BTK and PI3K Inhibitors Reveal Synergistic Inhibitory Anti-Tumoral Effects in Canine Diffuse Large B-Cell Lymphoma Cells
Abstract
:1. Introduction
2. Results
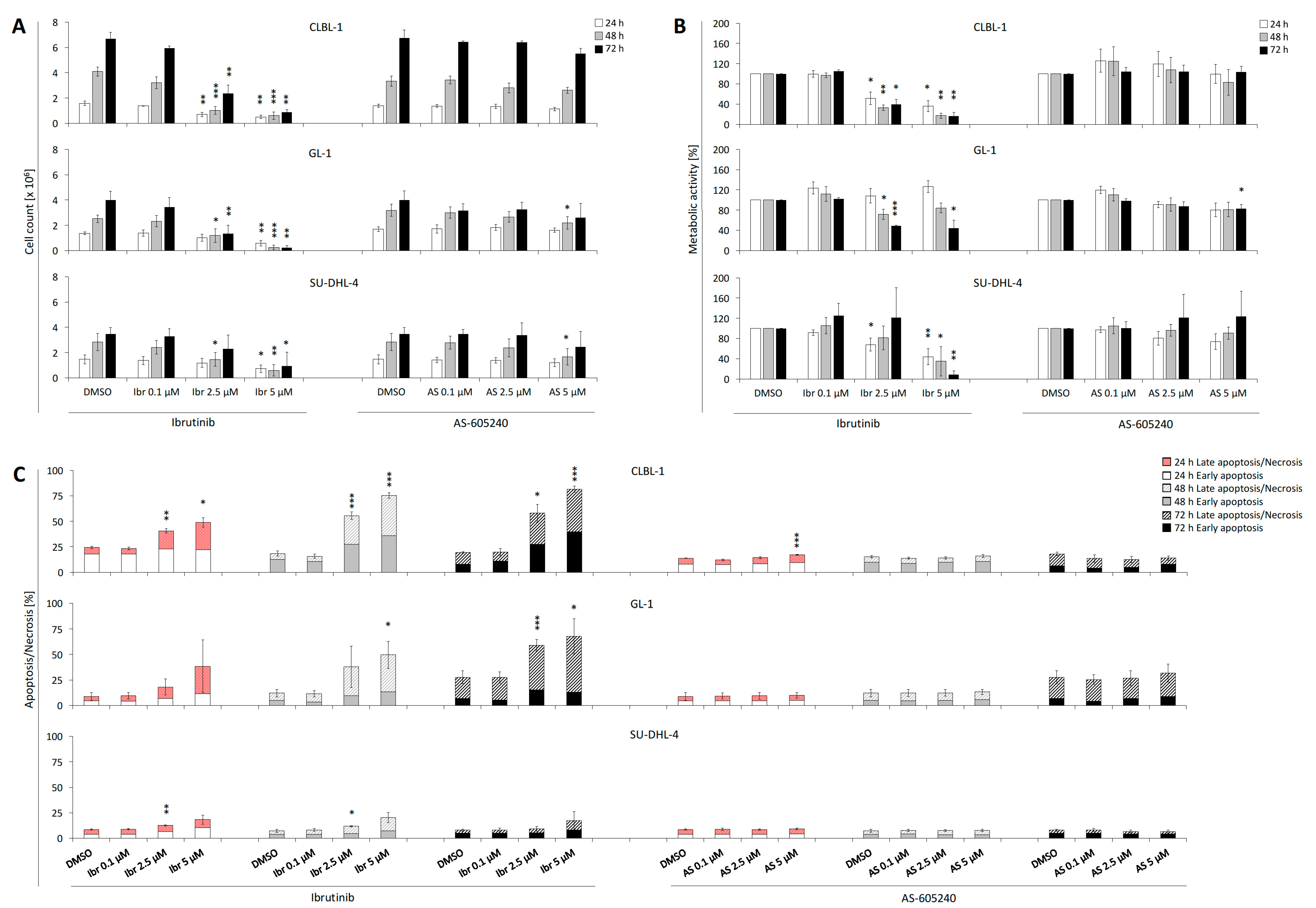
2.1. Single Treatment of Ibrutinib Exhibits Anti-proliferative Effects on CLBL-1, GL-1, and SU-DHL-4
2.2. Single Treatment of Ibrutinib Induces Apoptosis and Necrosis of CLBL-1, GL-1, and SU-DHL-4
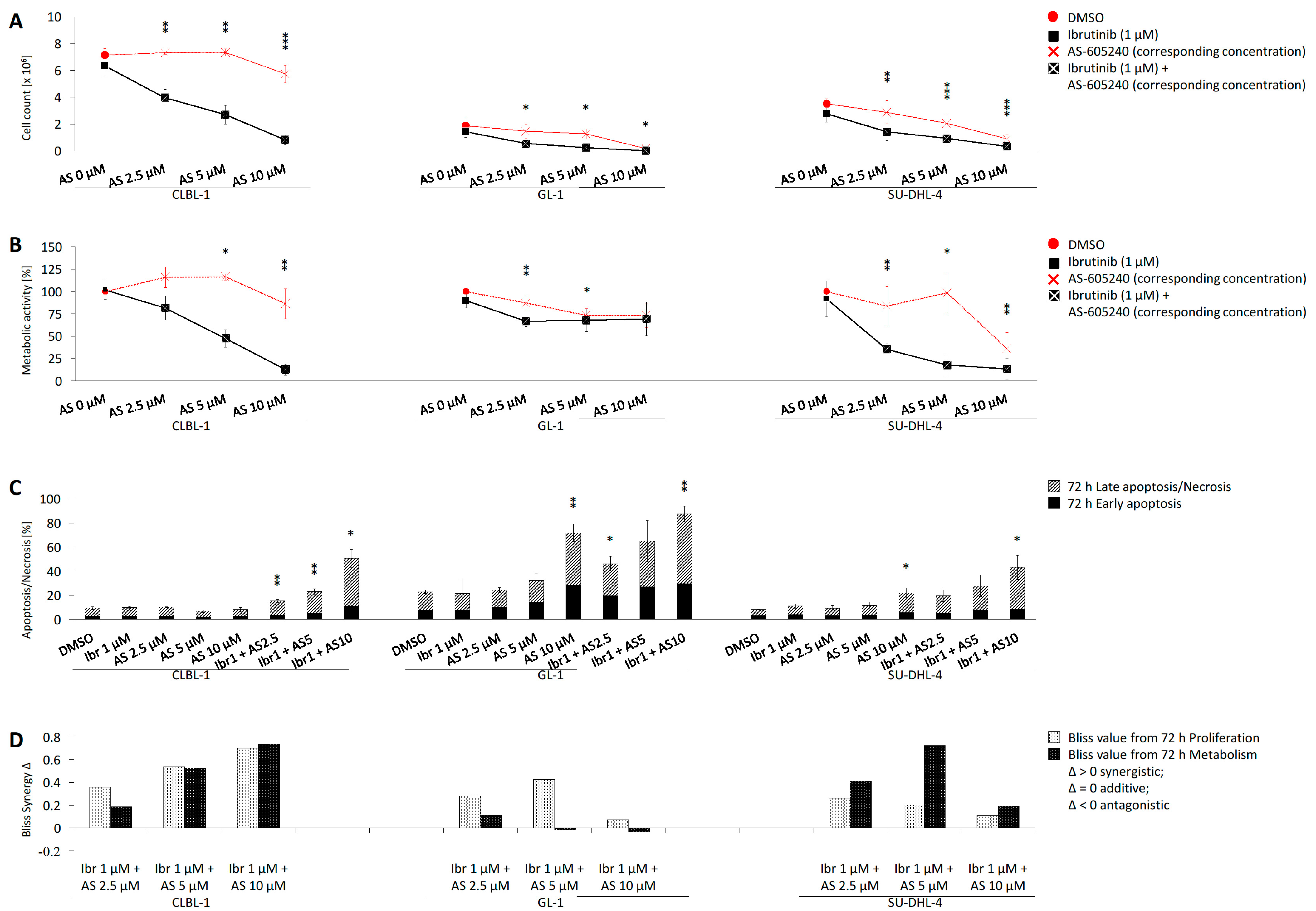
2.3. Combined Application of Ibrutinib and AS-605240 Enhances Inhibition of CLBL-1 Proliferation and Metabolic Activity
2.4. Combined Application of Ibrutinib and AS-605240 Induces Apoptosis and Necrosis of CLBL-1
2.5. Bliss Analysis Reveals Synergistic Effects of Ibrutinib and AS-605240
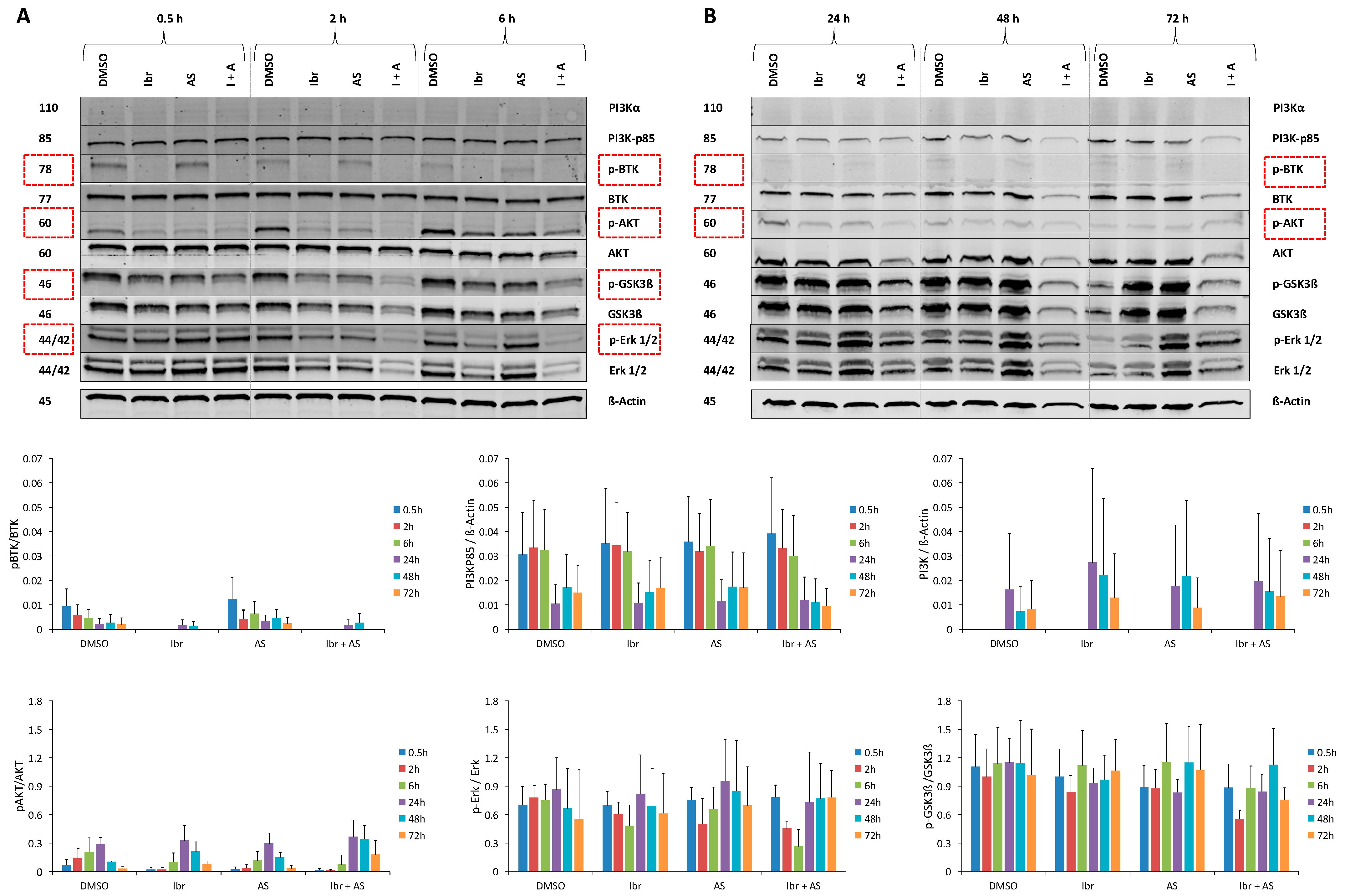
2.6. Single or Combined Ibrutinib and AS-605240 Reduce the Ratio of Phosphorylated and Total Forms of Target Proteins of CLBL-1
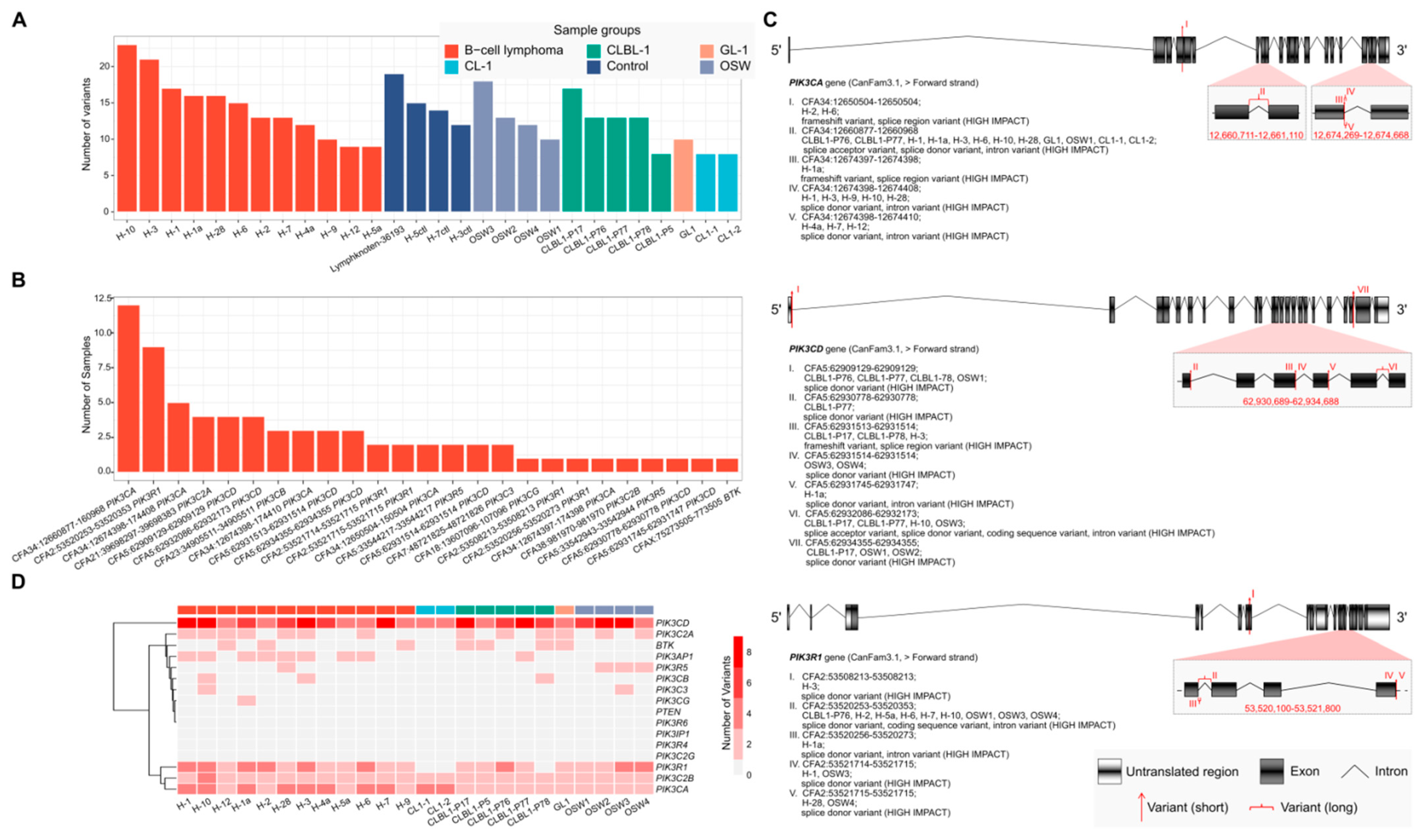
2.7. RNA-Seq Analysis of Canine B- and T-lymphoid Cell Lines and Primary Lymphoma Samples Reveals Potentially High-Impact Variants Predominantly in PI3K Transcripts
3. Discussion
4. Materials and Methods
4.1. Cell Lines and Culture Condition
4.2. Tyrosine Kinase Inhibitors
4.3. Single Drug Application Experiments
4.4. Combined Drug Application Experiments
4.5. Cell Proliferation and Metabolic Activity
4.6. Apoptosis and Necrosis Measurement
4.7. Detection of Synergistic Drug Combinations
4.8. Examination of Cell Morphology
4.9. Protein Expression Analyses Using Western Blot
4.10. Variant Calling on RNA-Seq Data
4.11. Statistical Analysis and CLBL-1 IC50 Identification
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- DeWeerdt, S. How dogs are teaching researchers new tricks for treating cancer. Nat. Cell Biol. 2018, 563, S50–S51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teske, E.; Rutteman, G.R.; van Heerde, P.; Misdorp, W. Polyethylene glycol-L-asparaginase versus native L-asparaginase in canine non-Hodgkin’s lymphoma. Eur. J. Cancer 1990, 26, 891–895. [Google Scholar] [CrossRef]
- Vail, D.M.; MacEwen, E.G. Spontaneously Occurring Tumors of Companion Animals as Models for Human Cancer. Cancer Investig. 2000, 18, 781–792. [Google Scholar] [CrossRef] [PubMed]
- Marconato, L.; Aresu, L.; Stefanello, D.; Comazzi, S.; Martini, V.; Ferrari, R.; Riondato, F.; Rouquet, N.; Frayssinet, P.; Sabattini, S. Opportunities and challenges of active immunotherapy in dogs with B-cell lymphoma: A 5-year experience in two veterinary oncology centers. J. Immunother. Cancer 2019, 7, 146. [Google Scholar] [CrossRef]
- Saba, C.F.; Vickery, K.R.; Clifford, C.A.; Burgess, K.E.; Phillips, B.; Vail, D.M.; Wright, Z.M.; Morges, M.A.; Fan, T.M.; Thamm, D.H. Rabacfosadine for relapsed canine B-cell lymphoma: Efficacy and adverse event profiles of 2 different doses. Veter- Comp. Oncol. 2017, 16, E76–E82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davis, R.E.; Ngo, V.; Lenz, G.; Tolar, P.; Young, R.M.; Romesser, P.; Kohlhammer, H.; Lamy, L.; Zhao, H.; Yang, Y.; et al. Chronic active B-cell-receptor signalling in diffuse large B-cell lymphoma. Nat. Cell Biol. 2010, 463, 88–92. [Google Scholar] [CrossRef] [Green Version]
- Wilson, W.H.; Young, R.M.; Schmitz, R.; Yang, Y.; Pittaluga, S.; Wright, G.; Lih, C.-J.; Williams, P.M.; Shaffer, A.L.; Gerecitano, J.; et al. Targeting B cell receptor signaling with ibrutinib in diffuse large B cell lymphoma. Nat. Med. 2015, 21, 922–926. [Google Scholar] [CrossRef] [PubMed]
- Honigberg, L.A.; Smith, A.M.; Sirisawad, M.; Verner, E.; Loury, D.; Chang, B.; Li, S.; Pan, Z.; Thamm, D.H.; Miller, R.A.; et al. The Bruton tyrosine kinase inhibitor PCI-32765 blocks B-cell activation and is efficacious in models of autoimmune disease and B-cell malignancy. Proc. Natl. Acad. Sci. USA 2010, 107, 13075–13080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harrington, B.K.; Gardner, H.L.; Izumi, R.; Hamdy, A.; Rothbaum, W.; Coombes, K.R.; Covey, T.; Kaptein, A.; Gulrajani, M.; Van Lith, B.; et al. Preclinical Evaluation of the Novel BTK Inhibitor Acalabrutinib in Canine Models of B-Cell Non-Hodgkin Lymphoma. PLoS ONE 2016, 11, e0159607. [Google Scholar] [CrossRef]
- Bojarczuk, K.; Wienand, K.; Ryan, J.A.; Chen, L.; Villalobos-Ortiz, M.; Mandato, E.; Shipp, M.A. Targeted inhibition of PI3Kalpha/delta is synergistic with BCL-2 blockade in genetically defined subtypes of DLBCL. Blood 2019, 133, 70–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scuoppo, C.; Wang, J.; Persaud, M.; Mittan, S.K.; Basso, K.; Pasqualucci, L.; Rabadan, R.; Inghirami, G.; Grandori, C.; Bosch, F.; et al. Repurposing dasatinib for diffuse large B cell lymphoma. Proc. Natl. Acad. Sci. USA 2019, 116, 16981–16986. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.T.; Tan, K.A.; Pang, L.Y.; Argyle, D.J. The class I PI3K/Akt pathway is critical for cancer cell survival in dogs and offers an opportunity for therapeutic intervention. BMC Vet. Res. 2012, 8, 73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gardner, H.L.; Rippy, S.B.; Bear, M.D.; Cronin, K.L.; Heeb, H.; Burr, H.; Cannon, C.M.; Penmetsa, K.V.; Viswanadha, S.; Vakkalanka, S.; et al. Phase I/II evaluation of RV1001, a novel PI3Kδ inhibitor, in spontaneous canine lymphoma. PLoS ONE 2018, 13, e0195357. [Google Scholar] [CrossRef] [Green Version]
- Niemann, C.U.; Mora-Jensen, H.I.; Dadashian, E.L.; Krantz, F.; Covey, T.; Chen, S.S.; Herman, S.E. Combined BTK and PI3Kdelta Inhibition with Acalabrutinib and ACP-319 Improves Survival and Tumor Control in CLL Mouse Model. Clin. Cancer Res. 2017, 23, 5814–5823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bichi, R.; Shinton, S.A.; Martin, E.S.; Koval, A.; Calin, G.; Cesari, R.; Russo, G.; Hardy, R.R.; Croce, C.M. Human chronic lymphocytic leukemia modeled in mouse by targeted TCL1 expression. Proc. Natl. Acad. Sci. USA 2002, 99, 6955–6960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kapoor, I.; Li, Y.; Sharma, A.; Zhu, H.; Bodo, J.; Xu, W.; Hsi, E.D.; Hill, B.T.; Almasan, A. Resistance to BTK inhibition by ibrutinib can be overcome by preventing FOXO3a nuclear export and PI3K/AKT activation in B-cell lymphoid malignancies. Cell Death Dis. 2019, 10, 924. [Google Scholar] [CrossRef] [PubMed]
- Woyach, J.A.; Furman, R.R.; Liu, T.M.; Ozer, H.G.; Zapatka, M.; Ruppert, A.S.; Byrd, J.C. Resistance mechanisms for the Bruton’s tyrosine kinase inhibitor ibrutinib. N. Engl. J. Med. 2014, 370, 2286–2294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Furman, R.R.; Cheng, S.; Lu, P.; Setty, M.; Perez, A.R.; Guo, A.; Racchumi, J.; Xu, G.; Wu, H.; Ma, J.; et al. Ibrutinib Resistance in Chronic Lymphocytic Leukemia. N. Engl. J. Med. 2014, 370, 2352–2354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ilic, N.; Utermark, T.; Widlund, H.R.; Roberts, T.M. PI3K-targeted therapy can be evaded by gene amplification along the MYC-eukaryotic translation initiation factor 4E (eIF4E) axis. Proc. Natl. Acad. Sci. USA 2011, 108, E699–E708. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.; Lu, P.; Lee, F.Y.; Chadburn, A.; Barrientos, J.C.; Leonard, J.P.; Ye, F.; Zhang, D.; Knowles, D.M.; Wang, Y.L. Tyrosine kinase inhibition in diffuse large B-cell lymphoma: Molecular basis for antitumor activity and drug resistance of dasatinib. Leukemia 2008, 22, 1755–1766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gamperl, S.; Stefanzl, G.; Peter, B.; Smiljkovic, D.; Bauer, K.; Willmann, M.; Valent, P.; Hadzijusufovic, E. Effects of ibrutinib on proliferation and histamine release in canine neoplastic mast cells. Veter- Comp. Oncol. 2019, 17, 553–561. [Google Scholar] [CrossRef]
- Imbruvica (ibrutinib): European Medicines Agency. 2014. Available online: https://www.ema.europa.eu/en/documents/assessment-report/imbruvica-epar-public-assessment-report_en.pdf (accessed on 14 December 2020).
- Furman, R.R.; Sharman, J.P.; Coutre, S.E.; Cheson, B.D.; Pagel, J.M.; Hillmen, P.; Barrientos, J.; Zelenetz, A.D.; Kipps, T.J.; Flinn, I.; et al. Idelalisib and Rituximab in Relapsed Chronic Lymphocytic Leukemia. N. Engl. J. Med. 2014, 370, 997–1007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- ZYDELIG (idelalisib): Food and Drug Administration; FDA. 2014. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/205858lbl.pdf (accessed on 14 December 2020).
- Zydeligidelalisib: European Medicines Agency. 2016. Available online: https://imedikament.de/zydelig (accessed on 14 December 2020).
- Silveira, A.; Laranjeira, A.B.A.; Rodrigues, G.O.L.; Leal, P.C.; Cardoso, B.; Barata, J.; Yunes, R.A.; Zanchin, N.; Brandalise, S.R.; Yunes, J.A. PI3K inhibition synergizes with glucocorticoids but antagonizes with methotrexate in T-cell acute lymphoblastic leukemia. Oncotarget 2015, 6, 13105–13118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rütgen, B.C.; Hammer, S.E.; Gerner, W.; Christian, M.; de Arespacochaga, A.G.; Willmann, M.; Kleiter, M.; Schwendenwein, I.; Saalmüller, A. Establishment and characterization of a novel canine B-cell line derived from a spontaneously occurring diffuse large cell lymphoma. Leuk. Res. 2010, 34, 932–938. [Google Scholar] [CrossRef] [PubMed]
- Griner, L.A.M.; Guha, R.; Shinn, P.; Young, R.M.; Keller, J.M.; Liu, D.; Goldlust, I.S.; Yasgar, A.; McKnight, C.; Boxer, M.B.; et al. High-throughput combinatorial screening identifies drugs that cooperate with ibrutinib to kill activated B-cell–like diffuse large B-cell lymphoma cells. Proc. Natl. Acad. Sci. USA 2014, 111, 2349–2354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, J.R. Ibrutinib in chronic lymphocytic leukemia and B cell malignancies. Leuk. Lymphoma 2014, 55, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Chang, B.Y.; Francesco, M.; De Rooij, M.F.M.; Magadala, P.; Steggerda, S.M.; Huang, M.M.; Kuil, A.; Herman, S.E.M.; Chang, S.; Pals, S.T.; et al. Egress of CD19+CD5+ cells into peripheral blood following treatment with the Bruton tyrosine kinase inhibitor ibrutinib in mantle cell lymphoma patients. Blood 2013, 122, 2412–2424. [Google Scholar] [CrossRef] [PubMed]
- Guo, A.; Lu, P.; Galanina, N.; Nabhan, C.; Smith, S.M.; Coleman, M.; Wang, Y.L. Heightened BTK-dependent cell proliferation in unmutated chronic lymphocytic leukemia confers increased sensitivity to ibrutinib. Oncotarget 2015, 7, 4598–4610. [Google Scholar] [CrossRef] [Green Version]
- Wei, X.; Han, J.; Chen, Z.-Z.; Qi, B.-W.; Wang, G.-C.; Ma, Y.-H.; Zheng, H.; Luo, Y.-F.; Wei, Y.-Q.; Chen, L.-J. A phosphoinositide 3-kinase-γ inhibitor, AS605240 prevents bleomycin-induced pulmonary fibrosis in rats. Biochem. Biophys. Res. Commun. 2010, 397, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Hurtz, C.; Koehrer, S.; Wang, Z.; Balasubramanian, S.; Chang, B.Y.; Müschen, M.; Davis, R.E.; Burger, J.A. Ibrutinib inhibits pre-BCR+ B-cell acute lymphoblastic leukemia progression by targeting BTK and BLK. Blood 2017, 129, 1155–1165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spitzenberg, V.; König, C.; Ulm, S.; Marone, R.; Röpke, L.; Müller, J.P.; Grün, M.; Bauer, R.; Rubio, I.; Wymann, M.; et al. Targeting PI3K in neuroblastoma. J. Cancer Res. Clin. Oncol. 2010, 136, 1881–1890. [Google Scholar] [CrossRef] [PubMed]
- Camps, M.; Rückle, T.; Ji, H.; Ardissone, V.; Rintelen, F.; Shaw, J.; Rommel, C. Blockade of PI3Kgamma suppresses joint inflammation and damage in mouse models of rheumatoid arthritis. Nat. Med. 2005, 11, 936–943. [Google Scholar] [CrossRef] [PubMed]
- Seda, V.; Mraz, M. B-cell receptor signalling and its crosstalk with other pathways in normal and malignant cells. Eur. J. Haematol. 2014, 94, 193–205. [Google Scholar] [CrossRef]
- He, Y.; Li, J.; Ding, N.; Wang, X.; Deng, L.; Xie, Y.; Ying, Z.; Liu, W.; Ping, L.; Zhang, C.; et al. Combination of Enzastaurin and Ibrutinib synergistically induces anti-tumor effects in diffuse large B cell lymphoma. J. Exp. Clin. Cancer Res. 2019, 38, 86. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Ma, J.; Guo, A.; Lu, P.; Leonard, J.P.; Coleman, M.P.; Liu, M.; Buggy, J.J.; Furman, R.R.; Wang, Y.L. BTK inhibition targets in vivo CLL proliferation through its effects on B-cell receptor signaling activity. Leukemia 2014, 28, 649–657. [Google Scholar] [CrossRef]
- Yoo, S.K.; Deng, Q.; Cavnar, P.J.; Wu, Y.I.; Hahn, K.; Huttenlocher, A. Differential Regulation of Protrusion and Polarity by PI(3)K during Neutrophil Motility in Live Zebrafish. Dev. Cell 2010, 18, 226–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakaichi, M.; Taura, Y.; Kanki, M.; Mamba, K.; Momoi, Y.; Tsujimoto, H.; Nakama, S. Establishment and Characterization of a New Canine B-Cell Leukemia Cell Line. J. Veter- Med Sci. 1996, 58, 469–471. [Google Scholar] [CrossRef] [Green Version]
- Epstein, A.L.; Kaplan, H.S. Feeder layer and nutritional requirements for the establishment and cloning of human malignant lymphoma cell lines. Cancer Res. 1979, 39, 1748–1759. [Google Scholar]
- Brauchle, E.; Thude, S.; Brucker, S.Y.; Schenke-Layland, K. Cell death stages in single apoptotic and necrotic cells monitored by Raman microspectroscopy. Sci. Rep. 2014, 4, 4698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roolf, C.; Richter, A.; Konkolefski, C.; Knuebel, G.; Sekora, A.; Krohn, S.; Stenzel, J.; Krause, B.J.; Vollmar, B.; Escobar, H.M.; et al. Decitabine demonstrates antileukemic activity in B cell precursor acute lymphoblastic leukemia with MLL rearrangements. J. Hematol. Oncol. 2018, 11, 62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foucquier, J.; Guedj, M. Analysis of drug combinations: Current methodological landscape. Pharmacol. Res. Perspect. 2015, 3, e00149. [Google Scholar] [CrossRef] [PubMed]
- Momoi, Y.; Okai, Y.; Watari, T.; Goitsuka, R.; Tsujimoto, H.; Hasegawa, A. Establishment and characterization of a canine T-lymphoblastoid cell line derived from malignant lymphoma. Veter- Immunol. Immunopathol. 1997, 59, 11–20. [Google Scholar] [CrossRef]
- Kisseberth, W.C.; Nadella, M.V.P.; Breen, M.; Thomas, R.; Duke, S.E.; Murahari, S.; Kosarek, C.E.; Vernau, W.; Avery, A.C.; Burkhard, M.J.; et al. A novel canine lymphoma cell line: A translational and comparative model for lymphoma research. Leuk. Res. 2007, 31, 1709–1720. [Google Scholar] [CrossRef] [PubMed]
- Taher, L.; Beck, J.; Liu, W.; Roolf, C.; Soller, J.T.; Rütgen, B.C.; Hammer, S.E.; Chodisetti, M.; Sender, S.; Sterenczak, K.A.; et al. Comparative High-Resolution Transcriptome Sequencing of Lymphoma Cell Lines and de novo Lymphomas Reveals Cell-Line-Specific Pathway Dysregulation. Sci. Rep. 2018, 8, 6279. [Google Scholar] [CrossRef] [PubMed]
- McKenna, A.; Hanna, M.; Banks, E.; Sivachenko, A.; Cibulskis, K.; Kernytsky, A.; Garimella, K.; Altshuler, D.; Gabriel, S.; Daly, M.; et al. The Genome Analysis Toolkit: A MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010, 20, 1297–1303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McLaren, W.; Gil, L.; Hunt, S.E.; Riat, H.S.; Ritchie, G.R.S.; Thormann, A.; Flicek, P.; Cunningham, F. The Ensembl Variant Effect Predictor. Genome Biol. 2016, 17, 122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kong, W.; Sender, S.; Taher, L.; Villa-Perez, S.; Ma, Y.; Sekora, A.; Ruetgen, B.C.; Brenig, B.; Beck, J.; Schuetz, E.; et al. BTK and PI3K Inhibitors Reveal Synergistic Inhibitory Anti-Tumoral Effects in Canine Diffuse Large B-Cell Lymphoma Cells. Int. J. Mol. Sci. 2021, 22, 12673. https://doi.org/10.3390/ijms222312673
Kong W, Sender S, Taher L, Villa-Perez S, Ma Y, Sekora A, Ruetgen BC, Brenig B, Beck J, Schuetz E, et al. BTK and PI3K Inhibitors Reveal Synergistic Inhibitory Anti-Tumoral Effects in Canine Diffuse Large B-Cell Lymphoma Cells. International Journal of Molecular Sciences. 2021; 22(23):12673. https://doi.org/10.3390/ijms222312673
Chicago/Turabian StyleKong, Weibo, Sina Sender, Leila Taher, Simon Villa-Perez, Yixuan Ma, Anett Sekora, Barbara C. Ruetgen, Bertram Brenig, Julia Beck, Ekkehard Schuetz, and et al. 2021. "BTK and PI3K Inhibitors Reveal Synergistic Inhibitory Anti-Tumoral Effects in Canine Diffuse Large B-Cell Lymphoma Cells" International Journal of Molecular Sciences 22, no. 23: 12673. https://doi.org/10.3390/ijms222312673
APA StyleKong, W., Sender, S., Taher, L., Villa-Perez, S., Ma, Y., Sekora, A., Ruetgen, B. C., Brenig, B., Beck, J., Schuetz, E., Junghanss, C., Nolte, I., & Murua Escobar, H. (2021). BTK and PI3K Inhibitors Reveal Synergistic Inhibitory Anti-Tumoral Effects in Canine Diffuse Large B-Cell Lymphoma Cells. International Journal of Molecular Sciences, 22(23), 12673. https://doi.org/10.3390/ijms222312673






