Disulfide Dimerization of Neuronal Calcium Sensor-1: Implications for Zinc and Redox Signaling
Abstract
:1. Introduction
2. Results
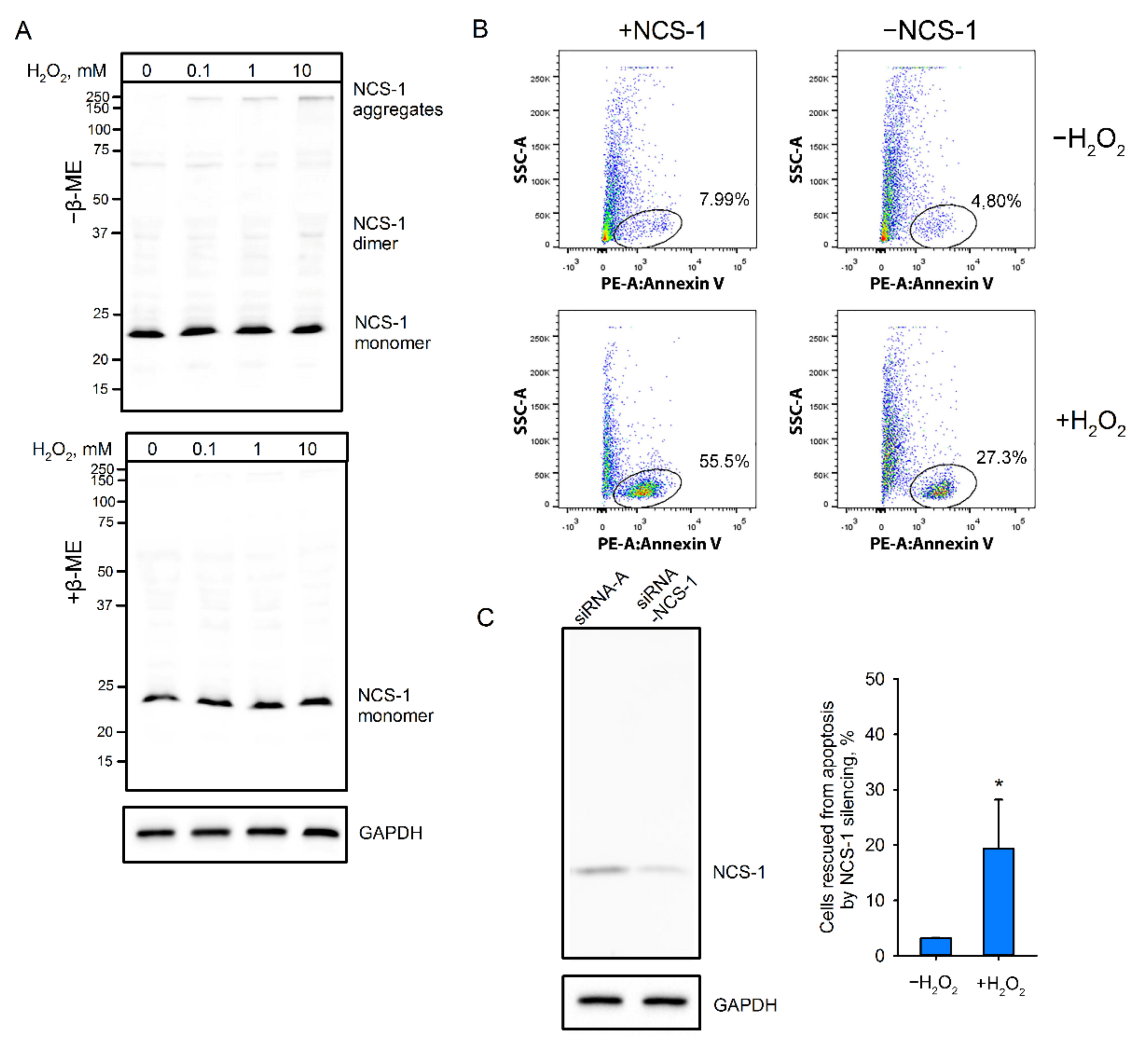
2.1. Disulfide Dimerization of NCS-1 in Cells
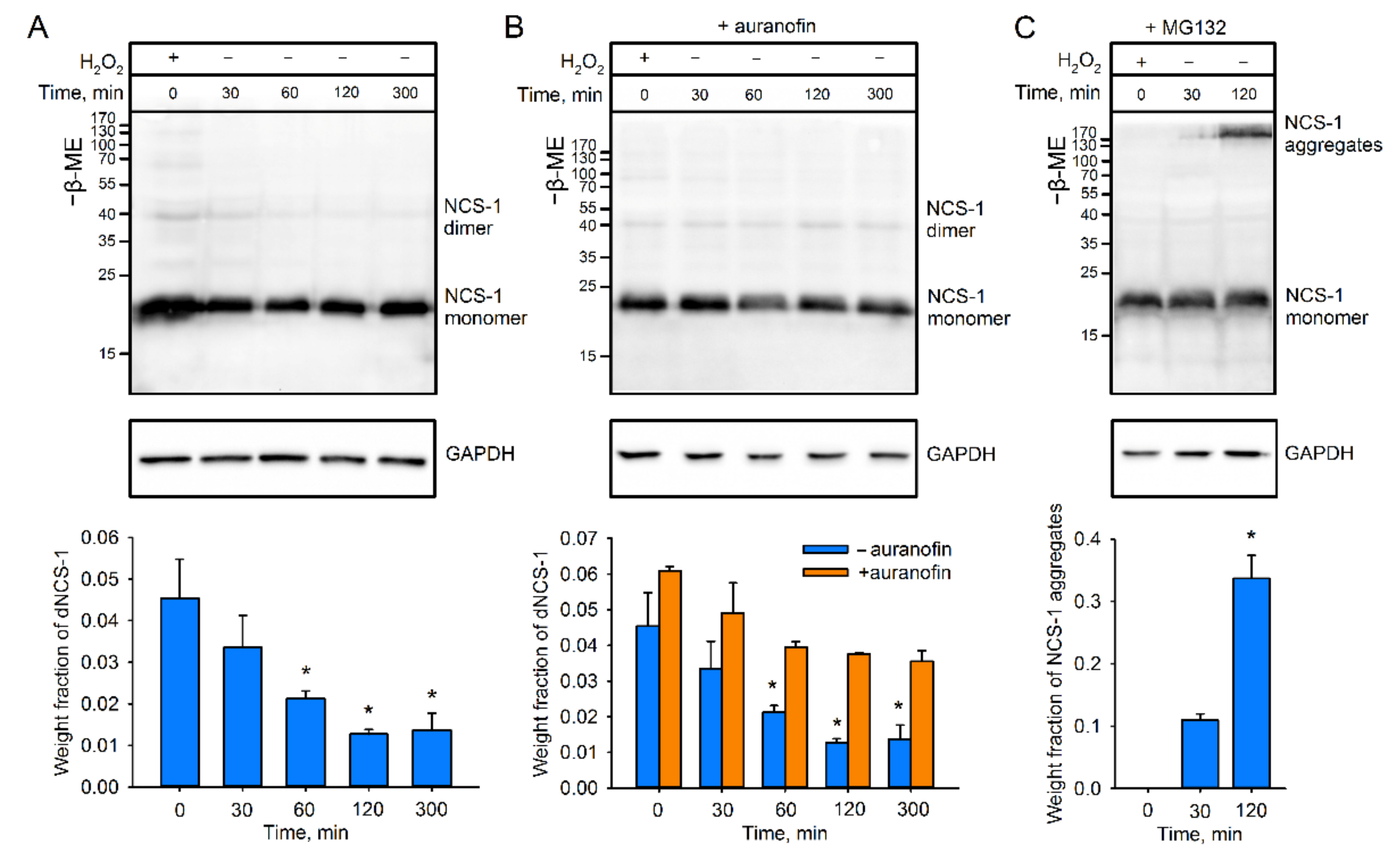
2.2. Recycling of dNCS-1 in Cells
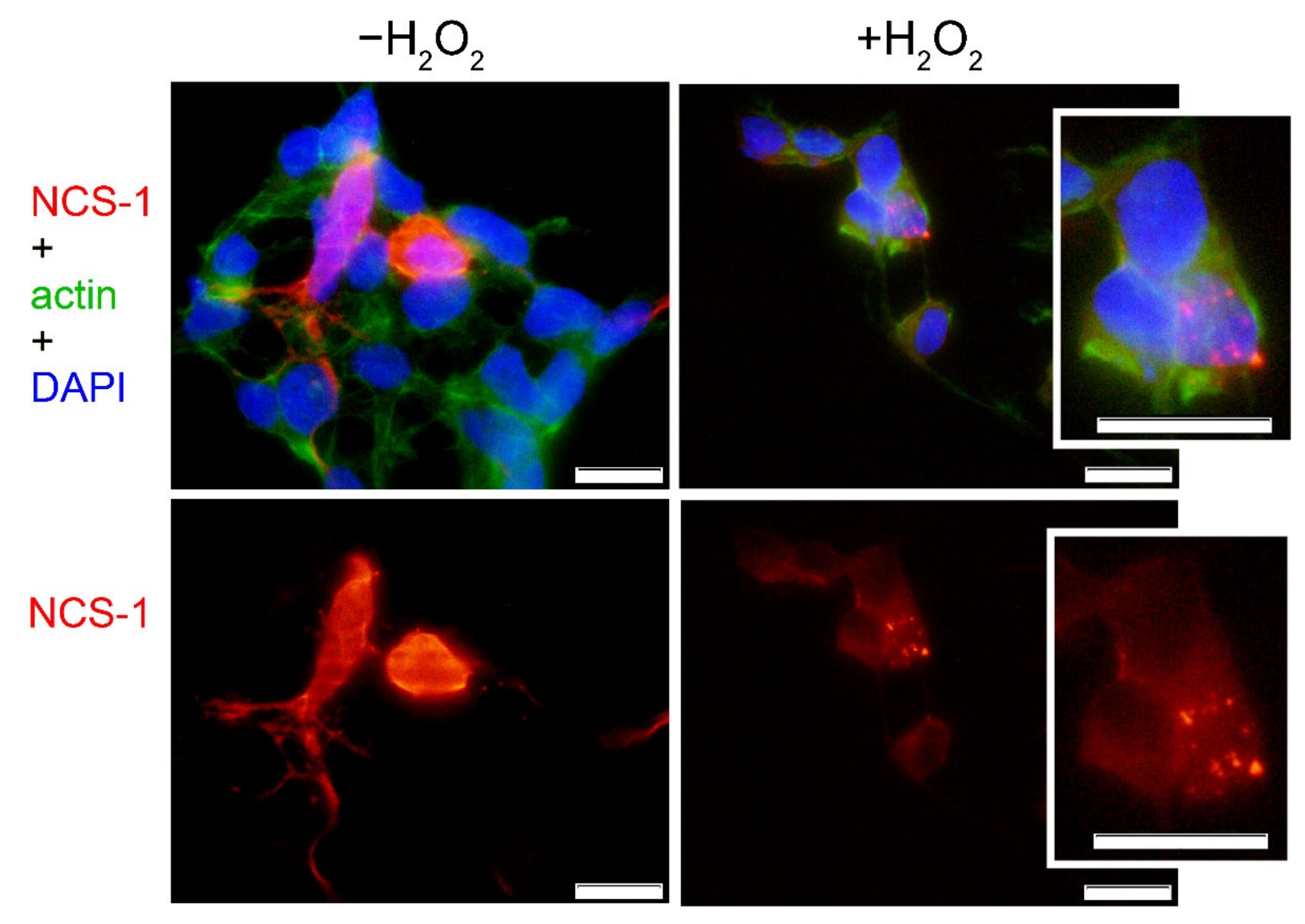
2.3. Cellular Localization of NCS-1 in Oxidative Stress
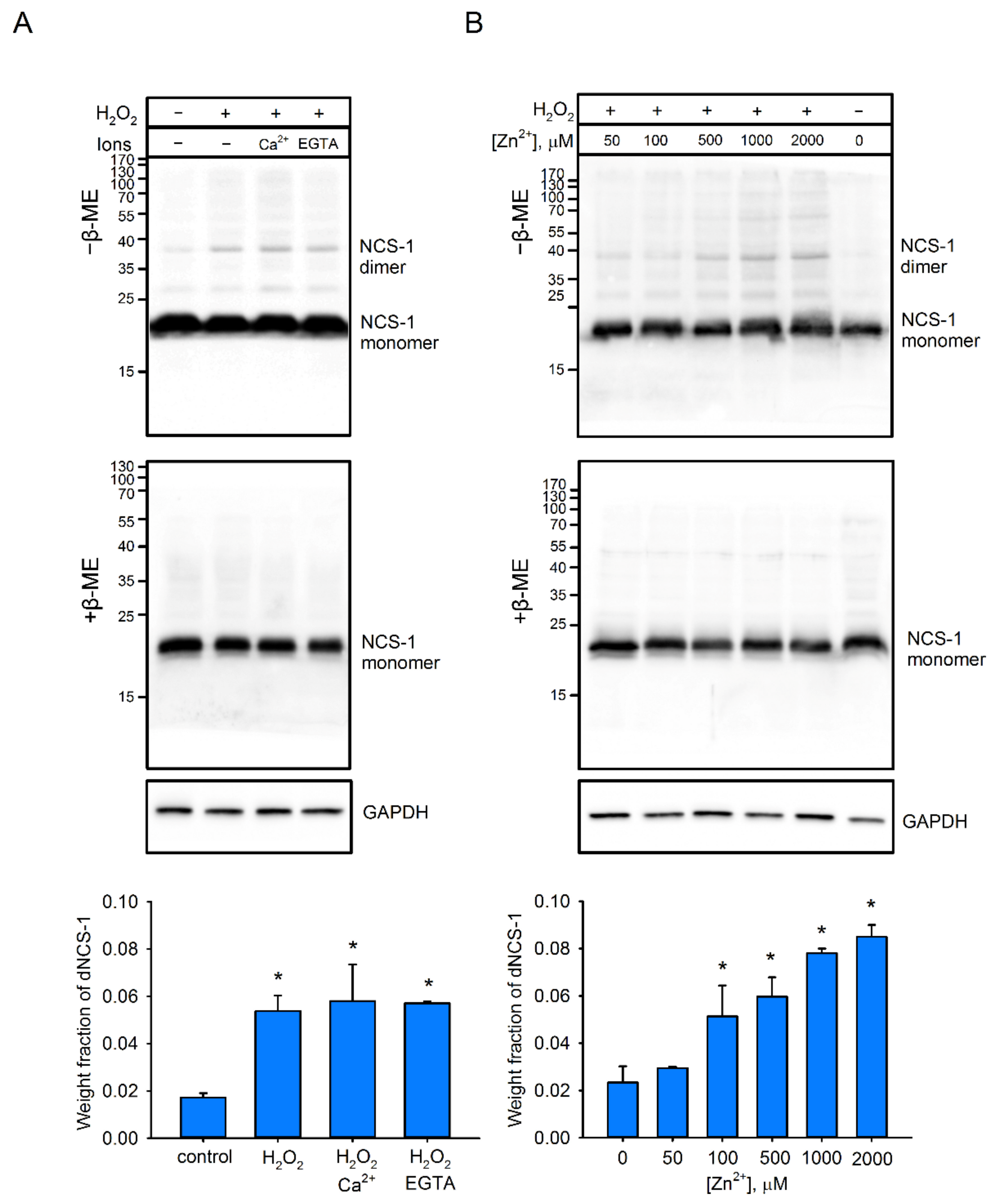
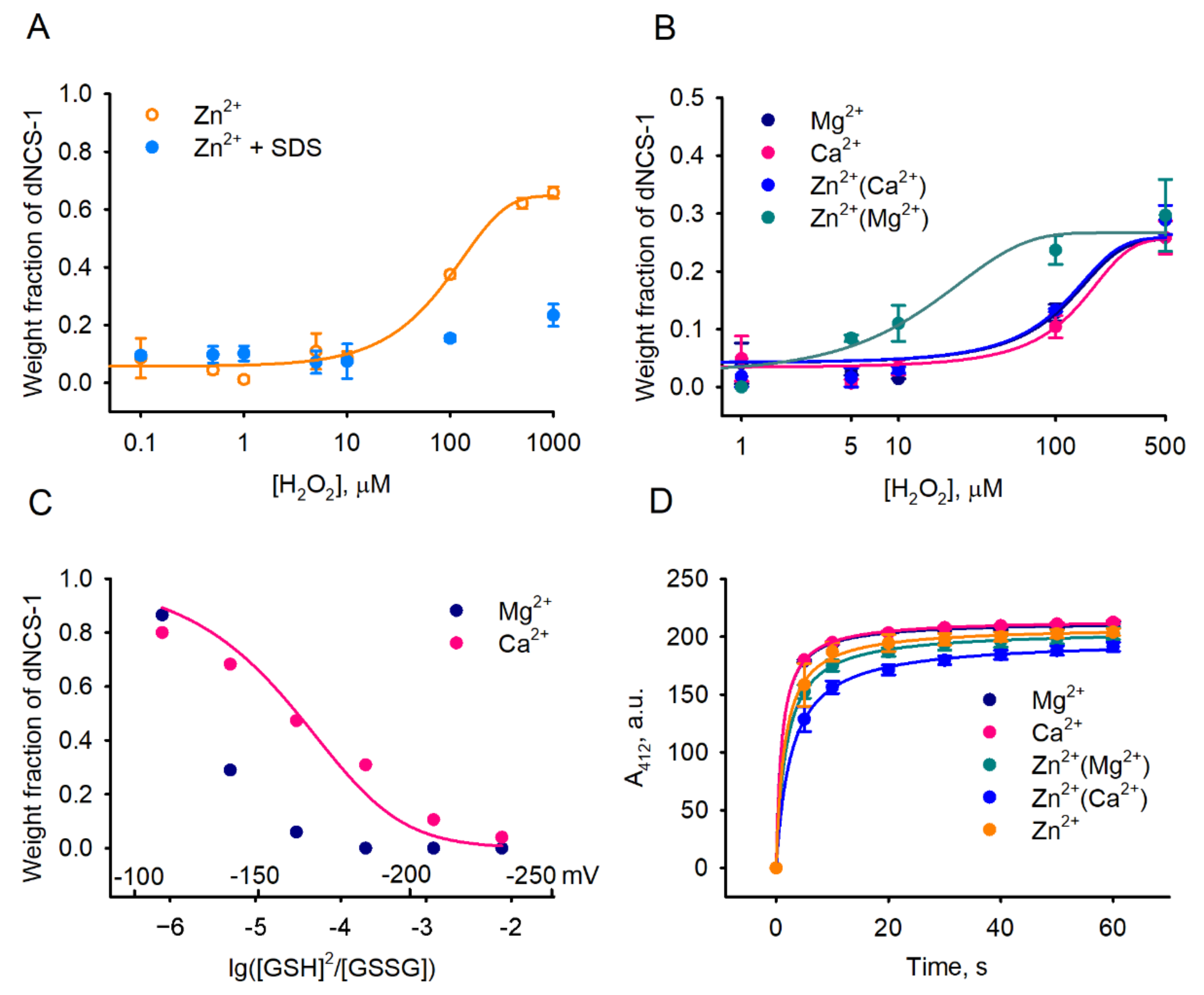
2.4. Factors Affecting dNCS-1 Formation
2.5. Calcium-Binding and Structural Properties of dNCS-1
2.6. Functional Properties of dNCS-1
2.7. Zinc-Binding Properties of dNCS-1
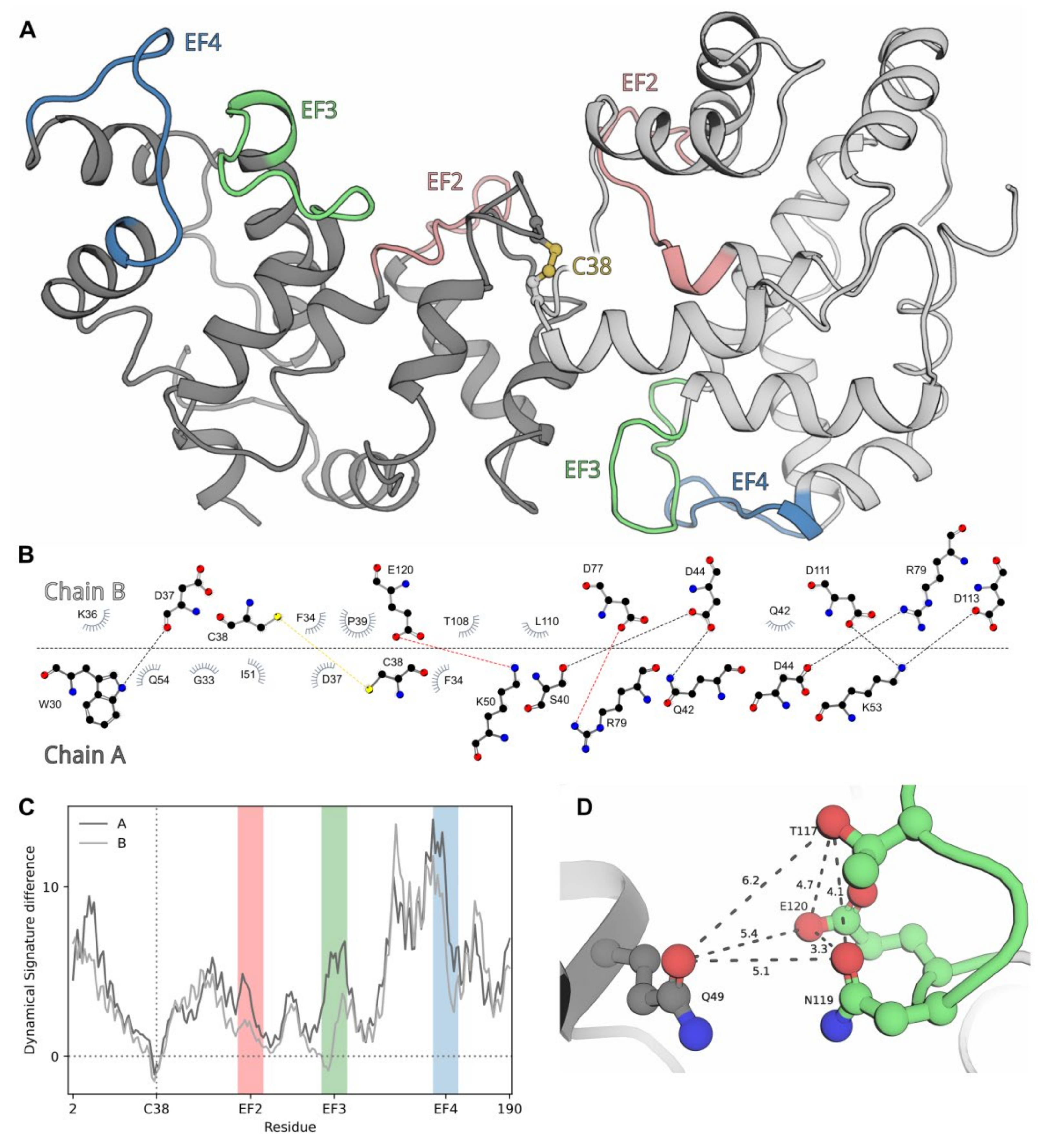
2.8. Modeling of dNCS-1 Structure
2.9. Role of NCS-1 Disulfides in Oxidative Stress-Induced Apoptosis
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Generation of Plasmid Constructs
4.3. Bacterial Expression and Purification of Recombinant Proteins
4.4. Oxidation of NCS-1 In Vitro
4.5. Ellmann’s Assay
4.6. Preparation of dNCS-1
4.7. Evaluation of Redox Potential of dNCS-1
4.8. Fluorescence Measurements
4.9. Circular Dichroism (CD) Measurements
4.10. Isothermal Titration Calorimetry (ITC)
4.11. Membrane Binding Assay
4.12. Rhodopsin Phosphorylation Assay
4.13. Surface Plasmon Resonance (SPR) Spectroscopy
4.14. Cell Culture and Treatments
4.15. Immunocytochemistry and Microscopy
4.16. Analysis of Apoptosis by Flow Cytometry
4.17. Western Blotting
4.18. Molecular Modeling
4.19. Statistics
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ames, J.B.; Lim, S. Molecular structure and target recognition of neuronal calcium sensor proteins. Biochim. Biophys. Acta 2012, 1820, 1205–1213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burgoyne, R.D. The neuronal calcium-sensor proteins. Biochim. Biophys. Acta 2004, 1742, 59–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ames, J.B.; Tanaka, T.; Stryer, L.; Ikura, M. Portrait of a myristoyl switch protein. Curr. Opin. Struct. Biol. 1996, 6, 432–438. [Google Scholar] [CrossRef]
- Ames, J.B.; Ishima, R.; Tanaka, T.; Gordon, J.I.; Stryer, L.; Ikura, M. Molecular mechanics of calcium-myristoyl switches. Nature 1997, 389, 198–202. [Google Scholar] [CrossRef]
- Beven, L.; Adenier, H.; Kichenama, R.; Homand, J.; Redeker, V.; Le Caer, J.P.; Ladant, D.; Chopineau, J. Ca2+-myristoyl switch and membrane binding of chemically acylated neurocalcins. Biochemistry 2001, 40, 8152–8160. [Google Scholar] [CrossRef]
- Hwang, J.Y.; Koch, K.W. Calcium- and myristoyl-dependent properties of guanylate cyclase-activating protein-1 and protein-2. Biochemistry 2002, 41, 13021–13028. [Google Scholar] [CrossRef]
- Spilker, C.; Braunewell, K.H. Calcium-myristoyl switch, subcellular localization, and calcium-dependent translocation of the neuronal calcium sensor protein VILIP-3, and comparison with VILIP-1 in hippocampal neurons. Mol. Cell. Neurosci. 2003, 24, 766–778. [Google Scholar] [CrossRef]
- Baksheeva, V.E.; Nazipova, A.A.; Zinchenko, D.V.; Serebryakova, M.V.; Senin, I.I.; Permyakov, S.E.; Philippov, P.P.; Li, Y.; Zamyatnin, A.A.; Zernii, E.Y.; et al. Ca2+ -myristoyl switch in neuronal calcium sensor-1: A role of C-terminal segment. CNS Neurol. Disord. Drug Targets 2015, 14, 437–451. [Google Scholar] [CrossRef] [Green Version]
- Burgoyne, R.D. Neuronal calcium sensor proteins: Generating diversity in neuronal Ca2+ signalling. Nat. Rev. Neurosci. 2007, 8, 182–193. [Google Scholar] [CrossRef] [Green Version]
- Baksheeva, V.E.; Nemashkalova, E.L.; Firsov, A.M.; Zalevsky, A.O.; Vladimirov, V.I.; Tikhomirova, N.K.; Philippov, P.P.; Zamyatnin, A.A.J.; Zinchenko, D.V.; Antonenko, Y.N.; et al. Membrane Binding of Neuronal Calcium Sensor-1: Highly Specific Interaction with Phosphatidylinositol-3-Phosphate. Biomolecules 2020, 10, 164. [Google Scholar] [CrossRef] [Green Version]
- O’Callaghan, D.W.; Haynes, L.P.; Burgoyne, R.D. High-affinity interaction of the N-terminal myristoylation motif of the neuronal calcium sensor protein hippocalcin with phosphatidylinositol 4,5-bisphosphate. Biochem. J. 2005, 391, 231–238. [Google Scholar] [CrossRef] [Green Version]
- Tsvetkov, P.O.; Roman, A.Y.; Baksheeva, V.E.; Nazipova, A.A.; Shevelyova, M.P.; Vladimirov, V.I.; Buyanova, M.F.; Zinchenko, D.V.; Zamyatnin, A.A., Jr.; Devred, F.; et al. Functional Status of Neuronal Calcium Sensor-1 Is Modulated by Zinc Binding. Front. Mol. Neurosci. 2018, 11, 459. [Google Scholar] [CrossRef] [Green Version]
- Chandra, K.; Ramakrishnan, V.; Sharma, Y.; Chary, K.V. N-terminal myristoylation alters the calcium binding pathways in neuronal calcium sensor-1. J. Biol. Inorg. Chem. JBIC: A Publ. Soc. Biol. Inorg. Chem. 2011, 16, 81–95. [Google Scholar] [CrossRef]
- Boeckel, G.R.; Ehrlich, B.E. NCS-1 is a regulator of calcium signaling in health and disease. Biochim. Biophys. Acta Mol. Cell Res. 2018, 1865, 1660–1667. [Google Scholar] [CrossRef]
- Weiss, J.L.; Hui, H.; Burgoyne, R.D. Neuronal calcium sensor-1 regulation of calcium channels, secretion, and neuronal outgrowth. Cell. Mol. Neurobiol. 2010, 30, 1283–1292. [Google Scholar] [CrossRef]
- Hilfiker, S. Neuronal calcium sensor-1: A multifunctional regulator of secretion. Biochem. Soc. Trans. 2003, 31, 828–832. [Google Scholar] [CrossRef]
- Burgoyne, R.D.; Helassa, N.; McCue, H.V.; Haynes, L.P. Calcium Sensors in Neuronal Function and Dysfunction. Cold Spring Harb. Perspect. Biol. 2019, 11, a035154. [Google Scholar] [CrossRef]
- Wang, D.; O’Halloran, D.; Goodman, M.B. GCY-8, PDE-2, and NCS-1 are critical elements of the cGMP-dependent thermotransduction cascade in the AFD neurons responsible for C. elegans thermotaxis. J. Gen. Physiol. 2013, 142, 437–449. [Google Scholar] [CrossRef] [Green Version]
- Todd, P.A.; McCue, H.V.; Haynes, L.P.; Barclay, J.W.; Burgoyne, R.D. Interaction of ARF-1.1 and neuronal calcium sensor-1 in the control of the temperature-dependency of locomotion in Caenorhabditis elegans. Sci. Rep. 2016, 6, 30023. [Google Scholar] [CrossRef] [Green Version]
- Vladimirov, V.I.; Zernii, E.Y.; Baksheeva, V.E.; Wimberg, H.; Kazakov, A.S.; Tikhomirova, N.K.; Nemashkalova, E.L.; Mitkevich, V.A.; Zamyatnin, A.A.J.; Lipkin, V.M.; et al. Photoreceptor calcium sensor proteins in detergent-resistant membrane rafts are regulated via binding to caveolin-1. Cell Calcium 2018, 73, 55–69. [Google Scholar] [CrossRef]
- Treloar, H.B.; Uboha, U.; Jeromin, A.; Greer, C.A. Expression of the neuronal calcium sensor protein NCS-1 in the developing mouse olfactory pathway. J. Comp. Neurol. 2005, 482, 201–216. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, J.C.; Ehrlich, B.E. Functional Interaction Between TRPV4 And NCS1 and the Effects of Paclitaxel. Mol. Pharmacol. 2021, 100, 258–270. [Google Scholar] [CrossRef] [PubMed]
- Bandura, J.; Feng, Z.P. Current Understanding of the Role of Neuronal Calcium Sensor 1 in Neurological Disorders. Mol. Neurobiol. 2019, 56, 6080–6094. [Google Scholar] [CrossRef] [PubMed]
- Apasu, J.E.; Schuette, D.; LaRanger, R.; Steinle, J.A.; Nguyen, L.D.; Grosshans, H.K.; Zhang, M.; Cai, W.L.; Yan, Q.; Robert, M.E.; et al. Neuronal calcium sensor 1 (NCS1) promotes motility and metastatic spread of breast cancer cells in vitro and in vivo. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2019, 33, 4802–4813. [Google Scholar] [CrossRef]
- Moore, L.M.; England, A.; Ehrlich, B.E.; Rimm, D.L. Calcium Sensor, NCS-1, Promotes Tumor Aggressiveness and Predicts Patient Survival. Mol. Cancer Res. 2017, 15, 942–952. [Google Scholar] [CrossRef] [Green Version]
- Ranaghan, M.J.; Kumar, R.P.; Chakrabarti, K.S.; Buosi, V.; Kern, D.; Oprian, D.D. A highly conserved cysteine of neuronal calcium-sensing proteins controls cooperative binding of Ca2+ to recoverin. J. Biol. Chem. 2013, 288, 36160–36167. [Google Scholar] [CrossRef] [Green Version]
- Zernii, E.Y.; Nazipova, A.A.; Nemashkalova, E.L.; Kazakov, A.S.; Gancharova, O.S.; Serebryakova, M.V.; Tikhomirova, N.K.; Baksheeva, V.E.; Vladimirov, V.I.; Zinchenko, D.V.; et al. Light-Induced Thiol Oxidation of Recoverin Affects Rhodopsin Desensitization. Front. Mol. Neurosci. 2018, 11, 474. [Google Scholar] [CrossRef]
- Permyakov, S.E.; Nazipova, A.A.; Denesyuk, A.I.; Bakunts, A.G.; Zinchenko, D.V.; Lipkin, V.M.; Uversky, V.N.; Permyakov, E.A. Recoverin as a redox-sensitive protein. J. Proteome Res. 2007, 6, 1855–1863. [Google Scholar] [CrossRef]
- Permyakov, S.E.; Zernii, E.Y.; Knyazeva, E.L.; Denesyuk, A.I.; Nazipova, A.A.; Kolpakova, T.V.; Zinchenko, D.V.; Philippov, P.P.; Permyakov, E.A.; Senin, I.I. Oxidation mimicking substitution of conservative cysteine in recoverin suppresses its membrane association. Amino Acids 2012, 42, 1435–1442. [Google Scholar] [CrossRef]
- Chen, K.C.; Wang, L.K.; Chang, L.S. Regulatory elements and functional implication for the formation of dimeric visinin-like protein-1. J. Pept. Sci. Off. Publ. Eur. Pept. Soc. 2009, 15, 89–94. [Google Scholar] [CrossRef]
- Liebl, M.P.; Kaya, A.M.; Tenzer, S.; Mittenzwei, R.; Koziollek-Drechsler, I.; Schild, H.; Moosmann, B.; Behl, C.; Clement, A.M. Dimerization of visinin-like protein 1 is regulated by oxidative stress and calcium and is a pathological hallmark of amyotrophic lateral sclerosis. Free Radic Biol. Med. 2014, 72, 41–54. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.K.; Simon, A.; Jessen, C.M.; Oliveira, C.L.; Mack, L.; Braunewell, K.H.; Ames, J.B.; Pedersen, J.S.; Hofmann, A. Divalent cations and redox conditions regulate the molecular structure and function of visinin-like protein-1. PLoS ONE 2011, 6, e26793. [Google Scholar] [CrossRef] [Green Version]
- Zernii, E.Y.; Nazipova, A.A.; Gancharova, O.S.; Kazakov, A.S.; Serebryakova, M.V.; Zinchenko, D.V.; Tikhomirova, N.K.; Senin, I.I.; Philippov, P.P.; Permyakov, E.A.; et al. Light-induced disulfide dimerization of recoverin under ex vivo and in vivo conditions. Free Radic Biol. Med. 2015, 83, 283–295. [Google Scholar] [CrossRef]
- Cox, J.A.; Durussel, I.; Comte, M.; Nef, S.; Nef, P.; Lenz, S.E.; Gundelfinger, E.D. Cation binding and conformational changes in VILIP and NCS-1, two neuron-specific calcium-binding proteins. J. Biol. Chem. 1994, 269, 32807–32813. [Google Scholar] [CrossRef]
- Nakamura, T.Y.; Jeromin, A.; Smith, G.; Kurushima, H.; Koga, H.; Nakabeppu, Y.; Wakabayashi, S.; Nabekura, J. Novel role of neuronal Ca2+ sensor-1 as a survival factor up-regulated in injured neurons. J. Cell Biol. 2006, 172, 1081–1091. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, T.Y.; Nakao, S.; Wakabayashi, S. Neuronal Ca(2+) sensor-1 contributes to stress tolerance in cardiomyocytes via activation of mitochondrial detoxification pathways. J. Mol. Cell. Cardiol. 2016, 99, 23–34. [Google Scholar] [CrossRef]
- Ye, H.; Ye, B.; Wang, D. Trace administration of vitamin E can retrieve and prevent UV-irradiation-and metal exposure-induced memory deficits in nematode Caenorhabditis elegans. Neurobiol. Learn. Mem. 2008, 90, 10–18. [Google Scholar] [CrossRef]
- Maret, W. Metals on the move: Zinc ions in cellular regulation and in the coordination dynamics of zinc proteins. Biometals 2011, 24, 411–418. [Google Scholar] [CrossRef]
- Bossy-Wetzel, E.; Talantova, M.V.; Lee, W.D.; Scholzke, M.N.; Harrop, A.; Mathews, E.; Gotz, T.; Han, J.H.; Ellisman, M.H.; Perkins, G.A.; et al. Crosstalk between nitric oxide and zinc pathways to neuronal cell death involving mitochondrial dysfunction and p38-activated K+ channels. Neuron 2004, 41, 351–365. [Google Scholar] [CrossRef] [Green Version]
- Ugarte, M.; Osborne, N.N. Recent advances in the understanding of the role of zinc in ocular tissues. Metallomics 2014, 6, 189–200. [Google Scholar] [CrossRef]
- Watt, N.T.; Whitehouse, I.J.; Hooper, N.M. The role of zinc in Alzheimer’s disease. Int. J. Alzheimer’s Dis. 2010, 2011, 971021. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Andereggen, L.; Yuki, K.; Omura, K.; Yin, Y.; Gilbert, H.Y.; Erdogan, B.; Asdourian, M.S.; Shrock, C.; de Lima, S.; et al. Mobile zinc increases rapidly in the retina after optic nerve injury and regulates ganglion cell survival and optic nerve regeneration. Proc. Natl. Acad. Sci. USA 2017, 114, E209–E218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maret, W.; Li, Y. Coordination dynamics of zinc in proteins. Chem. Rev. 2009, 109, 4682–4707. [Google Scholar] [CrossRef] [PubMed]
- Maret, W. Zinc coordination environments in proteins as redox sensors and signal transducers. Antioxid. Redox Signal. 2006, 8, 1419–1441. [Google Scholar] [CrossRef]
- Permyakov, S.E.; Cherskaya, A.M.; Wasserman, L.A.; Khokhlova, T.I.; Senin, I.I.; Zargarov, A.A.; Zinchenko, D.V.; Zernii, E.Y.; Lipkin, V.M.; Philippov, P.P.; et al. Recoverin is a zinc-binding protein. J. Proteome Res. 2003, 2, 51–57. [Google Scholar] [CrossRef]
- Xue, J.; Moyer, A.; Peng, B.; Wu, J.C.; Hannafon, B.N.; Ding, W.Q. Chloroquine Is a Zinc Ionophore. PLoS ONE 2014, 9, e109180. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, N.; Sawada, H.; Izumi, Y.; Kume, T.; Katsuki, H.; Shimohama, S.; Akaike, A. Proteasome inhibition induces glutathione synthesis and protects cells from oxidative stress: Relevance to Parkinson disease. J. Biol. Chem. 2007, 282, 4364–4372. [Google Scholar] [CrossRef] [Green Version]
- Taverna, E.; Francolini, M.; Jeromin, A.; Hilfiker, S.; Roder, J.; Rosa, P. Neuronal calcium sensor 1 and phosphatidylinositol 4-OH kinase beta interact in neuronal cells and are translocated to membranes during nucleotide-evoked exocytosis. J. Cell Sci. 2002, 115, 3909–3922. [Google Scholar] [CrossRef] [Green Version]
- Koizumi, S.; Rosa, P.; Willars, G.B.; Challiss, R.A.; Taverna, E.; Francolini, M.; Bootman, M.D.; Lipp, P.; Inoue, K.; Roder, J.; et al. Mechanisms underlying the neuronal calcium sensor-1-evoked enhancement of exocytosis in PC12 cells. J. Biol. Chem. 2002, 277, 30315–30324. [Google Scholar] [CrossRef] [Green Version]
- Krezel, A.; Maret, W. The biological inorganic chemistry of zinc ions. Arch. Biochem. Biophys. 2016, 611, 3–19. [Google Scholar] [CrossRef] [Green Version]
- Raghupathi, K.; Thayumanavan, S. Nano-Armoring of Enzymes: Rational Design of Polymer-Wrapped Enzymes. Methods Enzymol. 2017, 590, 381–411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Habeeb, A.F. [37] Reaction of protein sulfhydryl groups with Ellman’s reagent. Methods Enzymol. 1972, 25, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Greenfield, N.J. Using circular dichroism spectra to estimate protein secondary structure. Nat. Protoc. 2006, 1, 2876–2890. [Google Scholar] [CrossRef] [PubMed]
- Pandalaneni, S.; Karuppiah, V.; Saleem, M.; Haynes, L.P.; Burgoyne, R.D.; Mayans, O.; Derrick, J.P.; Lian, L.Y. Neuronal Calcium Sensor-1 Binds the D2 Dopamine Receptor and G-protein-coupled Receptor Kinase 1 (GRK1) Peptides Using Different Modes of Interactions. J. Biol. Chem. 2015, 290, 18744–18756. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.; Chen, J. Induction of the unfolded protein response by constitutive G-protein signaling in rod photoreceptor cells. J. Biol. Chem. 2014, 289, 29310–29321. [Google Scholar] [CrossRef] [Green Version]
- Lee, B.H.; Lee, M.J.; Park, S.; Oh, D.C.; Elsasser, S.; Chen, P.C.; Gartner, C.; Dimova, N.; Hanna, J.; Gygi, S.P.; et al. Enhancement of proteasome activity by a small-molecule inhibitor of USP14. Nature 2010, 467, 179–184. [Google Scholar] [CrossRef] [Green Version]
- Dantuma, N.P.; Bott, L.C. The ubiquitin-proteasome system in neurodegenerative diseases: Precipitating factor, yet part of the solution. Front. Mol. Neurosci. 2014, 7, 70. [Google Scholar] [CrossRef] [Green Version]
- Oshikawa, M.; Tsutsui, C.; Ikegami, T.; Fuchida, Y.; Matsubara, M.; Toyama, S.; Usami, R.; Ohtoko, K.; Kato, S. Full-length transcriptome analysis of human retina-derived cell lines ARPE-19 and Y79 using the vector-capping method. Investig. Ophthalmol. Vis. Sci. 2011, 52, 6662–6670. [Google Scholar] [CrossRef]
- Donato, R. Functional roles of S100 proteins, calcium-binding proteins of the EF-hand type. Biochim. Biophys. Acta 1999, 1450, 191–231. [Google Scholar] [CrossRef] [Green Version]
- Vologzhannikova, A.A.; Khorn, P.A.; Shevelyova, M.P.; Kazakov, A.S.; Emelyanenko, V.I.; Permyakov, E.A.; Permyakov, S.E. The Highly Conservative Cysteine of Oncomodulin as a Feasible Redox Sensor. Biomolecules 2021, 11, 66. [Google Scholar] [CrossRef]
- Sanagavarapu, K.; Weiffert, T.; Ni Mhurchu, N.; O’Connell, D.; Linse, S. Calcium Binding and Disulfide Bonds Regulate the Stability of Secretagogin towards Thermal and Urea Denaturation. PLoS ONE 2016, 11, e01657092016. [Google Scholar] [CrossRef]
- Ames, J.B. Dimerization of Neuronal Calcium Sensor Proteins. Front. Mol. Neurosci. 2018, 11, 397. [Google Scholar] [CrossRef]
- Davies, M.J. Protein oxidation and peroxidation. Biochem. J. 2016, 473, 805–825. [Google Scholar] [CrossRef] [Green Version]
- Myers, W.K.; Xu, X.; Li, C.; Lagerstedt, J.O.; Budamagunta, M.S.; Voss, J.C.; Britt, R.D.; Ames, J.B. Double electron-electron resonance probes Ca(2)(+)-induced conformational changes and dimerization of recoverin. Biochemistry 2013, 52, 5800–5808. [Google Scholar] [CrossRef] [Green Version]
- Ireland, S.M.; Martin, A.C.R. ZincBind-the database of zinc binding sites. Database J. Biol. Databases Curation 2019, 2019, baz006. [Google Scholar] [CrossRef]
- Moroz, O.V.; Wilson, K.S.; Bronstein, I.B. The role of zinc in the S100 proteins: Insights from the X-ray structures. Amino Acids 2011, 41, 761–772. [Google Scholar] [CrossRef]
- Abbas, S.; Marino, V.; Dell’Orco, D.; Koch, K.W. Molecular Recognition of Rhodopsin Kinase GRK1 and Recoverin Is Tuned by Switching Intra- and Intermolecular Electrostatic Interactions. Biochemistry 2019, 58, 4374–4385. [Google Scholar] [CrossRef]
- Chakrabarti, K.S.; Agafonov, R.V.; Pontiggia, F.; Otten, R.; Higgins, M.K.; Schertler, G.F.X.; Oprian, D.D.; Kern, D. Conformational Selection in a Protein-Protein Interaction Revealed by Dynamic Pathway Analysis. Cell Rep. 2016, 14, 32–42. [Google Scholar] [CrossRef] [Green Version]
- Fotiadis, D.; Liang, Y.; Filipek, S.; Saperstein, D.A.; Engel, A.; Palczewski, K. Atomic-force microscopy: Rhodopsin dimers in native disc membranes. Nature 2003, 421, 127–128. [Google Scholar] [CrossRef]
- Kemp, M.; Go, Y.M.; Jones, D.P. Nonequilibrium thermodynamics of thiol/disulfide redox systems: A perspective on redox systems biology. Free Radic Biol. Med. 2008, 44, 921–937. [Google Scholar] [CrossRef] [Green Version]
- Kirlin, W.G.; Cai, J.; Thompson, S.A.; Diaz, D.; Kavanagh, T.J.; Jones, D.P. Glutathione redox potential in response to differentiation and enzyme inducers. Free Radic Biol. Med. 1999, 27, 1208–1218. [Google Scholar] [CrossRef]
- Jones, D.P. Redox potential of GSH/GSSG couple: Assay and biological significance. Methods Enzymol. 2002, 348, 93–112. [Google Scholar] [CrossRef] [PubMed]
- Frederickson, C.J.; Bush, A.I. Synaptically released zinc: Physiological functions and pathological effects. Biometals 2001, 14, 353–366. [Google Scholar] [CrossRef] [PubMed]
- Kondo, N.; Nakamura, H.; Masutani, H.; Yodoi, J. Redox regulation of human thioredoxin network. Antioxid. Redox Signal. 2006, 8, 1881–1890. [Google Scholar] [CrossRef]
- Wilkinson, B.L.; Jeromin, A.; Roder, J.; Hyson, R.L. Activity-dependent regulation of the subcellular localization of neuronal calcium sensor-1 in the avian cochlear nucleus. Neuroscience 2003, 117, 957–964. [Google Scholar] [CrossRef] [Green Version]
- Grosshans, H.K.; Fischer, T.T.; Steinle, J.A.; Brill, A.L.; Ehrlich, B.E. Neuronal Calcium Sensor 1 is up-regulated in response to stress to promote cell survival and motility in cancer cells. Mol. Oncol. 2020, 14, 1134–1151. [Google Scholar] [CrossRef]
- Yip, P.K.; Wong, L.F.; Sears, T.A.; Yanez-Munoz, R.J.; McMahon, S.B. Cortical overexpression of neuronal calcium sensor-1 induces functional plasticity in spinal cord following unilateral pyramidal tract injury in rat. PLoS Biol. 2010, 8, e10003992010. [Google Scholar] [CrossRef]
- Zhao, X.; Varnai, P.; Tuymetova, G.; Balla, A.; Toth, Z.E.; Oker-Blom, C.; Roder, J.; Jeromin, A.; Balla, T. Interaction of neuronal calcium sensor-1 (NCS-1) with phosphatidylinositol 4-kinase beta stimulates lipid kinase activity and affects membrane trafficking in COS-7 cells. J. Biol. Chem. 2001, 276, 40183–40189. [Google Scholar] [CrossRef] [Green Version]
- Bong, A.H.L.; Robitaille, M.; Milevskiy, M.J.G.; Roberts-Thomson, S.J.; Monteith, G.R. NCS-1 expression is higher in basal breast cancers and regulates calcium influx and cytotoxic responses to doxorubicin. Mol. Oncol. 2020, 14, 87–104. [Google Scholar] [CrossRef]
- Kabbani, N.; Negyessy, L.; Lin, R.; Goldman-Rakic, P.; Levenson, R. Interaction with neuronal calcium sensor NCS-1 mediates desensitization of the D2 dopamine receptor. J. Neurosci. 2002, 22, 8476–8486. [Google Scholar] [CrossRef]
- Dragicevic, E.; Poetschke, C.; Duda, J.; Schlaudraff, F.; Lammel, S.; Schiemann, J.; Fauler, M.; Hetzel, A.; Watanabe, M.; Lujan, R.; et al. Cav1.3 channels control D2-autoreceptor responses via NCS-1 in substantia nigra dopamine neurons. Brain 2014, 137, 2287–2302. [Google Scholar] [CrossRef] [Green Version]
- De Castro, E.; Nef, S.; Fiumelli, H.; Lenz, S.E.; Kawamura, S.; Nef, P. Regulation of rhodopsin phosphorylation by a family of neuronal calcium sensors. Biochem. Biophys. Res. Commun. 1995, 216, 133–140. [Google Scholar] [CrossRef]
- Theccanat, T.; Philip, J.L.; Razzaque, A.M.; Ludmer, N.; Li, J.; Xu, X.; Akhter, S.A. Regulation of cellular oxidative stress and apoptosis by G protein-coupled receptor kinase-2; The role of NADPH oxidase 4. Cell. Signal. 2016, 28, 190–203. [Google Scholar] [CrossRef] [Green Version]
- Kawakami, M.; Hattori, M.; Ohashi, W.; Fujimori, T.; Hattori, K.; Takebe, M.; Tomita, K.; Yokoo, H.; Matsuda, N.; Yamazaki, M.; et al. Role of G protein-coupled receptor kinase 2 in oxidative and nitrosative stress-related neurohistopathological changes in a mouse model of sepsis-associated encephalopathy. J. Neurochem. 2018, 145, 474–488. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Premont, R.T.; Kontos, C.D.; Zhu, S.; Rockey, D.C. A crucial role for GRK2 in regulation of endothelial cell nitric oxide synthase function in portal hypertension. Nat. Med. 2005, 11, 952–958. [Google Scholar] [CrossRef]
- Lucas, J.J.; Hernandez, F.; Gomez-Ramos, P.; Moran, M.A.; Hen, R.; Avila, J. Decreased nuclear beta-catenin, tau hyperphosphorylation and neurodegeneration in GSK-3beta conditional transgenic mice. EMBO J. 2001, 20, 27–39. [Google Scholar] [CrossRef]
- Yang, L.; Wang, H.; Liu, L.; Xie, A. The Role of Insulin/IGF-1/PI3K/Akt/GSK3beta Signaling in Parkinson’s Disease Dementia. Front. Neurosci. 2018, 12, 73. [Google Scholar] [CrossRef] [Green Version]
- Mossuto, M.F. Disulfide bonding in neurodegenerative misfolding diseases. Int. J. Cell Biol. 2013, 2013, 318319. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, K.; Kelly, C.E.; Goh, K.Y.; Lim, K.B.; Ibanez, C.F. Death Domain Signaling by Disulfide-Linked Dimers of the p75 Neurotrophin Receptor Mediates Neuronal Death in the CNS. J. Neurosci. 2016, 36, 5587–5595. [Google Scholar] [CrossRef] [Green Version]
- Hill, S.E.; Nguyen, E.; Donegan, R.K.; Patterson-Orazem, A.C.; Hazel, A.; Gumbart, J.C.; Lieberman, R.L. Structure and Misfolding of the Flexible Tripartite Coiled-Coil Domain of Glaucoma-Associated Myocilin. Structure 2017, 25, 1697–1707.e5. [Google Scholar] [CrossRef] [Green Version]
- Lieven, C.J.; Ribich, J.D.; Crowe, M.E.; Levin, L.A. Redox Proteomic Identification of Visual Arrestin Dimerization in Photoreceptor Degeneration after Photic Injury. Investig. Opthalmol. Vis. Sci. 2012, 53, 3990–3998. [Google Scholar] [CrossRef] [PubMed]
- Garnier, C.; Devred, F.; Byrne, D.; Puppo, R.; Roman, A.Y.; Malesinski, S.; Golovin, A.V.; Lebrun, R.; Ninkina, N.N.; Tsvetkov, P.O. Zinc binding to RNA recognition motif of TDP-43 induces the formation of amyloid-like aggregates. Sci. Rep. 2017, 7, 6812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Radko, S.P.; Khmeleva, S.A.; Kaluzhny, D.N.; Kechko, O.I.; Kiseleva, Y.Y.; Kozin, S.A.; Mitkevich, V.A.; Makarov, A.A. The English (H6R) Mutation of the Alzheimer’s Disease Amyloid-beta Peptide Modulates Its Zinc-Induced Aggregation. Biomolecules 2020, 10, 961. [Google Scholar] [CrossRef] [PubMed]
- Roman, A.Y.; Devred, F.; Byrne, D.; La Rocca, R.; Ninkina, N.N.; Peyrot, V.; Tsvetkov, P.O. Zinc Induces Temperature-Dependent Reversible Self-Assembly of Tau. J. Mol. Biol 2019, 431, 687–695. [Google Scholar] [CrossRef] [Green Version]
- Dautzenberg, F.M.; Hauger, R.L. G-protein-coupled receptor kinase 3- and protein kinase C-mediated desensitization of the PACAP receptor type 1 in human Y-79 retinoblastoma cells. Neuropharmacology 2001, 40, 394–407. [Google Scholar] [CrossRef]
- Panieri, E.; Santoro, M.M. ROS homeostasis and metabolism: A dangerous liason in cancer cells. Cell Death Dis. 2016, 7, e2253. [Google Scholar] [CrossRef]
- Chen, Y.; McMillan-Ward, E.; Kong, J.; Israels, S.J.; Gibson, S.B. Oxidative stress induces autophagic cell death independent of apoptosis in transformed and cancer cells. Cell Death Differ. 2008, 15, 171–182. [Google Scholar] [CrossRef]
- Olzmann, J.A.; Li, L.; Chin, L.S. Aggresome formation and neurodegenerative diseases: Therapeutic implications. Curr. Med. Chem. 2008, 15, 47–60. [Google Scholar] [CrossRef] [Green Version]
- Saliba, R.S.; Munro, P.M.; Luthert, P.J.; Cheetham, M.E. The cellular fate of mutant rhodopsin: Quality control, degradation and aggresome formation. J. Cell Sci. 2002, 115, 2907–2918. [Google Scholar] [CrossRef]
- Ashrafi, G.; Schwarz, T.L. The pathways of mitophagy for quality control and clearance of mitochondria. Cell Death Differ. 2013, 20, 31–42. [Google Scholar] [CrossRef] [Green Version]
- Zhuang, N.; Li, L.; Chen, S.; Wang, T. PINK1-dependent phosphorylation of PINK1 and Parkin is essential for mitochondrial quality control. Cell Death Dis. 2016, 7, e2501. [Google Scholar] [CrossRef]
- Petko, J.A.; Kabbani, N.; Frey, C.; Woll, M.; Hickey, K.; Craig, M.; Canfield, V.A.; Levenson, R. Proteomic and functional analysis of NCS-1 binding proteins reveals novel signaling pathways required for inner ear development in zebrafish. BMC Neurosci. 2009, 10, 27. [Google Scholar] [CrossRef] [Green Version]
- Dautzenberg, F.M.; Braun, S.; Hauger, R.L. GRK3 mediates desensitization of CRF1 receptors: A potential mechanism regulating stress adaptation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2001, 280, R935–R946. [Google Scholar] [CrossRef] [Green Version]
- Kageyama, K.; Hanada, K.; Moriyama, T.; Nigawara, T.; Sakihara, S.; Suda, T. G protein-coupled receptor kinase 2 involvement in desensitization of corticotropin-releasing factor (CRF) receptor type 1 by CRF in murine corticotrophs. Endocrinology 2006, 147, 441–450. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Kuo, C.C.; Moghadam, S.H.; Monte, L.; Rice, K.C.; Rissman, R.A. Corticotropin-Releasing Factor Receptor-1 Antagonism Reduces Oxidative Damage in an Alzheimer’s Disease Transgenic Mouse Model. J. Alzheimer’s Dis. JAD 2015, 45, 639–650. [Google Scholar] [CrossRef] [Green Version]
- Vladimirov, V.I.; Baksheeva, V.E.; Mikhailova, I.V.; Ismailov, R.G.; Litus, E.A.; Tikhomirova, N.K.; Nazipova, A.A.; Permyakov, S.E.; Zernii, E.Y.; Zinchenko, D.V. A Novel Approach to Bacterial Expression and Purification of Myristoylated Forms of Neuronal Calcium Sensor Proteins. Biomolecules 2020, 10, 1025. [Google Scholar] [CrossRef]
- Kazakov, A.S.; Sokolov, A.S.; Vologzhannikova, A.A.A.; Permyakova, M.E.; Khorn, P.A.; Ismailov, R.G.; Denessiouk, K.A.; Denesyuk, A.I.; Rastrygina, V.A.; Baksheeva, V.E.; et al. Interleukin-11 binds specific EF-hand proteins via their conserved structural motifs. J. Biomol. Struct. Dyn. 2017, 35, 78–91. [Google Scholar] [CrossRef]
- Weber, G.; Farris, F.J. Synthesis and spectral properties of a hydrophobic fluorescent probe: 6-propionyl-2-(dimethylamino)naphthalene. Biochemistry 1979, 18, 3075–3078. [Google Scholar] [CrossRef]
- Sreerama, N.; Woody, R.W. Estimation of protein secondary structure from circular dichroism spectra: Comparison of CONTIN, SELCON, and CDSSTR methods with an expanded reference set. Anal. Biochem. 2000, 287, 252–260. [Google Scholar] [CrossRef]
- Grigoriev, I.I.; Senin, I.I.; Tikhomirova, N.K.; Komolov, K.E.; Permyakov, S.E.; Zernii, E.Y.; Koch, K.W.; Philippov, P.P. Synergetic effect of recoverin and calmodulin on regulation of rhodopsin kinase. Front. Mol. Neurosci. 2012, 5, 28. [Google Scholar] [CrossRef] [Green Version]
- Weiergraber, O.H.; Senin, I.I.; Zernii, E.Y.; Churumova, V.A.; Kovaleva, N.A.; Nazipova, A.A.; Permyakov, S.E.; Permyakov, E.A.; Philippov, P.P.; Granzin, J.; et al. Tuning of a neuronal calcium sensor. J. Biol. Chem. 2006, 281, 37594–37602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zernii, E.Y.; Komolov, K.E.; Permyakov, S.E.; Kolpakova, T.; Dell’orco, D.; Poetzsch, A.; Knyazeva, E.L.; Grigoriev, I.I.; Permyakov, E.A.; Senin, I.I.; et al. Involvement of the recoverin C-terminal segment in recognition of the target enzyme rhodopsin kinase. Biochem. J. 2011, 435, 441–450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Webb, B.; Sali, A. Comparative Protein Structure Modeling Using MODELLER. Curr. Protoc. Bioinform. 2016, 54, 5.6.1–5.6.37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reshetnikov, R.V.; Stolyarova, A.V.; Zalevsky, A.O.; Panteleev, D.Y.; Pavlova, G.V.; Klinov, D.V.; Golovin, A.V.; Protopopova, A.D. A coarse-grained model for DNA origami. Nucleic Acids Res. 2018, 46, 1102–1112. [Google Scholar] [CrossRef] [Green Version]
- Pierce, B.G.; Hourai, Y.; Weng, Z.P. Accelerating Protein Docking in ZDOCK Using an Advanced 3D Convolution Library. PLoS ONE 2011, 6, e24657. [Google Scholar] [CrossRef]
- Van Zundert, G.C.P.; Rodrigues, J.; Trellet, M.; Schmitz, C.; Kastritis, P.L.; Karaca, E.; Melquiond, A.S.J.; van Dijk, M.; de Vries, S.J.; Bonvin, A. The HADDOCK2.2 Web Server: User-Friendly Integrative Modeling of Biomolecular Complexes. J. Mol. Biol. 2016, 428, 720–725. [Google Scholar] [CrossRef] [Green Version]
- Adams, P.D.; Afonine, P.V.; Bunkoczi, G.; Chen, V.B.; Echols, N.; Headd, J.J.; Hung, L.W.; Jain, S.; Kapral, G.J.; Grosse Kunstleve, R.W.; et al. The Phenix software for automated determination of macromolecular structures. Methods 2011, 55, 94–106. [Google Scholar] [CrossRef] [Green Version]
- Mailhot, O.; Najmanovich, R. The NRGTEN Python package: An extensible toolkit for coarse-grained normal mode analysis of proteins, nucleic acids, small molecules and their complexes. Bioinformatics 2021, 10, btab189. [Google Scholar] [CrossRef]
- Laskowski, R.A.; Swindells, M.B. LigPlot+: Multiple ligand-protein interaction diagrams for drug discovery. J. Chem. Inf. Modeling 2011, 51, 2778–2786. [Google Scholar] [CrossRef]
- Hunter, J.D. Matplotlib: A 2D Graphics Environment. Comput. Sci. Eng. 2007, 9, 90–95. [Google Scholar] [CrossRef]
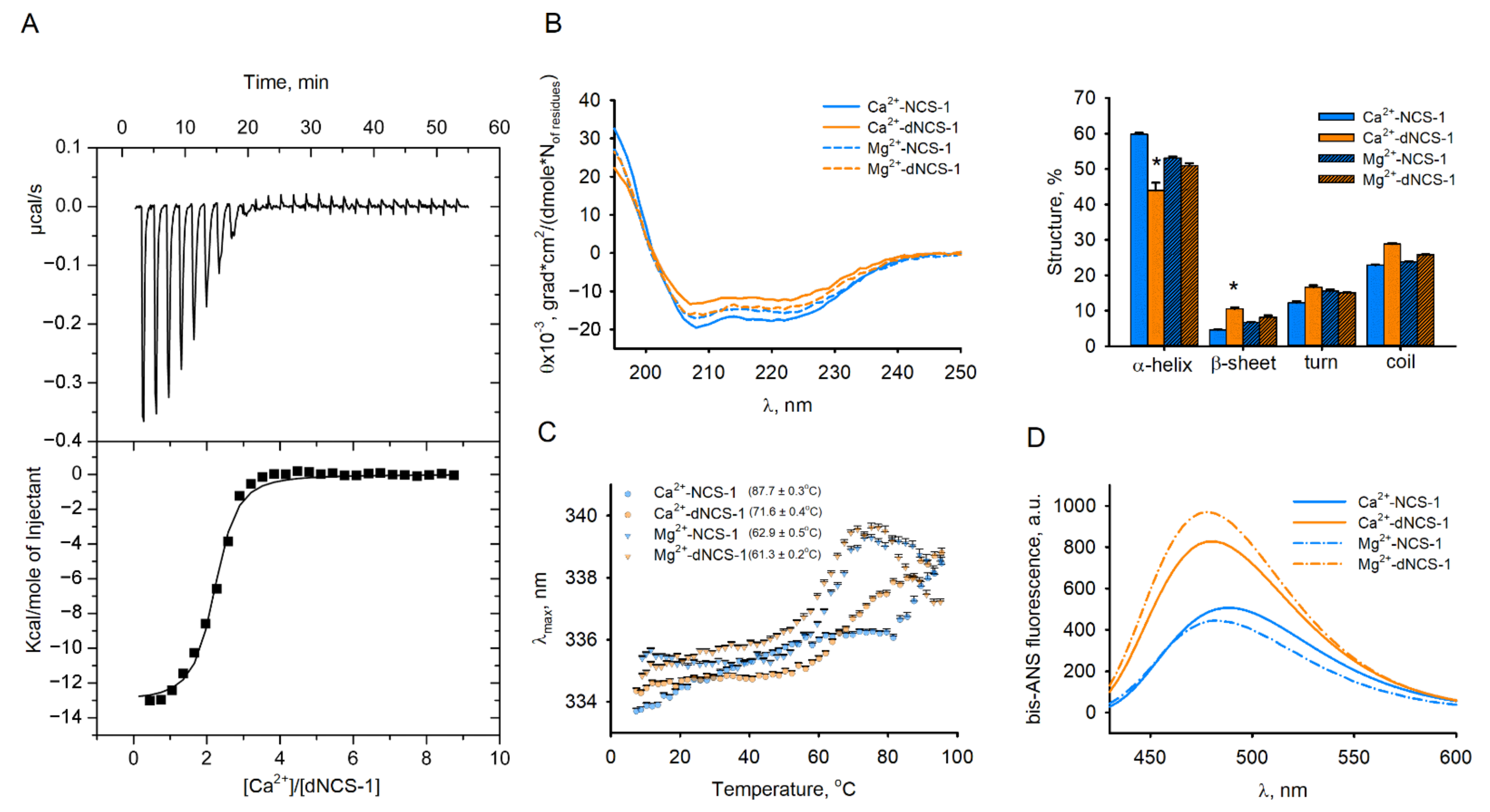
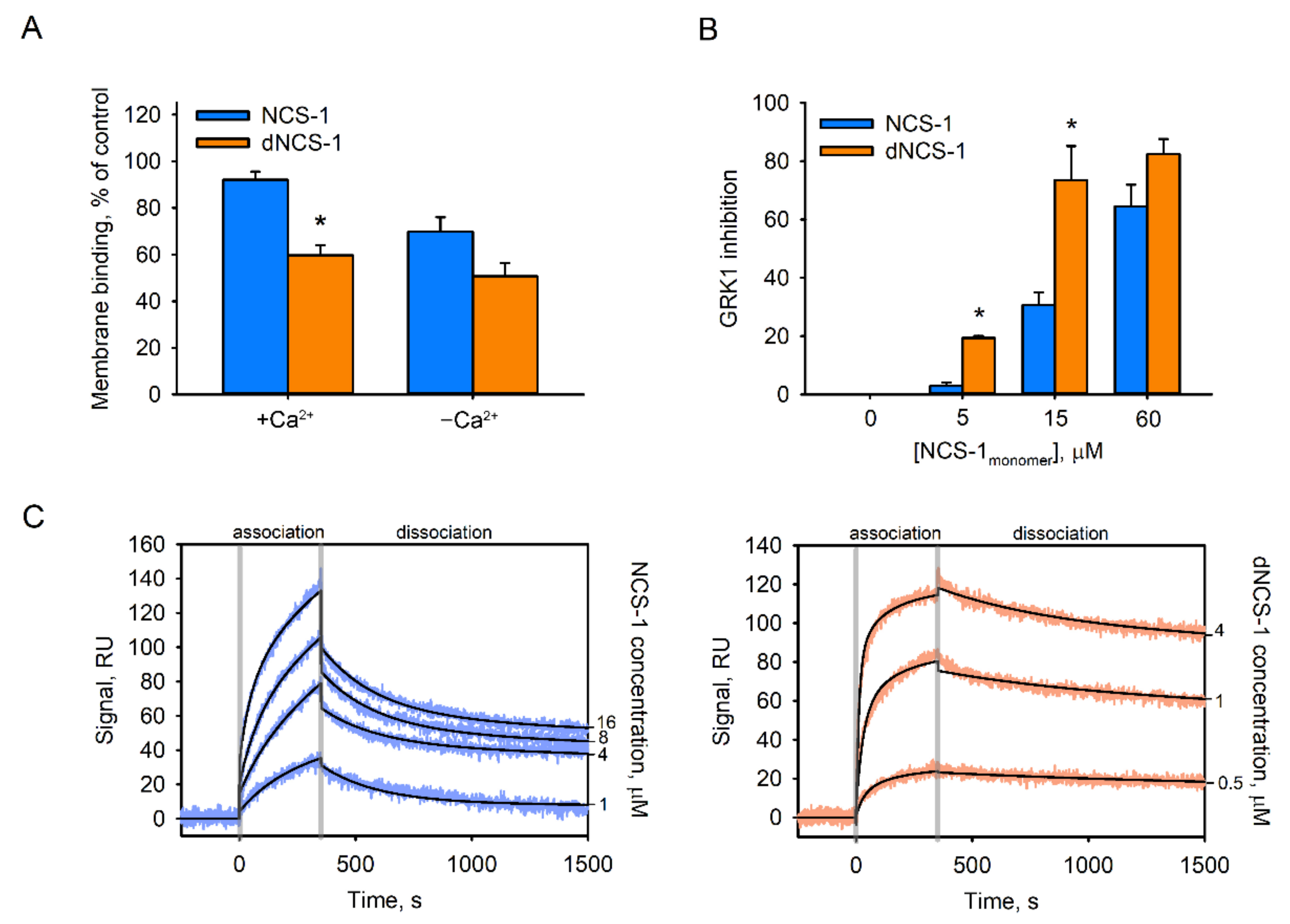

| Protein | KD 1, M | ΔH 1, kJ | KD 2, M | ΔH 2, kJ | KD 3, M | ΔH 3, kJ |
|---|---|---|---|---|---|---|
| NCS-1 1 | 2.3 × 10−7 | −10.1 | 5.0 × 10−6 | 1.4 | 2.9 × 10−7 | −17.8 |
| N 1 | KD 1, M | ΔH 1, kJ | N 2 | KD 2, M | ΔH 2, kJ | |
| dNCS-1 2,3 | 2.1 | (5.6 ± 0.8) × 10−7 | −13.1 ± 0.2 | - | - | - |
| Analyte | kon 1, s−1 M−1 | koff 1, s−1 | KD 1, M | kon 2, s−1 M−1 | koff 2, s−1 | KD 2, M |
|---|---|---|---|---|---|---|
| NCS-1 | 267 ± 22 | (1.6 ± 0.6) × 10−4 | (5.9 ± 2.0) × 10−7 | 1910 ± 650 | (4.4 ± 1.5) × 10−3 | (2.7 ± 1.8) × 10−6 |
| dNCS-1 1 | 3580 ± 2230 | (1.1 ± 0.2) × 10−4 | (4.8 ± 3.9) × 10−8 | 1150 ± 350 | (2.5 ± 0.3) × 10−3 | (2.2 ± 0.5) × 10−6 |
| dNCS-1 2 | 9780 ± 5700 | (2.3 ± 0.6) × 10−4 | (3.0 ± 1.8) × 10−8 | - | - | - |
| Interaction | N 1 | KD 1, M | ΔH 1, kJ | N 2 | KD 2, M | ΔH 2, kJ |
|---|---|---|---|---|---|---|
| NCS-1 1 | 0.7 | 4.3 × 10−6 | −11.8 | 2.0 | 1.1 × 10−7 | −7.3 |
| dNCS-1 2,3 | 1.3 | (4.7 ± 1.2) × 10−7 | −3.9 ± 0.4 | 2.1 | (1.2 ± 0.5) × 10−8 | −6.6 ± 0.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baksheeva, V.E.; Baldin, A.V.; Zalevsky, A.O.; Nazipova, A.A.; Kazakov, A.S.; Vladimirov, V.I.; Gorokhovets, N.V.; Devred, F.; Philippov, P.P.; Bazhin, A.V.; et al. Disulfide Dimerization of Neuronal Calcium Sensor-1: Implications for Zinc and Redox Signaling. Int. J. Mol. Sci. 2021, 22, 12602. https://doi.org/10.3390/ijms222212602
Baksheeva VE, Baldin AV, Zalevsky AO, Nazipova AA, Kazakov AS, Vladimirov VI, Gorokhovets NV, Devred F, Philippov PP, Bazhin AV, et al. Disulfide Dimerization of Neuronal Calcium Sensor-1: Implications for Zinc and Redox Signaling. International Journal of Molecular Sciences. 2021; 22(22):12602. https://doi.org/10.3390/ijms222212602
Chicago/Turabian StyleBaksheeva, Viktoriia E., Alexey V. Baldin, Arthur O. Zalevsky, Aliya A. Nazipova, Alexey S. Kazakov, Vasiliy I. Vladimirov, Neonila V. Gorokhovets, François Devred, Pavel P. Philippov, Alexandr V. Bazhin, and et al. 2021. "Disulfide Dimerization of Neuronal Calcium Sensor-1: Implications for Zinc and Redox Signaling" International Journal of Molecular Sciences 22, no. 22: 12602. https://doi.org/10.3390/ijms222212602
APA StyleBaksheeva, V. E., Baldin, A. V., Zalevsky, A. O., Nazipova, A. A., Kazakov, A. S., Vladimirov, V. I., Gorokhovets, N. V., Devred, F., Philippov, P. P., Bazhin, A. V., Golovin, A. V., Zamyatnin, A. A., Jr., Zinchenko, D. V., Tsvetkov, P. O., Permyakov, S. E., & Zernii, E. Y. (2021). Disulfide Dimerization of Neuronal Calcium Sensor-1: Implications for Zinc and Redox Signaling. International Journal of Molecular Sciences, 22(22), 12602. https://doi.org/10.3390/ijms222212602










