Analgesic Mechanisms of Steroid Ointment against Oral Ulcerative Mucositis in a Rat Model
Abstract
:1. Introduction
2. Results
2.1. Residual Property of Ointment Bases on the Oral Mucosa
2.2. Effects of a Steroid-Containing Ointment on Oral Ulcerative Mucositis Healing and Plasma Glucose Levels in OUM Rats
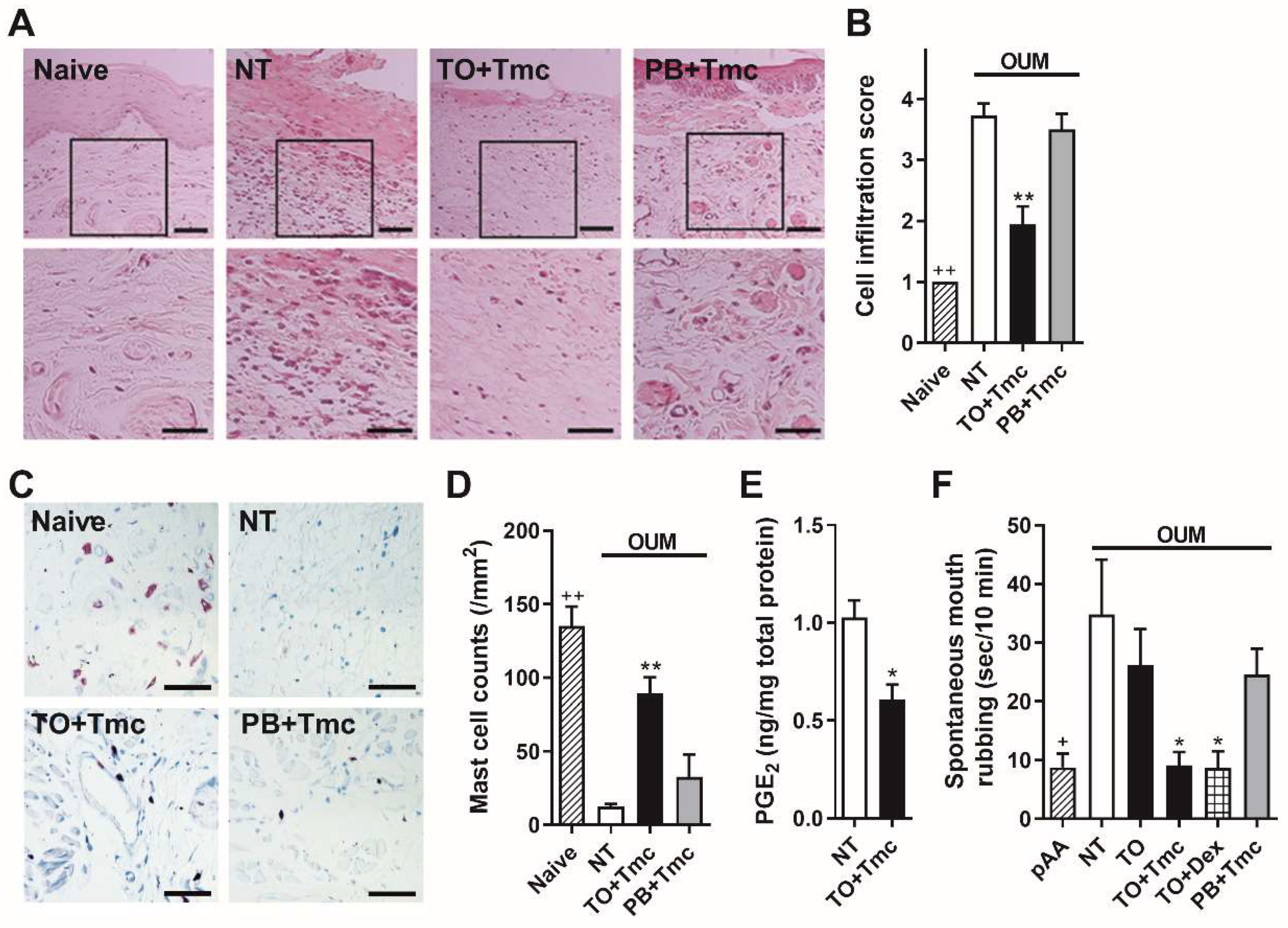
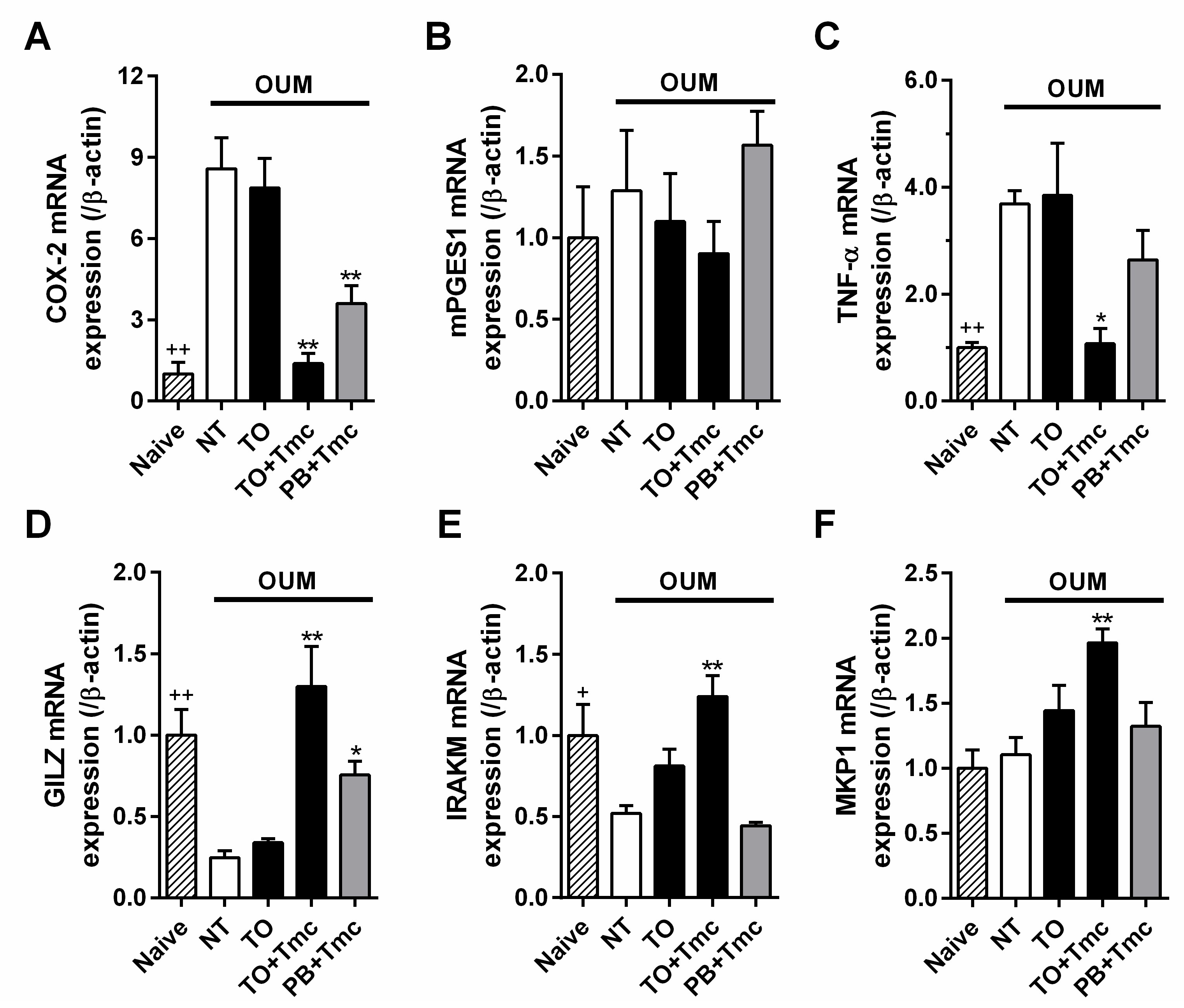
2.3. Anti-Inflammatory Effects of a Steroid-Containing Ointment on OUM Rats
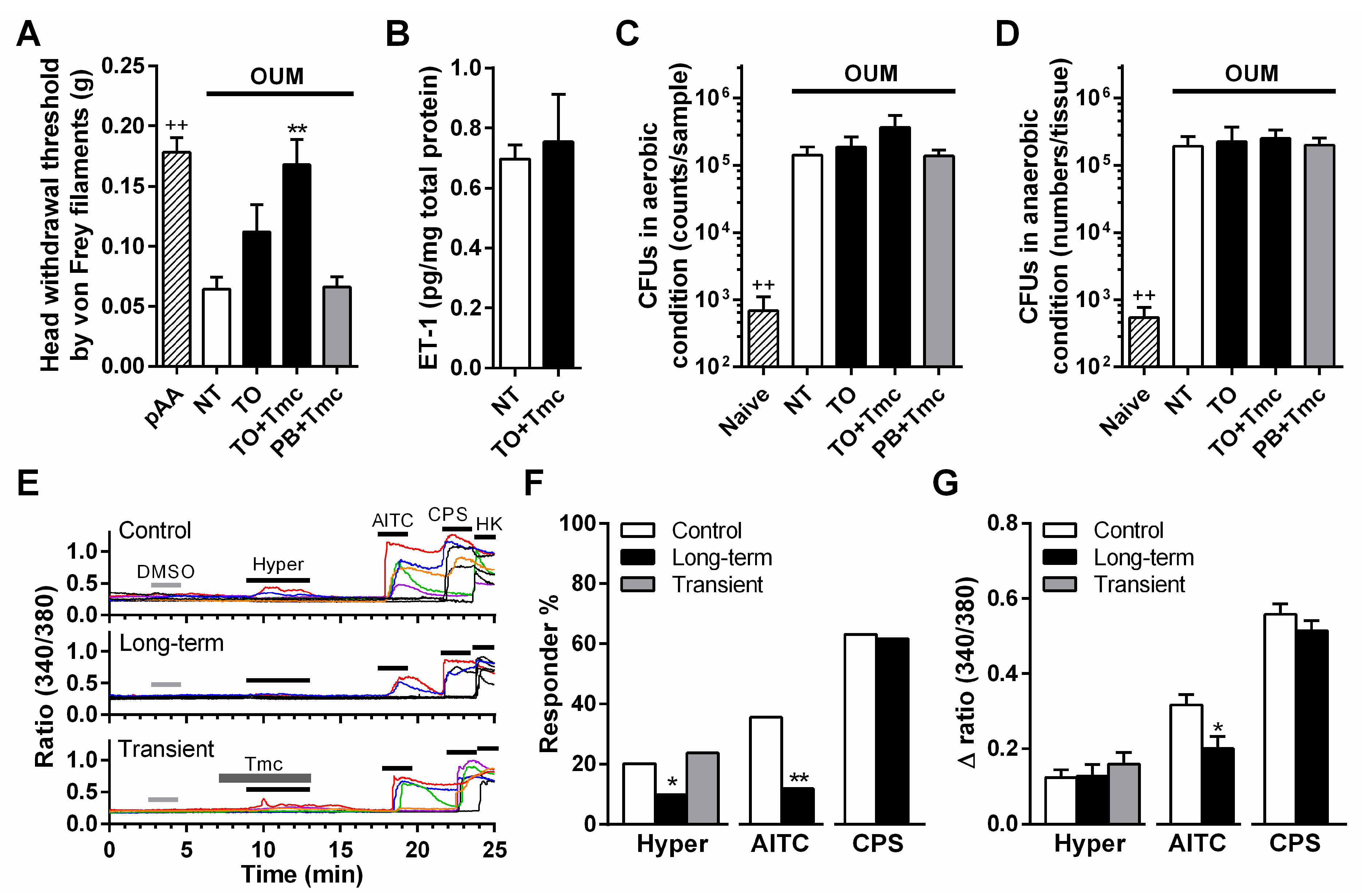
2.4. Anti-Allodynic Mechanism of a Steroid Containing Ointment in OUM Rats
3. Discussion
4. Materials and Methods
4.1. Ointments
4.2. Human Experiments
4.3. Animal Experiments
4.4. Rat Model of Oral Ulcerative Mucositis
4.5. Evaluation of Oral Ulcerative Mucositis Severity
4.6. Measurement of Blood Glucose Levels
4.7. Histology
4.8. Enzyme-linked Immunosorbent Assays (ELISAs)
4.9. Bacterial Counts
4.10. Quantitative Reserve-Transcription Polymerase Chain Reaction
4.11. Pain Evaluations
4.12. Ca2+ Imaging of Trigeminal Ganglion Neurons
4.13. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Edgar, N.R.; Saleh, D.; Miller, R.A. Recurrent Aphthous Stomatitis: A Review. J. Clin. Aesthet. Dermatol. 2017, 10, 26–36. [Google Scholar]
- Baricevic, M.; Mravak-Stipetic, M.; Majstorovic, M.; Baranovic, M.; Baricevic, D.; Loncar, B. Oral mucosal lesions during orthodontic treatment. Int. J. Paediatr. Dent. 2011, 21, 96–102. [Google Scholar] [CrossRef]
- Geckili, O.; Bektas-Kayhan, K.; Eren, P.; Bilgin, T.; Unur, M. The efficacy of a topical gel with triester glycerol oxide in denture-related mucosal injuries. Gerodontology 2012, 29, e715–e720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donnelly, J.P.; Bellm, L.A.; Epstein, J.B.; Sonis, S.T.; Symonds, R.P. Antimicrobial therapy to prevent or treat oral mucositis. Lancet Infect. Dis. 2003, 3, 405–412. [Google Scholar] [CrossRef]
- Lionel, D.; Christophe, L.; Marc, A.; Jean-Luc, C. Oral mucositis induced by anticancer treatments: Physiopathology and treatments. Ther. Clin. Risk Manag. 2006, 2, 159–168. [Google Scholar] [CrossRef] [Green Version]
- Sonis, S.T. Mucositis: The impact, biology and therapeutic opportunities of oral mucositis. Oral Oncol. 2009, 45, 1015–1020. [Google Scholar] [CrossRef] [PubMed]
- Natah, S.S.; Konttinen, Y.T.; Enattah, N.S.; Ashammakhi, N.; Sharkey, K.A.; Häyrinen-Immonen, R. Recurrent aphthous ulcers today: A review of the growing knowledge. Int. J. Oral Maxillofac. Surg. 2004, 33, 221–234. [Google Scholar] [CrossRef]
- Al-Omiri, M.K.; Karasneh, J.; Alhijawi, M.M.; Zwiri, A.M.A.; Scully, C.; Lynch, E. Recurrent aphthous stomatitis (RAS): A preliminary within-subject study of quality of life, oral health impacts and personality profiles. J. Oral Pathol. Med. 2015, 44, 278–283. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.S.; Park, E.Y.; Sohn, H.O. Oral Health Status and Oral Health-related Quality of Life According to Presence or Absence of Mucositis in Head and Neck Cancer Patients. J. Cancer Prev. 2019, 24, 43–47. [Google Scholar] [CrossRef] [Green Version]
- Burgess, J.A.; Johnson, B.D.; Sommers, E. Pharmacological management of recurrent oral mucosal ulceration. Drugs 1990, 39, 54–65. [Google Scholar] [CrossRef]
- Ship, J.A. Recurrent aphthous stomatitis. An update. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 1996, 81, 141–147. [Google Scholar] [CrossRef]
- Unur, M.; Ofluoglu, D.; Koray, M.; Mumcu, G.; Onal, A.E.; Tanyeri, H. Comparison of a New Medicinal Plant Extract and Triamcinolone Acetonide in Treatment of Recurrent Aphthous Stomatitis. Balk. J. Dent. Med. 2014, 18, 29–34. [Google Scholar] [CrossRef] [Green Version]
- Hermoso, M.A.; Cidlowski, J.A. Putting the brake on inflammatory responses: The role of glucocorticoids. IUBMB Life 2003, 55, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Vandewalle, J.; Luypaert, A.; Bosscher, K.D.; Libert, C. Therapeutic Mechanisms of Glucocorticoids. Trends Endocrinol. Metab. 2018, 29, 42–54. [Google Scholar] [CrossRef] [PubMed]
- Hitomi, S.; Ono, K.; Miyano, K.; Ota, Y.; Uezono, Y.; Matoba, M.; Kuramitsu, S.; Yamaguchi, K.; Matsuo, K.; Seta, Y.; et al. Novel methods of applying direct chemical and mechanical stimulation to the oral mucosa for traditional behavioral pain assays in conscious rats. J. Neurosci. Methods 2015, 239, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Nodai, T.; Hitomi, S.; Ono, K.; Masaki, C.; Harano, N.; Morii, A.; Sago-Ito, M.; Ujihara, I.; Hibino, T.; Terawaki, K.; et al. Endothelin-1 Elicits TRP-Mediated Pain in an Acid-Induced Oral Ulcer Model. J. Dent. Res. 2018, 97, 901–908. [Google Scholar] [CrossRef] [PubMed]
- Rhodus, N.L.; Bereuter, J. An evaluation of a chemical cautery agent and an anti-inflammatory ointment for the treatment of recurrent aphthous stomatitis: A pilot study. Quintessence Int. 1998, 29, 769–773. [Google Scholar] [PubMed]
- Filho, W.P.; Ribeiro, J.E.; Pinto, D.S. Safety and efficacy of Eupatorium laevigatum paste as therapy for buccal aphthae: Randomized, double-blind comparison with triamcinolone 0.1% orabase. Adv. Ther. 2000, 17, 272–281. [Google Scholar] [CrossRef]
- Liu, C.; Zhou, Z.; Liu, G.; Wang, Q.; Chen, J.; Wang, L.; Zhou, Y.; Dong, G.; Xu, X.; Wang, Y.; et al. Efficacy and safety of dexamethasone ointment on recurrent aphthous ulceration. Am. J. Med. 2012, 125, 292–301. [Google Scholar] [CrossRef]
- Sharma, R.; Pallagatti, S.; Aggarwal, A.; Sheikh, S.; Singh, R.; Gupta, D. A Randomized, Double-Blind, Placebo-Controlled Trial on Clinical Efficacy of Topical Agents in Reducing Pain and Frequency of Recurrent Aphthous Ulcers. Open Dent. J. 2018, 12, 700–713. [Google Scholar] [CrossRef]
- Miles, D.A.; Bricker, S.L.; Razmus, T.F.; Potter, R.H. Triamcinolone acetonide versus chlorhexidine for treatment of recurrent stomatitis. Oral Surg. Oral Med. Oral Pathol. 1993, 75, 397–402. [Google Scholar] [CrossRef]
- Alberti, L.R.; Vasconcellos, L.S.; Petroianu, A. Influence of local or systemic corticosteroids on skin wound healing resistance. Acta Cir. Bras. 2012, 27, 295–299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, A.S.; Armstrong, E.J.; Armstrong, A.W. Corticosteroids and wound healing: Clinical considerations in the perioperative period. Am. J. Surg. 2013, 206, 410–417. [Google Scholar] [CrossRef]
- Mitsuhashi, H.; Suemaru, K.; Li, B.; Cui, R.; Araki, H. Evaluation of topical external medicine for 5-fluorouracil-induced oral mucositis in hamsters. Eur. J. Pharmacol. 2006, 551, 152–155. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.; Coondoo, A. Systemic Side Effects of Topical Corticosteroids. In A Treatise on Topical Corticosteroids in Dermatology, 1st ed.; Lahiri, K., Ed.; Springer: Singapore, 2018; Chapter 25; pp. 241–249. [Google Scholar]
- Coondoo, A.; Phiske, M.; Verma, S.; Lahiri, K. Side-effects of topical steroids: A long overdue revisit. Indian Dermatol. Online J. 2014, 5, 416–425. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Ono, K.; Hitomi, S.; Ito, M.; Nodai, T.; Goto, T.; Harano, N.; Watanabe, S.; Inoue, H.; Miyano, K.; et al. Distinct TRPV1-and TRPA1-based mechanisms underlying enhancement of oral ulcerative mucositis-induced pain by 5-fluorouracil. Pain 2016, 157, 1004–1020. [Google Scholar] [CrossRef]
- Ayroldi, E.; Riccardi, C. Glucocorticoid-induced leucine zipper (GILZ): A new important mediator of glucocorticoid action. FASEB J. 2009, 23, 3649–3658. [Google Scholar] [CrossRef] [Green Version]
- Miyata, M.; Lee, J.Y.; Susuki-Miyata, S.; Wang, W.Y.; Xu, H.; Kai, H.; Kobayashi, K.S.; Flavell, R.A.; Li, J.D. Glucocorticoids suppress inflammation via the upregulation of negative regulator IRAK-M. Nat. Commun. 2015, 14, 6062. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vandevyver, S.; Dejager, L.; Bogaert, T.V.; Kleyman, A.; Liu, Y.; Tuckermann, J.; Libert, C. Glucocorticoid receptor dimerization induces MKP1 to protect against TNF-induced inflammation. J. Clin. Investig. 2012, 122, 2130–2140. [Google Scholar] [CrossRef] [Green Version]
- Meseguer, V.; Alpizar, Y.A.; Luis, E.; Tajada, S.; Denlinger, B.; Fajardo, O.; Manenschijn, J.A.; Fernández-Peña, C.; Talavera, A.; Kichko, T.; et al. TRPA1 channels mediate acute neurogenic inflammation and pain produced by bacterial endotoxins. Nat. Commun. 2014, 5, 3125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morii, A.; Miyamura, Y.; Sago-Ito, M.; Mizuhara, M.; Shikayama, T.; Naniwa, M.; Hitomi, S.; Ujihara, I.; Kuroishi-Nakao, K.; Gunjigake-Kometani, K.; et al. Orthodontic force-induced oxidative stress in the periodontal tissue and dental pulp elicits nociception via activation/sensitization of TRPA1 on nociceptive fibers. Free Radic. Biol. Med. 2020, 147, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, S.B.; de Araujo, A.A.; Araujo Junior, R.F.; Brito, G.A.C.; Leitao, R.C.; Barbosa, M.M.; Garcia, V.B.; Medeiros, A.C.; de Medeiros, C.A.C.X. Protective effect of dexamethasone on 5-FU-induced oral mucositis in hamsters. PLoS ONE 2017, 12, e0186511. [Google Scholar] [CrossRef]
- Ito, M.; Ono, K.; Hitomi, S.; Nodai, T.; Sago, T.; Yamaguchi, K.; Harano, N.; Gunnjigake, K.; Hosokawa, R.; Kawamoto, T.; et al. Prostanoid-dependent spontaneous pain and PAR2-dependent mechanical allodynia following oral mucosal trauma: Involvement of TRPV1, TRPA1 and TRPV4. Mol. Pain 2017, 13, 1744806917704138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitre-Aguilar, I.B.; Cabrera-Quintero, A.J.; Zentella-Dehesa, A. Genomic and non-genomic effects of glucocorticoids: Implications for breast cancer. Int. J. Clin. Exp. Pathol. 2015, 8, 1–10. [Google Scholar] [PubMed]
- Johnstone, W.M., 3rd; Honeycutt, J.L.; Deck, C.A.; Borski, R.J. Nongenomic glucocorticoid effects and their mechanisms of action in vertebrates. Int. Rev. Cell Mol. Biol. 2019, 346, 51–96. [Google Scholar] [CrossRef]
- Nummenmaa, E.; Hämäläinen, M.; Moilanen, L.J.; Moilanen, T.; Vuolteenaho, K.; Moilanen, E. TRPA1 expression is downregulated by dexamethasone and aurothiomalate in human chondrocytes: TRPA1 as a novel factor and drug target in arthritis. RMD Open 2017, 3, e000556. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.J.; Kim, H.S.; Spencer, C.A.; Brennan-Crispi, D.; Zheng, Y.; Johnson, N.M.; Rosenbach, M.; Miller, C.; Leung, D.H.; Cotsarelis, G.; et al. Activation of TRPA1 nociceptor promotes systemic adult mammalian skin regeneration. Sci. Immunol. 2020, 5, eaba5683. [Google Scholar] [CrossRef] [PubMed]
- Mogil, J.S. Sex differences in pain and pain inhibition: Multiple explanations of a controversial phenomenon. Nat. Rev. Neurosci. 2012, 13, 859–866. [Google Scholar] [CrossRef]
- de Araújo, A.A.; Varela, H.; de Medeiros, C.A.; de Castro Brito, G.A.; de Lima, K.C.; de Moura, L.M.; de Araújo Júnior, R.F. Azilsartan reduced TNF-α and IL-1β levels, increased IL-10 levels and upregulated VEGF, FGF, KGF, and TGF-α in an oral mucositis model. PLoS ONE 2015, 10, e0116799. [Google Scholar] [CrossRef]

| Vaseline | Plastibase | Traful | PRO-Quick | |
|---|---|---|---|---|
| Adhesiveness (N) | 2.2 ± 0.1 | 1.4 ± 0.1 | 2.9 ± 0.1 | 4.1 ± 0.1 |
| Hardness (N) | 1.1 ± 0.1 | 1.3 ± 0.0 | 2.9 ± 0.5 | 2.7 ± 0.4 |
| Viscosity, 1/s = 1 (mPa·s) | 12.6 ± 1.7 | 86.8 ± 1.9 | 163.9 ± 16.4 | 173.7 ± 16.8 |
| Viscosity, 1/s = 10 (mPa·s) | 1.3 ± 0.1 | 9.6 ± 0.4 | 17.4 ± 1.7 | 25.5 ± 3.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naniwa, M.; Nakatomi, C.; Hitomi, S.; Matsuda, K.; Tabuchi, T.; Sugiyama, D.; Kubo, S.; Miyamura, Y.; Yoshino, K.; Akifusa, S.; et al. Analgesic Mechanisms of Steroid Ointment against Oral Ulcerative Mucositis in a Rat Model. Int. J. Mol. Sci. 2021, 22, 12600. https://doi.org/10.3390/ijms222212600
Naniwa M, Nakatomi C, Hitomi S, Matsuda K, Tabuchi T, Sugiyama D, Kubo S, Miyamura Y, Yoshino K, Akifusa S, et al. Analgesic Mechanisms of Steroid Ointment against Oral Ulcerative Mucositis in a Rat Model. International Journal of Molecular Sciences. 2021; 22(22):12600. https://doi.org/10.3390/ijms222212600
Chicago/Turabian StyleNaniwa, Mako, Chihiro Nakatomi, Suzuro Hitomi, Kazunari Matsuda, Takuya Tabuchi, Daijiro Sugiyama, Sayaka Kubo, Yuichi Miyamura, Kenichi Yoshino, Sumio Akifusa, and et al. 2021. "Analgesic Mechanisms of Steroid Ointment against Oral Ulcerative Mucositis in a Rat Model" International Journal of Molecular Sciences 22, no. 22: 12600. https://doi.org/10.3390/ijms222212600
APA StyleNaniwa, M., Nakatomi, C., Hitomi, S., Matsuda, K., Tabuchi, T., Sugiyama, D., Kubo, S., Miyamura, Y., Yoshino, K., Akifusa, S., & Ono, K. (2021). Analgesic Mechanisms of Steroid Ointment against Oral Ulcerative Mucositis in a Rat Model. International Journal of Molecular Sciences, 22(22), 12600. https://doi.org/10.3390/ijms222212600







