The Role of Melatonin on Behavioral Changes and Concomitant Oxidative Stress in icvAβ1-42 Rat Model with Pinealectomy
Abstract
1. Introduction
2. Results
2.1. Effects of Pinealectomy and Melatonin Treatment on Behavioral Responses in icvAβ Rat Model
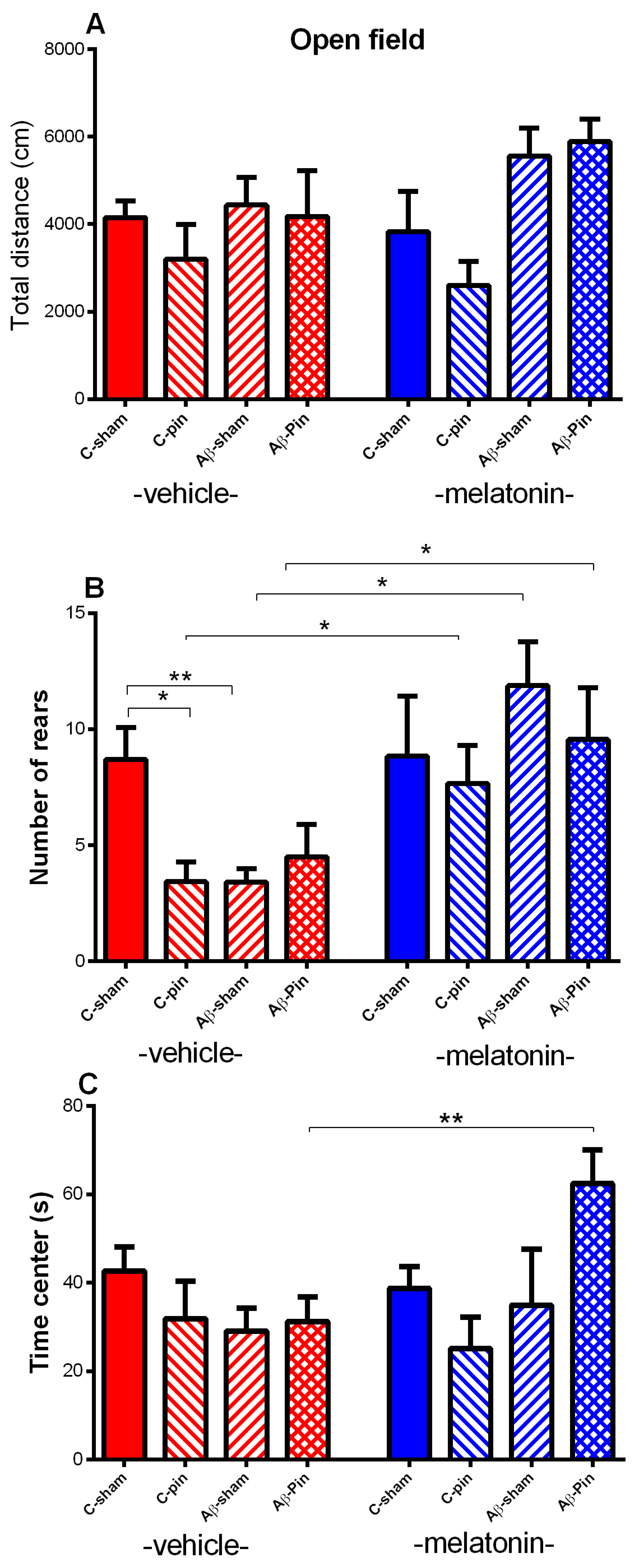
2.1.1. Open Field Test
2.1.2. Elevated plus Maze Test
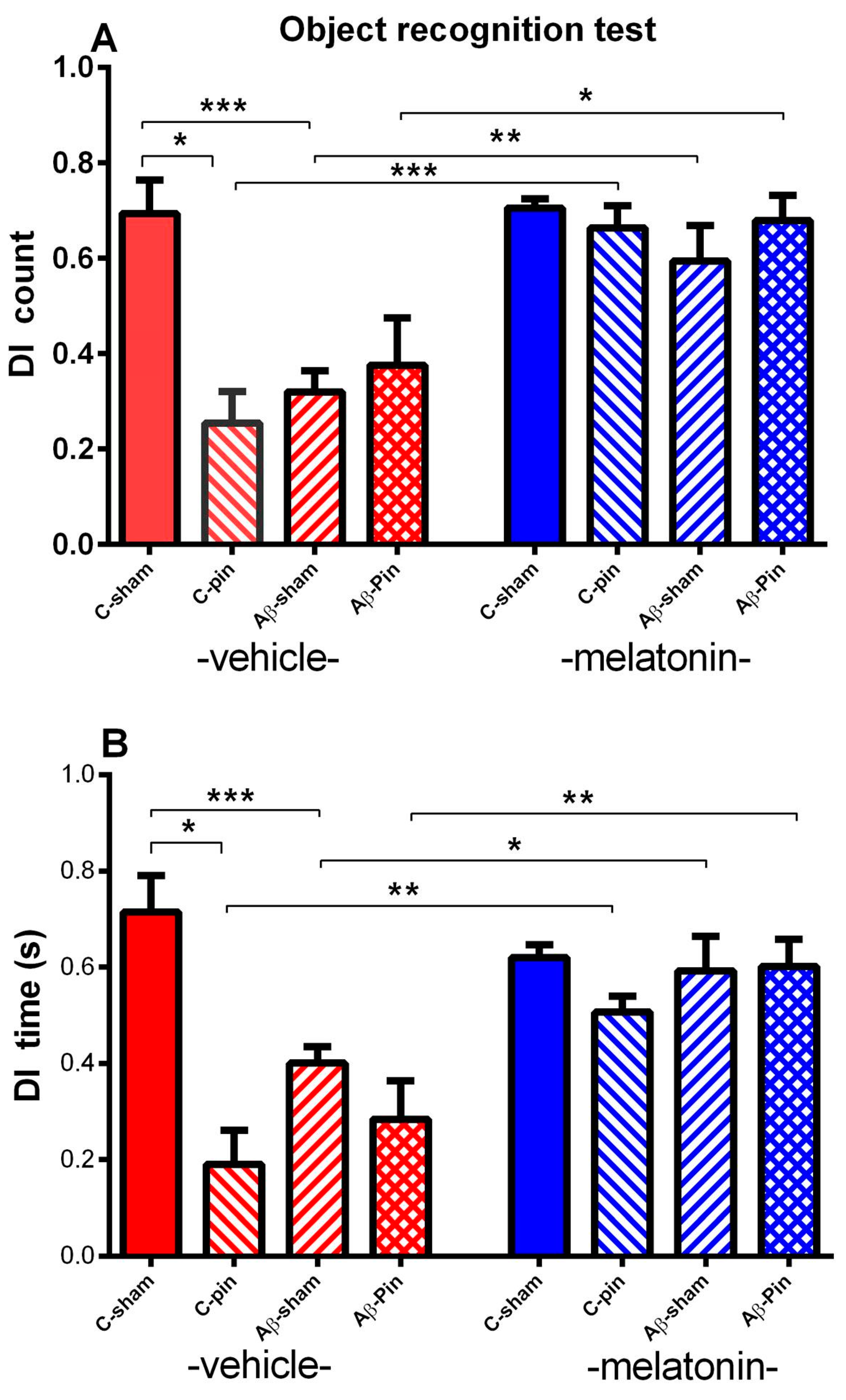
2.1.3. Object Recognition Test
2.2. Effects of Pinealectomy and Melatonin Treatment on Markers of Oxidative Stress in the Frontal Cortex and Hippocampus in icvAβ Rat Model
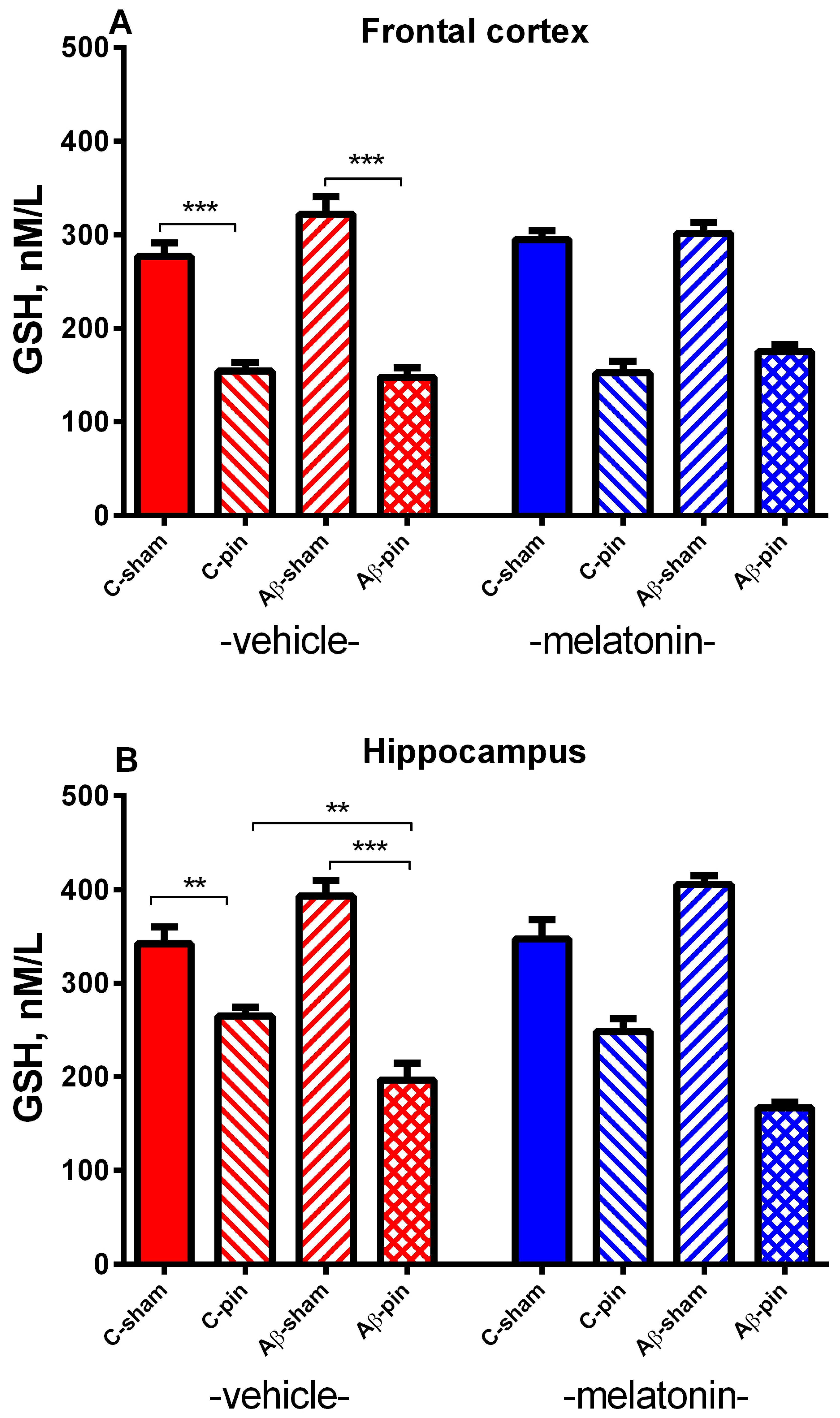
2.2.1. GSH Activity in the FC
2.2.2. GSH Activity in the Hippocampus
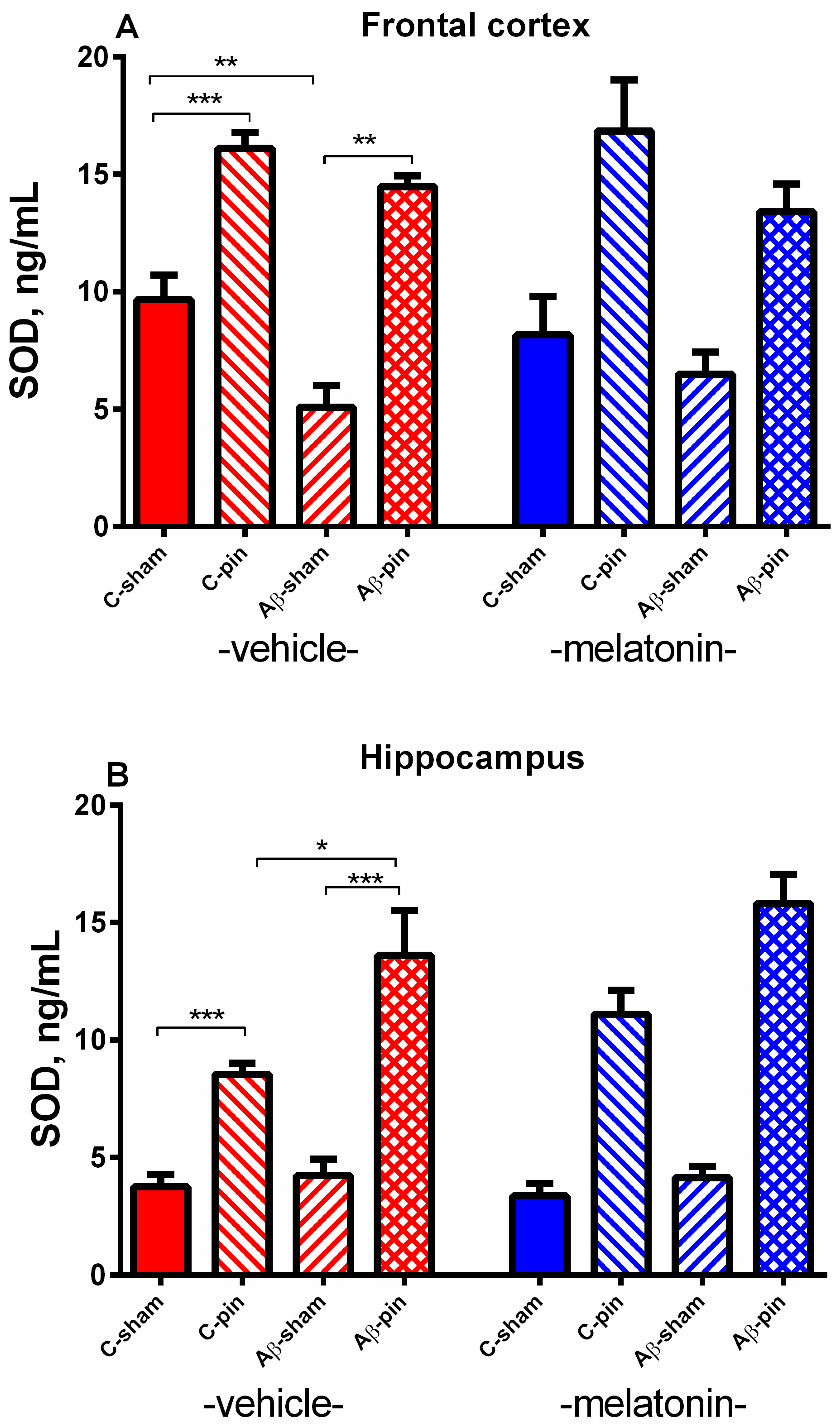
2.2.3. SOD Activity in the FC
2.2.4. SOD Activity in the Hippocampus
2.2.5. MDA Level in the FC
2.2.6. MDA Level in the Hippocampus
3. Discussion
4. Materials and Methods
4.1. Animals
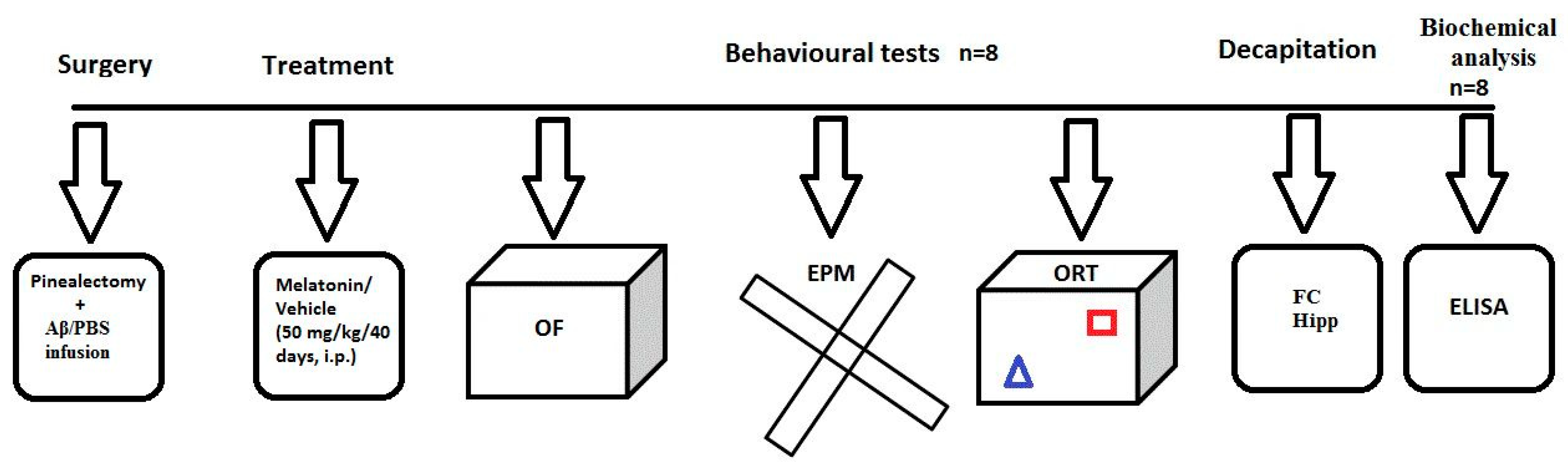
4.2. Experimental Design and Treatment with Melatonin
4.3. Surgery and icv Injection of Aβ1-42
4.4. Behavioral Tests
4.5. Detection of Markers Related to Oxidative Stress in the Homogenates from the Frontal Cortex and Hippocampus
4.5.1. Determination of GSH
4.5.2. Determination of SOD
4.5.3. Determination of MDA
4.5.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Jiang, T.; Sun, Q.; Chen, S. Oxidative stress: A major pathogenesis and potential therapeutic target of antioxidative agents in Parkinson’s disease and Alzheimer’s disease. Prog. Neurobiol. 2016, 147, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Markesbery, W.R.; Lovell, M.A. DNA oxidation in Alzheimer’s disease. Antioxid. Redox Signal. 2006, 8, 2039–2045. [Google Scholar] [CrossRef]
- Markesbery, W.R.; Lovell, M.A. Damage to lipids, proteins, DNA, and RNA in mild cognitive impairment. Arch. Neurol. 2007, 64, 954–956. [Google Scholar] [CrossRef] [PubMed]
- Moreira, P.I.; Nunomura, A.; Nakamura, M.; Takeda, A.; Shenk, J.C.; Aliev, G.; Smith, M.A.; Perry, G. Nucleic acid oxidation in Alzheimer disease. Free Radic. Biol. Med. 2008, 44, 1493–1505. [Google Scholar] [CrossRef] [PubMed]
- Oddo, S.; Caccamo, A.; Shepherd, J.D.; Murphy, M.P.; Golde, T.E.; Kayed, R.; Metherate, R.; Mattson, M.P.; Akbari, Y.; LaFerla, F.M. Triple-transgenic model of Alzheimer’s disease with plaques and tangles: Intracellular Abeta and synaptic dysfunction. Neuron 2003, 39, 409–421. [Google Scholar] [CrossRef]
- Halliwell, B. Reactive oxygen species and the central nervous system. J. Neurochem. 1992, 59, 1609–1623. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. Role of free radicals in the neurodegenerative diseases: Therapeutic implications for antioxidant treatment. Drugs Aging 2001, 18, 685–716. [Google Scholar] [CrossRef] [PubMed]
- Faraci, F.M. Reactive oxygen species: Influence on cerebral vascular tone. J. Appl. Physiol. 2006, 100, 739–743. [Google Scholar] [CrossRef] [PubMed]
- Mantha, A.K.; Moorthy, K.; Cowsik, S.M.; Baquer, N.Z. Neuroprotective role of neurokinin B (NKB) on beta-amyloid (25–35) induced toxicity in aging rat brain synaptosomes: Involvement in oxidative stress and excitotoxicity. Biogerontology 2006, 7, 1–17. [Google Scholar] [CrossRef]
- Jang, Y.C.; Rodriguez, K.; Lustgarten, M.S.; Muller, F.L.; Bhattacharya, A.; Pierce, A.; Choi, J.J.; Lee, N.H.; Chaudhuri, A.; Richardson, A.G.; et al. Superoxide-mediated oxidative stress accelerates skeletal muscle atrophy by synchronous activation of proteolytic systems. GeroScience 2020, 42, 1579–1591. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.C.; Lustgarten, M.S.; Liu, Y.; Muller, F.L.; Bhattacharya, A.; Liang, H.; Salmon, A.B.; Brooks, S.V.; Larkin, L.; Hayworth, C.R.; et al. Increased superoxide in vivo accelerates age-associated muscle atrophy through mitochondrial dysfunction and neuromuscular junction degeneration. FASEB J. 2010, 24, 1376–1390. [Google Scholar] [CrossRef] [PubMed]
- Praticò, D.; Delanty, N. Oxidative injury in diseases of the central nervous system: Focus on Alzheimer’s disease. Am. J. Med. 2000, 109, 577–585. [Google Scholar] [CrossRef]
- Floyd, R.A. Antioxidants, oxidative stress, and degenerative neurological disorders. Proc. Soc. Exp. Biol. Med. 1999, 222, 236–245. [Google Scholar] [CrossRef]
- Cetin, F. Role of oxidative stress in Aβ animal model of Alzheimer’s disease: Vicious circle of apoptosis, nitric oxide and age. In Neurodegenerative Diseases; Kishore, U., Ed.; IntechOpen: London, UK, 2013; pp. 76–99. [Google Scholar]
- Engelhart, M.J.; Geerlings, M.I.; Ruitenberg, A.; van Swieten, J.C.; Hofman, A.; Witteman, J.C.; Breteler, M.M. Dietary intake of antioxidants and risk of Alzheimer disease. JAMA 2002, 287, 3223–3229. [Google Scholar] [CrossRef]
- Morris, M.C.; Evans, D.A.; Bienias, J.L.; Tangney, C.C.; Bennett, D.A.; Aggarwal, N.; Wilson, R.S.; Scherr, P.A. Dietary intake of antioxidant nutrients and the risk of incident Alzheimer disease in a biracial community study. JAMA 2002, 287, 3230–3237. [Google Scholar] [CrossRef]
- Luchsinger, J.A.; Tang, M.X.; Shea, S.; Mayeux, R. Antioxidant vitamin intake and risk of Alzheimer disease. Arch. Neurol. 2003, 60, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Bubenik, G.A. Gastrointestinal melatonin: Localization, function, and clinical relevance. Dig. Dis. Sci. 2002, 47, 2336–2348. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Tan, D.X.; Osuna, C.; Gitto, E. Actions of melatonin in the reduction of oxidative stress. A review. J. Biomed. Sci. 2000, 7, 444–458. [Google Scholar] [CrossRef] [PubMed]
- Bondy, S.C.; Sharman, E.H. Melatonin and the aging brain. Neurochem. Int. 2007, 50, 571–580. [Google Scholar] [CrossRef] [PubMed]
- Morabito, R.; Remigante, A.; Marino, A. Melatonin Protects Band 3 Protein in Human Erythrocytes against H2O2-Induced Oxidative Stress. Molecules 2019, 24, 2741. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Huang, Q.X.; Yang, S.S.; Chu, J.; Wang, J.Z.; Tian, Q. Melatonin in Alzheimer’s disease. Int. J. Mol. Sci. 2013, 14, 14575–14593. [Google Scholar] [CrossRef]
- Ohashi, Y.; Okamoto, N.; Uchida, K.; Iyo, M.; Mori, N.; Morita, Y. Daily rhythm of serum melatonin levels and effect of light exposure in patients with dementia of the Alzheimer’s type. Biol. Psychiat. 1999, 45, 1646–1652. [Google Scholar] [CrossRef]
- Wu, Y.H.; Feenstra, M.G.; Zhou, J.N.; Liu, R.Y.; Torano, J.S.; van Kan, H.J.; Fischer, D.F.; Ravid, R.; Swaab, D.F. Molecular changes underlying reduced pineal melatonin levels in Alzheimer’s disease: Alterations in preclinical and clinical stages. J. Clin. Endocr. Metab. 2003, 88, 5898–5906. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.N.; Liu, R.Y.; Kamphorst, W.; Hofman, M.A.; Swaab, D.F. Early neuropathological Alzheimer’s changes in aged individuals are accompanied by decreased cerebrospinal fluid melatonin levels. J. Pineal Res. 2003, 35, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Savaskan, E.; Olivieri, G.; Meier, F.; Brydon, L.; Jockers, R.; Ravid, R.; Wirz-Justice, A.; Muller-Spahn, F. Increased melatonin 1a-receptor immunoreactivity in the hippocampus of Alzheimer’s disease patients. J. Pineal Res. 2002, 32, 59–62. [Google Scholar] [CrossRef] [PubMed]
- Savaskan, E.; Ayoub, M.A.; Ravid, R.; Angeloni, D.; Fraschini, F.; Meier, F.; Eckert, A.; Müller-Spahn, F.; Jockers, R. Reduced hippocampal MT2 melatonin receptor expression in Alzheimer’s disease. J. Pineal Res. 2005, 38, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Alluri, H.; Wilson, R.L.; Anasooya Shaji, C.; Wiggins-Dohlvik, K.; Patel, S.; Liu, Y.; Peng, X.; Beeram, M.R.; Davis, M.L.; Huang, J.H.; et al. Melatonin Preserves Blood-Brain Barrier Integrity and Permeability via Matrix Metalloproteinase-9 Inhibition. PLoS ONE 2016, 11, e0154427. [Google Scholar] [CrossRef] [PubMed]
- Namyen, J.; Permpoonputtana, K.; Nopparat, C.; Tocharus, J.; Tocharus, C.; Govitrapong, P. Protective Effects of Melatonin on Methamphetamine-Induced Blood-Brain Barrier Dysfunction in Rat Model. Neurotox. Res. 2020, 37, 640–660. [Google Scholar] [CrossRef] [PubMed]
- Brusco, L.I.; Márquez, M.; Cardinali, D.P. Melatonin treatment stabilizes chronobiologic and cognitive symptoms in Alzheimer’s disease. Neuroendocrinol. Lett. 2000, 21, 39–42. [Google Scholar] [PubMed]
- Cardinali, D.P.; Brusco, L.I.; Liberczuk, C.; Furio, A.M. The use of melatonin in Alzheimer’s disease. Neuroendocrinol. Lett. 2002, 23 (Suppl. 1), 20–23. [Google Scholar]
- Singer, C.; Tractenberg, R.E.; Kaye, J.; Schafer, K.; Gamst, A.; Grundman, M.; Thomas, R.; Thal, L.J.; Alzheimer’s Disease Cooperative Study. A multicenter, placebo-controlled trial of melatonin for sleep disturbance in Alzheimer’s disease. Sleep 2003, 26, 893–901. [Google Scholar] [CrossRef] [PubMed]
- O′Kelly, C.; Wang, X.; Raso, J.; Moreau, M.; Mahood, J.; Zhao, J.; Bagnall, K. The production of scoliosis after pinealectomy in young chickens, rats, and hamsters. Spine 1999, 24, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Beriwal, N.; Namgyal, T.; Sangay, P.; Al Quraan, A.M. Role of immune-pineal axis in neurodegenerative diseases, unraveling novel hybrid dark hormone therapies. Heliyon 2019, 5, e01190. [Google Scholar] [CrossRef] [PubMed]
- Cetin, F.; Dincer, S. The effect of intrahippocampal beta amyloid (1-42) peptide injection on oxidant and antioxidant status in rat brain. Ann. N. Y. Acad. Sci. 2007, 1100, 510–517. [Google Scholar] [CrossRef] [PubMed]
- Cioanca, O.; Hritcu, L.; Mihasan, M.; Hancianu, M. Cognitive-enhancing and antioxidant activities of inhaled coriander volatile oil in amyloid β(1-42) rat model of Alzheimer’s disease. Physiol. Behav. 2013, 120, 193–202. [Google Scholar] [CrossRef]
- Olariu, A.; Tran, M.H.; Yamada, K.; Mizuno, M.; Hefco, V.; Nabeshima, T. Memory deficits and increased emotionality induced by beta-amyloid (25-35) are correlated with the reduced acetylcholine release and altered phorbol dibutyrate binding in the hippocampus. J. Neural. Transm. 2001, 108, 1065–1079. [Google Scholar] [CrossRef] [PubMed]
- Ruan, L.; Kong, Y.; Wang, J.M.; Le, Y. Chemoattractants and receptors in Alzheimer’s disease. Front. Biosci. 2010, 2, 504–514. [Google Scholar] [CrossRef][Green Version]
- Butterfield, D.A.; Swomley, A.M.; Sultana, R. Amyloid β-Peptide (1-42)-Induced Oxidative Stress in Alzheimer Disease: Importance in Disease Pathogenesis and Progression. Antioxid. Redox Signal. 2013, 19, 823–835. [Google Scholar] [CrossRef]
- Wang, C.; Yang, X.M.; Zhuo, Y.Y.; Zhou, H.; Lin, H.B.; Cheng, Y.F.; Xu, J.P.; Zhang, H.T. The phosphodiesterase-4 inhibitor rolipram reverses Aβ-induced cognitive impairment and neuroinflammatory and apoptotic responses in rats. Int. J. Neuropsychopharmacol. 2012, 15, 749–766. [Google Scholar] [CrossRef] [PubMed]
- Jhoo, J.H.; Kim, H.C.; Nabeshima, T.; Yamada, K.; Shin, E.J.; Jhoo, W.K.; Kim, W.; Kang, K.S.; Jo, S.A.; Woo, J.I. Beta-amyloid (1-42)-induced learning and memory deficits in mice: Involvement of oxidative burdens in the hippocampus and cerebral cortex. Behav. Brain Res. 2004, 155, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Srivareerat, M.; Tran, T.T.; Salim, S.; Aleisa, A.M.; Alkadhi, K.A. Chronic nicotine restores normal Aβ levels and prevents short-term memory and E-LTP impairment in Aβ rat model of Alzheimer’s disease. Neurobiol. Aging 2011, 32, 834–844. [Google Scholar] [CrossRef] [PubMed]
- Eftekharzadeh, B.; Ramin, M.; Khodagholi, F.; Moradi, S.; Tabrizian, K.; Sharif, R.; Azami, K.; Beyer, C.; Sharifzadeh, M. Inhibition of PKA attenuates memory deficits induced by β-amyloid (1-42), and decreases oxidative stress and NF-κB transcription factors. Behav. Brain Res. 2012, 226, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Asadbegi, M.; Yaghmaei, P.; Salehi, I.; Ebrahim-Habibi, A.; Komaki, A. Neuroprotective effects of metformin against Aβ-mediated inhibition of long-term potentiation in rats fed a high-fat diet. Brain Res. Bull. 2016, 121, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Tchekalarova, J.; Atanasova, D.; Nenchovska, Z.; Atanasova, M.; Kortenska, L.; Gesheva, R.; Lazarov, N. Agomelatine protects against neuronal damage without preventing epileptogenesis in the kainate model of temporal lobe epilepsy. Neurobiol. Dis. 2017, 104, 1–14. [Google Scholar] [CrossRef]
- Ilieva, K.; Atanasova, M.; Atanasova, D.; Kortenska, L.; Tchekalarova, J. Chronic agomelatine treatment alleviates icvAβ-induced anxiety and depressive-like behavior through affecting Aβ metabolism in the hippocampus in a rat model of Alzheimer’s disease. Physiol. Behav. 2021, 239, 113525. [Google Scholar] [CrossRef] [PubMed]
- Schipper, H.M.; Chertkow, H.; Mehindate, K.; Frankel, D.; Melmed, C.; Bergman, H. Evaluation of heme oxygenase-1 as a systemic biological marker of sporadic AD. Neurology 2000, 54, 1297–1304. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.M.; Zhang, Y. Melatonin: A well-documented antioxidant with conditional pro-oxidant actions. J. Pineal Res. 2014, 57, 131–146. [Google Scholar] [CrossRef] [PubMed]
- Tchekalarova, J.; Nenchovska, Z.; Atanasova, D.; Atanasova, M.; Kortenska, L.; Stefanova, M.; Alova, L.; Lazarov, N. Consequences of long-term treatment with agomelatine on depressive-like behavior and neurobiological abnormalities in pinealectomized rats. Behav. Brain Res. 2016, 302, 11–28. [Google Scholar] [CrossRef] [PubMed]
- Appenrodt, E.; Schwarzberg, H. Central vasopressin administration failed to influence anxiety behavior after pinealectomy in rats. Physiol. Behav. 2000, 68, 735–739. [Google Scholar] [CrossRef]
- Bustamante-García, R.; Lira-Rocha, A.S.; Espejo-González, O.; Gómez-Martínez, A.E.; Picazo, O. Anxiolytic-like effects of a new 1-N substituted analog of melatonin in pinealectomized rats. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2014, 51, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Karakaş, A.; Coşkun, H.; Kaya, A.; Kücük, A.; Gündüz, B. The effects of the intraamygdalar melatonin injections on the anxiety like behavior and the spatial memory performance in male Wistar rats. Behav. Brain Res. 2011, 222, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Zahra, E.; Siham, O.; Abdelhalim, M.; Aboubakr, E.; Ali, O. Pinealectomy and exogenous melatonin regulate anxiety-like and depressive-like behaviors inmale and female wistar rats. Neurosci. Med. 2012, 3, 394–403. [Google Scholar] [CrossRef]
- Sharma, S.; Verma, S.; Kapoor, M.; Saini, A.; Nehru, B. Alzheimer’s disease like pathology induced six weeks after aggregated amyloid-beta injection in rats: Increased oxidative stress and impaired long-term memory with anxiety-like behavior. Neurol. Res. 2016, 38, 838–850. [Google Scholar] [CrossRef]
- Boyd-Kimball, D.; Sultana, R.; Mohmmad-Abdul, H.; Butterfield, D.A. Rodent Abeta(1-42) exhibits oxidative stress properties similar to those of human Abeta(1-42): Implications for proposed mechanisms of toxicity. J. Alzheimer’s Dis. 2004, 6, 515–525. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, C.; Zhang, J.H.; Cai, J.M.; Cao, Y.P.; Sun, X.J. Hydrogen-rich saline improves memory function in a rat model of amyloid-beta-induced Alzheimer’s disease by reduction of oxidative stress. Brain Res. 2010, 1328, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Saeed, K.; Shah, S.A.; Ullah, R.; Alam, S.I.; Park, J.S.; Saleem, S.; Jo, M.H.; Kim, M.W.; Hahm, J.R.; Kim, M.O. Quinovic Acid Impedes Cholesterol Dyshomeostasis, Oxidative Stress, and Neurodegeneration in an Amyloid-β-Induced Mouse Model. Oxidative Med. Cell. Longev. 2020, 2020, 9523758. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Orellana, A.; Kohler, I.; Frölich, L.; de Rojas, I.; Gil, S.; Boada, M.; Hernández, I.; Hausner, L.; Bakker, M.H.M.; et al. Association of lysophosphatidic acids with cerebrospinal fluid biomarkers and progression to Alzheimer’s disease. Alzheimer’s Res. Ther. 2020, 12, 124. [Google Scholar] [CrossRef]
- Tasdemir, S.; Samdanci, E.; Parlakpinar, H.; Polat, A.; Tasdemir, C.; Cengiz, N.; Sapmaz, H.; Acet, A. Effects of pinealectomy and exogenous melatonin on the brains, testes, duodena and stomachs of rats. Eur. Rev. Med. Pharmacol. Sci. 2012, 16, 860–866. [Google Scholar] [PubMed]
- Sahna, E.; Parlakpinar, H.; Vardi, N.; Ciğremis, Y.; Acet, A. Efficacy of melatonin as protectant against oxidative stress and structural changes in liver tissue in pinealectomized rats. Acta Histochem. 2004, 106, 331–336. [Google Scholar] [CrossRef] [PubMed]
- McCord, J.M. The evolution of free radicals and oxidative stress. Am. J. Med. 2000, 108, 652–659. [Google Scholar] [CrossRef]
- McCord, J.M. Superoxide dismutase, lipid peroxidation, and bell-shaped dose response curves. Dose-Response 2008, 6, 223–238. [Google Scholar] [CrossRef] [PubMed]
- Tchekalarova, J.; Stoyanova, T.; Nenchovska, Z.; Ivanova, N.; Atanasova, D.; Atanasova, M.; Georgieva, K. Effect of endurance training on diurnal rhythms of superoxide dismutase activity, glutathione and lipid peroxidation in plasma of pinealectomized rats. Neurosci. Lett. 2020, 716, 637. [Google Scholar] [CrossRef]
- Hoffman, R.A.; Reiter, R.J. Rapid pinealectomy in hamsters and other small rodents. Anat. Rec. 1965, 153, 19–21. [Google Scholar] [CrossRef] [PubMed]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates, 6th ed.; Academic Press: San Diego, CA, USA, 2007. [Google Scholar]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tzoneva, R.; Georgieva, I.; Ivanova, N.; Uzunova, V.; Nenchovska, Z.; Apostolova, S.; Stoyanova, T.; Tchekalarova, J. The Role of Melatonin on Behavioral Changes and Concomitant Oxidative Stress in icvAβ1-42 Rat Model with Pinealectomy. Int. J. Mol. Sci. 2021, 22, 12763. https://doi.org/10.3390/ijms222312763
Tzoneva R, Georgieva I, Ivanova N, Uzunova V, Nenchovska Z, Apostolova S, Stoyanova T, Tchekalarova J. The Role of Melatonin on Behavioral Changes and Concomitant Oxidative Stress in icvAβ1-42 Rat Model with Pinealectomy. International Journal of Molecular Sciences. 2021; 22(23):12763. https://doi.org/10.3390/ijms222312763
Chicago/Turabian StyleTzoneva, Rumiana, Irina Georgieva, Natasha Ivanova, Veselina Uzunova, Zlatina Nenchovska, Sonia Apostolova, Tzveta Stoyanova, and Jana Tchekalarova. 2021. "The Role of Melatonin on Behavioral Changes and Concomitant Oxidative Stress in icvAβ1-42 Rat Model with Pinealectomy" International Journal of Molecular Sciences 22, no. 23: 12763. https://doi.org/10.3390/ijms222312763
APA StyleTzoneva, R., Georgieva, I., Ivanova, N., Uzunova, V., Nenchovska, Z., Apostolova, S., Stoyanova, T., & Tchekalarova, J. (2021). The Role of Melatonin on Behavioral Changes and Concomitant Oxidative Stress in icvAβ1-42 Rat Model with Pinealectomy. International Journal of Molecular Sciences, 22(23), 12763. https://doi.org/10.3390/ijms222312763







