Manipulating Estrogenic/Anti-Estrogenic Activity of Triphenylethylenes towards Development of Novel Anti-Neoplastic SERMs
Abstract
1. Introduction
2. Results and Discussion
2.1. Chemistry Discussion
2.2. Anti-Estrogenic Assays
2.3. Estrogenic Assays
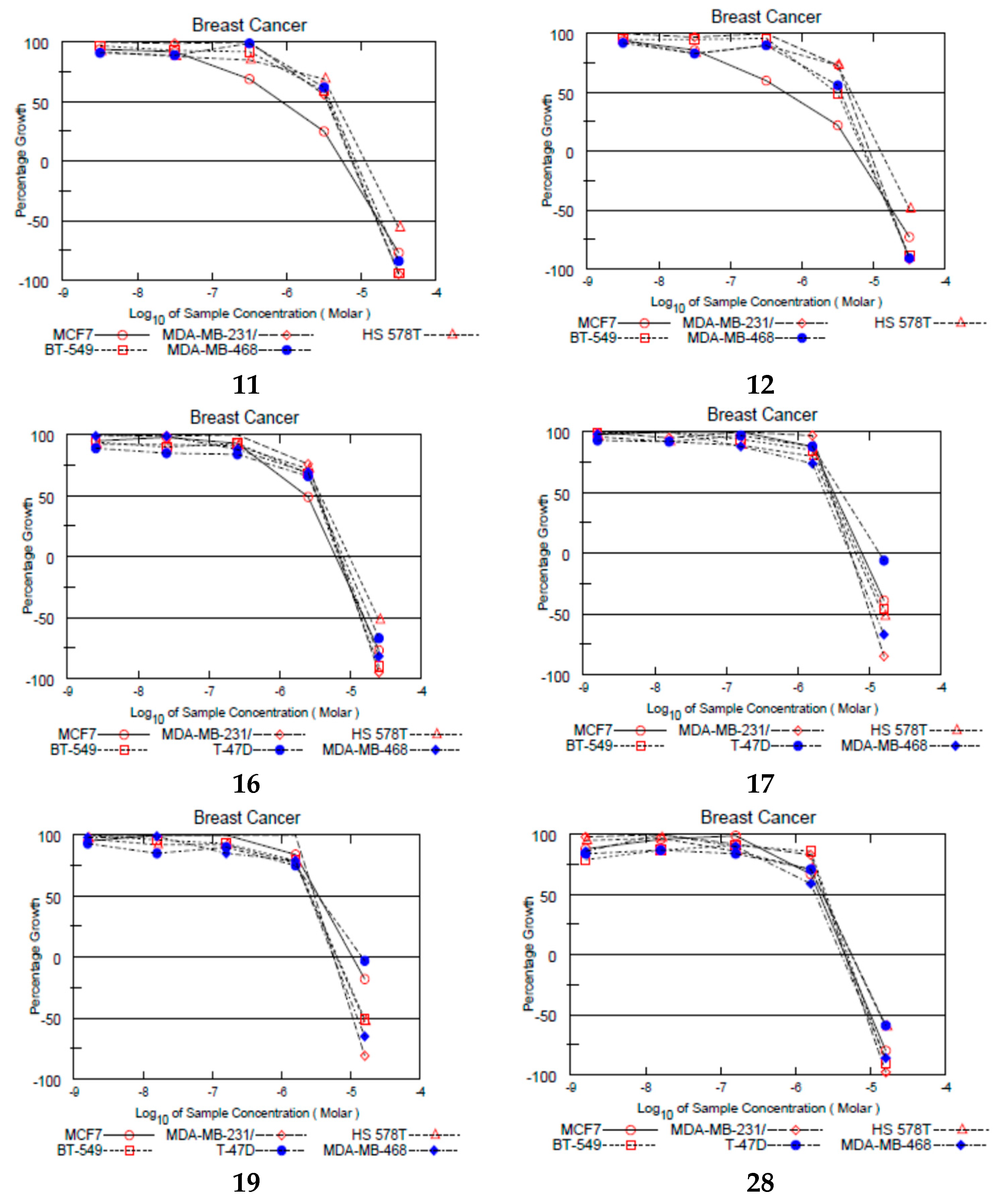
2.4. NCI Growth Inhibition Assays
2.5. Alkaline Phosphatase Activity in Ishikawa Cell Line
2.6. Uterotrophic Assay
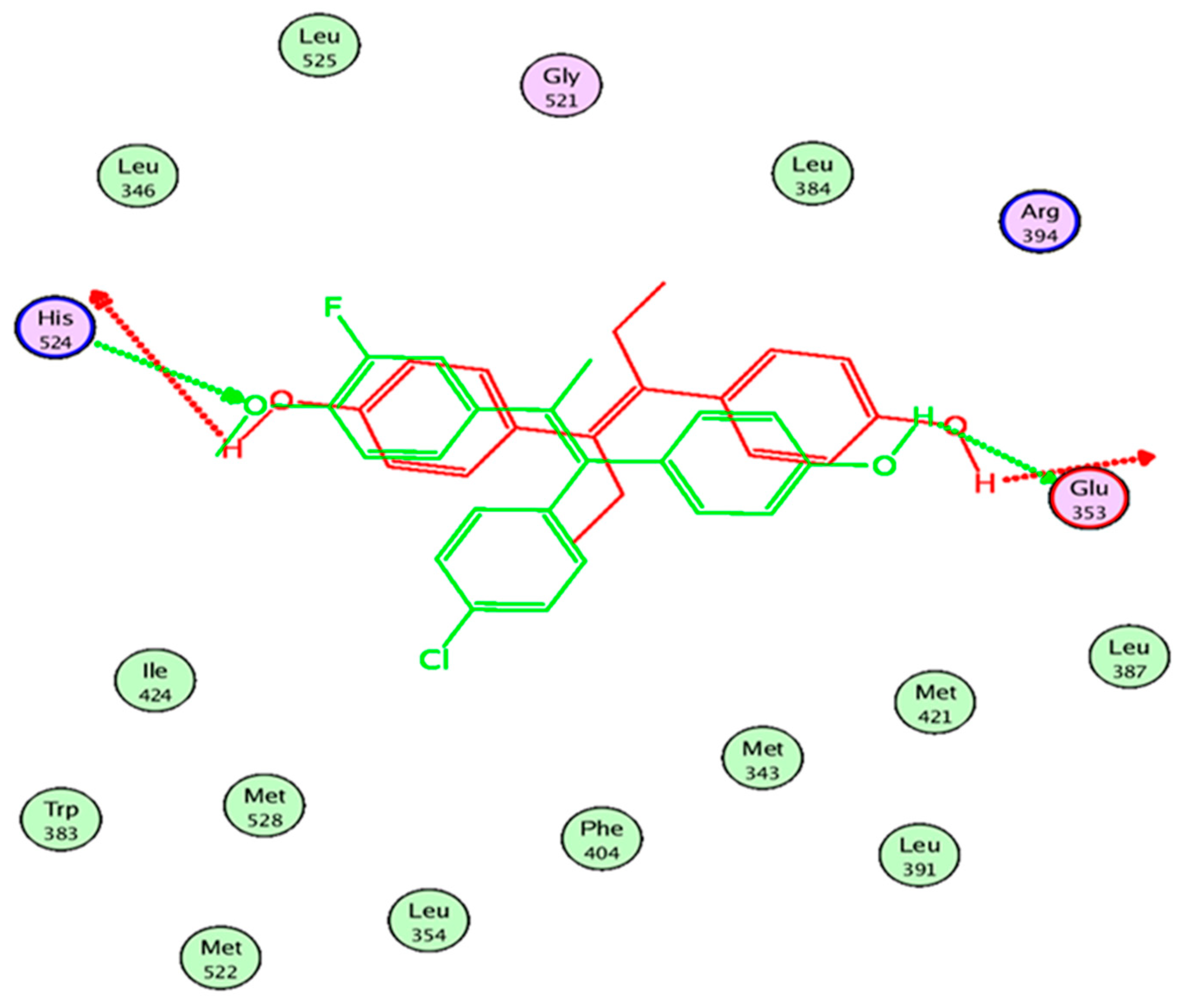
2.7. In Silico Study
3. Experimental Section
3.1. Chemistry
3.1.1. General Procedures for Preparation of Compound 1–4
E/Z-4-[1-(4-Chloro-phenyl)-2-phenylpropenyl]-phenol (1)
E/Z-4-[1-(4-Chloro-phenyl)-2-(4-methoxy-phenyl)-propenyl]-phenol (2)
E/Z-4-[1-(4-Chlorophenyl)-2-(3-fluoro-4-methoxyphenyl) propenyl]-phenol (3)
E/Z-4-[1-(4-Chlorophenyl)-2-(4-fluoro-3-methoxyphenyl) propenyl]-phenol (4)
3.1.2. General Procedures for Preparation of Compounds 5–28
E/Z-(3-{4-[1-(4-Chloro-phenyl)-2-phenyl-propenyl]-phenoxy}-propyl)-dimethyl-amine (5)
E/Z-1-(2-{4-[1-(4-Chloro-phenyl)-2-phenyl-propenyl]-phenoxy}-ethyl)-pyrrolidine (6)
E/Z-1-(2-{4-[1-(4-Chloro-phenyl)-2-phenyl-propenyl]-phenoxy}-ethyl)-piperidine (7)
E/Z-4-(2-{4-[1-(4-Chloro-phenyl)-2-phenyl-propenyl]-phenoxy}-ethyl)-morpholine (8)
E/Z-1-(2-{4-[1-(4-Chloro-phenyl)-2-phenyl-propenyl]-phenoxy}-ethyl)-azepane (9)
E/Z-(3-{4-[1-(4-Chloro-phenyl)-2-(4-methoxy-phenyl)-propenyl]-phenoxy}-propyl)-dimethyl-amine (10)
E/Z-1-(2-{4-[1-(4-Chloro-phenyl)-2-(4-methoxy-phenyl)-propenyl]-phenoxy}-ethyl)-pyrrolidine (11)
E/Z-1-(2-{4-[1-(4-Chloro-phenyl)-2-(4-methoxy-phenyl)-propenyl]-phenoxy}-ethyl)-piperidine (12)
E/Z-4-(2-{4-[1-(4-Chloro-phenyl)-2-(4-methoxy-phenyl)-propenyl]-phenoxy}-ethyl)-morpholine (13)
E/Z-1-(2-{4-[1-(4-Chloro-phenyl)-2-(4-methoxy-phenyl)-propenyl]-phenoxy}-ethyl)-azepane (14)
E/Z-(3-{4-[1-(4-Chlorophenyl)-2-(3-fluoro-4-methoxyphenyl)-propenyl]-phenoxy}-propyl)-dimethyl-amine (15)
E/Z-1-(2-{4-[1-(4-Chlorophenyl)-2-(3-fluoro-4-methoxyphenyl)-propenyl]-phenoxy}-ethyl)-pyrrolidine (16)
E/Z-1-(2-{4-[1-(4-Chlorophenyl)-2-(3-fluoro-4-methoxyphenyl)-propenyl]-phenoxy}-ethyl)-piperidine (17)
E/Z-4-(2-{4-[1-(4-Chlorophenyl)-2-(3-fluoro-4-methoxyphenyl)-propenyl]-phenoxy}-ethyl)-morpholine (18)
E/Z-1-(2-{4-[1-(4-Chlorophenyl)-2-(3-fluoro-4-methoxyphenyl)-propenyl]-phenoxy}-ethyl)-azepane (19)
E/Z-(2-{4-[1-(4-Chlorophenyl)-2-(3-fluoro-4-methoxyphenyl)-propenyl]-phenoxy}-ethyl)-dimethyl-amine (20)
E/Z-(2-{4-[1-(4-Chlorophenyl)-2-(3-fluoro-4-methoxyphenyl)-propenyl]-phenoxy}-ethyl)-diethyl-amine (21)
E/Z-(3-{4-[1-(4-Chlorophenyl)-2-(4-fluoro-3-methoxyphenyl)-propenyl]-phenoxy}-propyl)-dimethyl-amine (22)
E/Z-1-(2-{4-[1-(4-Chlorophenyl)-2-(4-fluoro-3-methoxyphenyl)-propenyl]-phenoxy}-ethyl)-pyrrolidine (23)
E/Z-1-(2-{4-[1-(4-Chlorophenyl)-2-(4-fluoro-3-methoxyphenyl)-propenyl]-phenoxy}-ethyl)-piperidine (24)
E/Z-4-(2-{4-[1-(4-Chlorophenyl)-2-(4-fluoro-3-methoxyphenyl)-propenyl]-phenoxy}-ethyl)-morpholine (25)
E/Z-1-(2-{4-[1-(4-Chlorophenyl)-2-(4-fluoro-3-methoxyphenyl)-propenyl]-phenoxy}-ethyl)-azepane (26)
E/Z-(2-{4-[1-(4-Chlorophenyl)-2-(4-fluoro-3-methoxyphenyl)-propenyl]-phenoxy}-ethyl)-dimethyl-amine (27)
E/Z-(2-{4-[1-(4-Chlorophenyl)-2-(4-fluoro-3-methoxyphenyl)-propenyl]-phenoxy}-ethyl)-diethyl-amine (28)
3.2. Biology
3.2.1. Yeast Estrogen Receptor Assay (YES)
3.2.2. NCI Anti-Cancer Screening
3.2.3. Alkaline Phosphatase Activity in Ishikawa Cells
3.2.4. Uterotrophic Assay
3.3. In Silico Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Riggs, B.L.; Hartmann, L.C. Selective estrogen-receptor modulators--mechanisms of action and application to clinical practice. N. Engl. J. Med. 2003, 348, 618–629. [Google Scholar] [CrossRef]
- Valéra, M.-C.; Fontaine, C.; Dupuis, M.; Noirrit-Esclassan, E.; Vinel, A.; Guillaume, M.; Gourdy, P.; Lenfant, F.; Arnal, J.-F. Towards optimization of estrogen receptor modulation in medicine. Pharmacol. Ther. 2018, 189, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Arnal, J.F.; Lenfant, F.; Metivier, R.; Flouriot, G.; Henrion, D.; Adlanmerini, M.; Katzenellenbogen, J. Membrane and Nuclear Estrogen Receptor Alpha Actions: From Tissue Specificity to Medical Implications. Physiol. Rev. 2017, 97, 1045–1087. [Google Scholar] [CrossRef] [PubMed]
- Maximov, P.Y.; McDaniel, R.E.; Jordan, V.C. (Eds.) Tamoxifen Goes Forward Alone BT-Tamoxifen: Pioneering Medicine in Breast Cancer; Springer: Basel, Switzerland, 2013; pp. 31–46. [Google Scholar]
- Crewe, H.K.; Notley, L.M.; Wunsch, R.M.; Lennard, M.S.; Gillam, E.M. Metabolism of tamoxifen by recombinant human cytochrome P450 enzymes: Formation of the 4-hydroxy, 4’-hydroxy and N-desmethyl metabolites and isomerization of trans-4-hydroxytamoxifen. Drug Metab. Dispos. 2002, 30, 869–874. [Google Scholar] [CrossRef] [PubMed]
- Goetz, M.P.; Rae, J.M.; Suman, V.J.; Safgren, S.L.; Ames, M.M.; Visscher, D.W.; Reynolds, C.; Couch, F.J.; Lingle, W.L.; Flockhart, D.A.; et al. Pharmacogenetics of Tamoxifen Biotransformation Is Associated With Clinical Outcomes of Efficacy and Hot Flashes. J. Clin. Oncol. 2005, 23, 9312–9318. [Google Scholar] [CrossRef]
- Brauch, H.; Schroth, W.; Goetz, M.P.; Mürdter, T.E.; Winter, S.; Ingle, J.N.; Schwab, M.; Eichelbaum, M. Tamoxifen Use in Postmenopausal Breast Cancer: CYP2D6 Matters. J. Clin. Oncol. 2013, 31, 176–180. [Google Scholar] [CrossRef] [PubMed]
- Schroth, W.; Goetz, M.P.; Hamann, U.; Fasching, P.A.; Schmidt, M.; Winter, S.; Fritz, P.; Simon, W.; Suman, V.J.; Ames, M.M.; et al. Association Between CYP2D6 Polymorphisms and Outcomes among Women with Early Stage Breast Cancer Treated with Tamoxifen. JAMA 2009, 302, 1429–1436. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.-H.; Jeon, J.; Kim, S.I. Personalized Medicine in Breast Cancer: A Systematic Review. J. Breast Cancer 2012, 15, 265–272. [Google Scholar] [CrossRef]
- Dehal, S.S.; Kupfer, D. CYP2D6 catalyzes tamoxifen 4-hydroxylation in human liver. Cancer Res. 1997, 57, 3402–3406. [Google Scholar]
- De Souza, J.A.; Olopade, O.I. CYP2D6 genotyping and tamoxifen: An unfinished story in the quest for personalized medicine. Semin. Oncol. 2011, 38, 263–273. [Google Scholar] [CrossRef]
- Gillis, E.P.; Eastman, K.J.; Hill, M.D.; Donnelly, D.; Meanwell, N. Applications of Fluorine in Medicinal Chemistry. J. Med. Chem. 2015, 58, 8315–8359. [Google Scholar] [CrossRef] [PubMed]
- McCague, R.; Leclercq, G.; Legros, N.; Goodman, J.; Blackburn, G.M.; Jarman, M.; Foster, A.B. Derivatives of tamoxifen. Dependence of antiestrogenicity on the 4-substituent. J. Med. Chem. 1989, 32, 2527–2533. [Google Scholar] [CrossRef] [PubMed]
- Knox, A.; Kalchschmid, C.; Schuster, D.; Gaggia, F.; Manzl, C.; Baecker, D.; Gust, R. Development of bivalent triarylalkene- and cyclofenil-derived dual estrogen receptor antagonists and downregulators. Eur. J. Med. Chem. 2020, 192, 112191. [Google Scholar] [CrossRef] [PubMed]
- Routledge, E.J.; Sumpter, J.P. Estrogenic activity of surfactants and some of their degradation products assessed using a recombinant yeast screen. Environ. Toxicol. Chem. 1996, 15, 241–248. [Google Scholar] [CrossRef]
- Arnold, S.F.; Robinson, M.K.; Notides, A.C.; Guillette, L.J., Jr.; McLachlan, J.A. A yeast estrogen screen for examining the relative exposure of cells to natural and xenoestrogens. Environ. Health Perspect. 1996, 104, 544–548. [Google Scholar] [CrossRef] [PubMed]
- Kretzschmar, G.; Zierau, O.; Wober, J.; Tischer, S.; Metz, P.; Vollmer, G. Prenylation has a compound specific effect on the estrogenicity of naringenin and genistein. J. Steroid Biochem. Mol. Biol. 2010, 118, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, S.; Gustafsson, J. Åke Estrogen receptor transcription and transactivation Basic aspects of estrogen action. Breast Cancer Res. 2000, 2, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Nishida, M.; Kasahara, K.; Kaneko, M.; Iwasaki, H.; Hayashi, K. Establishment of a new human endometrial adenocarcinoma cell line, Ishikawa cells, containing estrogen and progesterone receptors. Nihon Sanka Fujinka Gakkai Zasshi 1985, 37, 1103–1111. [Google Scholar]
- Holinka, C.F.; Hata, H.; Kuramoto, H.; Gurpide, E. Effects of steroid hormones and antisteroids on alkaline phosphatase activity in human endometrial cancer cells (Ishikawa line). Cancer Res. 1986, 46, 2771–2774. [Google Scholar]
- Wober, J.; Weißwange, I.; Vollmer, G. Stimulation of alkaline phosphatase activity in Ishikawa cells induced by various phytoestrogens and synthetic estrogens. J. Steroid Biochem. Mol. Biol. 2002, 83, 227–233. [Google Scholar] [CrossRef]
- Hata, H.; Holinka, C.F.; Pahuja, S.L.; Hochberg, R.B.; Kuramoto, H.; Gurpide, E. Estradiol metabolism in ishikawa endometrial cancer cells. J. Steroid Biochem. 1987, 26, 699–704. [Google Scholar] [CrossRef]
- Owens, J.W.; Ashby, J. Critical review and evaluation of the uterotrophic bioassay for the identification of possible estrogen agonists and antagonists: In support of the validation of the OECD uterotrophic protocols for the laboratory rodent. Organisation for Economic Co-oper. Crit. Rev. Toxicol. 2002, 32, 445–520. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, J.C.; Cook, J.C.; Marty, M.S.; Davis, L.G.; Kaplan, A.M.; Carney, E.W. Evaluation of Tier I screening approaches for detecting endocrine-active compounds (EACs). Crit. Rev. Toxicol. 2002, 32, 521–549. [Google Scholar] [CrossRef]
- Yoon, K.; Kwack, S.J.; Kim, H.S.; Lee, B.M. Estrogenic endocrine-disrupting chemicals: Molecular mechanisms of actions on putative human diseases. J. Toxicol. Environ. Health. Crit. Rev. 2014, 17, 127–174. [Google Scholar] [CrossRef] [PubMed]
- Kiyama, R.; Wada-Kiyama, Y. Estrogenic endocrine disruptors: Molecular mechanisms of action. Environ. Int. 2015, 83, 11–40. [Google Scholar] [CrossRef]
- Tyler, C.R.; Jobling, S.; Sumpter, J.P. Endocrine Disruption in Wildlife: A Critical Review of the Evidence. Crit. Rev. Toxicol. 1998, 28, 319–361. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency. Endocrine Disruptor Screening Program Test Guidelines OPPTS 890.1600: Uterotrophic Assay; Office of Prevention, Pesticides and Toxic Substances (OPPTS): Washington, DC, USA, 2009; pp. 1–21. [Google Scholar]
- United States Environmental Protection Agency. Uterotrophic Assay OCSPP Guideline 890.1600: Standard Evaluation Procedure (SEP); Endocrine Disruptor Screening Program, United States Environmental Protection Agency: Washington, DC, USA, 2011; pp. 1–19.
- Zierau, O.; Kretzschmar, G.; Möller, F.; Weigt, C.; Vollmer, G. Time dependency of uterine effects of naringenin type phytoestrogens in vivo. Mol. Cell. Endocrinol. 2008, 294, 92–99. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ahmed, N.S.; Elghazawy, N.H.; ElHady, A.K.; Engel, M.; Hartmann, R.W.; Abadi, A.H. Design and synthesis of novel tamoxifen analogues that avoid CYP2D6 metabolism. Eur. J. Med. Chem. 2016, 112, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Bedford, G.R.; Richardson, D.N. Preparation and Identification of cis and trans Isomers of a Substituted Triarylethylene. Nature 1966, 212, 733–734. [Google Scholar] [CrossRef]
- Coezy, E.; Borgna, J.L.; Rochefort, H. Tamoxifen and metabolites in MCF7 cells: Correlation between binding to estrogen receptor and inhibition of cell growth. Cancer Res. 1982, 42, 317–323. [Google Scholar] [PubMed]
- Kraft, K.S.; Ruenitz, A.P.C.; Bartlett, M.G. Carboxylic Acid Analogues of Tamoxifen: (Z)-2-[p-(1,2-Diphenyl-1-butenyl)phenoxy]-N,N-dimethylethylamine. Estrogen Receptor Affinity and Estrogen Antagonist Effects in MCF-7 Cells. J. Med. Chem. 1999, 42, 3126–3133. [Google Scholar] [CrossRef] [PubMed]
- Keely, N.O.; Carr, M.; Yassin, B.; Ana, G.; Lloyd, D.G.; Zisterer, D.; Meegan, M.J. Design, Synthesis and Biochemical Evaluation of Novel Selective Estrogen Receptor Ligand Conjugates Incorporating an Endoxifen-Combretastatin Hybrid Scaffold. Biomedicines 2016, 4, 15. [Google Scholar] [CrossRef] [PubMed]
- Lv, W.; Liu, J.; Skaar, T.C.; Flockhart, D.A.; Cushman, M. Design and Synthesis of Norendoxifen Analogues with Dual Aromatase Inhibitory and Estrogen Receptor Modulatory Activities. J. Med. Chem. 2015, 58, 2623–2648. [Google Scholar] [CrossRef] [PubMed]
- Maximov, P.Y.; McDaniel, R.E.; Fernandes, D.J.; Korostyshevskiy, V.R.; Bhatta, P.; Mürdter, T.E.; Jordan, V.C. Simulation with cells in vitro of tamoxifen treatment in premenopausal breast cancer patients with different CYP2D6 genotypes. Br. J. Pharmacol. 2014, 171, 5624–5635. [Google Scholar] [CrossRef] [PubMed]
- Brzozowski, A.M.; Pike, A.C.W.; Dauter, Z.; Hubbard, R.E.; Bonn, T.; Engström, O.; Öhman, L.; Greene, G.L.; Gustafsson, J.A.; Carlquist, M. Molecular basis of agonism and antagonism in the oestrogen receptor. Nature 1997, 389, 753–758. [Google Scholar] [CrossRef] [PubMed]
- Skehan, P.; Storeng, R.; Scudiero, D.; Monks, A.; McMahon, J.; Vistica, D.; Warren, J.T.; Bokesch, H.; Kenney, S.; Boyd, M.R. New Colorimetric Cytotoxicity Assay for Anticancer-Drug Screening. JNCI J. Natl. Cancer Inst. 1990, 82, 1107–1112. [Google Scholar] [CrossRef] [PubMed]
- Shiau, A.K.; Barstad, D.; Loria, P.M.; Cheng, L.; Kushner, P.J.; Agard, D.A.; Greene, G.L. The Structural Basis of Estrogen Receptor/Coactivator Recognition and the Antagonism of This Interaction by Tamoxifen. Cell 1998, 95, 927–937. [Google Scholar] [CrossRef]
- Shoemaker, R.H. The NCI60 human tumour cell line anticancer drug screen. Nat. Rev. Cancer 2006, 6, 813–823. [Google Scholar] [CrossRef]
- Littlefield, B.A.; Gurpide, E.; Markiewicz, L.; McKinley, B.; Hochberg, R.B. A simple and sensitive microtiter plate estrogen bioassay based on stimulation of alkaline phosphatase in Ishikawa cells: Estrogenic action of delta 5 adrenal steroids. Endocrinology 1990, 127, 2757–2762. [Google Scholar] [CrossRef]
- Saarinen, N.M.; Bingham, C.; Lorenzetti, S.; Mortensen, A.; Mäkelä, S.; Penttinen, P.; Zierau, O. Tools to evaluate estrogenic potency of dietary phytoestrogens: A consensus paper from the EU Thematic Network ‘Phytohealth’ (QLKI-2002-2453). Genes Nutr. 2006, 1, 143–158. [Google Scholar] [CrossRef][Green Version]





| Code | R1 | R2 | R3 |
|---|---|---|---|
| 1 | --- | H | H |
| 2 | --- | OCH3 | H |
| 3 | --- | OCH3 | F |
| 4 | --- | F | OCH3 |
| 5 | -CH2-N-(CH3)2 | H | H |
| 6 | -N-CH2-CH2-CH2-CH2- | H | H |
| 7 | -N-CH2-CH2-CH2-CH2-CH2- | H | H |
| 8 | -N-CH2-CH2-O-CH2-CH2- | H | H |
| 9 | -N-CH2-CH2-CH2-CH2-CH2-CH2- | H | H |
| 10 | -CH2-N-(CH3)2 | OCH3 | H |
| 11 | -N-CH2-CH2-CH2-CH2- | OCH3 | H |
| 12 | -N-CH2-CH2-CH2-CH2-CH2- | OCH3 | H |
| 13 | -N-CH2-CH2-O-CH2-CH2- | OCH3 | H |
| 14 | -N-CH2-CH2-CH2-CH2-CH2-CH2- | OCH3 | H |
| 15 | -CH2-N-(CH3)2 | OCH3 | F |
| 16 | -N-CH2-CH2-CH2-CH2- | OCH3 | F |
| 17 | -N-CH2-CH2-CH2-CH2-CH2- | OCH3 | F |
| 18 | -N-CH2-CH2-O-CH2-CH2- | OCH3 | F |
| 19 | -N-CH2-CH2-CH2-CH2-CH2-CH2- | OCH3 | F |
| 20 | -N-(CH3)2 | OCH3 | F |
| 21 | -N-(C2H5)2 | OCH3 | F |
| 22 | -CH2-N-(CH3)2 | F | OCH3 |
| 23 | -N-CH2-CH2-CH2-CH2- | F | OCH3 |
| 24 | -N-CH2-CH2-CH2-CH2-CH2- | F | OCH3 |
| 25 | -N-CH2-CH2-O-CH2-CH2- | F | OCH3 |
| 26 | -N-CH2-CH2-CH2-CH2-CH2-CH2- | F | OCH3 |
| 27 | -N-(CH3)2 | F | OCH3 |
| 28 | -N-(C2H5)2 | F | OCH3 |
| Code | Anti-Estrogenic Activity * | Code | Anti-Estrogenic Activity * |
|---|---|---|---|
| 5 | 1.34 ± 0.15 | 18 | n.d. |
| 6 | 1.20 ± 0.19 | 19 | n.d. |
| 7 | 1.35 ± 0.16 | 20 | n.d. |
| 8 | 1.11 ± 0.26 | 21 | n.d. |
| 9 | 1.99 ± 0.02 | 22 | 0.97 ± 0.03 |
| 10 | 3.92 ± 0.58 | 23 | 1.06 ± 0.05 |
| 11 | 1.55 ± 0.12 | 24 | 1.19 ± 0.10 |
| 12 | 2.55 ± 0.41 | 25 | 1.18 ± 0.03 |
| 13 | n.d. ** | 26 | 1.05 ± 0.12 |
| 14 | 1.53 ± 0.09 | 27 | 0.86 ± 0.04 |
| 15 | n.d. | 28 | 0.86 ± 0.07 |
| 16 | n.d. | TAM | 0.30 ± 0.08 |
| 17 | n.d. | 4-OH-TAM | 0.21 ± 0.004 |
| Code | Estrogenic Activity * | Code | Estrogenic Activity * |
|---|---|---|---|
| 3 | 12.83 ± 2.72 | 18 | 1.76 ± 0.56 |
| 4 | 6.62 ± 1.74 | 19 | 3.22 ± 1.22 |
| 5 | 2.25 ± 0.92 | 20 | 2.40 ± 0.64 |
| 6 | 2.88 ± 0.89 | 21 | 2.40 ± 0.77 |
| 7 | 2.87 ± 0.90 | 22 | 1.87 ± 0.74 |
| 8 | 1.85 ± 0.11 | 23 | 1.41 ± 1.01 |
| 9 | 6.74 ± 1.67 | 24 | 1.19 ± 0.20 |
| 10 | 11.61 ± 0.99 | 25 | 1.31 ± 0.30 |
| 11 | 1.98 ± 0.12 | 26 | 1.78 ± 0.64 |
| 12 | 3.65 ± 0.70 | 27 | 1.15 ± 0.17 |
| 13 | 12.41 ± 0.26 | 28 | 1.37 ± 0.39 |
| 14 | 1.80 ± 0.09 | TAM | 1.16 ± 0.13 |
| 15 | 7.77 ± 1.9 | 4-OH-TAM | 1.46 ± 0.21 |
| 16 | 2.19 ± 0.71 | E2 (10 nM) | 13 ± 2.90 |
| 17 | 7.28 ± 3.10 |
| Code | EC50 (nM) |
|---|---|
| 3 | 40.1 ± 0.5 |
| 4 | 258 ± 80 |
| 15 | 440 ± 10 |
| 16 | n.c. * |
| 17 | 252 ± 8 |
| 18 | n.c. |
| 19 | 407 ± 86 |
| 20 | n.c. |
| 21 | 735 ± 13 |
| E2 | 0.528 ± 0.051 |
| Code | Mean Growth Inhibition (%) | Growth Inhibition on MCF-7 (%) * | Code | Mean Growth Inhibition (%) | Growth Inhibition on MCF-7 (%) * |
|---|---|---|---|---|---|
| 3 | 2.34 | No inhibition | 17 | 69.12 | 85.01 |
| 4 | 15.41 | No inhibition | 18 | 4.23 | No inhibition |
| 5 | 15.45 | 54.99 | 19 | 60.79 | 75.88 |
| 6 | 37.82 | 66.24 | 20 | 46.97 | 68.25 |
| 7 | 29 | 56.39 | 21 | 31.21 | 29.69 |
| 8 | 9.4 | 30.94 | 22 | 18.62 | 33.18 |
| 9 | 25.41 | 24 | 23 | 46.89 | 73.52 |
| 10 | 33.44 | 63.07 | 24 | 29.19 | 49.32 |
| 11 | 67.76 | 86.96 | 25 | 12.77 | 2.75 |
| 12 | 55.21 | 79.65 | 26 | 28.33 | 36.51 |
| 13 | 7.86 | 1.21 | 27 | 47.17 | 68.34 |
| 14 | 47.33 | 63.49 | 28 | 92.33 | >100 |
| 15 | 47.91 | 86.98 | TAM | >100 | >100 |
| 16 | 77.24 | 90.04 |
| Code | 1 nM | 10 nM | 100 nM | 1 µM | 10 µM |
|---|---|---|---|---|---|
| E2 | n.d. ** | 6.86 ± 1.60 * | n.d. | n.d. | n.d. |
| Tam | n.d. | n.d. | n.d. | 1.40 ± 0.45 | n.d. |
| OH-Tam | n.d. | n.d. | n.d. | 1.47 ± 0.22 | n.d. |
| 5 | 0.93 ± 0.64 | 0.95 ± 0.37 | 1.05 ± 0.30 | 1.08 ± 0.33 | 0.21 ± 0.16 * |
| 11 | 1.25 ± 0.61 | 1.13 ± 0.31 | 1.75 ± 0.50 | 2.56 ± 0.83 * | 1.36 ± 0.38 |
| 12 | 0.95 ± 0.02 | 0.96 ± 0.01 | 1.14 ± 0.01 | 1.43 ± 0.07 * | 0.42 ± 0.13 * |
| 19 | 1.02 ± 0.13 | 1.06 ± 0.11 | 1.75 ± 0.08 * | 1.71 ± 0.08 * | 0.04 ± 0.04 * |
| Code | Mean ± SD g/kg BW |
|---|---|
| Vehicle | 0.61 ± 0.07 |
| E2 | 3.85 ± 0.71 |
| TAM | 1.42 ± 0.30 |
| 12 | 1.23 ± 0.18 |
| 19 | 1.15 ± 0.18 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elnakib, H.E.; Ramsis, M.M.; Albably, N.O.; Vector, M.A.; Weigand, J.J.; Schwedtmann, K.; Wober, J.; Zierau, O.; Vollmer, G.; Abadi, A.H.; et al. Manipulating Estrogenic/Anti-Estrogenic Activity of Triphenylethylenes towards Development of Novel Anti-Neoplastic SERMs. Int. J. Mol. Sci. 2021, 22, 12575. https://doi.org/10.3390/ijms222212575
Elnakib HE, Ramsis MM, Albably NO, Vector MA, Weigand JJ, Schwedtmann K, Wober J, Zierau O, Vollmer G, Abadi AH, et al. Manipulating Estrogenic/Anti-Estrogenic Activity of Triphenylethylenes towards Development of Novel Anti-Neoplastic SERMs. International Journal of Molecular Sciences. 2021; 22(22):12575. https://doi.org/10.3390/ijms222212575
Chicago/Turabian StyleElnakib, Heba E., Marian M. Ramsis, Nouran O. Albably, Merna A. Vector, Jan J. Weigand, Kai Schwedtmann, Jannette Wober, Oliver Zierau, Günter Vollmer, Ashraf H. Abadi, and et al. 2021. "Manipulating Estrogenic/Anti-Estrogenic Activity of Triphenylethylenes towards Development of Novel Anti-Neoplastic SERMs" International Journal of Molecular Sciences 22, no. 22: 12575. https://doi.org/10.3390/ijms222212575
APA StyleElnakib, H. E., Ramsis, M. M., Albably, N. O., Vector, M. A., Weigand, J. J., Schwedtmann, K., Wober, J., Zierau, O., Vollmer, G., Abadi, A. H., & Ahmed, N. S. (2021). Manipulating Estrogenic/Anti-Estrogenic Activity of Triphenylethylenes towards Development of Novel Anti-Neoplastic SERMs. International Journal of Molecular Sciences, 22(22), 12575. https://doi.org/10.3390/ijms222212575







