Anterior Cruciate Ligament Reconstruction: Is Biological Augmentation Beneficial?
Abstract
1. Introduction
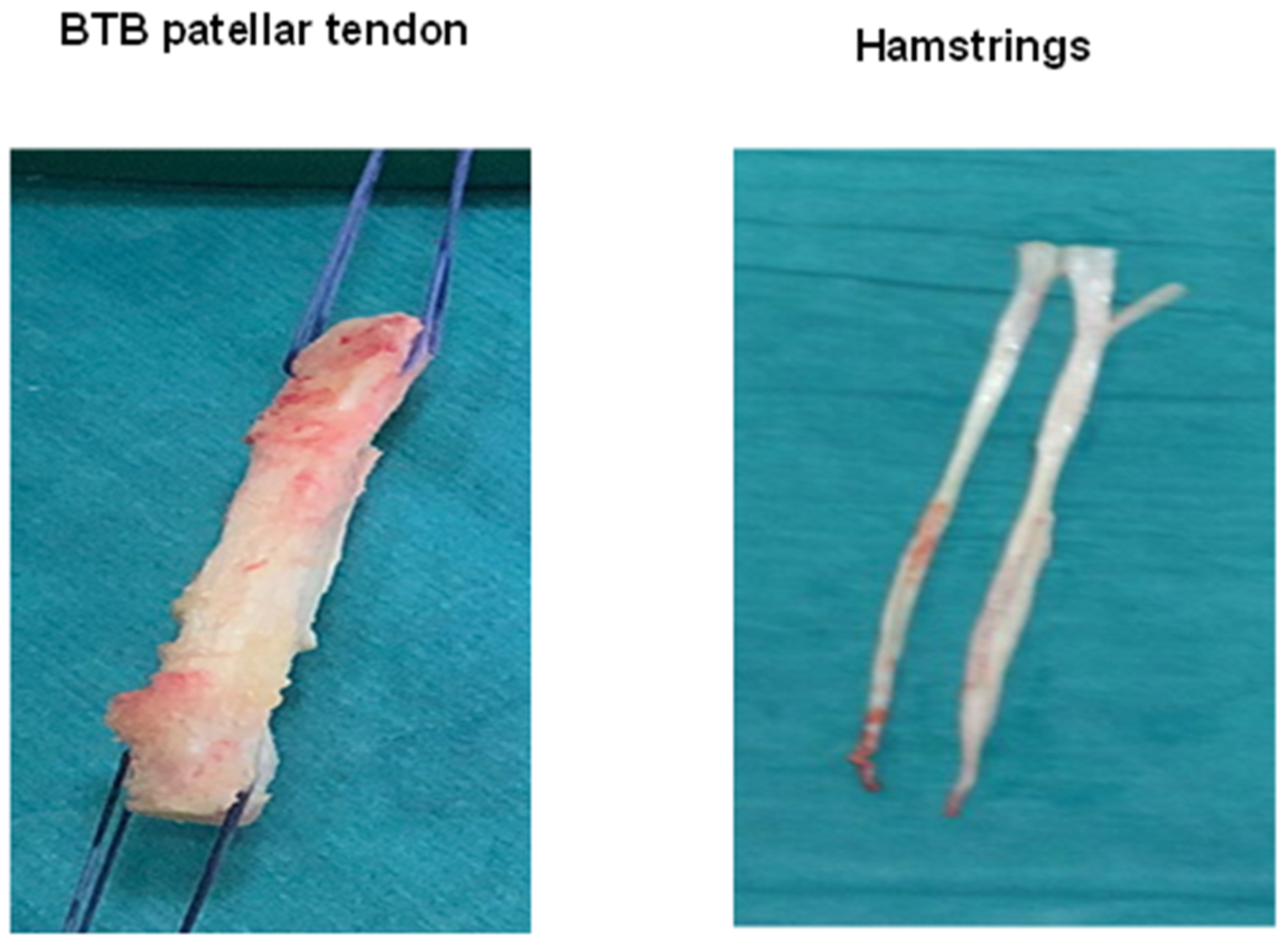
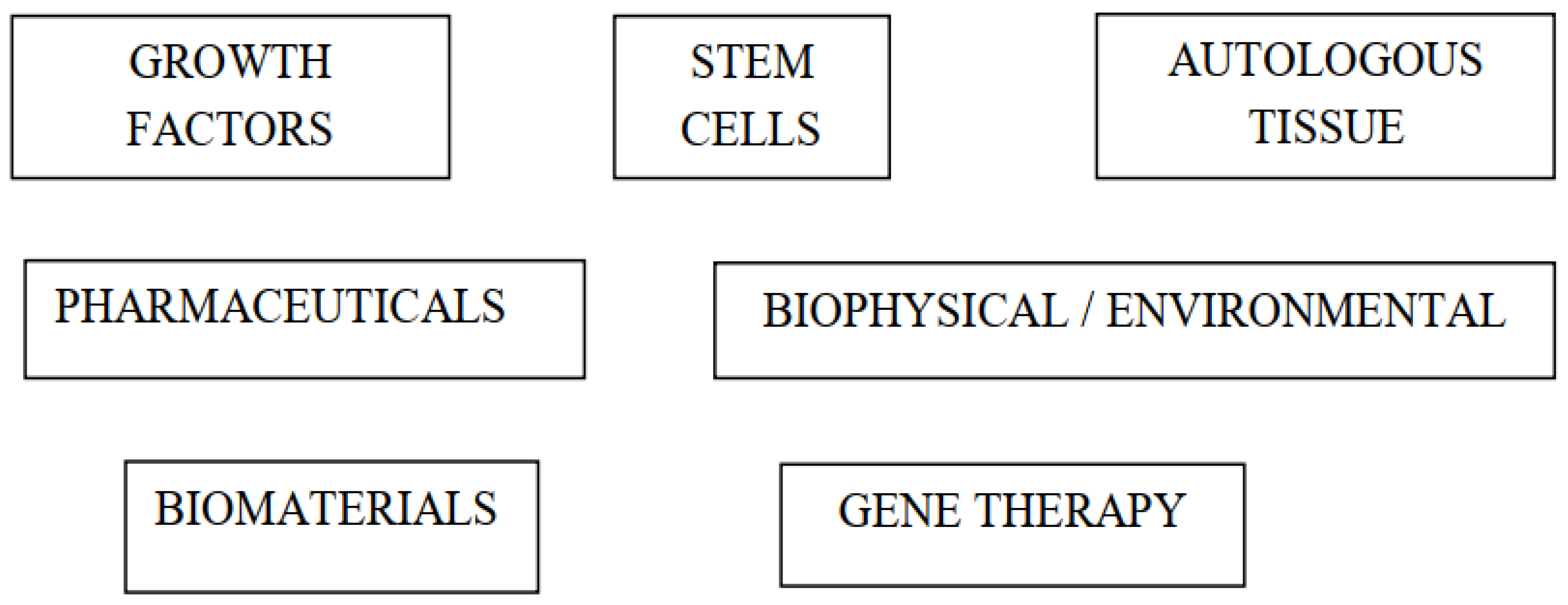
2. Types of Biological Augmentation Techniques in Anterior Cruciate Ligament Reconstruction
2.1. Animal Studies
2.2. Clinical Studies
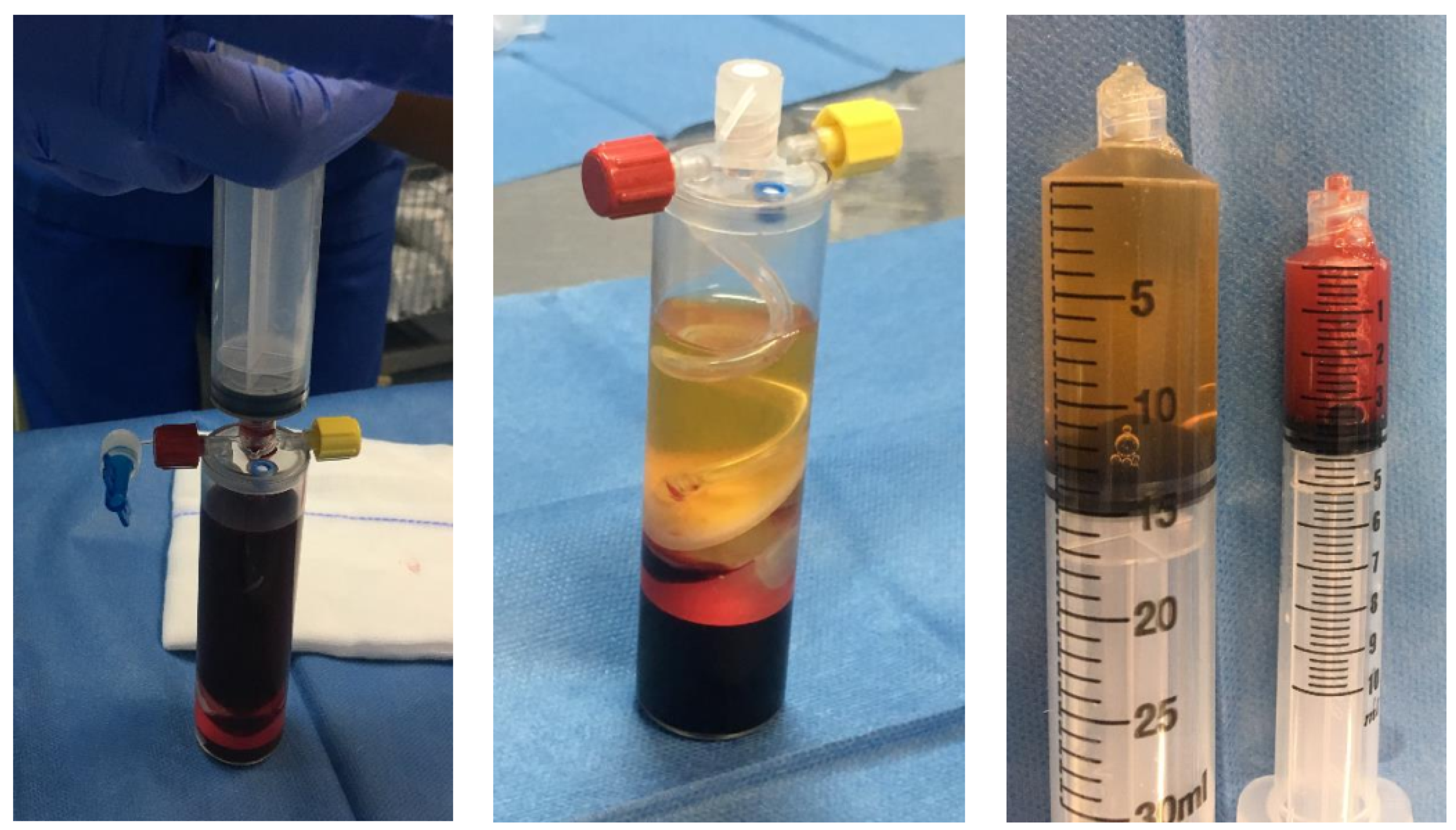
3. Growth Factors
4. Stem Cells
5. Autologous Tissue
6. Pharmaceuticals
7. Biophysical and Environmental
8. Biomaterials
8.1. Biological Fixation Methods
8.2. Biological Coatings
8.3. Biosynthetic Bone Substitutes
8.4. Osteoconductive Materials
9. Gene Therapy
10. Molecular Insights in ACLR
11. Discussion
12. Conclusions
Funding
Informed Consent Statement
Conflicts of Interest
References
- Prodromos, C.; Han, Y.; Rogowski, J.; Joyce, B.; Shi, K. A Meta-analysis of the Incidence of Anterior Cruciate Ligament Tears as a Function of Gender, Sport, and a Knee Injury–Reduction Regimen. Arthrosc. J. Arthrosc. Relat. Surg. 2007, 23, 1320–1325.e6. [Google Scholar] [CrossRef]
- Linko, E.; Harilainen, A.; Malmivaara, A.; Seitsalo, S. Surgical versus conservative interventions for anterior cruciate ligament ruptures in adults. Cochrane Database Syst. Rev. 2005, CD001356. [Google Scholar] [CrossRef]
- Śmigielski, R.; Zdanowicz, U.; Drwięga, M.; Ciszek, B.; Williams, A. The anatomy of the anterior cruciate ligament and its relevance to the technique of reconstruction. Bone Jt. J. 2016, 98-B, 1020–1026. [Google Scholar] [CrossRef]
- Shah, V.M.; Andrews, J.R.; Fleisig, G.S.; McMichael, C.S.; Lemak, L.J. Return to Play after Anterior Cruciate Ligament Reconstruction in National Football League Athletes. Am. J. Sports Med. 2010, 38, 2233–2239. [Google Scholar] [CrossRef] [PubMed]
- Kuršumović, K.; Charalambous, C. Graft salvage following infected anterior cruciate ligament reconstruction: A systematic review and meta-analysis. Bone Jt. J. 2016, 98-B, 608–615. [Google Scholar] [CrossRef]
- Ahmed, I.; Salmon, L.; Roe, J.; Pinczewski, L. The long-term clinical and radiological outcomes in patients who suffer recurrent injuries to the anterior cruciate ligament after reconstruction. Bone Jt. J. 2017, 99-B, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Gulotta, L.V.; Rodeo, S.A. Biology of Autograft and Allograft Healing in Anterior Cruciate Ligament Reconstruction. Clin. Sports Med. 2007, 26, 509–524. [Google Scholar] [CrossRef]
- Benjamin, M.; Toumi, H.; Ralphs, J.; Bydder, G.; Best, T.M.; Milz, S. Where tendons and ligaments meet bone: Attachment sites (‘entheses’) in relation to exercise and/or mechanical load. J. Anat. 2006, 208, 471–490. [Google Scholar] [CrossRef] [PubMed]
- Lazarides, A.L.; Eward, W.C.; Green, K.; Cardona, D.M.; Brigman, B.E.; Taylor, D.C. Histological Evaluation of Tendon-Bone Healing of an Anterior Cruciate Ligament Hamstring Graft in a 14-Year-Old Boy. Am. J. Sports Med. 2015, 43, 1935–1940. [Google Scholar] [CrossRef]
- LaPrade, R.F.; Dragoo, J.L.; Koh, J.L.; Murray, I.R.; Geeslin, A.G.; Chu, C.R. AAOS research symposium updates and consensus: Biologic treatment of orthopaedic injuries. J. Am. Acad. Orthop. Surg. 2016, 24, 62–78. [Google Scholar] [CrossRef]
- Sherman, S.L.; Calcei, J.; Ray, T.; A Magnussen, R.; Musahl, V.; Kaeding, C.C.; Clatworthy, M.; A Bergfeld, J.; Arnold, M.P. ACL Study Group presents the global trends in ACL reconstruction: Biennial survey of the ACL Study Group. J. ISAKOS 2021, 6, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Hexter, A.T.; Thangarajah, T.; Blunn, G.; Haddad, F.S. Biological augmentation of graft healing in anterior cruciate ligament reconstruction: A systematic review. Bone Jt. J. 2018, 100-B, 271–284. [Google Scholar] [CrossRef]
- Teng, C.; Zhou, C.; Xu, D.; Bi, F. Combination of platelet-rich plasma and bone marrow mesenchymal stem cells enhances tendon–bone healing in a rabbit model of anterior cruciate ligament reconstruction. J. Orthop. Surg. Res. 2016, 11, 1–9. [Google Scholar] [CrossRef]
- Hensler, D.; Illingworth, K.D.; Musahl, V.; Working, Z.M.; Kobayashi, T.; Miyawaki, M.; Lorenz, S.; Witt, M.; Irrgang, J.J.; Huard, J.; et al. Does fibrin clot really enhance graft healing after double-bundle ACL reconstruction in a caprine model? Knee Surg. Sports Traumatol. Arthrosc. 2015, 23, 669–679. [Google Scholar] [CrossRef]
- Darabos, N.; Haspl, M.; Moser, C.; Darabos, A.; Bartolek, D.; Groenemeyer, D.H.W. Intraarticular application of autologous conditioned serum (ACS) reduces bone tunnel widening after ACL reconstructive surgery in a randomized controlled trial. Knee Surg. Sports Traumatol. Arthrosc. 2011, 19, 36–46. [Google Scholar] [CrossRef]
- Momaya, A.M.; McGee, A.S.; Dombrowsky, A.R.; Wild, A.J.; Faroqui, N.M.; Waldrop, R.P.; He, J.K.; Brabston, E.W.; Ponce, B.A. The Cost Variability of Orthobiologics. Sports Health 2020, 12, 94–98. [Google Scholar] [CrossRef]
- Sun, X.; Liu, W.; Cheng, G.; Qu, X.; Bi, H.; Cao, Z.; Yu, Q. The influence of connective tissue growth factor on rabbit ligament injury repair. Bone Jt. Res. 2017, 6, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Bailey, L.; Weldon, M.; Kleihege, J.; Lauck, K.; Syed, M.; Mascarenhas, R.; Lowe, W.R. Platelet-Rich Plasma Augmentation of Meniscal Repair in the Setting of Anterior Cruciate Ligament Reconstruction. Am. J. Sports Med. 2021. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Fan, J.; Sun, L.; Liu, X.; Cheng, P.; Fan, H. Functional regeneration of ligament-bone interface using a triphasic silk-based graft. Biomaterials 2016, 106, 180–192. [Google Scholar] [CrossRef]
- Teuschl, A.; Heimel, P.; Nürnberger, S.; van Griensven, M.; Redl, H.; Nau, T. A novel silk fiber-based scaffold for regeneration of the anterior cruciate ligament: Histological results from a study in sheep. Am. J. Sports Med. 2016, 44, 1547–1557. [Google Scholar] [CrossRef] [PubMed]
- Kouroupis, D.; Kyrkou, A.; Triantafyllidi, E.; Katsimpoulas, M.; Chalepakis, G.; Goussia, A.; Georgoulis, A.; Murphy, C.; Fotsis, T. Generation of stem cell-based bioartificial anterior cruciate ligament (ACL) grafts for effective ACL rupture repair. Stem Cell Res. 2016, 17, 448–457. [Google Scholar] [CrossRef]
- Kosaka, M.; Nakase, J.; Hayashi, K.; Tsuchiya, H. Adipose-derived regenerative Cells Promote Tendon-Bone Healing in a Rabbit Model. Arthroscopy 2016, 32, 851–859. [Google Scholar] [CrossRef] [PubMed]
- Jang, K.-M.; Lim, H.C.; Jung, W.Y.; Moon, S.W.; Wang, J.H. Efficacy and Safety of Human Umbilical Cord Blood–Derived Mesenchymal Stem Cells in Anterior Cruciate Ligament Reconstruction of a Rabbit Model: New Strategy to Enhance Tendon Graft Healing. Arthrosc. J. Arthrosc. Relat. Surg. 2015, 31, 1530–1539. [Google Scholar] [CrossRef]
- Mifune, Y.; Matsumoto, T.; Takayama, K.; Terada, S.; Sekiya, N.; Kuroda, R.; Kurosaka, M.; Fu, F.H.; Huard, J. Tendon graft revitalization using adult anterior cruciate ligament (ACL)-derived CD34+ cell sheets for ACL reconstruction. Biomaterials 2013, 34, 5476–5487. [Google Scholar] [CrossRef] [PubMed]
- Lui, P.P.Y.; Wong, O.T.; Lee, Y.W. Application of Tendon-Derived Stem Cell Sheet for the Promotion of Graft Healing in Anterior Cruciate Ligament Reconstruction. Am. J. Sports Med. 2014, 42, 681–689. [Google Scholar] [CrossRef]
- Lim, J.K.; Hui, J.; Li, L.; Thambyah, A.; Goh, J.; Lee, E.H. Enhancement of tendon graft osteointegration using mesenchymal stem cells in a rabbit model of anterior cruciate ligament reconstruction. Arthroscopy 2004, 20, 899–910. [Google Scholar] [CrossRef]
- Takahashi, T.; Kondo, E.; Yasuda, K.; Miyatake, S.; Kawaguchi, Y.; Onodera, J.; Kitamura, N. Effects of remnant tissue preservation on the tendon graft in anterior cruciate ligament reconstruction: A biomechanical and histological study. Am. J. Sports Med. 2016, 44, 1708–1716. [Google Scholar] [CrossRef]
- Song, G.-Y.; Zhang, J.; Li, X.; Li, Y.; Feng, H. Biomechanical and Biological Findings Between Acute Anterior Cruciate Ligament Reconstruction With and Without an Augmented Remnant Repair: A Comparative in Vivo Animal Study. Arthrosc. J. Arthrosc. Relat. Surg. 2016, 32, 307–319. [Google Scholar] [CrossRef]
- Ouanezar, H.; Blakeney, W.G.; Fernandes, L.R.; Borade, A.; Latrobe, C.; Temponi, E.F.; Sonnery-Cottet, B. Clinical Outcomes of Single Anteromedial Bundle Biologic Augmentation Technique for Anterior Cruciate Ligament Reconstruction With Consideration of Tibial Remnant Size. Arthrosc. J. Arthrosc. Relat. Surg. 2018, 34, 714–722. [Google Scholar] [CrossRef] [PubMed]
- Karaoglu, S.; Celik, C.; Korkusuz, P. The effects of bone marrow or periosteum on tendon-to-bone tunnel healing in a rabbit model. Knee Surg. Sports Traumatol. Arthrosc. 2009, 17, 170–178. [Google Scholar] [CrossRef]
- Demirag, B.; Sarisozen, B.; Ozer, O.; Kaplan, T.; Ozturk, C. Enhancement of Tendon-Bone Healing of Anterior Cruciate Ligament Grafts by Blockage of Matrix Metalloproteinases. J. Bone Jt. Surg.-Am. Vol. 2005, 87-A, 2401–2410. [Google Scholar] [CrossRef]
- Lui, P.P.Y.; Lee, Y.W.; Mok, T.Y.; Cheuk, Y.C. Local administration of alendronate reduced peri-tunnel bone loss and promoted graft-bone tunnel healing with minimal systemic effect on bone in contralateral knee. J. Orthop. Res. 2013, 31, 1897–1906. [Google Scholar] [CrossRef] [PubMed]
- Bi, F.; Shi, Z.; Jiang, S.; Guo, P.; Yan, S. Intermittently Administered Parathyroid Hormone [1–34] Promotes Tendon-Bone Healing in a Rat Model. Int. J. Mol. Sci. 2014, 15, 17366–17379. [Google Scholar] [CrossRef]
- Yeh, W.-L.; Lin, S.-S.; Yuan, L.-J.; Lee, K.-F.; Lee, M.-Y.; Ueng, S.W.N. Effects of hyperbaric oxygen treatment on tendon graft and tendon-bone integration in bone tunnel: Biochemical and histological analysis in rabbits. J. Orthop. Res. 2007, 25, 636–645. [Google Scholar] [CrossRef]
- Papatheodorou, L.K.; Malizos, K.N.; Poultsides, L.A.; Hantes, M.E.; Grafanaki, K.; Giannouli, S.; Ioannou, M.G.; Koukoulis, G.K.; Protopappas, V.C.; Fotiadis, D.I.; et al. Effect of Transosseous Application of Low-Intensity Ultrasound at the Tendon Graft-Bone Interface Healing: Gene Expression and Histological Analysis in Rabbits. Ultrasound Med. Biol. 2009, 35, 576–584. [Google Scholar] [CrossRef]
- Wang, C.-J.; Wang, F.-S.; Yang, K.D.; Weng, L.-H.; Sun, Y.-C.; Yang, Y.-J. The effect of shock wave treatment at the tendon–bone interface—An histomorphological and biomechanical study in rabbits. J. Orthop. Res. 2005, 23, 274–280. [Google Scholar] [CrossRef]
- Cheng, P.; Han, P.; Zhao, C.; Zhang, S.; Zhang, X.; Chai, Y. Magnesium inference screw supports early graft incorporation with inhibition of graft degradation in anterior cruciate ligament reconstruction. Sci. Rep. 2016, 6, 26434. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xu, J.; Fu, W.; Cheng, W.; Chan, K.; Yung, P.S.-H.; Qin, L. Biodegradable Magnesium Screws Accelerate Fibrous Tissue Mineralization at the Tendon-Bone Insertion in Anterior Cruciate Ligament Reconstruction Model of Rabbit. Sci. Rep. 2017, 7, 40369. [Google Scholar] [CrossRef]
- Chou, Y.-C.; Yeh, W.-L.; Chao, C.-L.; Hsu, Y.-H.; Yu, Y.-H.; Chen, J.-K.; Liu, S.-J. Enhancement of tendon–bone healing via the combination of biodegradable collagen-loaded nanofibrous membranes and a three-dimensional printed bone-anchoring bolt. Int. J. Nanomed. 2016, 11, 4173–4186. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Yamada, T.; Yasukawa, A.; Koyama, Y.; Muneta, T.; Takakuda, K. Anterior Cruciate Ligament Reconstruction Using Chitin-coated Fabrics in a Rabbit Model. Artif. Organs 2010, 34, 55–64. [Google Scholar] [CrossRef]
- Li, H.; Chen, S.; Wu, Y.; Jiang, J.; Ge, Y.; Gao, K.; Zhang, P.; Wu, L. Enhancement of the osseointegration of a polyethylene terephthalate artificial ligament graft in a bone tunnel using 58S bioglass. Int. Orthop. 2011, 36, 191–197. [Google Scholar] [CrossRef]
- Cho, S.; Li, H.; Chen, C.; Jiang, J.; Tao, H.; Chen, S. Cationised gelatin and hyaluronic acid coating enhances polyethylene terephthalate artificial ligament graft osseointegration in porcine bone tunnels. Int. Orthop. 2012, 37, 507–513. [Google Scholar] [CrossRef][Green Version]
- Vaquette, C.; Viateau, V.; Guérard, S.; Anagnostou, F.; Manassero, M.; Castner, D.G.; Migonney, V. The effect of polystyrene sodium sulfonate grafting on polyethylene terephthalate artificial ligaments on in vitro mineralisation and in vivo bone tissue integration. Biomaterials 2013, 34, 7048–7063. [Google Scholar] [CrossRef]
- Bi, F.; Shi, Z.; Liu, A.; Guo, P.; Yan, S. Anterior Cruciate Ligament Reconstruction in a Rabbit Model Using Silk-Collagen Scaffold and Comparison with Autograft. PLoS ONE 2015, 10, e0125900. [Google Scholar] [CrossRef]
- Han, F.; Zhang, P.; Sun, Y.; Lin, C.; Zhao, P.; Chen, J. Hydroxyapatite-doped polycaprolactone nanofiber membrane improves tendon–bone interface healing for anterior cruciate ligament reconstruction. Int. J. Nanomed. 2015, 10, 7333–7343. [Google Scholar] [CrossRef]
- Hsu, S.-L.; Wang, C.-J. The use of demineralized bone matrix for anterior cruciate ligament reconstruction: A radiographic, histologic, and immunohistochemical study in rabbits. J. Surg. Res. 2013, 187, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Elnikety, S.; Pendegrass, C.J.; De Godoy, R.F.; Holden, C.; Blunn, G.W. Augmentation and repair of tendons using demineralised cortical bone. BMC Musculoskelet. Disord. 2016, 17, 483. [Google Scholar] [CrossRef] [PubMed]
- Thangarajah, T.; Shahbazi, S.; Pendegrass, C.J.; Lambert, S.; Alexander, S.; Blunn, G.W. Tendon Reattachment to Bone in an Ovine Tendon Defect Model of Retraction Using Allogenic and Xenogenic Demineralised Bone Matrix Incorporated with Mesenchymal Stem Cells. PLoS ONE 2016, 11, e0161473. [Google Scholar] [CrossRef] [PubMed]
- Thangarajah, T.; Henshaw, F.; Sanghani-Kerai, A.; Lambert, S.M.; Blunn, G.W.; Pendegrass, C.J. The effectiveness of demineralized cortical bone matrix in a chronic rotator cuff tear model. J. Shoulder Elb. Surg. 2017, 26, 619–626. [Google Scholar] [CrossRef]
- Lovric, V.; Chen, N.; Yu, Y.; Oliver, R.A.; Genin, F.; Walsh, W.R. Effects of Demineralized Bone Matrix on Tendon-Bone Healing in an Intra-articular Rodent Model. Am. J. Sports Med. 2012, 40, 2365–2374. [Google Scholar] [CrossRef]
- Mutsuzaki, H.; Fujie, H.; Nakajima, H.; Fukagawa, M.; Nomura, S.; Sakane, M. Effect of Calcium Phosphate–Hybridized Tendon Graft in Anatomic Single-Bundle ACL Reconstruction in Goats. Orthop. J. Sports Med. 2016, 4. [Google Scholar] [CrossRef]
- Kuang, G.M.; Yau, W.P.; Lu, W.W.; Chiu, K.Y. Local application of strontium in a calcium phosphate cement system accelerates healing of soft tissue tendon grafts in anterior cruciate ligament reconstruction experiment using a rabbit model. Am. J. Sports Med 2014, 42, 2996–3002. [Google Scholar] [CrossRef]
- Chen, B.; Li, B.; Qi, Y.-J.; Ni, Q.-B.; Pan, Z.-Q.; Wang, H.; Chen, L.-B. Enhancement of tendon-to-bone healing after anterior cruciate ligament reconstruction using bone marrow-derived mesenchymal stem cells genetically modified with bFGF/BMP2. Sci. Rep. 2016, 6, 25940. [Google Scholar] [CrossRef]
- Kawakami, Y.; Takayama, K.; Matsumoto, T.; Tang, Y.; Wang, B.; Mifune, Y.; Cummins, J.H.; Warth, R.J.; Kuroda, R.; Kurosaka, M.; et al. Anterior Cruciate Ligament–Derived Stem Cells Transduced With BMP2 Accelerate Graft-Bone Integration After ACL Reconstruction. Am. J. Sports Med. 2016, 45, 584–597. [Google Scholar] [CrossRef]
- Hao, Z.-C.; Wang, S.-Z.; Zhang, X.-J.; Lu, J. Stem cell therapy: A promising biological strategy for tendon-bone healing after anterior cruciate ligament reconstruction. Cell Prolif. 2016, 49, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Radice, F.; Yánez, R.; Gutiérrez, V.; Rosales, J.; Pinedo, M.; Coda, S. Comparison of Magnetic Resonance Imaging Findings in Anterior Cruciate Ligament Grafts With and Without Autologous Platelet-Derived Growth Factors. Arthrosc. J. Arthrosc. Relat. Surg. 2010, 26, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Rupreht, M.; Jevtič, V.; Serša, I.; Vogrin, M.; Jevšek, M. Evaluation of the tibial tunnel after intraoperatively administered platelet-rich plasma gel during anterior cruciate ligament reconstruction using diffusion weighted and dynamic contrast-enhanced MRI. J. Magn. Reson. Imaging 2012, 37, 928–935. [Google Scholar] [CrossRef]
- Vogrin, M.; Rupreht, M.; Dinevski, D.; Hašpl, M.; Kuhta, M.; Jevsek, M.; Knežević, M.; Rožman, P. Effects of a Platelet Gel on Early Graft Revascularization after Anterior Cruciate Ligament Reconstruction: A Prospective, Randomized, Double-Blind, Clinical Trial. Eur. Surg. Res. 2010, 45, 77–85. [Google Scholar] [CrossRef]
- Seijas, R.; Ares, O.; Catala, J.; Díaz, P.; Cusco, X.; Cugat, R. Magnetic Resonance Imaging Evaluation of Patellar Tendon Graft Remodelling after Anterior Cruciate Ligament Reconstruction with or without Platelet-Rich Plasma. J. Orthop. Surg. 2013, 21, 10–14. [Google Scholar] [CrossRef]
- Orrego, M.; Larrain, C.; Rosales, J.; Valenzuela, L.; Matas, J.; Durruty, J.; Sudy, H.; Mardones, R. Effects of Platelet Concentrate and a Bone Plug on the Healing of Hamstring Tendons in a Bone Tunnel. Arthrosc. J. Arthrosc. Relat. Surg. 2008, 24, 1373–1380. [Google Scholar] [CrossRef] [PubMed]
- Vadala, A.; Iorio, R.; De Carli, A.; Ferretti, M.; Paravani, D.; Caperna, L.; Iorio, C.; Gatti, A.; Ferretti, A. Platelet-rich plasma: Does it help reduce tunnel widening after ACL reconstruction? Knee Surg. Sports Traumatol. Arthrosc. 2013, 21, 824–829. [Google Scholar] [CrossRef]
- Mirzatolooei, F.; Alamdari, M.T.; Khalkhali, H.R. The impact of platelet-rich plasma on the prevention of tunnel widening in anterior cruciate ligament reconstruction using quadrupled autologous hamstring tendon. Bone Jt. J. 2013, 95-B, 65–69. [Google Scholar] [CrossRef]
- Valenti Azcarate, A.; Lamo-Espinosa, J.; Aquerreta Beola, J.D.; Hernandez Gonzalez, M.; Mora Gasque, G.; Valentí Nin, J.R. Comparison between two different platelet-rich plasma preparations and control applied during anterior cruciate ligament reconstruction: Is there any evidence to support their use? Injury 2014, 4, S36–S41. [Google Scholar] [CrossRef]
- Komzák, M.; Hart, R.; Šmíd, P.; Puskeiler, M.; Jajtner, P. [The effect of platelet-rich plasma on graft healing in reconstruction of the anterior cruciate ligament of the knee joint: Prospective study]. Acta Chir. Orthop. Traumatol. Cechoslov. 2015, 82, 135–179. [Google Scholar]
- Kroon, M.E.; Van Schie, M.L.; Van Der Vecht, B.; Van Hinsbergh, V.W.; Koolwijk, P. Collagen type 1 retards tube formation by human microvascular endothelial cells in a fibrin matrix. Angiogenesis 2002, 5, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Berdis, A.S.; Veale, K.; Fleissner, P.R., Jr. Outcomes of Anterior Cruciate Ligament Reconstruction Using Biologic Augmentation in Patients 21 Years of Age and Younger. Arthrosc. J. Arthrosc. Relat. Surg. 2019, 35, 3107–3113. [Google Scholar] [CrossRef] [PubMed]
- Wiggins, A.J.; Grandhi, R.K.; Schneider, D.K.; Stanfield, D.; Webster, K.E.; Myer, G.D. Risk of Secondary Injury in Younger Athletes After Anterior Cruciate Ligament Reconstruction. Am. J. Sports Med. 2016, 44, 1861–1876. [Google Scholar] [CrossRef]
- Heckmann, N.; Auran, R.; Mirzayan, R. Application of Amniotic Tissue in Orthopedic Surgery. Am. J. Orthop. 2016, 45, E421–E425. [Google Scholar]
- Woodall, B.M.; Elena, N.; Gamboa, J.T.; Shin, E.C.; Pathare, N.; McGahan, P.J.; Chen, J.L. Anterior Cruciate Ligament Reconstruction With Amnion Biological Augmentation. Arthrosc. Tech. 2018, 7, e355–e360. [Google Scholar] [CrossRef]
- Lavender, C.; Bishop, C. The Fertilized Anterior Cruciate Ligament: An All-Inside Anterior Cruciate Ligament Reconstruction Augmented With Amnion, Bone Marrow Concentrate, and a Suture Tape. Arthrosc. Tech. 2019, 8, e555–e559. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. Bio ACL Reconstruction With Amnion Collagen Matrix Wrap And Stem Cells Case Series. Available online: https://clinicaltrials.gov/ct2/show/NCT03294720 (accessed on 21 October 2021).
- Hexter, A.T.; Sanghani-Kerai, A.; Heidari, N.; Kalaskar, D.M.; Boyd, A.; Pendegrass, C.; Rodeo, S.A.; Haddad, F.S.; Blunn, G.W. Mesenchymal stromal cells and platelet-rich plasma promote tendon allograft healing in ovine anterior cruciate ligament reconstruction. Knee Surg. Sports Traumatol. Arthrosc. 2020, 29, 3678–3688. [Google Scholar] [CrossRef]
- Hexter, A.T.; Pendegrass, C.; Haddad, F.; Blunn, G. Demineralized bone matrix to augment tendon-bone healing: A systematic review. Orthop. J. Sports Med. 2017, 5, 2325967117734517. [Google Scholar] [CrossRef]
- Dai, Z.; Bao, W.; Li, S.; Li, H.; Jiang, J.; Chen, S. Enhancement of Polyethylene Terephthalate Artificial Ligament Graft Osseointegration using a Periosteum Patch in a Goat Model. Int. J. Sports Med. 2016, 37, 493–499. [Google Scholar] [CrossRef]
- Yu, H.; Fu, F.; Yao, S.; Luo, H.; Xu, T.; Jin, H.; Tong, P.; Chen, D.; Wu, C.; Ruan, H. Biomechanical, histologic, and molecular characteristics of graft-tunnel healing in a murine modified ACL reconstruction model. J. Orthop. Transl. 2020, 24, 103–111. [Google Scholar] [CrossRef]
- Atherton, C.M.; Spencer, S.J.; McCall, K.; Garcia-Melchor, E.; Leach, W.J.; Mullen, M.; Rooney, B.P.; Walker, C.; McInnes, I.B.; Millar, N.L.; et al. Vancomycin Wrap for Anterior Cruciate Ligament Surgery: Molecular Insights. Am. J. Sports Med. 2021, 49, 426–434. [Google Scholar] [CrossRef] [PubMed]
- Iorio, R.; Di Sanzo, V.; Vadalà, A.; Mazza, D.; Valeo, L.; A Messano, G.; Redler, A.; Iorio, C.; Bolle, G.; Conteduca, F.; et al. Nanohydroxyapatite-based bone graft substitute in tunnel enlargement after ACL surgery: RMN study. La Clin. Ter. 2013, 164, e101–e116. [Google Scholar]
- Mutsuzaki, H.; Kanamori, A.; Ikeda, K.; Hioki, S.; Kinugasa, T.; Sakane, M. Effect of calcium phosphate-hybridized tendon graft in anterior cruciate ligament reconstruction: A randomized controlled trial. Am. J. Sports Med. 2012, 40, 1772–1780. [Google Scholar] [CrossRef] [PubMed]
- Mutsuzaki, H.; Kinugasa, T.; Ikeda, K.; Sakane, M. Morphological changes in the femoral and tibial bone tunnels after anatomic single-bundle anterior cruciate ligament reconstruction using a calcium phosphate-hybridized tendon graft in 2 years of follow-up. Orthop. Traumatol. Surg. Res. 2019, 105, 653–660. [Google Scholar] [CrossRef]
- Lv, Z.-T.; Zhang, J.-M.; Pang, Z.-Y.; Wang, Z.; Huang, J.-M.; Zhu, W.-T. The efficacy of platelet rich plasma on anterior cruciate ligament reconstruction: A systematic review and meta-analysis. Platelets 2021, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Looney, A.M.; Leider, J.D.; Horn, A.R.; Bodendorfer, B.M. Bioaugmentation in the surgical treatment of anterior cruciate ligament injuries: A review of current concepts and emerging techniques. SAGE Open Med. 2020, 8, 2050312120921057. [Google Scholar] [CrossRef]
- Mc Millan, S.; Thorn, D.; Ford, E. A Novel Approach to Augmenting Allograft Hamstring Anterior Cruciate Ligament Reconstructions Utilizing a Resorbable Type I Collagen Matrix with Platelet Rich Plasma. Case Rep. Orthop. 2021, 2021, 5574676. [Google Scholar] [CrossRef] [PubMed]
| Bone Morphogenetic Growth Proteins |
| Basic fibroblast growth factor |
| Epidermal growth factor |
| Granulocyte colony-stimulating factor |
| Hepatocyte growth factor |
| Transforming growth factor-β |
| Vascular endothelial growth factor |
| Platelet concentrates: +Platelet-rich plasma (PRP) +Fibrin clots +Autologous conditioned serum |
| Adipose-Derived Stem Cells |
| Bone marrow-derived stem cells |
| Induced pluripotent stem cells |
| Umbilical cord-derived mesenchymal stem cells |
| Tendon-derived stem cells |
| CD34+ ACL-derived stem cells |
| Stem cells seeded on scaffold (in the form of sheets or applied locally to grafts) |
| Autologous Tissue Over Cultured Stem Cells |
| Attachment of the ACL remnant to the graft: single anteromedial bundle biological augmentation technique |
| Periosteal grafts |
| Matrix Metalloproteinase-Inhibitor Alpha-2-Macroglobulin: Intra-Articular Injection |
| Bisphosphonates (alendronate): local or systematic administration |
| Subcutaneous parathyroid hormone |
| Hyperbaric Oxygen |
| Low-intensity pulsed ultrasound |
| Extracorporeal shockwave therapy applied to the tibial tunnel |
| Biological Fixation Methods +Magnesium-Based Interference Screws +Biodegradable Polylactide Bolts As The Bone Anchor +Poly(D,L-Lactide-Co-Glycolide) Nanofibrous Membrane At The Graft-Tunnel Interface |
| Biological coatings +Chitin +Bioglass +Gelatin and hyaluronic acid +Polystyrene sodium sulfonate: collagen matrix; hydroxyapatite-doped polycaprolactone nanofiber membrane wrapped around autograft hamstring tendons |
| Biosynthetic bone substitutes +Demineralized bone matrix +Recombinant bone xenograft (nanohydroxyapatite bone-based graft). |
| Osteoconductive materials +Calcium phosphate |
| Tendon Graft Infected In Vitro With Adenovirus-BMP-2 |
| BMP-2 gene-transfected normal rat kidney cells at the tendon-bone interface |
| Transfecting stem cells with growth factors such as BMP-2, platelet-derived growth factor subunit and transforming growth factor beta |
| Implantation of genetically modified mesenchymal stem cells with basic fibroblast growth factor and BMP-2 at the graft-tunnel interface |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Merchán, E.C. Anterior Cruciate Ligament Reconstruction: Is Biological Augmentation Beneficial? Int. J. Mol. Sci. 2021, 22, 12566. https://doi.org/10.3390/ijms222212566
Rodríguez-Merchán EC. Anterior Cruciate Ligament Reconstruction: Is Biological Augmentation Beneficial? International Journal of Molecular Sciences. 2021; 22(22):12566. https://doi.org/10.3390/ijms222212566
Chicago/Turabian StyleRodríguez-Merchán, Emerito Carlos. 2021. "Anterior Cruciate Ligament Reconstruction: Is Biological Augmentation Beneficial?" International Journal of Molecular Sciences 22, no. 22: 12566. https://doi.org/10.3390/ijms222212566
APA StyleRodríguez-Merchán, E. C. (2021). Anterior Cruciate Ligament Reconstruction: Is Biological Augmentation Beneficial? International Journal of Molecular Sciences, 22(22), 12566. https://doi.org/10.3390/ijms222212566






