Highly Effective Protocol for Differentiation of Induced Pluripotent Stem Cells (iPS) into Melanin-Producing Cells
Abstract
:1. Introduction
2. Results
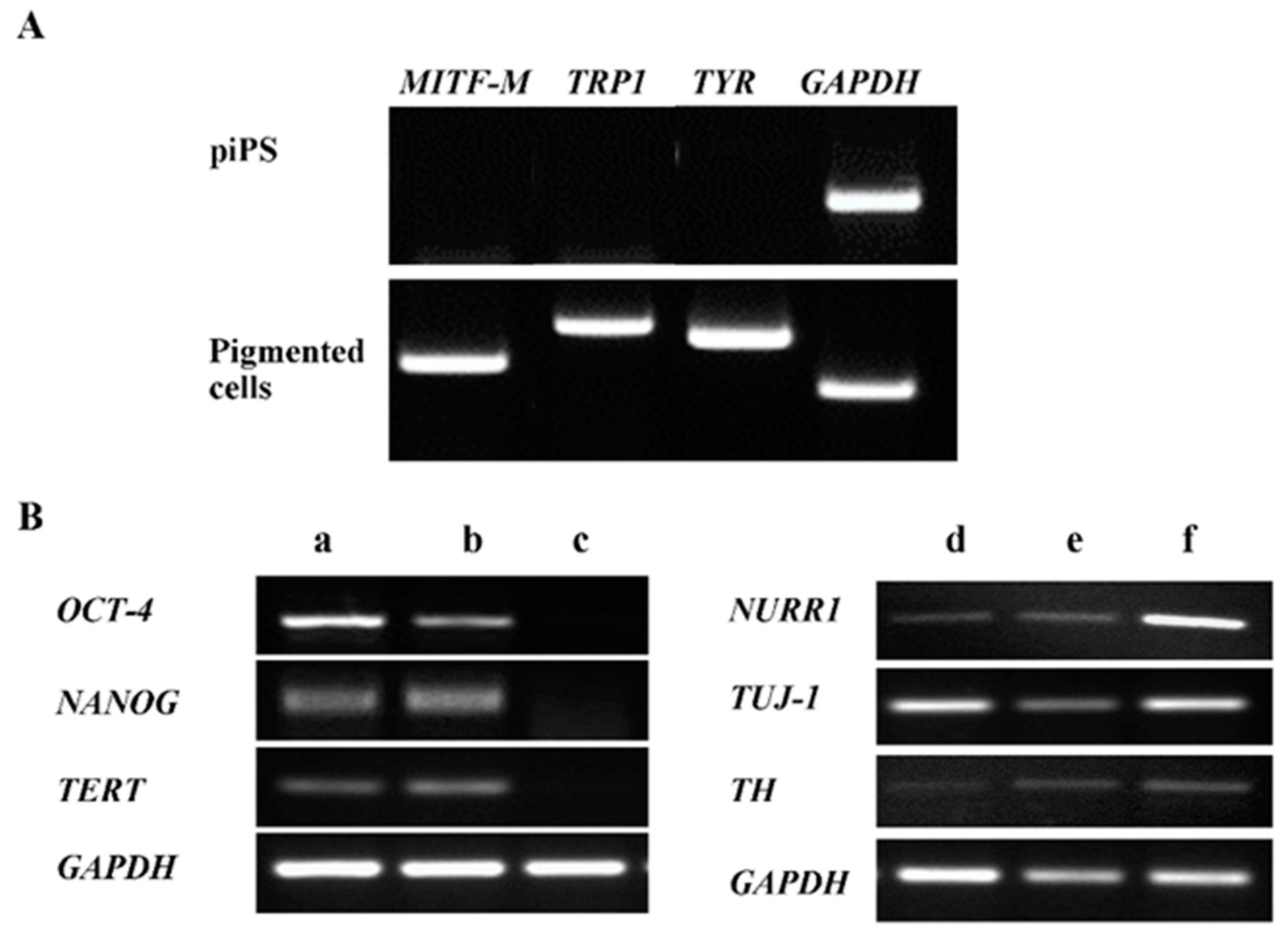
2.1. The Induced Pluripotent Stem Cell Differentiation into Pigment-Containing Cells

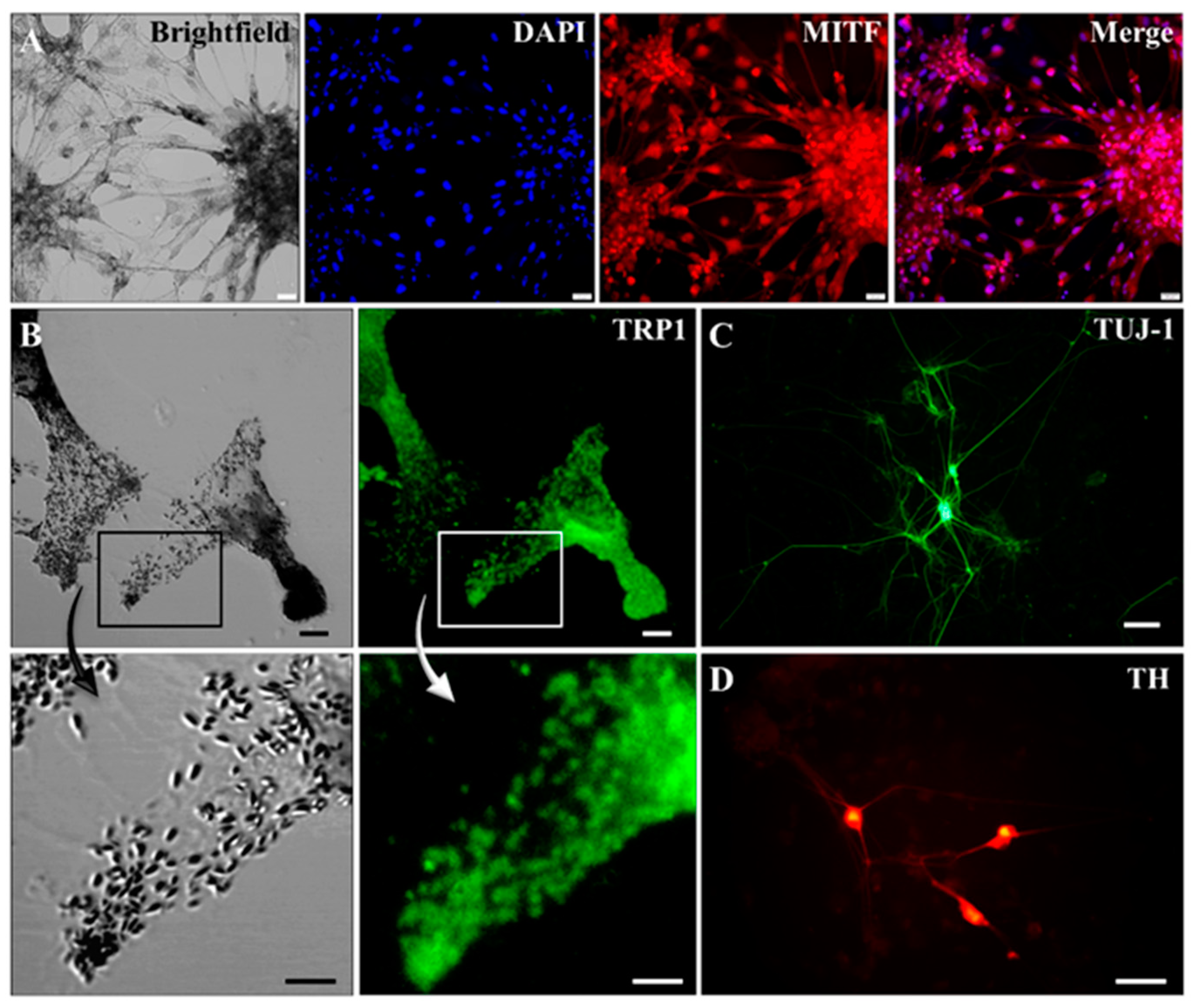
2.2. Characterization of Pigmented Cells
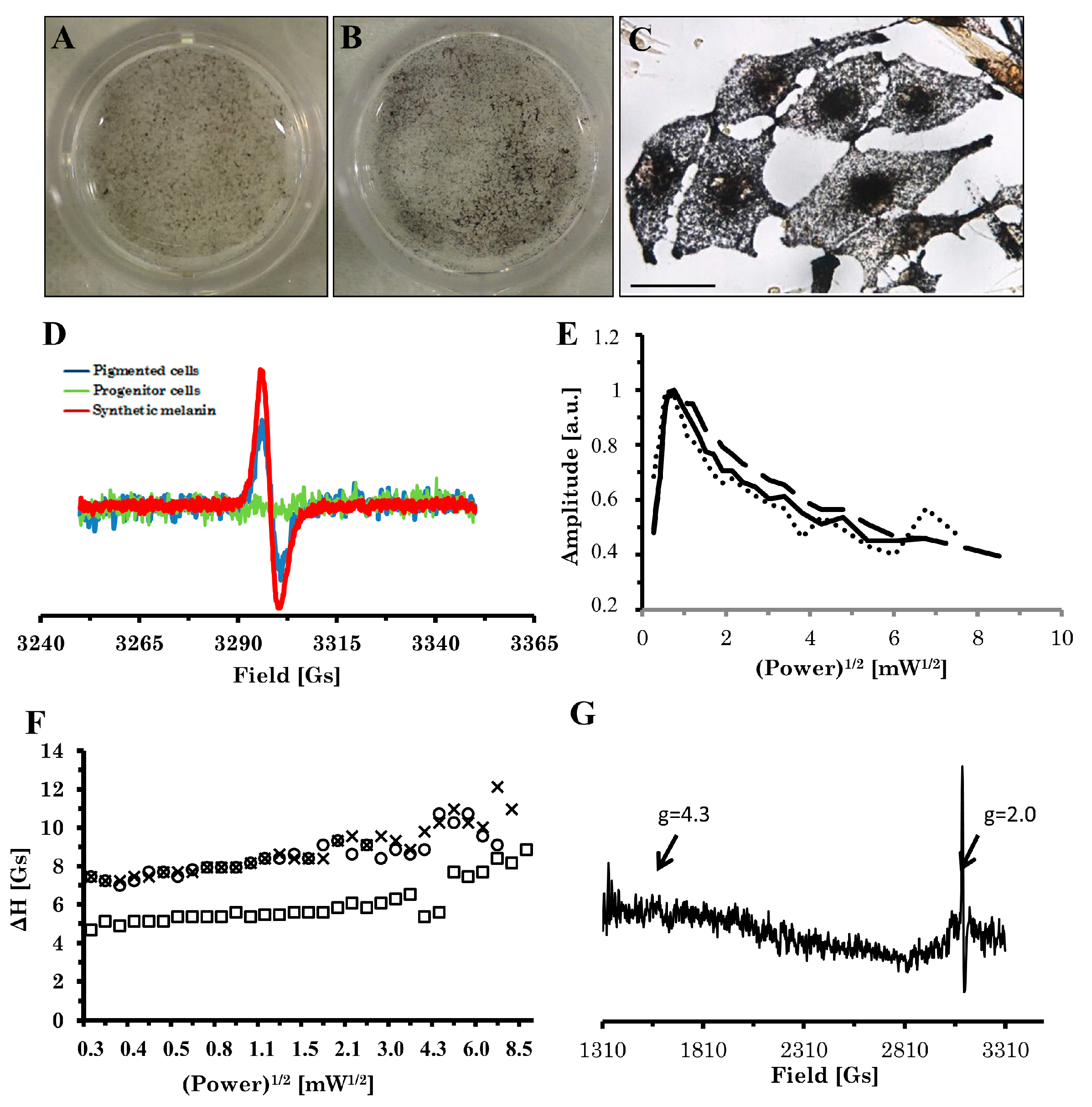
2.3. Electron Paramagnetic Resonance (EPR) Analysis of Pigment
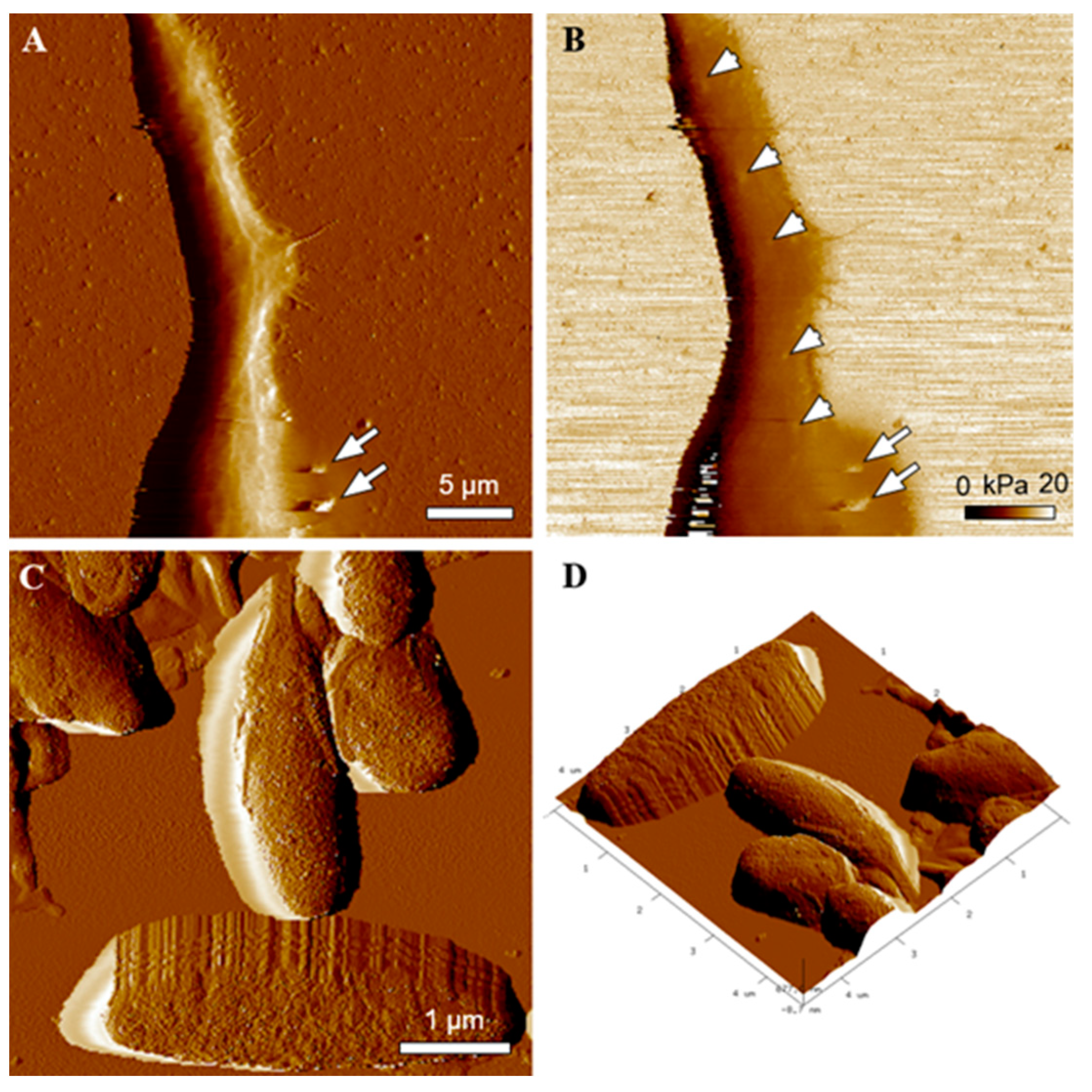
2.4. Transmission Electron Microscopy (TEM) and Atomic Force Microscopy (AFM) Characterization of Melanin-Containing Structures
3. Discussion
4. Materials and Methods
4.1. Cell Culture and EBs Formation
Differentiation in N1, N2, and N3 Media
4.2. RT-PCR
4.3. Immunocytochemistry
4.4. Modified Fontana–Masson Staining for Melanin
4.5. EPR Measurements
4.6. TEM Imaging
4.7. AFM Imaging and Elasticity Measurements
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Danés, A.; Richaud-Patin, Y.; Carballo-Carbajal, I.; Jiménez-Delgado, S.; Caig, C.; Mora, S.; di Guglielmo, C.; Ezquerra, M.; Patel, B.; Giralt, A.; et al. Disease-specific phenotypes in dopamine neurons from human iPS-based models of genetic and sporadic Parkinson’s disease. EMBO Mol. Med. 2012, 4, 380–395. [Google Scholar] [CrossRef]
- Fatima, A.; Kaifeng, S.; Dittmann, S.; Xu, G.; Gupta, M.K.; Linke, M.; Zechner, U.; Nguemo, F.; Milting, H.; Farr, M.; et al. The disease-specific phenotype in cardiomyocytes derived from induced pluripotent stem cells of two long QT syndrome type 3 Patients. PLoS ONE 2013, 8, e83005. [Google Scholar] [CrossRef] [Green Version]
- Ebert, A.D.; Yu, J.; Rose, F.F., Jr.; Mattis, V.B.; Lorson, C.L.; Thomson, J.A.; Svendsen, C.N. Induced pluripotent stem cells from a spinal muscular atrophy patient. Nature 2009, 457, 277–280. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, R. Induced pluripotent stem cells are Japanese brand sources for therapeutic cells to pretrial clinical research. Progress Stem Cell. 2020, 7, 296–303. [Google Scholar] [CrossRef]
- Slominski, A.; Tobin, D.J.; Shibahara, S.; Wortsman, J. Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol Rev. 2004, 84, 1155–1228. [Google Scholar] [CrossRef]
- Marks, M.S.; Seabra, M.C. The melanosome: Membrane dynamics in black and white. Nat. Rev. Mol. Cell Biol. 2001, 2, 738–748. [Google Scholar] [CrossRef] [PubMed]
- Ohta, S.; Imaizumi, Y.; Okada, Y.; Akamatsu, W.; Kuwahara, R.; Ohyama, M.; Amagai, M.; Matsuzaki, Y.; Yamanaka, S.; Okano, H.; et al. Generation of human melanocytes from induced pluripotent stem cells. PLoS ONE 2011, 6, e16182. [Google Scholar] [CrossRef]
- Jones, J.C.; Sabatini, K.; Liao, X.; Tran, H.T.; Lynch, C.L.; Morey, R.E.; Glenn-Pratola, V.; Boscolo, F.S.; Yang, Q.; Parast, M.M.; et al. Melanocytes derived from transgene-free human induced pluripotent stem cells. J. Investig. Dermatol. 2013, 133, 2104–2108. [Google Scholar] [CrossRef] [Green Version]
- Yang, R.; Jiang, M.; Kumar, S.M.; Xu, T.; Wang, F.; Xiang, L.; Xu, X. Generation of melanocytes from induced pluripotent stem cells. J. Investig. Dermatol. 2011, 131, 2458–2466. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.-H.; Lumelsky, N.; Studer, L.; Auerbach, J.M.; McKay, R.D. Efficient generation of midbrain and hindbrain neurons from mouse embryonic stem cells. Nat. Biotechnol. 2000, 18, 675–679. [Google Scholar] [CrossRef]
- Chlebanowska, P.; Sułkowski, M.; Skrzypek, K.; Tejchman, A.; Muszyńska, A.; Noroozi, R.; Majka, M. Origin of the Induced Pluripotent Stem Cells Affects Their Differentiation into Dopaminergic Neurons. Int. J. Mol. Sci. 2020, 21, 5705. [Google Scholar] [CrossRef] [PubMed]
- D'ischia, M.; Wakamatsu, K.; Cicoira, F.; di Mauro, E.; Garcia-Borron, J.C.; Commo, S.; Galván, I.; Ghanem, G.; Kenzo, K.; Meredith, P.; et al. Melanins and melanogenesis: From pigment cells to human health and technological applications. Pigment Cell Melanoma Res. 2015, 5, 520–544. [Google Scholar] [CrossRef] [Green Version]
- Zucca, F.A.; Segura-Aguilar, J.; Ferrari, E.; Muñoz, P.; Paris, I.; Sulzer, D.; Sarna, T.; Casella, L.; Zecca, L. Interactions of iron, dopamine and neuromelanin pathways in brain aging and Parkinson's disease. Prog. Neurobiol. 2017, 155, 96–119. [Google Scholar] [CrossRef] [PubMed]
- Bharti, K.; Nguyen, M.T.T.; Skuntz, S.; Bertuzzi, S.; Arnheiter, H. The other pigment cell: Specification and development of the pigmented epithelium of the vertebrate eye. Pigment Cell Res. 2006, 19, 380–394. [Google Scholar] [CrossRef] [Green Version]
- Płonka, P.; Michalczyk, D.; Popik, M.; Handjiski, B.; Slominski, A.; Paus, R. Splenic eumelanin differs from hair eumelanin in C57BL/6 mice. Acta Biochim. Pol. 2005, 52, 433–441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ko, J.-Y.; Park, C.-H.; Koh, H.-C.; Cho, Y.-H.; Kyhm, J.-H.; Kim, Y.-S.; Lee, I.; Lee, Y.-S.; Lee, S.-H. Human embryonic stem cell-derived neural precursors as a continuous, stable, and on-demand source for human dopamine neurons. J. Neurochem. 2007, 103, 1417–1429. [Google Scholar] [CrossRef]
- Biesemeier, A.; Eibl, O.; Eswara, S.; Audinot, J.; Wirtz, T.; Pezzoli, G.; Zucca, F.A.; Zecca, L.; Schraermeyer, U. Elemental mapping of neuromelanin organelles of human Substantia Nigra: Correlative ultrastructural and chemical analysis by analytical transmission electron microscopy and nano-secondary ion mass spectrometry. J. Neurochem. 2016, 138, 339–353. [Google Scholar] [CrossRef]
- Zucca, F.A.; Vanna, R.; Cupaioli, F.A.; Bellei, C.; De Palma, A.; Di Silvestre, D.; Mauri, P.; Grassi, S.; Prinetti, A.; Casella, L.; et al. Neuromelanin organelles are specialized autolysosomes that accumulate undegraded proteins and lipids in aging human brain and are likely involved in Parkinson's disease. NPJ Parkinson's Dis. 2018, 4, 17. [Google Scholar] [CrossRef]
- Raposo, G.; Marks, M.S. Melanosomes—Dark organelles enlighten endosomal membrane transport. Nat. Rev. Mol. Cell Biol. 2007, 8, 786–797. [Google Scholar] [CrossRef] [Green Version]
- Sarna, M.; Zadlo, A.; Pilat, A.; Olchawa, M.; Gkogkolou, P.; Burda, K.; Böhm, M.; Sarna, T. Nanomechanical analysis of pigmented human melanoma cells. Pigment Cell Melanoma Res. 2013, 26, 727–730. [Google Scholar] [CrossRef]
- Sarna, M.; Zadlo, A.; Czuba-Pelech, B.; Urbanska, K. Nanomechanical phenotype of melanoma cells depends solely on the amount of endogenous pigment in the cells. Int. J. Mol. Sci. 2018, 19, 607. [Google Scholar] [CrossRef] [Green Version]
- Sarna, M.; Zadlo, A.; Hermanowicz, P.; Madeja, Z.; Burda, K.; Sarna, T. Cell elasticity is an important indicator of the metastatic phenotype of melanoma cells. Exp. Dermatol. 2014, 23, 813–818. [Google Scholar] [CrossRef] [PubMed]
- Sarna, M.; Olchawa, M.; Zadlo, A.; Wnuk, D.; Sarna, T. The nanomechanical role of melanin granules in the retinal pigment epithelium. Nanomedicine 2017, 3, 801–807. [Google Scholar] [CrossRef] [PubMed]
- Sarna, M.; Krzykawska-Serda, M.; Jakubowska, M.; Zadlo, A.; Urbanska, K.; Sarna, M.; Krzykawska-Serda, M.; Jakubowska, M.; Zadlo, A.; Urbanska, K. Melanin presence inhibits melanoma cell spread in mice in a unique mechanical fashion. Sci. Rep. 2019, 9, 9280. [Google Scholar] [CrossRef] [PubMed]
- Zadlo, A.; Pilat, A.; Sarna, M.; Pawlak, A.; Sarna, T. Redox active transition metal ions make melanin susceptible to chemical degradation induced by organic peroxide. Cell Biochem. Bioph. 2017, 75, 319–333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laurent, L.C.; Ulitsky, I.; Slavin, I.; Tran, H.; Schork, A.; Morey, R.; Lynch, C.; Harness, J.V.; Lee, S.; Barrero, M.; et al. Dynamic changes in the copy number of pluripotency and cell proliferation genes in human ESCs and iPSCs during reprogramming and time in culture. Cell Stem Cell 2011, 8, 106–118. [Google Scholar] [CrossRef] [Green Version]
- Hill, H.Z.; Hill, G.J.; Cieszka, K.; Plonka, P.M.; Mitchell, D.L.; Meyenhofer, M.F.; Xin, P.; Boissy, R.E. Comparative action spectrum for ultraviolet light killing of mouse melanocytes from different genetic coat color backgrounds. Photochem. Photobiol. 1997, 65, 983–989. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Fujitam, K. Microanalysis of eumelanin and pheomelanin in hair and melanomas by chemical degradation and liquid chromatography. Anal. Biochem. 1985, 144, 527–536. [Google Scholar] [CrossRef]
- Grabacka, M.; Wieczorek, J.; Michalczyk-Wetula, D.; Malinowski, M.; Wolan, N.; Wojcik, K.; Plonka, P.M. Peroxisome proliferator-activated receptor α (PPARα) contributes to control of melanogenesis in B16 F10 melanoma cells. Arch. Dermatol. Res. 2017, 309, 141–157. [Google Scholar] [CrossRef]
- Wolnicka-Glubisz, A.; Nogal, K.; Żądło, A.; Płonka, P.M. Curcumin does not switch melanin synthesis towards pheomelanin in B16F10 cells. Arch. Dermatol. Res. 2015, 307, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Zecca, L.; Casella, L.; Albertini, A.; Bellei, C.; Zucca, F.A.; Engelen, M.; Zadlo, A.; Szewczyk, G.; Zareba, M.; Sarna, T. Neuromelanin can protect against iron-mediated oxidative damage in system modeling iron overload in brain aging and Parkinson’s disease. J. Neurochem. 2008, 106, 1866–1875. [Google Scholar]
- Sulzer, D.; Bogulavsky, J.; Larsen, K.E.; Behr, G.; Karatekin, E.; Kleinman, M.H.; Turro, N.; Krantz, D.; Edwards, R.H.; Greene, L.A.; et al. Neuromelanin biosynthesis is driven by excess cytosolic catecholamines not accumulated by synaptic vesicles. Proc. Natl. Acad. Sci. USA 2000, 97, 11869–11874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zadlo, A.C.; Sarna, T. Interaction of iron ions with melanin. Acta Biochim. Pol. 2019, 66, 459–462. [Google Scholar] [PubMed]
- Okazaki, M.; Kuwata, K.; Miki, Y.; Shiga, S.; Shiga, T. Electron spin relaxation of synthetic melanin and melanin-containing human tissues as studied by electron spin echo and electron spin resonance. Arch. Biochem. Biophys. 1985, 242, 197–205. [Google Scholar] [CrossRef]
- Green, S.; Simoes-Costa, M.; Bronner, M.E. Evolution of vertebrates: A view from the crest. Nature 2015, 520, 474–482. [Google Scholar] [CrossRef] [Green Version]
- D’Ischia, M.; Wakamatsu, K.; Napolitano, A.; Briganti, S.; Garcia-Borron, J.C.; Kovacs, D.; Meredith, P.; Pezzella, A.; Picardo, M.; Sarna, T.; et al. Melanins and melanogenesis: Methods, standards, protocols. Pigment Cell Melanoma Res. 2013, 26, 616–633. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.-P.; Li, Y.-M.; Guo, N.-N.; Li, S.; Ma, X.; Zhang, Y.-X.; Gao, Y.; Huang, J.-L.; Zheng, D.-X.; Wang, L.-Y.; et al. Therapeutic Potential of Patient iPSC-Derived Melanocytes in Autologous Transplantation. Cell Rep. 2019, 27, 455–466. [Google Scholar] [CrossRef] [Green Version]
- Huang, W.-S.; Wei, L.-G.; Li, J.-K.; Fu, K.-Y.; Huang, T.-C.; Hsieh, P.-S.; Huang, N.-C.; Dai, L.-G.; Chang, F.-W.; Loh, S.-H.; et al. Melanocyte differentiation from induced pluripotent stem cells derived from human adipose-derived stem cells. Ann. Plast. Surg. 2019, 82, 119–125. [Google Scholar] [CrossRef]
- Cruz-Martinez, P.; Martinez-Ferre, A.; Jaramillo-Merchán, J.; Estirado, A.; Martinez, S.; Jones, J. FGF8 activates proliferation and migration in mouse post-natal oligodendrocyte progenitor cells. PLoS ONE 2014, 9, e108241. [Google Scholar]
- Vogel-Hopker, A.; Momoseb, T.; Rohrerc, H.; Yasudab, K. Multiple functions of fibroblast growth factor-8 (FGF-8) in chick eye development. Mech. Dev. 2000, 94, 25–36. [Google Scholar] [CrossRef]
- Newman, A.M.; Cooper, J.B. Lab-specific gene expression signatures in pluripotent stem cells. Cell Stem Cell 2010, 7, 258–262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, M.; Liu, G.H.; Belmonte, J.C.I. Navigating the epigenetic landscape of pluripotent stem cells. Nat. Rev. Mol. Cell Biol. 2012, 13, 524–535. [Google Scholar] [CrossRef]
- Kim, K.; Doi, A.; Wen, B.; Ng, K.; Zhao, R.; Cahan, P.; Kim, J.; Aryee, M.J.; Ji, H.; Ehrlich, L.I.R.; et al. Epigenetic memory in induced pluripotent stem cells. Nature 2010, 467, 285–290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chambers, S.M.; Fasano, C.A.; Papapetrou, E.P.; Tomishima, M.; Sadelain, M.; Studer, L. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat. Biotechnol. 2009, 2, 275–280. [Google Scholar] [CrossRef] [Green Version]
- Kawasaki, H.; Suemori, H.; Mizuseki, K.; Watanabe, K.; Urano, F.; Ichinose, H.; Haruta, M.; Takahashi, M.; Yoshikawa, K.; Nishikawa, S.-I.; et al. Generation of dopaminergic neurons and pigmented epithelia from primate ES cells by stromal cell-derived inducing activity. Proc. Natl. Acad. Sci. USA 2002, 99, 1580–1585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ikemoto, K.; Nagatsu, I.; Ito, S.; A King, R.; Nishimura, A.; Nagatsu, T. Does tyrosinase exist in neuromelanin-pigmented neurons in the human substantia nigra? Neurosci. Lett. 1998, 253, 198–200. [Google Scholar] [CrossRef]
- Tribl, F.; Arzberger, T.; Riederer, P.; Gerlach, M. Tyrosinase is not detected in human catecholaminergic neurons by immunohistochemistry and Western blot analysis. J. Neural. Transm. Suppl. 2007, 72, 51–55. [Google Scholar]
- Kim, D.; Kim, C.-H.; Moon, J., II; Chung, Y.-G.; Chang, M.-Y.; Han, B.-S.; Ko, S.; Yang, E.; Cha, K.Y.; Lanza, R.; et al. Generation of human induced pluripotent stem cells by direct delivery of reprogramming proteins. Cell Stem Cell. 2009, 4, 472–476. [Google Scholar] [CrossRef] [Green Version]
- Michalczyk-Wetula, D.; Salwiński, A.; Popik, M.; Jakubowska, M.; Płonka, P.M. Splenic melanosis during normal murine C57BL/6 hair cycle and after chemotherapy. Acta Biochim. Pol. 2013, 60, 313–321. [Google Scholar] [CrossRef]
- Hermanowicz, P.; Sarna, M.; Burda, K.; Gabryś, H. AtomicJ: An open source software for analysis of force curves. Rev. Sci. Instrum. 2014, 85, 063703. [Google Scholar] [CrossRef] [PubMed]
- Sarna, M.; Wojcik, K.A.; Hermanowicz, P.; Wnuk, D.; Burda, K.; Sanak, M.; Czyż, J.; Michalik, M. Undifferentiated bronchial fibroblasts derived from asthmatic patients display higher elastic modulus than their non-asthmatic counterparts. PLoS ONE 2015, 10, e0116840. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| MITF-M | ACCTTCTCTTTGCCAGTCCATCT | GGACATGCAAGCTCAGGACT |
| TRP1 | TGTAACAGCACCGAGGATGG | TGTCCAATAGGGGCATTTTCCA |
| TYR | GAATGCTCCTGGCTGTTTTG | AGTCGTCTCTCTGTGCAGT |
| OCT-4 | ATGGCGGGACACCTGGCTT | GGGAGAGCCCAGAGTGGTGACG |
| Nanog | TGAACCTCAGCTACAAACAG | TGGTGGTAGGAAGAGTAAAG |
| TERT | TGTGCACCAACATCTACAAG | GCGTTCTTGGCTTTCAGGAT |
| GAPDH | CAAAGTTGTCATGGATGACC | CCATGGAGAAGGCTGGGG |
| TUJ-1 | TGGATTCGGTCCTGGATGTG | ACCTTGCTGATGAGCAACG |
| TH | GAGTACACCGCCGAGGAGATTG | GCGGATATACTGGGTGCACTGG |
| NURR1 | CCCAGTGGAGGGTAAACTCA | AATGCAGGAGAAGGCAGAAA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sułkowski, M.; Kot, M.; Badyra, B.; Paluszkiewicz, A.; Płonka, P.M.; Sarna, M.; Michalczyk-Wetula, D.; Zucca, F.A.; Zecca, L.; Majka, M. Highly Effective Protocol for Differentiation of Induced Pluripotent Stem Cells (iPS) into Melanin-Producing Cells. Int. J. Mol. Sci. 2021, 22, 12787. https://doi.org/10.3390/ijms222312787
Sułkowski M, Kot M, Badyra B, Paluszkiewicz A, Płonka PM, Sarna M, Michalczyk-Wetula D, Zucca FA, Zecca L, Majka M. Highly Effective Protocol for Differentiation of Induced Pluripotent Stem Cells (iPS) into Melanin-Producing Cells. International Journal of Molecular Sciences. 2021; 22(23):12787. https://doi.org/10.3390/ijms222312787
Chicago/Turabian StyleSułkowski, Maciej, Marta Kot, Bogna Badyra, Anna Paluszkiewicz, Przemysław M. Płonka, Michał Sarna, Dominika Michalczyk-Wetula, Fabio A. Zucca, Luigi Zecca, and Marcin Majka. 2021. "Highly Effective Protocol for Differentiation of Induced Pluripotent Stem Cells (iPS) into Melanin-Producing Cells" International Journal of Molecular Sciences 22, no. 23: 12787. https://doi.org/10.3390/ijms222312787
APA StyleSułkowski, M., Kot, M., Badyra, B., Paluszkiewicz, A., Płonka, P. M., Sarna, M., Michalczyk-Wetula, D., Zucca, F. A., Zecca, L., & Majka, M. (2021). Highly Effective Protocol for Differentiation of Induced Pluripotent Stem Cells (iPS) into Melanin-Producing Cells. International Journal of Molecular Sciences, 22(23), 12787. https://doi.org/10.3390/ijms222312787









