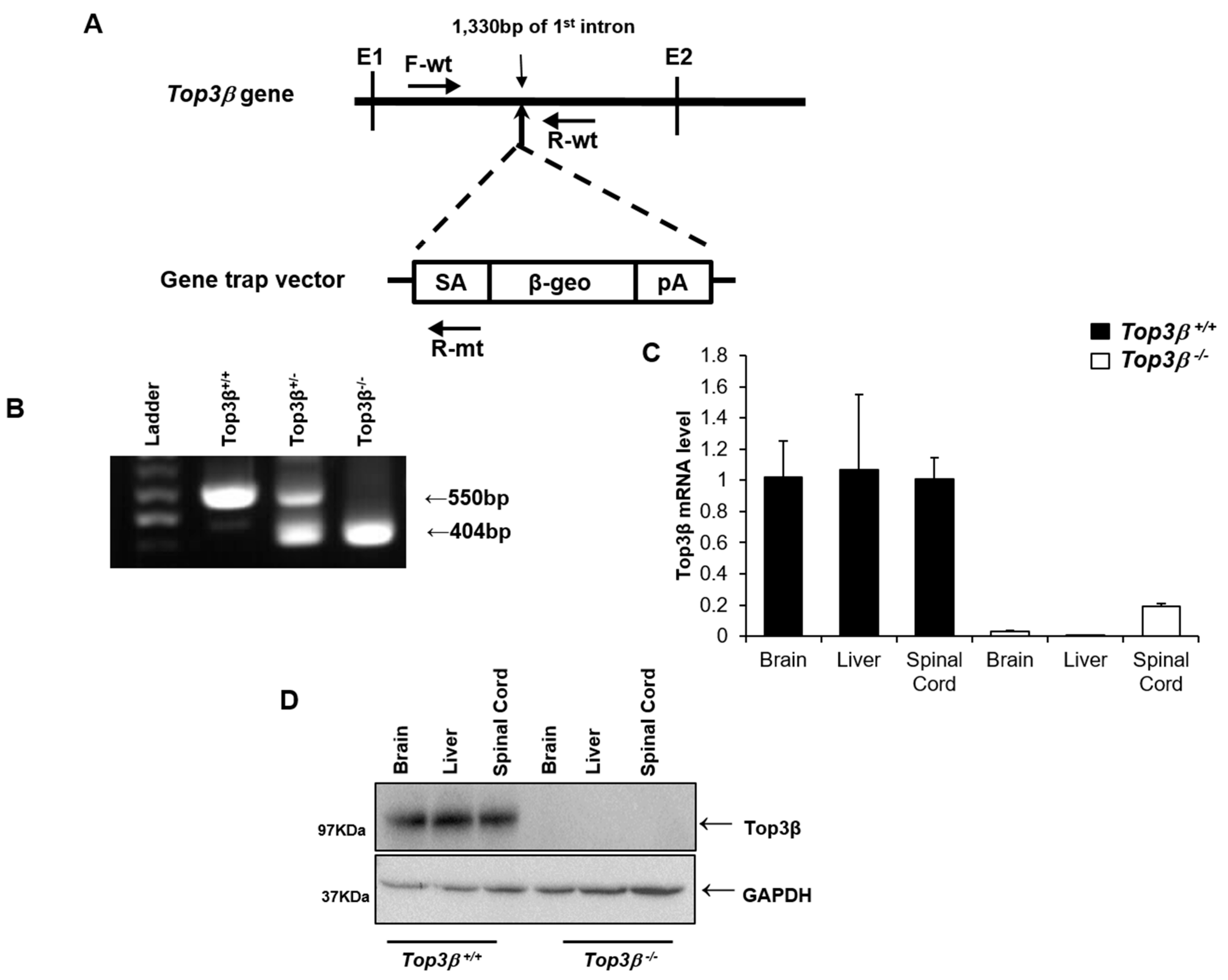
2.1. Generation of Top3β Mutant Mice
2.2. Deficiency of Top3β Does Not Affect Mouse Body Weight Gain
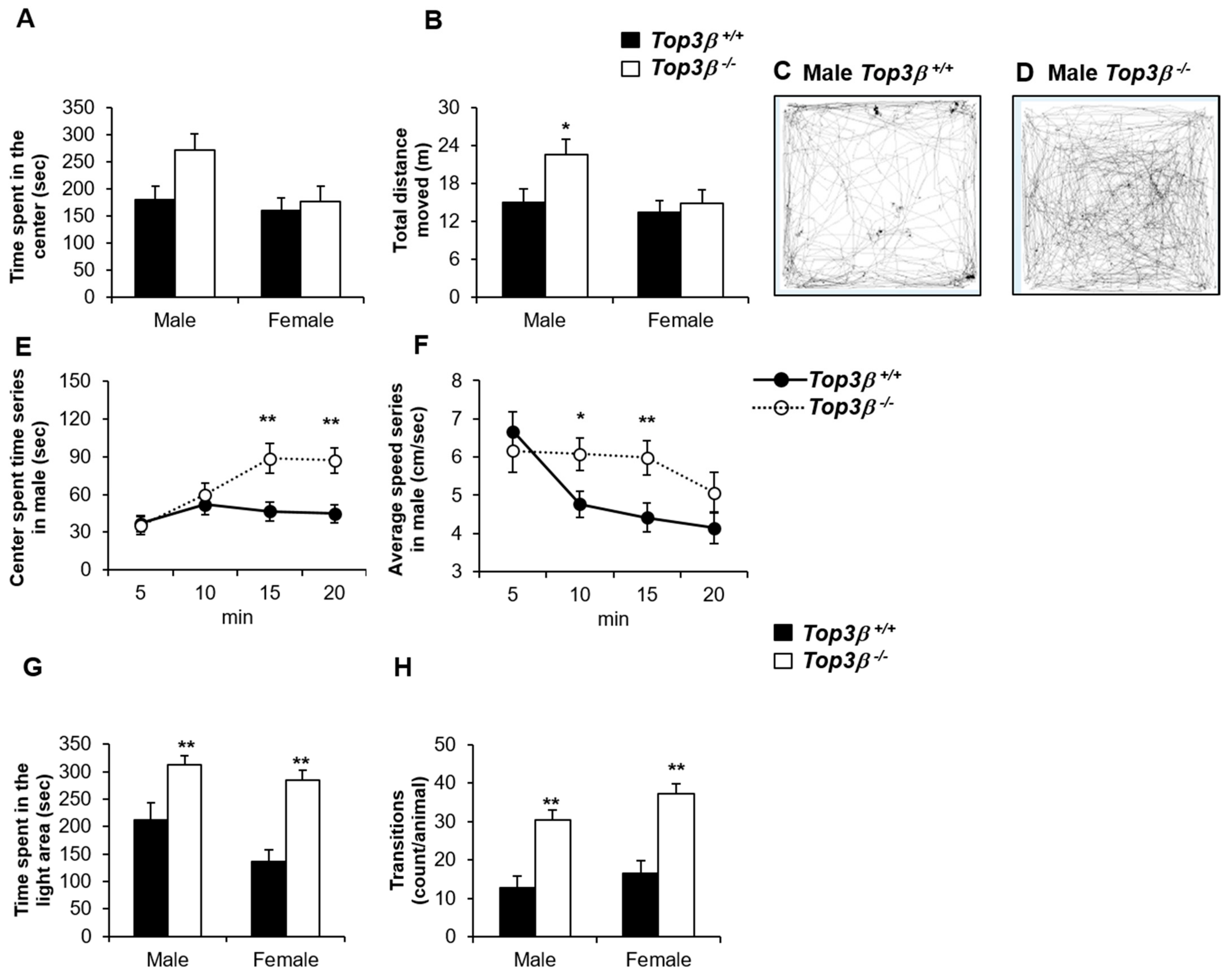
2.3. Top3β−/− Mice Show Less Anxiety-like Behavior
2.4. Top3β−/− Mice Exhibit Hyperactivity Based on the Light-Dark Box Test
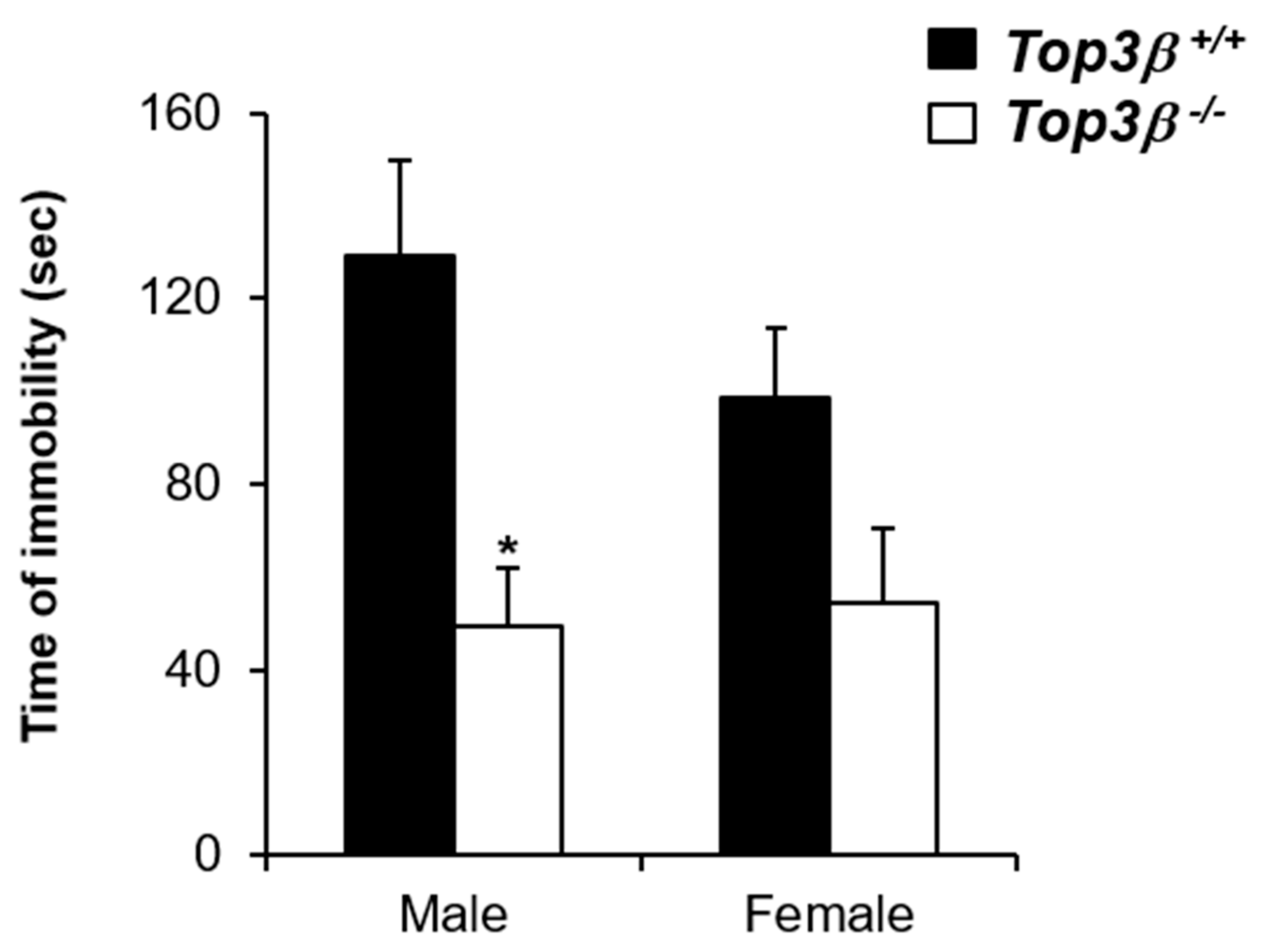
2.5. Top3β−/− Mice Show Decreased Depression-like Behavior
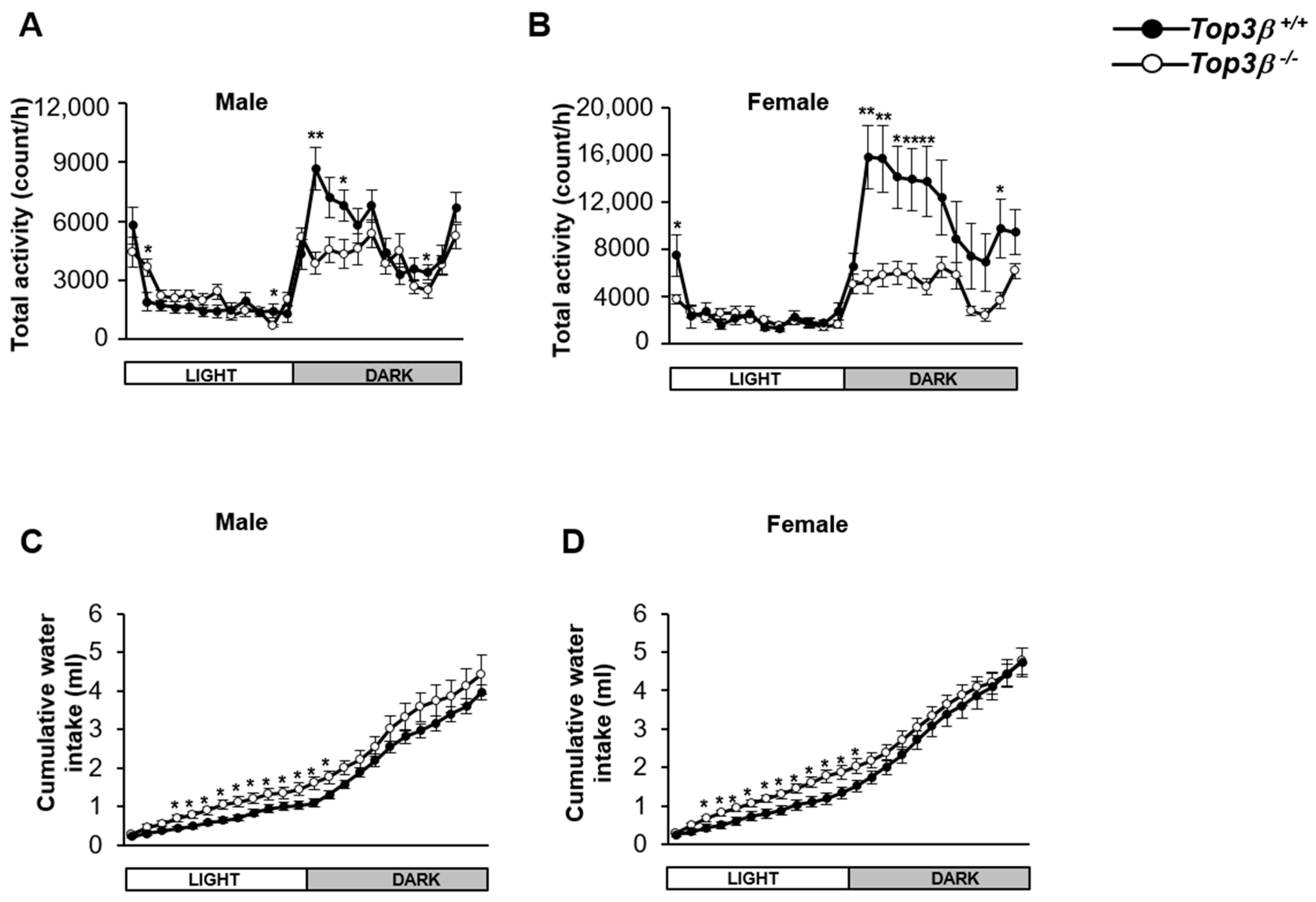
2.6. Top3β Deficiency Induces Alteration in Circadian Activity
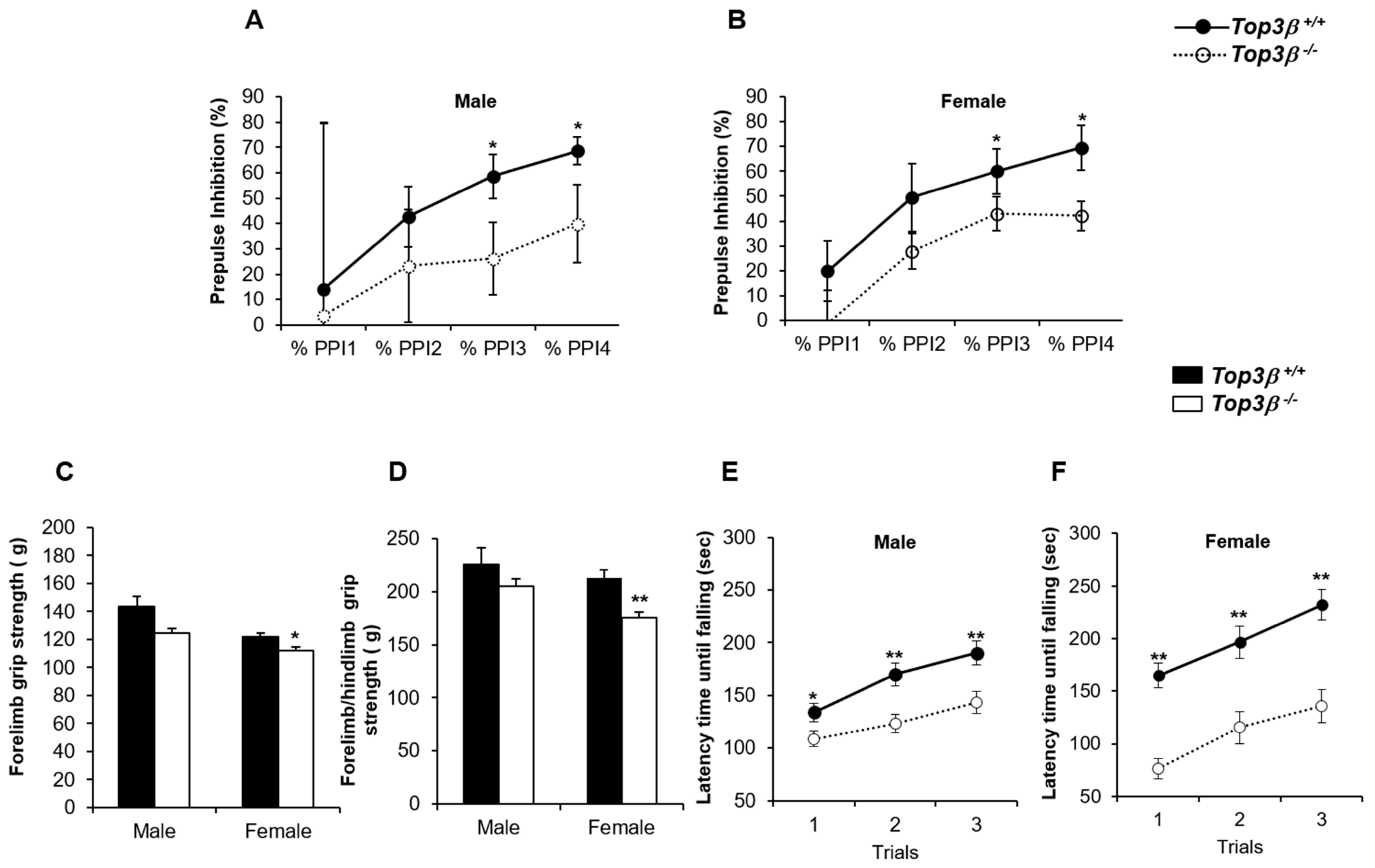
2.7. Top3β−/− Mice Exhibit Neurological Defects and Altered Neuromuscular Functions
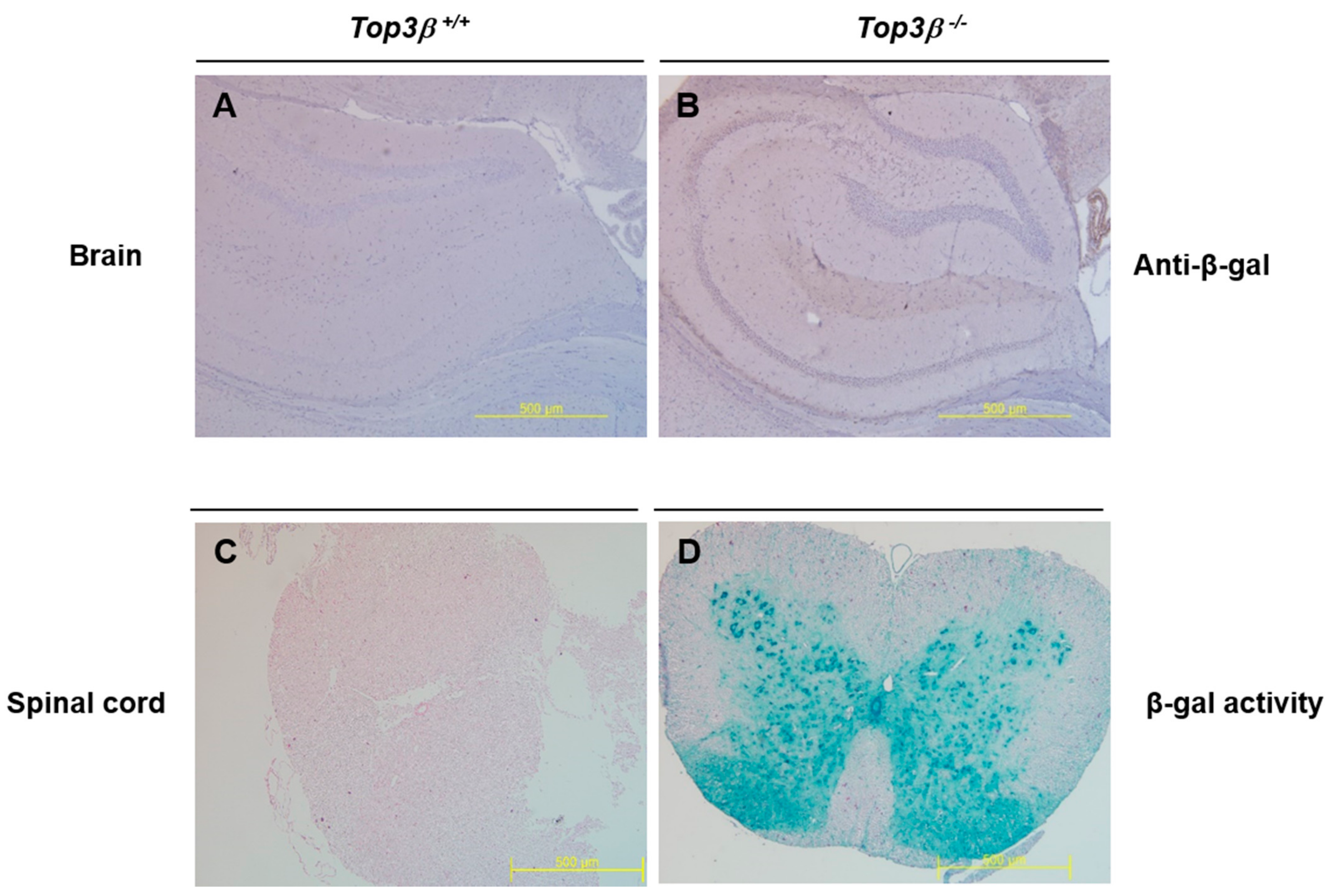
2.8. Central Nervous Tissues Display Top3β Gene Expression
2.9. Deficiency of Top3β Provokes Altered Connectivity in Brains of Top3β−/− Mice
3. Discussion
4. Materials and Methods
4.1. Generation of Top3β Mutant Mice
4.2. Real-Time Quantitative Polymerase Chain Reaction (q-PCR)
4.3. Western Blot Analysis
4.4. Open Field Test
4.5. Light-Dark Box Test
4.6. Rotarod Test
4.7. Forced Swim Test
4.8. Y-Maze, Fear Conditioning, and Acoustic Startle and Pre-Pulse Inhibition Tests
4.9. X-Gal (5-Bromo-4-chloro-3-indolyl-b–galactopyranoside) Staining
4.10. Immunohistochemical Staining against β-Galactosidase
4.11. PET Image Acquisition
4.12. Image Processing
4.13. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, J.C. Cellular roles of DNA topoisomerases: A molecular perspective. Nat. Rev. Mol. Cell Biol. 2002, 3, 430–440. [Google Scholar] [CrossRef] [PubMed]
- Champoux, J.J. DNA topoisomerases: Structure, function, and mechanism. Annu. Rev. Biochem. 2001, 70, 369–413. [Google Scholar] [CrossRef]
- Pommier, Y.; Sun, Y.; Huang, S.N.; Nitiss, J.L. Roles of eukaryotic topoisomerases in transcription, replication and genomic stability. Nat. Rev. Mol. Cell Biol. 2016, 17, 703–721. [Google Scholar] [CrossRef]
- McKinnon, P.J. Topoisomerases and the regulation of neural function. Nat. Rev. Neurosci. 2016, 17, 673–679. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, K.; Minoshima, S.; Nakato, E.; Shibuya, K.; Shintani, A.; Schmeits, J.L.; Wang, J.; Shimizu, N. One-megabase sequence analysis of the human immunoglobulin lambda gene locus. Genome Res. 1997, 7, 250–261. [Google Scholar] [CrossRef]
- Seki, T.; Seki, M.; Onodera, R.; Katada, T.; Enomoto, T. Cloning of cDNA encoding a novel mouse DNA topoisomerase III (Topo IIIbeta) possessing negatively supercoiled DNA relaxing activity, whose message is highly expressed in the testis. J. Biol. Chem. 1998, 273, 28553–28556. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Shen, W.; Guo, R.; Xue, Y.; Peng, W.; Sima, J.; Yang, J.; Sharov, A.; Srikantan, S.; Yang, J.; et al. Top3β is an RNA topoisomerase that works with fragile X syndrome protein to promote synapse formation. Nat. Neurosci. 2013, 16, 1238–1247. [Google Scholar] [CrossRef]
- Stoll, G.; Pietiläinen, O.P.H.; Linder, B.; Suvisaari, J.; Brosi, C.; Hennah, W.; Leppä, V.; Torniainen, M.; Ripatti, S.; Ala-Mello, S.; et al. Deletion of TOP3β, a component of FMRP-containing mRNPs, contributes to neurodevelopmental disorders. Nat. Neurosci. 2013, 16, 1228–1237. [Google Scholar] [CrossRef]
- Iossifov, I.; Ronemus, M.; Levy, D.; Wang, Z.; Hakker, I.; Rosenbaum, J.; Yamrom, B.; Lee, Y.H.; Narzisi, G.; Leotta, A.; et al. De novo gene disruptions in children on the autistic spectrum. Neuron 2012, 74, 285–299. [Google Scholar] [CrossRef]
- Xu, B.; Ionita-Laza, I.; Roos, J.L.; Boone, B.; Woodrick, S.; Sun, Y.; Levy, S.; Gogos, J.A.; Karayiorgou, M. De novo gene mutations highlight patterns of genetic and neural complexity in schizophrenia. Nat. Genet. 2012, 44, 1365–1369. [Google Scholar] [CrossRef]
- Tarsitano, M.; Ceglia, C.; Novelli, A.; Capalbo, A.; Lombardo, B.; Pastore, L.; Fioretti, G.; Vicari, L.; Pisanti, M.A.; Friso, P.; et al. Microduplications in 22q11.2 and 8q22.1 associated with mild mental retardation and generalized overgrowth. Gene 2014, 536, 213–216. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Shen, W.; Li, W.; Xue, Y.; Zou, S.; Xu, D.; Wang, W. Topoisomerase 3β is the major topoisomerase for mRNAs and linked to neurodevelopment and mental dysfunction. Nucleic Acids Res. 2017, 45, 2704–2713. [Google Scholar] [CrossRef] [PubMed]
- Daghsni, M.; Lahbib, S.; Fradj, M.; Sayeb, M.; Kelmemi, W.; Kraoua, L.; Kchaou, M.; Maazoul, F.; Echebbi, S.; Ben Ali, N.; et al. TOP3B: A novel candidate gene in juvenile myoclonic epilepsy? Cytogenet. Genome Res. 2018, 154, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, C.S.; Genovese, A.; Butler, M.G. Deletion of TOP3B is associated with cognitive impairment and facial dysmorphism. Cytogenet. Genome Res. 2016, 150, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Garavelli, L.; Rosato, S.; Wischmeijer, A.; Gelmini, C.; Esposito, A.; Mazzanti, L.; Franchi, F.; De Crescenzo, A.; Palumbo, O.; Carella, M.; et al. 22q11.2 Distal deletion syndrome: Description of a new case with truncus arteriosus type 2 and review. Mol. Syndromol. 2011, 2, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Kwan, K.Y.; Wang, J.C. Mice lacking DNA topoisomerase IIIbeta develop to maturity but show a reduced mean lifespan. Proc. Natl. Acad. Sci. USA 2001, 98, 5717–5721. [Google Scholar] [CrossRef] [PubMed]
- Kwan, K.Y.; Greenwald, R.J.; Mohanty, S.; Sharpe, A.H.; Shaw, A.C.; Wang, J.C. Development of autoimmunity in mice lacking DNA topoisomerase 3beta. Proc. Natl. Acad. Sci. USA 2007, 104, 9242–9247. [Google Scholar] [CrossRef]
- Joo, Y.; Xue, Y.; Wang, Y.; McDevitt, R.A.; Sah, N.; Bossi, S.; Su, S.; Lee, S.K.; Peng, W.; Xie, A.; et al. Topoisomerase 3β knockout mice show transcriptional and behavioural impairments associated with neurogenesis and synaptic plasticity. Nat. Commun. 2020, 11, 3143. [Google Scholar] [CrossRef]
- Li, W.; Wang, J.C. Mammalian DNA topoisomerase IIIalpha is essential in early embryogenesis. Proc. Natl. Acad. Sci. USA 1998, 95, 1010–1013. [Google Scholar] [CrossRef] [PubMed]
- Powell, T.R.; Fernandes, C.; Schalkwyk, L.C. Depression-related behavioral tests. Curr. Protoc. Mouse Biol. 2012, 2, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Salgado-Delgado, R.; Tapia Osorio, A.; Saderi, N.; Escobar, C. Disruption of circadian rhythms: A crucial factor in the etiology of depression. Depress. Res. Treat. 2011, 2011, 839743. [Google Scholar] [CrossRef] [PubMed]
- Kronfeld-Schor, N.; Einat, H. Circadian rhythms and depression: Human psychopathology and animal models. Neuropharmacology 2012, 62, 101–114. [Google Scholar] [CrossRef] [PubMed]
- Braff, D.L.; Grillon, C.; Geyer, M.A. Gating and habituation of the startle reflex in schizophrenic patients. Arch. Gen. Psychiatry 1992, 49, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Shoji, H.; Miyakawa, T. Relationships between the acoustic startle response and prepulse inhibition in C57BL/6J mice: A large-scale meta-analytic study. Mol. Brain 2018, 11, 42. [Google Scholar] [CrossRef] [PubMed]
- Mena, A.; Ruiz-Salas, J.C.; Puentes, A.; Dorado, I.; Ruiz-Veguilla, M.; De la Casa, L.G. Reduced prepulse inhibition as a biomarker of schizophrenia. Front. Behav. Neurosci. 2016, 10, 202. [Google Scholar] [CrossRef] [PubMed]
- Nelson, C.E.; Hakim, C.H.; Ousterout, D.G.; Thakore, P.I.; Moreb, E.A.; Castellanos Rivera, R.M.; Madhavan, S.; Pan, X.; Ran, F.A.; Yan, W.X.; et al. In vivo genome editing improves muscle function in a mouse model of Duchenne muscular dystrophy. Science 2016, 351, 403–407. [Google Scholar] [CrossRef] [PubMed]
- Hakim, C.H.; Wasala, N.B.; Duan, D. Evaluation of muscle function of the extensor digitorum longus muscle ex vivo and tibialis anterior muscle in situ in mice. J. Vis. Exp. 2013, 21, 50183. [Google Scholar] [CrossRef] [PubMed]
- Dunnett, S.B.; Torres, E.M.; Annett, L.E. A lateralised grip strength test to evaluate unilateral nigrostriatal lesions in rats. Neurosci. Lett. 1998, 246, 1–4. [Google Scholar] [CrossRef]
- Shiotsuki, H.; Yoshimi, K.; Shimo, Y.; Funayama, M.; Takamatsu, Y.; Ikeda, K.; Takahashi, R.; Kitazawa, S.; Hattori, N. A rotarod test for evaluation of motor skill learning. J. Neurosci. Methods 2010, 189, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Deacon, R.M. Measuring motor coordination in mice. J. Vis. Exp. 2013, 75, e2609. [Google Scholar] [CrossRef]
- Attems, J.; Walker, L.; Jellinger, K.A. Olfactory bulb involvement in neurodegenerative diseases. Acta Neuropathol. 2014, 127, 459–475. [Google Scholar] [CrossRef] [PubMed]
- Rey, N.L.; Wesson, D.W.; Brundin, P. The olfactory bulb as the entry site for prion-like propagation in neurodegenerative diseases. Neurobiol. Dis. 2018, 109, 226–248. [Google Scholar] [CrossRef] [PubMed]
- Turetsky, B.I.; Hahn, C.-G.; Borgmann-Winter, K.; Moberg, P.J. Scents and nonsense: Olfactory dysfunction in schizophrenia. Schizophr. Bull. 2009, 35, 1117–1131. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Schumann, C.M.; Bauman, M.D.; Amaral, D.G. Abnormal structure or function of the amygdala is a common component of neurodevelopmental disorders. Neuropsychologia 2011, 49, 745–759. [Google Scholar] [CrossRef]
- Heim, C.; Nemeroff, C.B. The role of childhood trauma in the neurobiology of mood and anxiety disorders: Preclinical and clinical studies. Biol. Psychiatry 2001, 49, 1023–1039. [Google Scholar] [CrossRef]
- François, C.; Grabli, D.; McCairn, K.; Jan, C.; Karachi, C.; Hirsch, E.C.; Féger, J.; Tremblay, L. Behavioural disorders induced by external globus pallidus dysfunction in primates II. Anatomical study. Brain 2004, 127, 2055–2070. [Google Scholar] [CrossRef]
- Tse-Dinh, Y.C. Bacterial and archeal type I topoisomerases. Biochim. Biophys. Acta 1998, 1400, 19–27. [Google Scholar] [CrossRef]
- Kwan, K.Y.; Moens, P.B.; Wang, J.C. Infertility and aneuploidy in mice lacking a type IA DNA topoisomerase III beta. Proc. Natl. Acad. Sci. USA 2003, 100, 2526–2531. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.K.; Wang, W. Roles of topoisomerases in heterochromatin, aging, and diseases. Genes 2019, 10, 884. [Google Scholar] [CrossRef] [PubMed]
- Kokras, N.; Dalla, C. Sex differences in animal models of psychiatric disorders. Br. J. Pharmacol. 2014, 171, 4595–4619. [Google Scholar] [CrossRef]
- Braff, D.L.; Geyer, M.A.; Swerdlow, N.R. Human studies of prepulse inhibition of startle: Normal subjects, patient groups, and pharmacological studies. Psychopharmacology 2001, 156, 234–258. [Google Scholar] [CrossRef] [PubMed]
- Swerdlow, N.R.; Weber, M.; Qu, Y.; Light, G.A.; Braff, D.L. Realistic expectations of prepulse inhibition in translational models for schizophrenia research. Psychopharmacology 2008, 199, 331–388. [Google Scholar] [CrossRef] [PubMed]
- Hickie, I.B.; Naismith, S.L.; Robillard, R.; Scott, E.M.; Hermens, D.F. Manipulating the sleep-wake cycle and circadian rhythms to improve clinical management of major depression. BMC Med. 2013, 11, 79. [Google Scholar] [CrossRef] [PubMed]
- Robillard, R.; Carpenter, J.S.; Rogers, N.L.; Fares, S.; Grierson, A.B.; Hermens, D.F.; Naismith, S.L.; Mullin, S.J.; Feilds, K.L.; Glozier, N.; et al. Circadian rhythms and psychiatric profiles in young adults with unipolar depressive disorders. Transl. Psychiatry 2018, 8, 213. [Google Scholar] [CrossRef] [PubMed]
- Coles, M.E.; Schubert, J.R.; Nota, J.A. Sleep, circadian rhythms, and anxious traits. Curr. Psychiatry Rep. 2015, 17, 73. [Google Scholar] [CrossRef]
- Cameron, O.G.; Lee, M.A.; Kotun, J.; McPhee, K.M. Circadian symptom fluctuations in people with anxiety disorders. J. Affect. Disord. 1986, 11, 213–218. [Google Scholar] [CrossRef]
- Liberman, A.R.; Kwon, S.B.; Vu, H.T.; Filipowicz, A.; Ay, A.; Ingram, K.K. Circadian clock model supports molecular link between PER3 and human anxiety. Sci. Rep. 2017, 7, 9893. [Google Scholar] [CrossRef]
- Ma, L.L.; Kong, D.G.; Qi, X.W.; Wang, L.X. Generalized anxiety disorder and the circadian rhythm of blood pressure in patients with hypertension. Int. J. Psychiatry Clin. Pract. 2008, 12, 292–295. [Google Scholar] [CrossRef]
- Reddy, S.; Reddy, V.; Sharma, S. Physiology, circadian rhythm. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Nott, A.; Tsai, L.H. The Top3β way to untangle RNA. Nat. Neurosci. 2013, 16, 1163–1164. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Iturza, N.; Lo, A.C.; Shah, D.; Armendáriz, M.; Vannelli, A.; Mercaldo, V.; Trusel, M.; Li, K.W.; Gastaldo, D.; Santos, A.R.; et al. The autism- and schizophrenia-associated protein CYFIP1 regulates bilateral brain connectivity and behaviour. Nat. Commun. 2019, 10, 3454. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Allen, E.A.; Sui, J.; Arbabshirani, M.R.; Pearlson, G.; Calhoun, V.D. Brain connectivity networks in schizophrenia underlying resting state functional magnetic resonance imaging. Curr. Top. Med. Chem. 2012, 12, 2415–2425. [Google Scholar] [CrossRef]
- Dawson, N.; Kurihara, M.; Thomson, D.M.; Winchester, C.L.; McVie, A.; Hedde, J.R.; Randall, A.D.; Shen, S.; Seymour, P.A.; Hughes, Z.A.; et al. Altered functional brain network connectivity and glutamate system function in transgenic mice expressing truncated Disrupted-in-Schizophrenia 1. Transl. Psychiatry 2015, 5, e569. [Google Scholar] [CrossRef]
- Nejad, A.B.; Ebdrup, B.H.; Glenthøj, B.Y.; Siebner, H.R. Brain connectivity studies in schizophrenia: Unravelling the effects of antipsychotics. Curr. Neuropharmacol. 2012, 10, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.M.; Zamaleeva, A.I.; Lee, Y.W.; Ahmed, M.R.; Kim, E.; Lee, H.R.; Pothineni, V.R.; Tao, J.; Rhee, S.; Jayakumar, M.; et al. Characterization of brain dysfunction induced by bacterial lipopeptides that alter neuronal activity and network in rodent brains. J. Neurosci. 2018, 38, 10672–10691. [Google Scholar] [CrossRef] [PubMed]
- Tropea, D.; Hardingham, N.; Millar, K.; Fox, K. Mechanisms underlying the role of DISC1 in synaptic plasticity. J. Physiol. 2018, 596, 2747–2771. [Google Scholar] [CrossRef] [PubMed]
- Zoghbi, H.Y.; Bear, M.F. Synaptic dysfunction in neurodevelopmental disorders associated with autism and intellectual disabilities. Cold Spring Harb. Perspect. Biol. 2012, 4, a009886. [Google Scholar] [CrossRef] [PubMed]
- Sidorov, M.S.; Auerbach, B.D.; Bear, M.F. Fragile X mental retardation protein and synaptic plasticity. Mol. Brain 2013, 6, 15. [Google Scholar] [CrossRef] [PubMed]
- Kiparizoska, S.; Ikuta, T. Disrupted olfactory integration in schizophrenia: Functional connectivity study. Int. J. Neuropsychopharmacol. 2017, 20, 740–746. [Google Scholar] [CrossRef] [PubMed]
- Nakahara, M.; Tateyama, H.; Araki, M.; Nakagata, N.; Yamamura, K.; Araki, K. Gene-trap mutagenesis using Mol/MSM-1 embryonic stem cells from MSM/Ms mice. Mamm. Genome 2013, 24, 228–239. [Google Scholar] [CrossRef] [PubMed]
- Taniwaki, T.; Haruna, K.; Nakamura, H.; Sekimoto, T.; Oike, Y.; Imaizumi, T.; Saito, F.; Muta, M.; Soejima, Y.; Utoh, A.; et al. Characterization of an exchangeable gene trap using pU-17 carrying a stop codon-beta geo cassette. Dev. Growth Differ. 2005, 47, 163–172. [Google Scholar] [CrossRef]
- Liu, L.M.; Xiong, D.D.; Lin, P.; Yang, H.; Dang, Y.W.; Chen, G. DNA topoisomerase 1 and 2A function as oncogenes in liver cancer and may be direct targets of nitidine chloride. Int. J. Oncol. 2018, 53, 1897–1912. [Google Scholar] [CrossRef] [PubMed]
- Douarre, C.; Sourbier, C.; Dalla Rosa, I.; Brata Das, B.; Redon, C.E.; Zhang, H.; Neckers, L.; Pommier, Y. Mitochondrial topoisomerase I is critical for mitochondrial integrity and cellular energy metabolism. PLoS ONE 2012, 7, e41094. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, V.K.; Burger, L.; Nikoletopoulou, V.; Deogracias, R.; Thakurela, S.; Wirbelauer, C.; Kaut, J.; Terranova, R.; Hoerner, L.; Mielke, C.; et al. Target genes of Topoisomerase IIβ regulate neuronal survival and are defined by their chromatin state. Proc. Natl. Acad. Sci. USA 2012, 109, E934–E943. [Google Scholar] [CrossRef]
- Guiraldelli, M.F.; Eyster, C.; Pezza, R.J. Genome instability and embryonic developmental defects in RMI1 deficient mice. DNA Repair 2013, 12, 835–843. [Google Scholar] [CrossRef] [PubMed]
- Crusio, W.E. Genetic dissection of mouse exploratory behaviour. Behav. Brain Res. 2001, 125, 127–132. [Google Scholar] [CrossRef]
- Choleris, E.; Thomas, A.W.; Kavaliers, M.; Prato, F.S. A detailed ethological analysis of the mouse open field test: Effects of diazepam, chlordiazepoxide and an extremely low frequency pulsed magnetic field. Neurosci. Biobehav. Rev. 2001, 25, 235–260. [Google Scholar] [CrossRef]
- Takao, K.; Miyakawa, T. Light/dark transition test for mice. J. Vis. Exp. JoVE 2006, 104. [Google Scholar] [CrossRef]
- Jones, B.J.; Roberts, D.J. A rotarod suitable for quantitative measurements of motor incoordination in naive mice. Naunyn Schmiedebergs Arch. Exp. Pathol. Pharmakol. 1968, 259, 211. [Google Scholar] [CrossRef] [PubMed]
- Porsolt, R.D.; Le Pichon, M.; Jalfre, M. Depression: A new animal model sensitive to antidepressant treatments. Nature 1977, 266, 730–732. [Google Scholar] [CrossRef]
- Cryan, J.F.; Markou, A.; Lucki, I. Assessing antidepressant activity in rodents: Recent developments and future needs. Trends Pharmacol. Sci. 2002, 23, 238–245. [Google Scholar] [CrossRef]
- Dorr, A.; Sled, J.G.; Kabani, N. Three-dimensional cerebral vasculature of the CBA mouse brain: A magnetic resonance imaging and micro computed tomography study. Neuroimage 2007, 35, 1409–1423. [Google Scholar] [CrossRef] [PubMed]
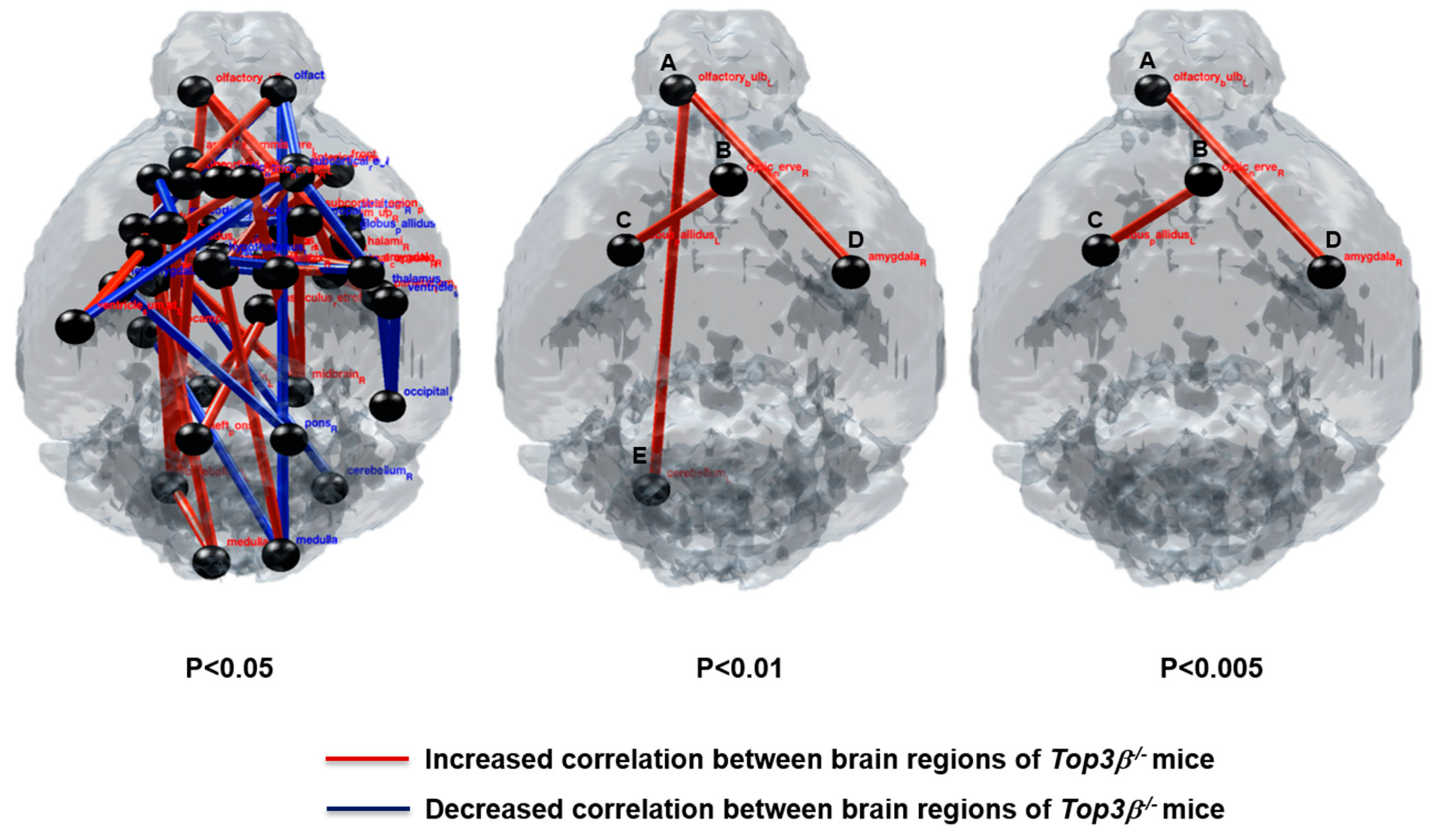
| Increased Correlation between Two Nodes | Decreased Correlation between Two Nodes | p-Value | ||
|---|---|---|---|---|
| optic_nerve_R | frontal_lobe_R | optic_nerve_R | optic_nerve_L | p < 0.05 |
| optic_nerve_R | midbrain_R | olfactory_bulb_R | anterior_commissure_R | |
| optic_nerve_R | globus_pallidus_L | olfactory_bulb_R | medulla_R | |
| olfactory_bulb_R | subcortical_region_frontal_L | olfactory_bulb_R | striatum_L | |
| anterior_commissure_R | midbrain_R | anterior_commissure_R | ventricle_sum_lat_L | |
| anterior_commissure_R | mamilothalamic_tract_L | occipital_cortex_R | thalamus_R | |
| anterior_commissure_R | corpus_callosum_L | occipital_cortex_R | ventricle_sum_lat_R | |
| frontal_lobe_R | stria_medullaris_thalami_L | subcortical_region_frontal_R | globus_pallidus_R | |
| parietal_temporal_cortex_R | stria_medullaris_thalami_R | subcortical_region_frontal_R | amygdala_R | |
| subcortical_region_parieto_temopal_R | internal_capsule_R | subcortical_region_frontal_R | corpus_callosum_L | |
| fasciculus_retroflexus_R | cerebellum_L | striatum_R | ventricle_sum_sup_L | |
| fasciculus_retroflexus_R | left_pons_L | internal_capsule_R | hypothalamus_L | |
| fasciculus_retroflexus_R | midbrain_L | mammilothalamic_R | corpus_callosum_L | |
| fornix_R | internal_capsule_R | amygdala_R | corpus_callosum_L | |
| globus_pallidus_R | internal_capsule_R | medulla_R | hippocampus_L | |
| stria_medullaris_thalami_R | amygdala_R | pons_R | left_amygdala_L | |
| midbrain_R | corpus_callosum_L | cerebellum_R | corpus_callosum_L | |
| amygdala_R | olfactory_bulb_L | ventricle_sum_sup_R | corpus_callosum_L | |
| medulla_R | mamilothalamic_tract_L | subcortical_region_parieto_temopal_L | frontal_lobe_L | |
| ventricle_sum_sup_R | olfactory_bulb_L | |||
| ventricle_sum_lat_L | globus_pallidus_L | |||
| cerebellum_L | medulla_L | |||
| cerebellum_L | internal_capsule_L | |||
| cerebellum_L | subcortical_region_parieto_temopal_L | |||
| cerebellum_L | frontal_lobe_L | |||
| cerebellum_L | olfactory_bulb_L | |||
| medulla_L | internal_capsule_L | |||
| hippocampus_L | midbrain_L | |||
| globus_pallidus_L | striatum_L | |||
| subcortical_region_frontal_L | anterior_commissure_L | |||
| optic_nerve_R | globus_pallidus_L | |||
| amygdala_R | olfactory_bulb_L | |||
| Cerebellum_L | olfactory_bulb_L | p < 0.01 | ||
| Optic_nerve_R | globus_pallidus_L | p < 0.005 | ||
| amygdala_R | olfactory_bulb_L | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahman, F.U.; Kim, Y.-R.; Kim, E.-K.; Kim, H.-r.; Cho, S.-M.; Lee, C.-S.; Kim, S.J.; Araki, K.; Yamamura, K.-i.; Lee, M.-N.; et al. Topoisomerase IIIβ Deficiency Induces Neuro-Behavioral Changes and Brain Connectivity Alterations in Mice. Int. J. Mol. Sci. 2021, 22, 12806. https://doi.org/10.3390/ijms222312806
Rahman FU, Kim Y-R, Kim E-K, Kim H-r, Cho S-M, Lee C-S, Kim SJ, Araki K, Yamamura K-i, Lee M-N, et al. Topoisomerase IIIβ Deficiency Induces Neuro-Behavioral Changes and Brain Connectivity Alterations in Mice. International Journal of Molecular Sciences. 2021; 22(23):12806. https://doi.org/10.3390/ijms222312806
Chicago/Turabian StyleRahman, Faiz Ur, You-Rim Kim, Eun-Kyeung Kim, Hae-rim Kim, Sang-Mi Cho, Chin-Soo Lee, Su Jin Kim, Kimi Araki, Ken-ichi Yamamura, Mi-Ni Lee, and et al. 2021. "Topoisomerase IIIβ Deficiency Induces Neuro-Behavioral Changes and Brain Connectivity Alterations in Mice" International Journal of Molecular Sciences 22, no. 23: 12806. https://doi.org/10.3390/ijms222312806
APA StyleRahman, F. U., Kim, Y.-R., Kim, E.-K., Kim, H.-r., Cho, S.-M., Lee, C.-S., Kim, S. J., Araki, K., Yamamura, K.-i., Lee, M.-N., Park, S. G., Yoon, W.-K., Lee, K., Won, Y.-S., Kim, H.-C., Lee, Y., Lee, H.-Y., & Nam, K.-H. (2021). Topoisomerase IIIβ Deficiency Induces Neuro-Behavioral Changes and Brain Connectivity Alterations in Mice. International Journal of Molecular Sciences, 22(23), 12806. https://doi.org/10.3390/ijms222312806






