Blood–Brain Barrier in Brain Tumors: Biology and Clinical Relevance
Abstract
1. Blood-Brain Barrier: Structure and Function
- They are returned to the blood flow through the action of active transporters: P-glycoprotein (P-gp, ABCB1), breast-cancer-resistance protein (BCRP, ABCG2) and multidrug resistance-associated proteins (MRP1, -4, -5, ABCC1, -4, -5). Efflux pumps are expressed at the luminal side of the endothelium and transport a variety of molecules with wide structural diversity, showing a significant overlap in their substrates [14].
- They enter the cerebrospinal fluid or the lymphatic system, and then back to the blood flow.
- They are metabolized by enzymes of phase I (monoamine oxydases A and B and Cytochromes P450) or phase II (UDP-glucuronosyltransferases and methyl transferases), which make them sufficiently polar to be excreted from the CNS by the above-mentioned pathways.
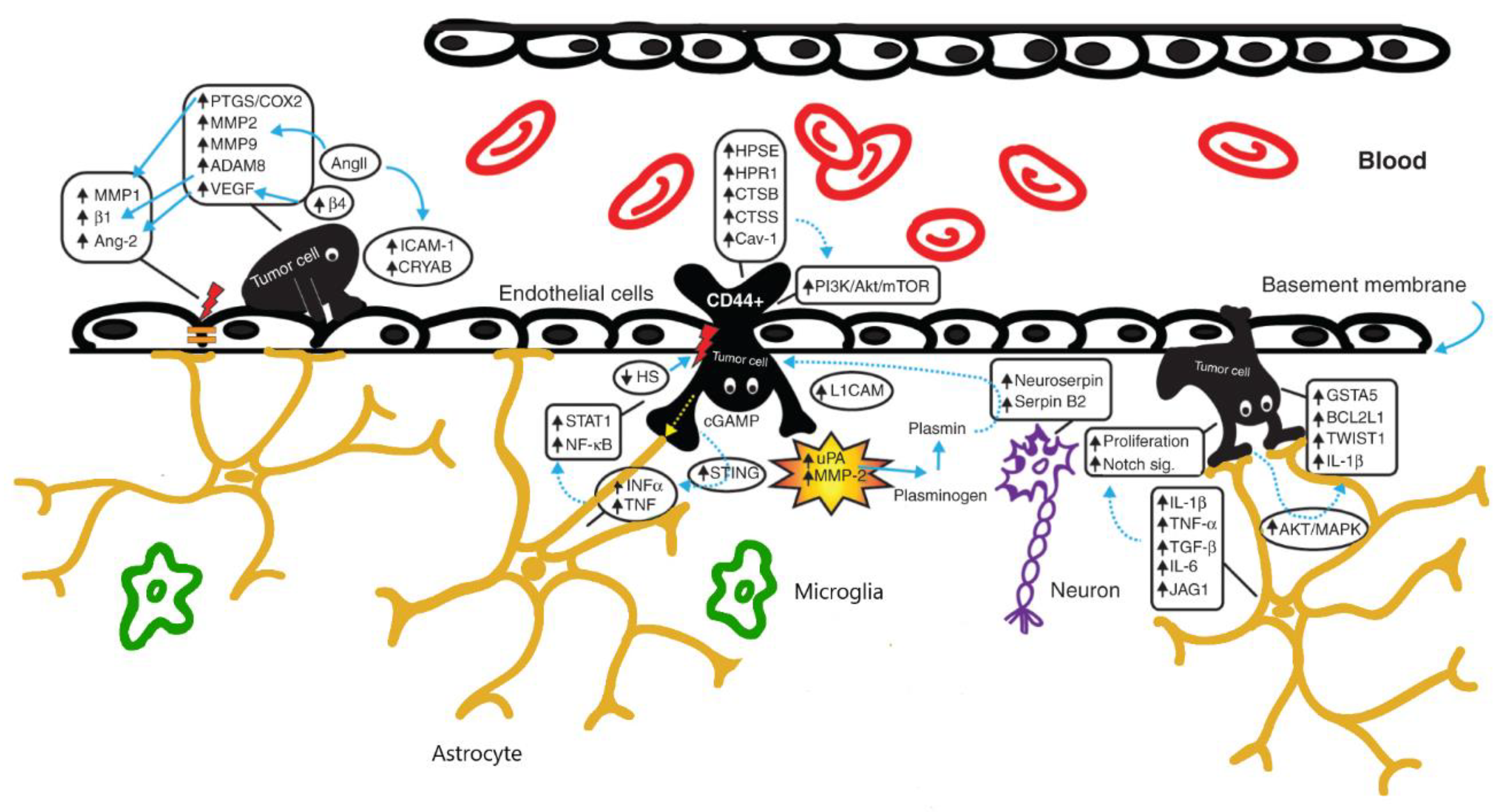
2. BBB Alterations in Brain Tumors
3. Strategies to By-Pass BBB
3.1. Intratumoral, Intranasal or Intrathecal Administration of Drugs
3.2. Chemical Modification of Drugs
3.3. Chemical Modification of BBB
3.4. Targeting of Efflux Transporters and Tight Junctions
3.5. Physical Disruption of BBB
3.6. The Role of Stem Cells
4. BBB and Drug Delivery: The Model of Brain Metastases
5. Clinical Relevance and Limitations of the Current Strategies for Bypassing the BBB
6. Future Perspectives and Conclusions
Funding
Conflicts of Interest
References
- Abbott, N.J.; Patabendige, A.A.; Dolman, D.E.; Yusof, S.R.; Begley, D.J. Structure and function of the blood-brain barrier. Neurobiol. Dis. 2010, 37, 13–25. [Google Scholar] [CrossRef]
- Daneman, R.; Engelhardt, B. Brain barriers in health and disease. Neurobiol. Dis. 2017, 107, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Saunders, N.R.; Dreifuss, J.J.; Dziegielewska, K.M.; Johansson, P.A.; Habgood, M.D.; Møllgård, K.; Bauer, H.C. The rights and wrongs of blood-brain barrier permeability studies: A walk through 100 years of history. Front. Neurosci. 2014, 8, 404. [Google Scholar] [CrossRef] [PubMed]
- Pardridge, W.M. CSF, blood-brain barrier, and brain drug delivery. Expert Opin. Drug Deliv. 2016, 13, 963–975. [Google Scholar] [CrossRef] [PubMed]
- Cecchelli, R.; Berezowski, V.; Lundquist, S.; Culot, M.; Renftel, M.; Dehouck, M.P.; Fenart, L. Modelling of the blood-brain barrier in drug discovery and development. Nat. Rev. Drug Discov. 2007, 6, 650–661. [Google Scholar] [CrossRef]
- Chowdhury, E.A.; Noorani, B.; Alqahtani, F.; Bhalerao, A.; Raut, S.; Sivandzade, F.; Cucullo, L. Understanding the brain uptake and permeability of small molecules through the BBB: A technical overview. J. Cereb. Blood Flow Metab. 2021, 41, 1797–1820. [Google Scholar] [CrossRef]
- Butt, A.M.; Jones, H.C.; Abbott, N.J. Electrical resistance across the blood-brain barrier in anaesthetized rats: A developmental study. J. Physiol. 1990, 429, 47–62. [Google Scholar] [CrossRef]
- Irudayanathan, F.J.; Wang, N.; Wang, X.; Nangia, S. Architecture of the paracellular channels formed by claudins of the blood-brain barrier tight junctions. Ann. N. Y. Acad. Sci. 2017, 1405, 131–146. [Google Scholar] [CrossRef] [PubMed]
- Banks, W.A. Characteristics of compounds that cross the blood-brain barrier. BMC Neurol. Vol. 2009, 9 (Suppl. 1), S3. [Google Scholar] [CrossRef]
- Sweeney, M.D.; Zhao, Z.; Montagne, A.; Nelson, A.R.; Zlokovic, B.V. Blood-Brain Barrier: From Physiology to Disease and Back. Physiol. Rev. 2019, 99, 21–78. [Google Scholar] [CrossRef]
- Lim, J.P.; Gleeson, P.A. Macropinocytosis: An endocytic pathway for internalising large gulps. Immunol. Cell Biol. 2011, 89, 836–843. [Google Scholar] [CrossRef]
- De Bock, M.; Van Haver, V.; Vandenbroucke, R.E.; Decrock, E.; Wang, N.; Leybaert, L. Into rather unexplored terrain-transcellular transport across the blood-brain barrier. Glia 2016, 64, 1097–1123. [Google Scholar] [CrossRef]
- Kaksonen, M.; Roux, A. Mechanisms of clathrin-mediated endocytosis. Nat. Rev. Mol. Cell Biol. 2018, 19, 313–326. [Google Scholar] [CrossRef]
- Pahnke, J.; Wolkenhauer, O.; Krohn, M.; Walker, L.C. Clinico-Pathologic Function of Cerebral ABC Transporters—Implications for the Pathogenesis of Alzheimer’s Disease. Curr. Alzheimer Res. 2008, 5, 396–405. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef]
- Mathiisen, T.M.; Lehre, K.P.; Danbolt, N.C.; Ottersen, O.P. The perivascular astroglial sheath provides a complete covering of the brain microvessels: An electron microscopic 3D reconstruction. Glia 2010, 58, 1094–1103. [Google Scholar] [CrossRef]
- Attwell, D.; Mishra, A.; Hall, C.N.; O’Farrell, F.M.; Dalkara, T. What is a pericyte? J. Cereb. Blood Flow Metab. 2016, 36, 451–455. [Google Scholar] [CrossRef] [PubMed]
- Hall, C.N.; Reynell, C.; Gesslein, B.; Hamilton, N.B.; Mishra, A.; Sutherland, B.A.; O’Farrell, F.M.; Buchan, A.M.; Lauritzen, M.; Attwell, D. Capillary pericytes regulate cerebral blood flow in health and disease. Nature 2014, 508, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Kaya, M.; Ahishali, B. Basic physiology of the blood-brain barrier in health and disease: A brief overview. Tissue Barriers 2021, 9, 1840913. [Google Scholar] [CrossRef] [PubMed]
- Takano, T.; Tian, G.F.; Peng, W.; Lou, N.; Libionka, W.; Han, X.; Nedergaard, M. Astrocyte-mediated control of cerebral blood flow. Nat. Neurosci. 2006, 9, 260–267. [Google Scholar] [CrossRef]
- Stankovic, N.D.; Teodorczyk, M.; Ploen, R.; Zipp, F.; Schmidt, M.H. Microglia-blood vessel interactions: A double-edged sword in brain pathologies. Acta Neuropathol. 2016, 131, 347–363. [Google Scholar] [CrossRef]
- Thurgur, H.; Pinteaux, E. Microglia in the Neurovascular Unit: Blood-Brain Barrier-microglia Interactions After Central Nervous System Disorders. Neuroscience 2019, 405, 55–67. [Google Scholar] [CrossRef]
- Sumi, N.; Nishioku, T.; Takata, F.; Matsumoto, J.; Watanabe, T.; Shuto, H.; Yamauchi, A.; Dohgu, S.; Kataoka, Y. Lipopolysaccharide-activated microglia induce dysfunction of the blood-brain barrier in rat microvascular endothelial cells co-cultured with microglia. Cell Mol. Neurobiol. 2010, 30, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Nirwane, A.; Yao, Y. Basement membrane and blood-brain barrier. Stroke Vasc. Neurol. 2018, 4, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Muoio, V.; Persson, P.B.; Sendeski, M.M. The neurovascular unit—Concept review. Acta Physiol. 2014, 210, 790–798. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.K.; Tsai, C.L.; Huang, Y.; Hynynen, K. Focused Ultrasound and Microbubbles-Mediated Drug Delivery to Brain Tumor. Pharmaceutics 2021, 13, 15. [Google Scholar] [CrossRef] [PubMed]
- Arvanitis, C.D.; Ferraro, G.B.; Jain, R.K. The blood-brain barrier and blood-tumour barrier in brain tumours and metastases. Nat. Rev. Cancer. 2020, 20, 26–41. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Shusta, E.V. Blood-Brain Barrier Modulation to Improve Glioma Drug Delivery. Pharmaceutics 2020, 12, 1085. [Google Scholar] [CrossRef]
- Phoenix, T.M.; Patmore, D.M.; Boop, S.; Boulos, N.; Jacus, M.O.; Patel, Y.T.; Roussel, M.F.; Finkelstein, D.; Goumnerova, L.; Perreault, S.; et al. Medulloblastoma Genotype Dictates Blood Brain Barrier Phenotype. Cancer Cell 2016, 29, 508–522. [Google Scholar] [CrossRef]
- Chapouly, C.; Guimbal, S.; Hollier, P.L. and Renault, M.A. Role of Hedgehog Signaling in Vasculature Development, Differentiation, and Maintenance. Int. J. Mol. Sci. 2019, 20, 3076. [Google Scholar] [CrossRef]
- Yonemori, K.; Tsuta, K.; Ono, M.; Shimizu, C.; Hirakawa, A.; Hasegawa, T.; Hatanaka, Y.; Narita, Y.; Shibui, S.; Fujiwara, Y. Disruption of the blood brain barrier by brain metastases of triple-negative and basal-type breast cancer but not HER2/neu-positive breast cancer. Cancer 2010, 116, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Pedrosa, R.M.S.M.; Mustafa, D.A.; Soffietti, R.; Kros, J.M. Breast cancer brain metastasis: Molecular mechanisms and directions for treatment. Neuro-Oncology 2018, 20, 1439–1449. [Google Scholar] [CrossRef] [PubMed]
- Griffith, J.I.; Rathi, S.; Zhang, W.; Zhang, W.; Drewes, L.R.; Sarkaria, J.N.; Elmquist, W.F. Addressing BBB Heterogeneity: A New Paradigm for Drug Delivery to Brain Tumors. Pharmaceutics 2020, 12, 1205. [Google Scholar] [CrossRef] [PubMed]
- Buahin, K.G.; Brem, H. Interstitial chemotherapy of experimental brain tumors: Comparison of intratumoral injection versus polymeric controlled release. J. Neurooncol. 1995, 26, 103–110. [Google Scholar] [CrossRef]
- Sukumar, U.K.; Bose, R.J.C.; Malhotra, M.; Babikir, H.A.; Afjei, R.; Robinson, E.; Zeng, Y.; Chang, E.; Habte, F.; Sinclair, R.; et al. Intranasal delivery of targeted polyfunctional gold-iron oxide nanoparticles loaded with therapeutic microRNAs for combined theranostic multimodality imaging and presensitization of glioblastoma to temozolomide. Biomaterials 2019, 218, 119342. [Google Scholar] [CrossRef]
- Ommaya, A.K. Subcutaneous reservoir and pump for sterile access to ventricular cerebrospinal fluid. Lancet 1963, 2, 983–984. [Google Scholar] [CrossRef]
- Pluchart, H.; Jacquet, E.; Charlety, D.; Allenet, B.; Bedouch, P.; Mousseau, M. Long-Term Survivor with Intrathecal and Intravenous Trastuzumab Treatment in Metastatic Breast Cancer. Target. Oncol. 2016, 11, 687–691. [Google Scholar] [CrossRef]
- Gulia, S.; Gupta, S.; Singh, A. Intrathecal trastuzumab for leptomeningeal carcinomatosis in patients with human epidermal growth factor receptor 2 positive breast cancer. Indian J. Med. Paediatr. Oncol. 2016, 37, 196–198. [Google Scholar] [CrossRef]
- Niwińska, A.; Rudnicka, H.; Murawska, M. Breast cancer leptomeningeal metastasis: The results of combined treatment and the comparison of methotrexate and liposomal cytarabine as intra-cerebrospinal fluid chemotherapy. Clin. Breast Cancer 2015, 15, 66–72. [Google Scholar] [CrossRef]
- Assi, H.I.; Mahmoud, T.; Saadeh, F.S.; Darsa, H.E. Management of leptomeningeal metastasis in breast cancer. Clin. Neurol. Neurosurg. 2018, 172, 151–159. [Google Scholar] [CrossRef]
- Chamberlain, M.; Soffietti, R.; Raizer, J.; Rudà, R.; Brandsma, D.; Boogerd, W.; Taillibert, S.; Groves, M.D.; Le Rhun, E.; Junck, L.; et al. Leptomeningeal metastasis: A Response Assessment in Neuro-oncology critical review of endpoints and response criteria of published randomized clinical trials. Neuro-Oncology 2014, 16, 1176–1185. [Google Scholar] [CrossRef] [PubMed]
- Jabbour, E.; O’Brien, S.; Kantarjian, H.; Garcia-Manero, G.; Ferrajoli, A.; Ravandi, F.; Cabanillas, M.; Thomas, D.A. Neurologic complications associated with intrathecal liposomal cytarabine given prophylactically in combination with high-dose methotrexate and cytarabine to patients with acute lymphocytic leukemia. Blood 2007, 109, 3214–3218. [Google Scholar] [CrossRef]
- Pan, Y.; Wang, C.; Wang, H.; Tao, Q.; Xiong, S.; Zhai, Z. Transverse myelopathy occurring with intrathecal administration of methotrexate and cytarabine chemotherapy: A case report. Oncol. Lett. 2016, 11, 4066–4068. [Google Scholar] [CrossRef] [PubMed]
- Le Rhun, E.; Wallet, J.; Mailliez, A.; Le Deley, M.C.; Rodrigues, I.; Boulanger, T.; Lorgis, V.; Barriere, J.; Robin, Y.M.; Weller, M.; et al. Intrathecal liposomal cytarabine plus systemic therapy versus systemic chemotherapy alone for newly diagnosed leptomeningeal metastasis from breast cancer. Neuro-Oncology 2020, 22, 524–538. [Google Scholar] [CrossRef] [PubMed]
- Rice, A.; Liu, Y.; Michaelis, M.L.; Himes, R.H.; Georg, G.I.; Audus, K.L. Chemical modification of paclitaxel (Taxol) reduces P-glycoprotein interactions and increases permeation across the blood-brain barrier in vitro and in situ. J. Med. Chem. 2005, 48, 832–838. [Google Scholar] [CrossRef] [PubMed]
- Greig, N.H.; Genka, S.; Daly, E.M.; Sweeney, D.J.; Rapoport, S.I. Physicochemical and pharmacokinetic parameters of seven lipophilic chlorambucil esters designed for brain penetration. Cancer Chemother. Pharmacol. 1990, 25, 311–319. [Google Scholar] [CrossRef]
- Adkins, C.E.; Nounou, M.I.; Hye, T.; Mohammad, A.S.; Terrell-Hall, T.; Mohan, N.K.; Eldon, M.A.; Hoch, U.; Lockman, P.R. NKTR-102 Efficacy versus irinotecan in a mouse model of brain metastases of breast cancer. BMC Cancer 2015, 15, 685. [Google Scholar] [CrossRef]
- Sachdev, J.C.; Munster, P.; Northfelt, D.W.; Han, H.S.; Ma, C.; Maxwell, F.; Wang, T.; Belanger, B.; Zhang, B.; Moore, Y.; et al. Phase I study of liposomal irinotecan in patients with metastatic breast cancer: Findings from the expansion phase. Breast Cancer Res. Treat. 2021, 185, 759–771. [Google Scholar] [CrossRef]
- Decuzzi, P.; Godin, B.; Tanaka, T.; Lee, S.Y.; Chiappini, C.; Liu, X.; Ferrari, M. Size and shape effects in the biodistribution of intravascularly injected particles. J. Control. Release 2010, 141, 320–327. [Google Scholar] [CrossRef]
- Fisher, D.G.; Price, R.J. Recent Advances in the Use of Focused Ultrasound for Magnetic Resonance Image-Guided Therapeutic Nanoparticle Delivery to the Central Nervous System. Front. Pharmacol. 2019, 10, 1348. [Google Scholar] [CrossRef]
- Zhang, H. Molecularly Imprinted Nanoparticles for Biomedical Applications. Adv. Mater. 2020, 32, e1806328. [Google Scholar] [CrossRef] [PubMed]
- Wiwatchaitawee, K.; Quarterman, J.C.; Geary, S.M.; Salem, A.K. Enhancement of Therapies for Glioblastoma (GBM) Using Nanoparticle-based Delivery Systems. AAPS PharmSciTech 2021, 22, 71. [Google Scholar] [CrossRef] [PubMed]
- Siegal, T.; Horowitz, A.; Gabizon, A. Doxorubicin encapsulated in sterically stabilized liposomes for the treatment of a brain tumor model: Biodistribution and therapeutic efficacy. J. Neurosurg. 1995, 83, 1029–1037. [Google Scholar] [CrossRef] [PubMed]
- Fabel, K.; Dietrich, J.; Hau, P.; Wismeth, C.; Winner, B.; Przywara, S.; Steinbrecher, A.; Ullrich, W.; Bogdahn, U. Long-term stabilization in patients with malignant glioma after treatment with liposomal doxorubicin. Cancer 2001, 92, 1936–1942. [Google Scholar] [CrossRef]
- Hau, P.; Fabel, K.; Baumgart, U.; Rümmele, P.; Grauer, O.; Bock, A.; Dietmaier, C.; Dietmaier, W.; Dietrich, J.; Dudel, C.; et al. Pegylated liposomal doxorubicin-efficacy in patients with recurrent high-grade glioma. Cancer 2004, 100, 1199–1207. [Google Scholar] [CrossRef]
- Glas, M.; Koch, H.; Hirschmann, B.; Jauch, T.; Steinbrecher, A.; Herrlinger, U.; Bogdahn, U.; Hau, P. Pegylated liposomal doxorubicin in recurrent malignant glioma: Analysis of a case series. Oncology 2007, 72, 302–307. [Google Scholar] [CrossRef]
- Nishiyama, N.; Matsumura, Y.; Kataoka, K. Development of polymeric micelles for targeting intractable cancers. Cancer Sci. 2016, 107, 867–874. [Google Scholar] [CrossRef]
- Stenström, P.; Manzanares, D.; Zhang, Y.; Ceña, V.; Malkoch, M. Evaluation of Amino-Functional Polyester Dendrimers Based on Bis-MPA as Nonviral Vectors for siRNA Delivery. Molecules 2018, 23, 2028. [Google Scholar] [CrossRef]
- Dhanikula, R.S.; Argaw, A.; Bouchard, J.F.; Hildgen, P. Methotrexate loaded polyether-copolyester dendrimers for the treatment of gliomas: Enhanced efficacy and intratumoral transport capability. Mol. Pharm. 2008, 5, 105–116. [Google Scholar] [CrossRef]
- Bobyk, L.; Edouard, M.; Daman, P.; Vautrin, M.; Pernet-Gallay, K.; Delaroche, J.; Adam, J.F.; Estève, F.; Ravanat, J.L.; Elleaume, H. Photoactivation of gold nanoparticles for glioma treatment. Nanomedicine 2013, 9, 1089–1097. [Google Scholar] [CrossRef]
- Ningaraj, N.S.; Rao, M.K.; Black, K.L. Adenosine 5′-triphosphate-sensitive potassium channel-mediated blood-brain tumor barrier permeability increase in a rat brain tumor model. Cancer Res. 2003, 63, 8899–8911. [Google Scholar] [PubMed]
- Ningaraj, N.S.; Rao, M.; Black, K.L. Calcium-dependent potassium channels as a target protein for modulation of the blood-brain tumor barrier. Drug News Perspect. 2003, 16, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Black, K.L.; Yin, D.; Konda, B.M.; Wang, X.; Hu, J.; Ko, M.K.; Bayan, J.A.; Sacapano, M.R.; Espinoza, A.J.; Ong, J.M.; et al. Different effects of KCa and KATP agonists on brain tumor permeability between syngeneic and allogeneic rat models. Brain Res. 2008, 1227, 198–206. [Google Scholar] [CrossRef] [PubMed][Green Version]
- D’Amico, R.S.; Khatri, D.; Reichman, N.; Patel, N.V.; Wong, T.; Fralin, S.R.; Li, M.; Ellis, J.A.; Ortiz, R.; Langer, D.J.; et al. Super selective intra-arterial cerebral infusion of modern chemotherapeutics after blood-brain barrier disruption: Where are we now, and where we are going. J. Neurooncol. 2020, 147, 261–278. [Google Scholar] [CrossRef] [PubMed]
- Salaroglio, I.C.; Abate, C.; Rolando, B.; Battaglia, L.; Gazzano, E.; Colombino, E.; Costamagna, C.; Annovazzi, L.; Mellai, M.; Berardi, F.; et al. Validation of Thiosemicarbazone Compounds as P-Glycoprotein Inhibitors in Human Primary Brain-Blood Barrier and Glioblastoma Stem Cells. Mol. Pharm. 2019, 16, 3361–3373. [Google Scholar] [CrossRef]
- Pinzón-Daza, M.I.; Garzón, R.; Couraud, P.; Romero, I.; Weksler, B.; Ghigo, D.; Bosia, A.; Riganti, C. The association of statins plus LDL receptor-targeted liposome-encapsulated doxorubicin increases in vitro drug delivery across blood-brain barrier cells. Br. J. Pharmacol. 2012, 167, 1431–1447. [Google Scholar] [CrossRef]
- Durmus, S.; Sparidans, R.W.; Wagenaar, E.; Beijnen, J.H.; Schinkel, A.H. Oral availability and brain penetration of the B-RAFV600E inhibitor vemurafenib can be enhanced by the P-GLYCOprotein (ABCB1) and breast cancer resistance protein (ABCG2) inhibitor elacridar. Mol. Pharm. 2012, 9, 3236–3245. [Google Scholar] [CrossRef]
- Tachibana, K.; Iwashita, Y.; Wakayama, E.; Nishino, I.; Nishikaji, T.; Kondoh, M. Tight Junction Modulating Bioprobes for Drug Delivery System to the Brain: A Review. Pharmaceutics 2020, 12, 1236. [Google Scholar] [CrossRef]
- Sonoda, N.; Furuse, M.; Sasaki, H.; Yonemura, S.; Katahira, J.; Horiguchi, Y.; Tsukita, S. Clostridium perfringens enterotoxin fragment removes specific claudins from tight junction strands: Evidence for direct involvement of claudins in tight junction barrier. J. Cell Biol. 1999, 147, 195–204. [Google Scholar] [CrossRef]
- Huang, L.Y.; Stuart, C.; Takeda, K.; D’Agnillo, F.; Golding, B. Poly(I:C) Induces Human Lung Endothelial Barrier Dysfunction by Disrupting Tight Junction Expression of Claudin-5. PLoS ONE 2016, 11, e0160875. [Google Scholar] [CrossRef]
- Jia, Y.; Qin, T.; Zhang, X.; Liu, S.; Liu, Z.; Zhang, C.; Wang, J.; Li, K. Effect of bevacizumab on the tight junction proteins of vascular endothelial cells. Am. J. Transl. Res. 2019, 11, 5546–5559. [Google Scholar] [PubMed]
- Zeniya, S.; Kuwahara, H.; Daizo, K.; Watari, A.; Kondoh, M.; Yoshida-Tanaka, K.; Kaburagi, H.; Asada, K.; Nagata, T.; Nagahama, M.; et al. Angubindin-1 opens the blood-brain barrier in vivo for delivery of antisense oligonucleotide to the central nervous system. J. Control. Release 2018, 283, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Greene, C.; Campbell, M. Tight junction modulation of the blood brain barrier: CNS delivery of small molecules. Tissue Barriers 2016, 4, e1138017. [Google Scholar] [CrossRef]
- Guerit, S.; Liebner, S. Blood-Brain Barrier Breakdown Determines Differential Therapeutic Outcome in Genetically Diverse Forms of Medulloblastoma. Cancer Cell 2016, 29, 427–429. [Google Scholar] [CrossRef]
- Neuwelt, E.A. Reversible osmotic blood-brain barrier disruption in humans: Implications for the chemotherapy of malignant brain tumors. Neurosurgery 1980, 7, 204. [Google Scholar] [CrossRef]
- Neuwelt, E.A.; Goldman, D.L.; Dahlborg, S.A.; Crossen, J.; Ramsey, F.; Roman-Goldstein, S.; Braziel, R.; Dana, B. Primary CNS lymphoma treated with osmotic blood-brain barrier disruption: Prolonged survival and preservation of cognitive function. J. Clin. Oncol. 1991, 9, 1580–1590. [Google Scholar] [CrossRef]
- Sırav, B.; Seyhan, N. Effects of GSM modulated radio-frequency electromagnetic radiation on permeability of blood-brain barrier in male & female rats. J. Chem. Neuroanat. 2016, 75 (Pt B), 123–127. [Google Scholar]
- Sabel, M.; Rommel, F.; Kondakci, M.; Gorol, M.; Willers, R.; Bilzer, T. Locoregional opening of the rodent blood-brain barrier for paclitaxel using Nd:YAG laser-induced thermo therapy: A new concept of adjuvant glioma therapy? Lasers Surg. Med. 2003, 33, 75–80. [Google Scholar] [CrossRef]
- Qin, D.; Ou, G.; Mo, H.; Song, Y.; Kang, G.; Hu, Y.; Gu, X. Improved efficacy of chemotherapy for glioblastoma by radiation-induced opening of blood-brain barrier: Clinical results. Int. J. Radiat. Oncol. Biol. Phys. 2001, 51, 959–962. [Google Scholar] [CrossRef]
- Cao, Y.; Tsien, C.I.; Shen, Z.; Tatro, D.S.; Haken, R.T.; Kessler, M.L.; Chenevert, T.L.; Lawrence, T.S. Use of magnetic resonance imaging to assess blood-brain/blood-glioma barrier opening during conformal radiotherapy. J. Clin. Oncol. 2005, 23, 4127–4136. [Google Scholar] [CrossRef]
- Sándor, N.; Walter, F.R.; Bocsik, A.; Sántha, P.; Schilling-Tóth, B.; Léner, V.; Varga, Z.; Kahán, Z.; Deli, M.A.; Sáfrány, G.; et al. Low dose cranial irradiation-induced cerebrovascular damage is reversible in mice. PLoS ONE 2014, 9, e112397. [Google Scholar] [CrossRef] [PubMed]
- Sheikov, N.; McDannold, N.; Vykhodtseva, N.; Jolesz, F.; Hynynen, K. Cellular mechanisms of the blood-brain barrier opening induced by ultrasound in presence of microbubbles. Ultrasound Med. Biol. 2004, 30, 979–989. [Google Scholar] [CrossRef]
- Fan, C.H.; Liu, H.L.; Ting, C.Y.; Lee, Y.H.; Huang, C.Y.; Ma, Y.J.; Wei, K.C.; Yen, T.C.; Yeh, C.K. Submicron-bubble-enhanced focused ultrasound for blood-brain barrier disruption and improved CNS drug delivery. PLoS ONE 2014, 9, e96327. [Google Scholar] [CrossRef]
- McMahon, D.; Hynynen, K. Acute Inflammatory Response Following Increased Blood-Brain Barrier Permeability Induced by Focused Ultrasound is Dependent on Microbubble Dose. Theranostics 2017, 7, 3989–4000. [Google Scholar] [CrossRef] [PubMed]
- Tsushima, Y.; Endo, K. Hypointensities in the brain on T2*-weighted gradient-echo magnetic resonance imaging. Curr. Probl. Diagn. Radiol. 2006, 35, 140–150. [Google Scholar] [CrossRef]
- Aryal, M.; Vykhodtseva, N.; Zhang, Y.Z.; Park, J.; McDannold, N. Multiple treatments with liposomal doxorubicin and ultrasound-induced disruption of blood-tumor and blood-brain barriers improve outcomes in a rat glioma model. J. Control. Release 2013, 169, 103–111. [Google Scholar] [CrossRef]
- O’Reilly, M.A.; Chinnery, T.; Yee, M.L.; Wu, S.K.; Hynynen, K.; Kerbel, R.S.; Czarnota, G.J.; Pritchard, K.I.; Sahgal, A. Preliminary Investigation of Focused Ultrasound-Facilitated Drug Delivery for the Treatment of Leptomeningeal Metastases. Sci. Rep. 2018, 8, 9013. [Google Scholar] [CrossRef]
- Alli, S.; Figueiredo, C.A.; Golbourn, B.; Sabha, N.; Wu, M.Y.; Bondoc, A.; Luck, A.; Coluccia, D.; Maslink, C.; Smith, C.; et al. Brainstem blood brain barrier disruption using focused ultrasound: A demonstration of feasibility and enhanced doxorubicin delivery. J. Control. Release 2018, 281, 29–41. [Google Scholar] [CrossRef] [PubMed]
- McDannold, N.; Zhang, Y.; Supko, J.G.; Power, C.; Sun, T.; Peng, C.; Vykhodtseva, N.; Golby, A.J.; Reardon, D.A. Acoustic feedback enables safe and reliable carboplatin delivery across the blood-brain barrier with a clinical focused ultrasound system and improves survival in a rat glioma model. Theranostics 2019, 9, 6284–6299. [Google Scholar] [CrossRef]
- Mainprize, T.; Lipsman, N.; Huang, Y.; Meng, Y.; Bethune, A.; Ironside, S.; Heyn, C.; Alkins, R.; Trudeau, M.; Sahgal, A.; et al. Blood-Brain Barrier Opening in Primary Brain Tumors with Non-invasive MR-Guided Focused Ultrasound: A Clinical Safety and Feasibility Study. Sci. Rep. 2019, 9, 321. [Google Scholar] [CrossRef]
- Park, S.H.; Kim, M.J.; Jung, H.H.; Chang, W.S.; Choi, H.S.; Rachmilevitch, I.; Zadicario, E.; Chang, J.W. Safety and feasibility of multiple blood-brain barrier disruptions for the treatment of glioblastoma in patients undergoing standard adjuvant chemotherapy. J. Neurosurg. 2020, 134, 475–483. [Google Scholar] [CrossRef] [PubMed]
- McDannold, N.; Vykhodtseva, N.; Hynynen, K. Use of Ultrasound Pulses Combined with Definity for Targeted Blood-Brain Barrier Disruption. In Proceedings of the 6th International Symposium on Therapeutic Ultrasound. Proceedings Held at Oxford, United Kingdom, 30 August–2 September 2006. AIP Conference Proceedings Volume 911; Coussios, C.C., Haar, G.T., Eds.; American Institute of Physics: Melville, NY, USA, 2006; pp. 547–553. [Google Scholar]
- Wu, S.K.; Chu, P.C.; Chai, W.Y.; Kang, S.T.; Tsai, C.H.; Fan, C.H.; Yeh, C.K.; Liu, H.L. Characterization of Different Microbubbles in Assisting Focused Ultrasound-Induced Blood-Brain Barrier Opening. Sci. Rep. 2017, 7, 46689. [Google Scholar] [CrossRef] [PubMed]
- Bobo, R.H.; Laske, D.W.; Akbasak, A.; Morrison, P.F.; Dedrick, R.L.; Oldfield, E.H. Convection-enhanced delivery of macromolecules in the brain. Proc. Natl. Acad. Sci. USA 1994, 91, 2076–2080. [Google Scholar] [CrossRef] [PubMed]
- Sampson, J.H.; Archer, G.; Pedain, C.; Wembacher-Schröder, E.; Westphal, M.; Kunwar, S.; Vogelbaum, M.A.; Coan, A.; Herndon, J.E.; Raghavan, R.; et al. Poor drug distribution as a possible explanation for the results of the PRECISE trial. J. Neurosurg. 2010, 113, 301–309. [Google Scholar] [CrossRef]
- Pandit, R.; Chen, L.; Gotz, J. The blood-brain barrier: Physiology and strategies for drug delivery. Adv. Drug Deliv. Rev. 2020, 165–166, 1–14. [Google Scholar] [CrossRef]
- Bovenberg, M.S.S.; Degeling, M.H.; Tannous, B.A. Advances in stem cell therapy against gliomas. Trends Mol. Med. 2013, 19, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Mooney, R.; Majid, A.A.; Mota, D.; He, A.; Aramburo, S.; Flores, L.; Covello-Batalla, J.; Machado, D.; Gonzaga, J.; Aboody, K.S. Bcl-2 Overexpression Improves Survival and Efficacy of Neural Stem Cell-Mediated Enzyme Prodrug Therapy. Stem Cells Int. 2018, 2018, 7047496. [Google Scholar] [CrossRef] [PubMed]
- Stuckey, D.W.; Shah, K. Stem cell-based therapies for cancer treatment: Separating hope from hype. Nat. Rev. Cancer 2014, 14, 683–691. [Google Scholar] [CrossRef]
- Portnow, J.; Synold, T.W.; Badie, B.; Tirughana, R.; Lacey, S.F.; D’Apuzzo, M.; Metz, M.Z.; Najbauer, J.; Bedell, V.; Vo, T.; et al. Neural Stem Cell-Based Anticancer Gene Therapy: A First-in-Human Study in Recurrent High-Grade Glioma Patients. Clin. Cancer Res. 2017, 23, 2951–2960. [Google Scholar] [CrossRef]
- Nayak, L.; Lee, E.Q.; Wen, P.Y. Epidemiology of brain metastases. Curr. Oncol. Rep. 2012, 14, 48–54. [Google Scholar] [CrossRef]
- Brastianos, P.; Davies, M.A.; Margolin, K.; Yu, H.A. Modern Management of Central Nervous System Metastases in the Era of Targeted Therapy and Immune Oncology. Am. Soc. Clin. Oncol. Educ. Book 2019, 39, e59–e69. [Google Scholar] [CrossRef] [PubMed]
- Soffietti, R.; Ahluwalia, M.; Lin, N.; Rudà, R. Management of brain metastases according to molecular subtypes. Nat. Rev. Neurol. 2020, 16, 557–574. [Google Scholar] [CrossRef]
- Kim, M.; Kizilbash, S.H.; Laramy, J.K.; Gampa, G.; Parrish, K.E.; Sarkaria, J.N.; Elmquist, W.F. Barriers to Effective Drug Treatment for Brain Metastases: A Multifactorial Problem in the Delivery of Precision Medicine. Pharm. Res. 2018, 35, 177. [Google Scholar] [CrossRef] [PubMed]
- Harrison, P.T.; Vyse, S.; Huang, P.H. Rare epidermal growth factor receptor (EGFR) mutations in non-small cell lung cancer. Semin. Cancer Biol. 2020, 61, 167–179. [Google Scholar] [CrossRef]
- Herbst, R.S.; Morgensztern, D.; Boshoff, C. The biology and management of non-small cell lung cancer. Nature 2018, 553, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Reungwetwattana, T.; Nakagawa, K.; Cho, B.C.; Cobo, M.; Cho, E.K.; Bertolini, A.; Bohnet, S.; Zhou, C.; Lee, K.H.; Nogami, N.; et al. CNS Response to Osimertinib Versus Standard Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors in Patients With Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2018, 36, 3290–3297. [Google Scholar] [CrossRef]
- Peters, S.; Camidge, D.R.; Shaw, A.T.; Gadgeel, S.; Ahn, J.S.; Kim, D.W.; Ou, S.I.; Pérol, M.; Dziadziuszko, R.; Rosell, R.; et al. Alectinib versus Crizotinib in Untreated ALK-Positive Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2017, 377, 829–838. [Google Scholar] [CrossRef]
- Solomon, B.J.; Besse, B.; Bauer, T.M.; Felip, E.; Soo, R.A.; Camidge, D.R.; Chiari, R.; Bearz, A.; Lin, C.C.; Gadgeel, S.M.; et al. Lorlatinib in patients with ALK-positive non-small-cell lung cancer: Results from a global phase 2 study. Lancet Oncol. 2018, 19, 1654–1667. [Google Scholar] [CrossRef]
- Bendell, J.C.; Domchek, S.M.; Burstein, H.J.; Harris, L.; Younger, J.; Kuter, I.; Bunnell, C.; Rue, M.; Gelman, R.; Winer, E. Central nervous system metastases in women who receive trastuzumab-based therapy for metastatic breast carcinoma. Cancer 2003, 97, 2972–2977. [Google Scholar] [CrossRef]
- Freedman, R.A.; Gelman, R.S.; Anders, C.K.; Melisko, M.E.; Parsons, H.A.; Cropp, A.M.; Silvestri, K.; Cotter, C.M.; Componeschi, K.P.; Marte, J.M.; et al. TBCRC 022: A Phase II Trial of Neratinib and Capecitabine for Patients With Human Epidermal Growth Factor Receptor 2-Positive Breast Cancer and Brain Metastases. J. Clin. Oncol. 2019, 37, 1081–1089. [Google Scholar] [CrossRef]
- Murthy, R.K.; Loi, S.; Okines, A.; Paplomata, E.; Hamilton, E.; Hurvitz, S.A.; Lin, N.U.; Borges, V.; Abramson, V.; Anders, C.; et al. Tucatinib, Trastuzumab, and Capecitabine for HER2-Positive Metastatic Breast Cancer. N. Engl. J. Med. 2020, 382, 597–609. [Google Scholar] [CrossRef] [PubMed]
- Fabi, A.; Alesini, D.; Valle, E.; Moscetti, L.; Caputo, R.; Caruso, M.; Carbognin, L.; Ciccarese, M.; La Verde, N.; Arpino, G.; et al. T-DM1 and brain metastases: Clinical outcome in HER2-positive metastatic breast cancer. Breast 2018, 41, 137–143. [Google Scholar] [CrossRef]
- Li, F.; Tang, S.C. Targeting metastatic breast cancer with ANG1005, a novel peptide-paclitaxel conjugate that crosses the blood-brain-barrier (BBB). Genes Dis. 2017, 4, 1–3. [Google Scholar] [CrossRef]
- James, J.; Tang, K.; Wei, T. Tesetaxel, a novel, oral taxane, crosses intact blood-brain barrier (BBB) at therapeutically relevant concentrations. Cancer Res. 2019, 79, 3078. [Google Scholar]
- Mittapalli, R.K.; Vaidhyanathan, S.; Dudek, A.Z.; Elmquist, W.F. Mechanisms limiting distribution of the threonine-protein kinase B-RaF(V600E) inhibitor dabrafenib to the brain: Implications for the treatment of melanoma brain metastases. J. Pharmacol. Exp. Ther. 2013, 344, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Davies, M.A.; Flaherty, K.T. Melanoma in 2017: Moving treatments earlier to move further forwards. Nat. Rev. Clin. Oncol. 2018, 15, 75–76. [Google Scholar] [CrossRef]
- Tawbi, H.A.; Forsyth, P.A.; Algazi, A.; Hamid, O.; Hodi, F.S.; Moschos, S.J.; Khushalani, N.I.; Lewis, K.; Lao, C.D.; Postow, M.A.; et al. Combined Nivolumab and Ipilimumab in Melanoma Metastatic to the Brain. N. Engl. J. Med. 2018, 379, 722–730. [Google Scholar] [CrossRef]
- Oberoi, R.K.; Mittapalli, R.K.; Elmquist, W.F. Pharmacokinetic assessment of efflux transport in sunitinib distribution to the brain. J. Pharmacol. Exp. Ther. 2013, 347, 755–764. [Google Scholar] [CrossRef]
- Idbaih, A.; Canney, M.; Belin, L.; Desseaux, C.; Vignot, A.; Bouchoux, G.; Asquier, N.; Law-Ye, B.; Leclercq, D.; Bissery, A.; et al. Safety and feasibility of repeated and transient blood-brain barrier disruption by pulsed ultrasound in patients with recurrent glioblastoma. Clin. Cancer Res. 2019, 25, 3793–3801. [Google Scholar] [CrossRef]
- Sevenich, L. Turning “Cold” Into “Hot” Tumors-Opportunities and Challenges for Radio-Immunotherapy Against Primary and Metastatic Brain Cancers. Front. Oncol. 2019, 9, 163. [Google Scholar] [CrossRef]
- Curley, C.T.; Sheybani, N.D.; Bullock, T.N.; Price, R.J. Focused Ultrasound Immunotherapy for Central Nervous System Pathologies: Challenges and Opportunities. Theranostics 2017, 7, 3608–3623. [Google Scholar] [CrossRef] [PubMed]
- Akhavan, D.; Alizadeh, D.; Wang, D.; Weist, M.R.; Shepphird, J.K.; Brown, C.E. CAR T cells for brain tumors: Lessons learned and road ahead. Immunol. Rev. 2019, 290, 60–84. [Google Scholar] [CrossRef] [PubMed]
| Route of Administration | Type of Drug | Clinical Relevance |
|---|---|---|
| Intratumoral | Carmustine Cyclophosphamide | Studies in mice—no impact on OS [34] |
| Intranasal | Gold-iron oxide nanoparticles (plus systemic temozolomide) | Studies in mice—increased OS in the group treated with nanoparticles in addition to temozolomide respect to temozolomide alone [35] |
| Intrathecal | Trastuzumab ± cytarabine or methotrexateLyposomal cytarabine | No clear benefit of trastuzumab alone—better disease control when combined with cytarabine or methotrexate in Her2 breast cancers [39,40,41] Improvement in PFS when combined with systemic chemotherapy respect to chemotherapy alone [44] |
| Mechanism of Action/Targeted Pathway | Type of Drug | Clinical Relevance |
|---|---|---|
| Reduced interaction with MDR1 | Tx67 (paclitaxel with a succinate group in C10 position) | Animal models and in vitro—increased penetration across BBB [45] |
| Increased liphophilicity and plasmatic half-life | Chlorambucil-tertiary butyl ester | Animal models—higher concentrations in the brain than chlorambucil [46] |
| Increased plasmatic half-life and CNS penetration | Etirinotecan pegol (NKTR-102) | Studies in mice—increased overall survival compared to conventional irinotecan [47] |
| Increased liphophilicity | Liposomal irinotecan | Phase I study in metastatic breast cancer—intracranial objective response rate (ORR) in 30% of patients [48] |
| Increased plasmatic half-life and selective accumulation in GBM | Liposomal doxorubicine | Retrospective and prospective nonrandomized studies—moderate effect on PFS and OS with long-term stabilization of gliomas [54,55,56] |
| Increased BBB permeability and drug endocytosis | Methotrexate loaded polyether-copolyester (PEPE) dendrimers | In vitro studies—higher antitumoral activity [59] |
| Strategy to Bypass BBB | Mechanism Involved | Molecules Used |
|---|---|---|
| Direct injection of drugs | Intranasal, intratumoral (by a catheter connected to a reservoir) or intrathecal administration | Intratumoral: carmustine, cyclophosphamide [34] Intrathecal: Trastuzumab +/− cytarabine or methotrexate [39,40,41], lyposomal cytarabine [44] |
| Chemical modification of drugs | Conjugation with succinate or ester groups and encapsulation in nanoparticles | Tx67 (paclitaxel with a succinate group in C10 position) [45], chlorambucil-tertiary butyl ester [46], etirinotecan pegol (NKTR-102) [47], liposomal irinotecan [48], liposomal doxorubicine [54,55,56], methotrexate loaded polyether-copolyester (PEPE) dendrimers [59] |
| Chemical modification of BBB | Increasing BBB permeability by expression of caveolin-1 and downregulation of TJ proteins, stimulation of endocytic process, activation of cGMP and bradykinin B2 receptors | Minoxidil sulfate [61], NS1619 [62], vardenafil [63], cereport [64] |
| Targeting tight junctions and efflux transporters | Inhibition of Pgp and BCRP, inhibition of claudins -3, -4, -5 | Thiosemicarbazone and tetrahydroisoquinoline derivatives [65], statins [66], clostridium perfringens enterotoxin (CPE) [67], polyinosinic-polycytidylic acid (poly IC) [68], bevacizumab [69], angubindin 1 [72], mannitol [75] |
| Physical disruption of BBB | Radiofrequency electromagnetic radiation (EMP), laser-induced thermal therapy (LITT), microbeam radiation therapy (MRT), focused ultrasound (FUS) with sonicated microbubbles (Definity, SonoVue, Optison) or implantable devices, convection-enhanced delivery (CED) | Combined treatment with chemotherapeutic drugs (paclitaxel [78], doxorubicin [86], temozolomide [87], carboplatin [88] |
| Stem cells | Ability to cross the BBB endothelium | Engineered to carry anticancer proteins, antiangiogenetic factors or immunosupportive factors like IL-12 [100] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mo, F.; Pellerino, A.; Soffietti, R.; Rudà, R. Blood–Brain Barrier in Brain Tumors: Biology and Clinical Relevance. Int. J. Mol. Sci. 2021, 22, 12654. https://doi.org/10.3390/ijms222312654
Mo F, Pellerino A, Soffietti R, Rudà R. Blood–Brain Barrier in Brain Tumors: Biology and Clinical Relevance. International Journal of Molecular Sciences. 2021; 22(23):12654. https://doi.org/10.3390/ijms222312654
Chicago/Turabian StyleMo, Francesca, Alessia Pellerino, Riccardo Soffietti, and Roberta Rudà. 2021. "Blood–Brain Barrier in Brain Tumors: Biology and Clinical Relevance" International Journal of Molecular Sciences 22, no. 23: 12654. https://doi.org/10.3390/ijms222312654
APA StyleMo, F., Pellerino, A., Soffietti, R., & Rudà, R. (2021). Blood–Brain Barrier in Brain Tumors: Biology and Clinical Relevance. International Journal of Molecular Sciences, 22(23), 12654. https://doi.org/10.3390/ijms222312654









