A Talk between Flavonoids and Hormones to Reorient the Growth of Gymnosperms
Abstract
1. Introduction
2. Gravitropism in Gymnosperms
3. Hormones and the Gravitropic Response in Gymnosperms
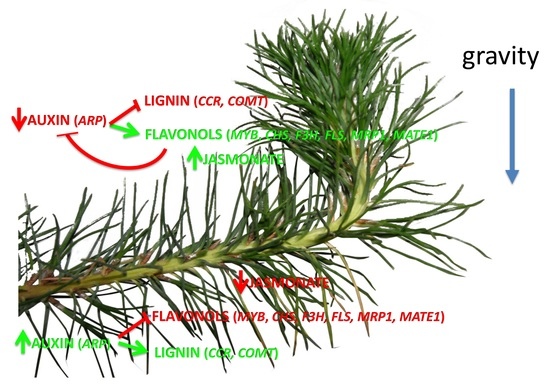
4. Hormone and Flavonoid Metabolism during the Gravitropic Response
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Welker, C.M.; Balasubramanian, V.K.; Petti, C.; Rai, K.M.; DeBolt, S.; Mendu, V. Engineering Plant Biomass Lignin Content and Composition for Biofuels and Bioproducts. Energies 2015, 8, 7654–7676. [Google Scholar] [CrossRef]
- Harfouche, A.; Meilan, R.; Altmane, A. Tree genetic engineering and applications to sustainable forestry and biomass production. Trends Biotechnol. 2011, 29, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Fritsche, S.; Klocko, A.L.; Boron, A.; Brunner, A.M.; Thorlby, G. Strategies for Engineering Reproductive Sterility in Plantation Forests. Front Plant Sci. 2018, 9, 1671. [Google Scholar] [CrossRef] [PubMed]
- Shekhawat, J.K.; Rai, M.K.; Shekhawat, N.S.; Kataria, V. Exploring genetic variability in Prosopis cineraria using two gene targeted CAAT box-derived polymorphism (CBDP) and start codon targeted (SCoT) polymorphism markers. Mol. Biol. Rep. 2018, 45, 2359–2367. [Google Scholar] [CrossRef]
- Clifton-Brown, J.; Harfouche, A.; Casler, M.D.; Dylan Jones, H.; Macalpine, W.J.; Murphy-Bokern, D.; Smart, L.B.; Adler, A.; Ashman, C.; Awty-Carroll, D.; et al. Breeding progress and preparedness for mass-scale deployment of perennial lignocellulosic biomass crops switchgrass, miscanthus, willow and poplar. Glob. Chang. Biol. Bioenergy 2019, 11, 118–151. [Google Scholar] [CrossRef]
- Muday, G.K. Auxins and tropisms. J. Plant Growth Regul. 2001, 20, 226–243. [Google Scholar] [CrossRef]
- Rodrigo, G.; Jaramillo, A.; Blázquez, M.A. Integral control of plant gravitropism through the interplay of hormone signaling and gene regulation. Biophys. J. 2011, 101, 757–763. [Google Scholar] [CrossRef]
- Herranz, R.; Valbuena, M.A.; Youssef, K.; Medina, F.J. Mechanisms of disruption of meristematic competence by micro-gravity in Arabidopsis seedlings. Plant Signal. Behav. 2014, 9, e28289. [Google Scholar] [CrossRef]
- Kumari, S.; Panigrahi, K.C.S. Light and auxin signaling cross-talk programme root development in plants. J. Biosci. 2019, 44, 26. [Google Scholar] [CrossRef]
- Tasaka, M.; Kato, T.; Fukaki, H. The endodermis and shoot gravitropism. Trends Plant Sci. 1999, 4, 103–107. [Google Scholar] [CrossRef]
- Ruelle, J. Morphology, Anatomy and Ultrastructure of Reaction Wood. In The Biology of Reaction Wood; Springer: Berlin/Heidelberg, Germany, 2014; pp. 13–35. [Google Scholar]
- Fukaki, H.; Tasaka, M. Gravity perception and gravitropic response of inflorescence stems in Arabidopsis thaliana. Adv. Space Res. 1999, 24, 763–770. [Google Scholar] [CrossRef]
- Zobel, B.J.; van Buijtenen, J.P. Wood Variation: Its Causes and Control; Springer: Berlin/Heidelberg, Germany, 1989. [Google Scholar]
- Mellerowicz, E.J.; Gorshkova, T.A. Tensional stress generation in gelatinous fibres: A review and possible mechanism based on cell-wall structure and composition. J. Exp. Bot. 2012, 63, 551–565. [Google Scholar] [CrossRef]
- Allona, I.; Quinn, M.; Shoop, E.; Swope, K.; Cyr, S.S.; Carlis, J.; Riedl, J.; Retzel, E.; Campbell, M.M.; Sederoff, R.; et al. Analysis of xylem formation in pine by cDNA sequencing. Proc. Natl. Acad. Sci. USA 1998, 95, 9693–9698. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Sederoff, R.R.; Allona, I. Differential expression of genes encoding cell wall proteins in vascular tissues from vertical and bent loblolly pine trees. Tree Physiol. 2000, 20, 457–466. [Google Scholar] [CrossRef]
- Le Provost, G.; Paiva, J.; Pot, D.; Brach, J.; Plomion, C. Seasonal variation in transcript accumulation in wood-forming tissues of maritime pine (Pinus pinaster Ait.) with emphasis on a cell wall glycine-rich protein. Planta 2003, 217, 820–830. [Google Scholar] [CrossRef]
- Gion, J.-M.; Lalanne, C.; Le Provost, G.; Ferry-Dumazet, H.; Paiva, J.; Chaumeil, P.; Frigerio, J.-M.; Brach, J.; Barré, A.; de Daruvar, A.; et al. The proteome of maritime pine wood forming tissue. Proteomics 2005, 5, 3731–3751. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, S.; Yoshida, M.; Yamamoto, H.; Okuyama, T. Screening genes that change expression during compression wood formation in Chamaecyparis obtusa. Tree Physiol. 2008, 28, 1331–1340. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, S.; Yoshida, M.; Yamamoto, H. Relationship between development of compression wood and gene expression. Plant Sci. 2009, 176, 729–735. [Google Scholar] [CrossRef]
- Ramos, P.; Le Provost, G.; Gantz, C.; Plomion, C.; Herrera, R. Transcriptional analysis of differentially expressed genes in response to stem inclination in young seedlings of pine. Plant Biol. 2012, 14, 923–933. [Google Scholar] [CrossRef]
- Li, X.; Yang, X.; Wu, H.X. Transcriptome profiling of radiata pine branches reveals new insights into reaction wood formation with implications in plant gravitropism. BMC Genom. 2013, 14, 768. [Google Scholar] [CrossRef]
- Salazar, R.; Pollmann, S.; Morales-Quintana, L.; Herrera, R.; Caparrós-Ruiz, D.; Ramos, P. In seedlings of Pinus radiata, jasmonic acid and auxin are differentially distributed on opposite sides of tilted stems affecting lignin monomer biosynthesis and composition. Plant Physiol. Biochem. 2019, 135, 215–223. [Google Scholar] [CrossRef]
- Savidge, R.A.; Mutumba, G.M.C.; Heald, J.K.; Wareing, P.F. Gas Chromatography-Mass Spectroscopy Identification of 1-Aminocyclopropane-1-carboxylic Acid in Compression Wood Vascular Cambium of Pinus contorta Dougl. Plant Physiol. 1983, 71, 434–436. [Google Scholar] [CrossRef][Green Version]
- Ingemarsson, B.S.M.; Eklund, L.; Eliasson, L. Ethylene effects on cambial activity and cell wall formation in hypocotyls of Picea abies seedlings. Physiol. Plant 1991, 82, 219–224. [Google Scholar] [CrossRef]
- Little, C.H.A.; Eklund, L. Ethylene in relation to compression wood formation in Abies balsamea shoots. Trees 1999, 13, 173–177. [Google Scholar] [CrossRef]
- Plomion, C.; Pionneau, C.; Brach, J.; Costa, P.; Baillères, H. Compression wood-responsive proteins in developing xylem of maritime pine (Pinus pinaster Ait.). Plant Physiol. 2000, 123, 959–969. [Google Scholar] [CrossRef] [PubMed]
- Klintborg, A.; Eklund, L.; Little, C.H.A. Ethylene metabolism in Scots pine (Pinus sylvestris) shoots during the year. Tree Physiol. 2002, 22, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.-H.; van Zyl, L.; No, E.-G.; Loopstra, C.A. Microarray analysis of genes preferentially expressed in differentiating xylem of loblolly pine (Pinus taeda). Plant Sci. 2004, 166, 1185–1195. [Google Scholar] [CrossRef]
- Ramos, P.; Valenzuela, C.; Le Provost, G.; Plomion, C.; Gantz, C.; Moya-León, M.A.; Herrera, R. ACC Oxidase and ACC Synthase Expression Profiles after Leaning of Young Radiata (P. radiata D. Don) and Maritime Pine (P. pinaster Ait.) Seedlings. J. Plant Growth Regul. 2012, 31, 382–391. [Google Scholar] [CrossRef]
- Ramos, P.; Herrera, R. Anatomical changes of xylem cells in stem of Pinus radiata seedlings exposed to inclination and ethylene. Biol. Plant 2013, 57, 525–530. [Google Scholar] [CrossRef]
- Ramos, P.; Guajardo, J.; Moya-León, M.A.; Herrera, R. A differential distribution of auxin and flavonols in radiata pine stem seedlings exposed to inclination. Tree Genet. Genomes 2016, 12, 42. [Google Scholar] [CrossRef]
- Philosoph-Hadas, S.; Friedman, H.; Meir, S. Gravitropic bending and plant hormones. Vitam. Horm. 2005, 72, 31–78. [Google Scholar] [CrossRef]
- Vandenbrink, J.P.; Kiss, J.Z. Plant responses to gravity. Semin. Cell Dev. Biol. 2019, 92, 122–125. [Google Scholar] [CrossRef]
- Plomion, C.; Leprovost, G.; Stokes, A. Wood formation in trees. Plant Physiol. 2001, 127, 1513–1523. [Google Scholar] [CrossRef]
- Andersson-Gunnerås, S.; Hellgren, J.M.; Björklund, S.; Regan, S.; Moritz, T.; Sundberg, B. Asymmetric expression of a poplar ACC oxidase controls ethylene production during gravitational induction of tension wood. Plant J. 2003, 34, 339–349. [Google Scholar] [CrossRef] [PubMed]
- Hellgren, J.M.; Olofsson, K.; Sundberg, B. Patterns of Auxin Distribution during Gravitational Induction of Reaction Wood in Poplar and Pine. Plant Physiol. 2004, 135, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Kende, H. Ethylene Biosynthesis. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1993, 44, 283–307. [Google Scholar] [CrossRef]
- Bleecker, A.B.; Kende, H. Ethylene: A Gaseous Signal Molecule in Plants. Annu. Rev. Cell Dev. Biol. 2000, 16, 1–18. [Google Scholar] [CrossRef]
- Wang, K.L.-C.; Li, H.; Ecker, J.R. Ethylene biosynthesis and signaling networks. Plant Cell 2002, 14, 131–151. [Google Scholar] [CrossRef]
- Dolan, L. The role of ethylene in the development of plant form. J. Exp. Bot. 1997, 48, 201–210. [Google Scholar] [CrossRef]
- Seyfferth, C.; Wessels, B.; Jokipii-Lukkari, S.; Sundberg, B.; Delhomme, N.; Felten, J.; Tuominen, H. Ethylene-Related Gene Expression Networks in Wood Formation. Front Plant Sci. 2018, 9, 272. [Google Scholar] [CrossRef]
- Wasternack, C. Jasmonates: An Update on Biosynthesis, Signal Transduction and Action in Plant Stress Response, Growth and Development. Ann. Bot. 2007, 100, 681–697. [Google Scholar] [CrossRef]
- Shan, X.; Zhang, Y.; Peng, W.; Wang, Z.; Xie, D. Molecular mechanism for jasmonate-induction of anthocyanin accumulation in Arabidopsis. J. Exp. Bot. 2009, 60, 3849–3860. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, X.; Wang, J.; Zou, G.; Wang, L.; Li, X. Two responses to MeJA induction of R2R3-MYB transcription factors regulate flavonoid accumulation in Glycyrrhiza uralensis Fisch. PLoS ONE 2020, 15, e0236565. [Google Scholar] [CrossRef] [PubMed]
- Swarup, R.; Perry, P.; Hagenbeek, D.; Van Der Straeten, D.; Beemster, G.T.S.; Sandberg, G.; Bhalerao, R.; Ljung, K.; Bennett, M.J. Ethylene upregulates auxin biosynthesis in Arabidopsis seedlings to enhance inhibition of root cell elongation. Plant Cell 2007, 19, 2186–2196. [Google Scholar] [CrossRef] [PubMed]
- Stepanova, A.N.; Yun, J.; Likhacheva, A.V.; Alonso, J.M. Multilevel Interactions between Ethylene and Auxin in Arabidopsis Roots. Plant Cell 2007, 19, 2169–2185. [Google Scholar] [CrossRef] [PubMed]
- Negi, S.; Ivanchenko, M.G.; Muday, G.K. Ethylene regulates lateral root formation and auxin transport in Arabidopsis thaliana. Plant J. 2008, 55, 175–187. [Google Scholar] [CrossRef]
- Yang, Y.; Hammes, U.Z.; Taylor, C.G.; Schachtman, D.P.; Nielsen, E. High-Affinity Auxin Transport by the AUX1 Influx Carrier Protein. Curr. Biol. 2006, 16, 1123–1127. [Google Scholar] [CrossRef]
- Zazimalova, E.; Murphy, A.S.; Yang, H.; Hoyerova, K.; Hosek, P. Auxin Transporters—Why So Many? Cold Spring Harb. Perspect. Biol. 2010, 2, a001552. [Google Scholar] [CrossRef]
- Peer, W.A.; Blakeslee, J.J.; Yang, H.; Murphy, A.S. Seven Things We Think We Know about Auxin Transport. Mol. Plant 2011, 4, 487–504. [Google Scholar] [CrossRef]
- Went, F.W. Reflections and Speculations. Annu. Rev. Plant Physiol. 1974, 25, 1–27. [Google Scholar] [CrossRef]
- Iino, M.; Tarui, Y.; Uematsu, C. Gravitropism of maize and rice coleoptiles: Dependence on the stimulation angle. Plant Cell Environ. 1996, 19, 1160–1168. [Google Scholar] [CrossRef]
- Godbolé, R.; Michalke, W.; Nick, P.; Hertel, R. Cytoskeletal Drugs and Gravity-Induced Lateral Auxin Transport in Rice Coleoptiles. Plant Biol. 2000, 2, 176–181. [Google Scholar] [CrossRef]
- Friml, J.; Wiśniewska, J.; Benková, E.; Mendgen, K.; Palme, K. Lateral relocation of auxin efflux regulator PIN3 mediates tropism in Arabidopsis. Nature 2002, 415, 806–809. [Google Scholar] [CrossRef] [PubMed]
- Benjamins, R.; Quint, A.; Weijers, D.; Hooykaas, P.; Offringa, R. The PINOID protein kinase regulates organ development in Arabidopsis by enhancing polar auxin transport. Development 2001, 128, 4057–4067. [Google Scholar] [CrossRef]
- DeLong, A.; Mockaitis, K.; Christensen, S. Protein phosphorylation in the delivery of and response to auxin signals. Plant Mol. Biol. 2002, 49, 285–303. [Google Scholar] [CrossRef]
- Friml, J.; Yang, X.; Michniewicz, M.; Weijers, D.; Quint, A.; Tietz, O.; Benjamins, R.; Ouwerkerk, P.B.F.; Ljung, K.; Sandberg, G.; et al. A PINOID-Dependent Binary Switch in Apical-Basal PIN Polar Targeting Directs Auxin Efflux. Science 2004, 306, 862–865. [Google Scholar] [CrossRef] [PubMed]
- Murphy, A.; Peer, W.A.; Taiz, L. Regulation of auxin transport by aminopeptidases and endogenous flavonoids. Planta 2000, 211, 315–324. [Google Scholar] [CrossRef]
- Brown, D.E.; Rashotte, A.M.; Murphy, A.S.; Normanly, J.; Tague, B.W.; Peer, W.A.; Taiz, L.; Muday, G.K. Flavonoids act as negative regulators of auxin transport in vivo in arabidopsis. Plant Physiol. 2001, 126, 524–535. [Google Scholar] [CrossRef]
- Buer, C.S.; Muday, G.K. The transparent testa4 mutation prevents flavonoid synthesis and alters auxin transport and the response of Arabidopsis roots to gravity and light. Plant Cell 2004, 16, 1191–1205. [Google Scholar] [CrossRef]
- Santelia, D.; Henrichs, S.; Vincenzetti, V.; Sauer, M.; Bigler, L.; Klein, M.; Bailly, A.; Lee, Y.; Friml, J.; Geisler, M.; et al. Flavonoids redirect PIN-mediated polar auxin fluxes during root gravitropic responses. J. Biol. Chem. 2008, 283, 31218–31226. [Google Scholar] [CrossRef]
- Mierziak, J.; Kostyn, K.; Kulma, A. Flavonoids as important molecules of plant interactions with the environment. Molecules 2014, 19, 16240–16265. [Google Scholar] [CrossRef]
- Winkel-Shirley, B. Biosynthesis of flavonoids and effects of stress. Curr. Opin. Plant Biol. 2002, 5, 218–223. [Google Scholar] [CrossRef]
- Peer, W.A.; Bandyopadhyay, A.; Blakeslee, J.J.; Makam, S.N.; Chen, R.J.; Masson, P.H.; Murphy, A.S. Variation in expression and protein localization of the PIN family of auxin efflux facilitator proteins in flavonoid mutants with altered auxin transport in Arabidopsis thaliana. Plant Cell 2004, 16, 1898–1911. [Google Scholar] [CrossRef]
- Buer, C.S.; Djordjevic, M.A. Architectural phenotypes in the transparent testa mutants of Arabidopsis thaliana. J. Exp. Bot. 2009, 60, 751–763. [Google Scholar] [CrossRef] [PubMed]
- Geisler, M.; Murphy, A.S. The ABC of auxin transport: The role of p-glycoproteins in plant development. FEBS Lett. 2006, 580, 1094–1102. [Google Scholar] [CrossRef] [PubMed]
- Blakeslee, J.J.; Bandyopadhyay, A.; Lee, O.R.; Mravec, J.; Titapiwatanakun, B.; Sauer, M.; Makam, S.N.; Cheng, Y.; Bouchard, R.; Adamec, J.; et al. Interactions among PIN-FORMED and P-Glycoprotein Auxin Transporters in Arabidopsis. Plant Cell 2007, 19, 131–147. [Google Scholar] [CrossRef]
- Bouchard, R.; Bailly, A.; Blakeslee, J.J.; Oehring, S.C.; Vincenzetti, V.; Lee, O.R.; Paponov, I.; Palme, K.; Mancuso, S.; Murphy, A.S.; et al. Immunophilin-like TWISTED DWARF1 Modulates Auxin Efflux Activities of Arabidopsis P-glycoproteins. J. Biol. Chem. 2006, 281, 30603–30612. [Google Scholar] [CrossRef]
- Kuhn, B.M.; Geisler, M.; Bigler, L.; Ringli, C. Flavonols accumulate asymmetrically and affect auxin transport in Arabidopsis. Plant Physiol. 2011, 156, 585–595. [Google Scholar] [CrossRef]
- Kuhn, B.M.; Errafi, S.; Bucher, R.; Dobrev, P.; Geisler, M.; Bigler, L.; Zažímalová, E.; Ringli, C. 7-Rhamnosylated Flavonols Modulate Homeostasis of the Plant Hormone Auxin and Affect Plant Development. J. Biol. Chem. 2016, 291, 5385–5395. [Google Scholar] [CrossRef]
- Kuhn, B.M.; Nodzyński, T.; Errafi, S.; Bucher, R.; Gupta, S.; Aryal, B.; Dobrev, P.; Bigler, L.; Geisler, M.; Zažímalová, E.; et al. Flavonol-induced changes in PIN2 polarity and auxin transport in the Arabidopsis thaliana rol1-2 mutant require phosphatase activity. Sci. Rep. 2017, 7, 41906. [Google Scholar] [CrossRef] [PubMed]
- Bailly, A.; Sovero, V.; Vincenzetti, V.; Santelia, D.; Bartnik, D.; Koenig, B.W.; Mancuso, S.; Martinoia, E.; Geisler, M. Modulation of P-glycoproteins by Auxin Transport Inhibitors Is Mediated by Interaction with Immunophilins. J. Biol. Chem. 2008, 283, 21817–21826. [Google Scholar] [CrossRef] [PubMed]
- Besseau, S.; Hoffmann, L.; Geoffroy, P.; Lapierre, C.; Pollet, B.; Legrand, M. Flavonoid accumulation in Arabidopsis repressed in lignin synthesis affects auxin transport and plant growth. Plant Cell 2007, 19, 148–162. [Google Scholar] [CrossRef] [PubMed]
- Pourcel, L.; Irani, N.G.; Koo, A.J.K.; Bohorquez-Restrepo, A.; Howe, G.A.; Grotewold, E. A chemical complementation approach reveals genes and interactions of flavonoids with other pathways. Plant J. 2013, 74, 383–397. [Google Scholar] [CrossRef] [PubMed]
- Grotewold, E. The challenges of moving chemicals within and out of cells: Insights into the transport of plant natural products. Planta 2004, 219, 906–909. [Google Scholar] [CrossRef]
- Zhao, J.; Dixon, R.A. MATE Transporters Facilitate Vacuolar Uptake of Epicatechin 3’-O-Glucoside for Proanthocyanidin Biosynthesis in Medicago truncatula and Arabidopsis. Plant Cell 2009, 21, 2323–2340. [Google Scholar] [CrossRef]
- Marinova, K.; Pourcel, L.; Weder, B.; Schwarz, M.; Barron, D.; Routaboul, J.-M.; Debeaujon, I.; Klein, M. The Arabidopsis MATE transporter TT12 acts as a vacuolar flavonoid/H+ -antiporter active in proanthocyanidin-accumulating cells of the seed coat. Plant Cell 2007, 19, 2023–2038. [Google Scholar] [CrossRef]
- Zhao, J.; Huhman, D.; Shadle, G.; He, X.-Z.; Sumner, L.W.; Tang, Y.; Dixon, R.A. MATE2 Mediates Vacuolar Sequestration of Flavonoid Glycosides and Glycoside Malonates in Medicago truncatula. Plant Cell 2011, 23, 1536–1555. [Google Scholar] [CrossRef] [PubMed]
- Behrens, C.E.; Smith, K.E.; Iancu, C.V.; Choe, J.Y.; Dean, J.V. Transport of Anthocyanins and other Flavonoids by the Arabidopsis ATP-Binding Cassette Transporter AtABCC2. Sci. Rep. 2019, 9, 437. [Google Scholar] [CrossRef]
- Yazaki, K. ABC transporters involved in the transport of plant secondary metabolites. FEBS Lett. 2006, 580, 1183–1191. [Google Scholar] [CrossRef]
- Francisco, R.M.; Regalado, A.; Ageorges, A.; Burla, B.J.; Bassin, B.; Eisenach, C.; Zarrouk, O.; Vialet, S.; Marlin, T.; Chaves, M.M.; et al. ABCC1, an ATP Binding Cassette Protein from Grape Berry, Transports Anthocyanidin 3-O-Glucosides. Plant Cell 2013, 25, 1840–1854. [Google Scholar] [CrossRef]
- Baxter, I.R.; Young, J.C.; Armstrong, G.; Foster, N.; Bogenschutz, N.; Cordova, T.; Peer, W.A.; Hazen, S.P.; Murphy, A.S.; Harper, J.F. A plasma membrane H+-ATPase is required for the formation of proanthocyanidins in the seed coat endothelium of Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2005, 102, 2649–2654. [Google Scholar] [CrossRef] [PubMed]
- Goodman, C.D.; Casati, P.; Walbot, V. A Multidrug Resistance-Associated Protein Involved in Anthocyanin Transport in Zea mays. Plant Cell 2004, 16, 1812–1826. [Google Scholar] [CrossRef]
- Buer, C.S.; Sukumar, P.; Muday, G.K. Ethylene modulates flavonoid accumulation and gravitropic responses in roots of Arabidopsis. Plant Physiol. 2006, 140, 1384–1396. [Google Scholar] [CrossRef] [PubMed]
- Lewis, D.R.; Ramirez, M.V.; Miller, N.D.; Vallabhaneni, P.; Ray, W.K.; Helm, R.F.; Winkel, B.S.; Muday, G.K. Auxin and ethylene induce flavonol accumulation through distinct transcriptional networks. Plant Physiol. 2011, 156, 144–164. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Dixon, R.A. The ‘ins’ and ‘outs’ of flavonoid transport. Trends Plant Sci. 2010, 15, 72–80. [Google Scholar] [CrossRef]
- Gomez, R.; Gonzalez, J.; Herrera, R.; Ramos, P. MYB Transcription Factors and a Putative Flavonoid Transporter ABCC-Like are Differentially Expressed in Radiata Pine Seedlings Exposed to Inclination. J. Plant Growth Regul. 2018, 37, 64–75. [Google Scholar] [CrossRef]
- Morales-Quintana, L.; Bustos, D.; González, J.; Urbina, D.C.; Herrera, R.; Ramos, P. PrMATE1 Is Differentially Expressed in Radiata Pine Exposed to Inclination and the Deduced Protein Displays High Affinity to Proanthocyanidin Substrates by a Computational Approach. J. Plant Growth Regul. 2019, 38, 14–29. [Google Scholar] [CrossRef]
- Lepiniec, L.; Debeaujon, I.; Routaboul, J.-M.; Baudry, A.; Pourcel, L.; Nesi, N.; Caboche, M. Genetics and biochemistry of seed flavonoids. Annu. Rev. Plant Biol. 2006, 57, 405–430. [Google Scholar] [CrossRef]
- Dubos, C.; Stracke, R.; Grotewold, E.; Weisshaar, B.; Martin, C.; Lepiniec, L. MYB transcription factors in Arabidopsis. Trends Plant Sci. 2010, 15, 573–581. [Google Scholar] [CrossRef]

| Species | Genes/Proteins Families and Biological Processes | Reference |
|---|---|---|
| Pinus contorta | Ethylene biosynthesis—ACC accumulation | [24] |
| Picea abies | Ethylene biosynthesis—ethylene induce changes in cell walls composition | [25] |
| Pinus taeda L. | Lignin biosynthesis—Phenylalanine amonio lyase (PAL), Cinnamate-4-hydroxylase (C4H), O-methyltransferase (OMT), 4-Coumarate-CoA ligase (4CL), and Cinnamyl alcohol dehydrogenase (CAD). Cell wall carbohydrate metabolism—Xyloglucan endotransglycosylases (XET). Transcription factors—MADS box, homeodomain, LIM-domain proteins | [15] |
| Abies balsamea | Ethylene biosyntesis—differential ethylene accumulation in tilted seedlings and tracheid production | [26] |
| Pinus taeda L. | Arabinogalactan proteins (AGPs)—differential accumulation of secondary cell walls remodelling proteins | [16] |
| Pinus pinaster Ait. | Ethylene and lignin biosynthesis—ACC oxidase, caffeic O-methyltransferase and caffeoyl CoA-O-methyltransferase. Nitrogen and carbon assimilation—glutamine synthetase and fructokinase. | [27] |
| Pinus sylvestris | Ethylene biosynthesis—ACC synthase and ACC oxidase activity | [28] |
| Pinus pinaster Ait. | Cell wall formation—glycine-rich protein (GRP) and UDP-glucose pyrophosphorylase. | [17] |
| Pinus taeda L. | Cell wall-related proteins—cellulose synthase, expansin, xyloglucan endotransglycosylases (XET), glucanase, laccase, arabinogalactan-proteins (AGPs). Intermediate metabolism—12-OXO-phytodiennoate reductase, UDP-glucosyltransferase, Short-chain type dehydrogenase/reductase, Myo-inositol-1-phosphate synthase, UDP-glucose pyrophosphorylase. | [29] |
| Pinus pinaster Ait. | Defense, carbohydrates and amino acid metabolisms, genes and proteins expression, cytoskeleton, cell wall biosynthesis, secondary and primary metabolisms. | [18] |
| Chamaecyparis obtusa | Cell wall modification proteins—β-1,3-glucanase-like protein. Lignin biosynthesis—laccase. | [19,20] |
| Pinus radiata D. Don | Hormone signaling—EIN3-like protein, Auxin-repressed protein. Phenylpropanoid pathway—Phenylalanine amonio lyase (PAL), chalcone synthase (CHS), flavanone 3-hydroxylase (F3H). | [21] |
| Pinus radiata D. Don | Ethylene biosynthesis—ACC oxidase and ACC synthase differetially expressed. | [30] |
| Pinus radiata D. Don | Cell division, cellulose biosynthesis, lignin deposition, microtubules. | [22] |
| Pinus radiata D. Don | Ethylene signaling—Induction of tracheids with compression wood phenotypes in seedlings treated with ethylene biosynthesis precursor. | [31] |
| Pinus radiata D. Don | Lignin and flavonoid biosynthesis—Chalcone synthase (CHS), Flavanone 3-hydroxylase (F3H), Flavonol synthase (FLS), Caffeic acid O-methyl transferase (COMT), Cinnamoyl-CoA reductase (CCR), auxin signaling—Auxin repressed-protein (ARP). | [32] |
| Pinus radiata D. Don | Auxin transporters—ABCB1, ABCB2, AUX1-1, AUX1-2, AUX1-3 and PIN1, lignin biosynthesis—analysis of lignin content and monomeric composition. Auxin and jasmonate content and distribution. | [23] |
| 1 Month | ||
|---|---|---|
| Stem lower half | Stem upper half | |
| KL (mg/gAIR) | 417.5 ± 18.4 a | 367.4 ± 5.7 b |
| %H | 47.9 ± 5.1 a | 16.9 ± 0.3 b |
| %G | 52.1 ± 5.1 b | 83.1 ± 0.3 a |
| G/H | 1.1 | 4.9 |
| Species | Genes/Proteins Involved in Biological Processes | Reference |
|---|---|---|
| Pinus radiata D. Don | Phenylalanine amonio lyase (PAL), chalcone synthase (CHS), flavanone 3-hydroxylase (F3H), transparent testa 12 (TT12)—inclination response and flavonoids homeostasis | [21] |
| Pinus radiata D. Don | Chalcone synthase (CHS), Flavanone 3-hydroxylase (F3H), Flavonol synthase (FLS), Caffeic acid O-methyl transferase (COMT), Cinnamoyl-CoA reductase(CCR)—lignin and flavonols biosynthesis. | [32] |
| Pinus radiata D. Don | ABCC-flavonoid transporter (MRP1), MYB2, MYB5, MYB6, MYB10—flavonoid biosynthesis and homeostasis. | [88] |
| Pinus radiata D. Don | MATE-flavonoid transporter (MATE1)—intracellular flavonoid homeostasis | [89] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morales-Quintana, L.; Ramos, P. A Talk between Flavonoids and Hormones to Reorient the Growth of Gymnosperms. Int. J. Mol. Sci. 2021, 22, 12630. https://doi.org/10.3390/ijms222312630
Morales-Quintana L, Ramos P. A Talk between Flavonoids and Hormones to Reorient the Growth of Gymnosperms. International Journal of Molecular Sciences. 2021; 22(23):12630. https://doi.org/10.3390/ijms222312630
Chicago/Turabian StyleMorales-Quintana, Luis, and Patricio Ramos. 2021. "A Talk between Flavonoids and Hormones to Reorient the Growth of Gymnosperms" International Journal of Molecular Sciences 22, no. 23: 12630. https://doi.org/10.3390/ijms222312630
APA StyleMorales-Quintana, L., & Ramos, P. (2021). A Talk between Flavonoids and Hormones to Reorient the Growth of Gymnosperms. International Journal of Molecular Sciences, 22(23), 12630. https://doi.org/10.3390/ijms222312630