Possible Treatment of Myocardial Infarct Based on Tissue Engineering Using a Cellularized Solid Collagen Scaffold Functionalized with Arg-Glyc-Asp (RGD) Peptide
Abstract
:1. Introduction
2. Different Approaches for Cardiac Cell Delivery after MI
2.1. Use of Cells Associated with a 3D Scaffold for Tissue Engineering and Cell Transfer
2.2. Possible Transfer of Cells in a 3D Construct without a 3D Scaffold
2.2.1. Culture of Cells In Vitro as a 3D Cell Cluster: The “Spheroid Approach”
2.2.2. Cell Sheet Technology
3. Different Three-Dimensional Materials for Tissue Engineering and Contractilities Observed after Seeding with Cells with Contractile Potential
3.1. Different States of Three-Dimensional Materials: Hydrogel, Gel or Solid for Tissue Engineering Contractile Tissues
3.2. Composition and Key Parameters of the Three-Dimensional Materials for Engineering a Contractile Tissue: Polymer Composition, Adhesion Binding Sites, Porosity, Alignment, Architecture, Microarchitecture, Stiffness, Viscoelasticity
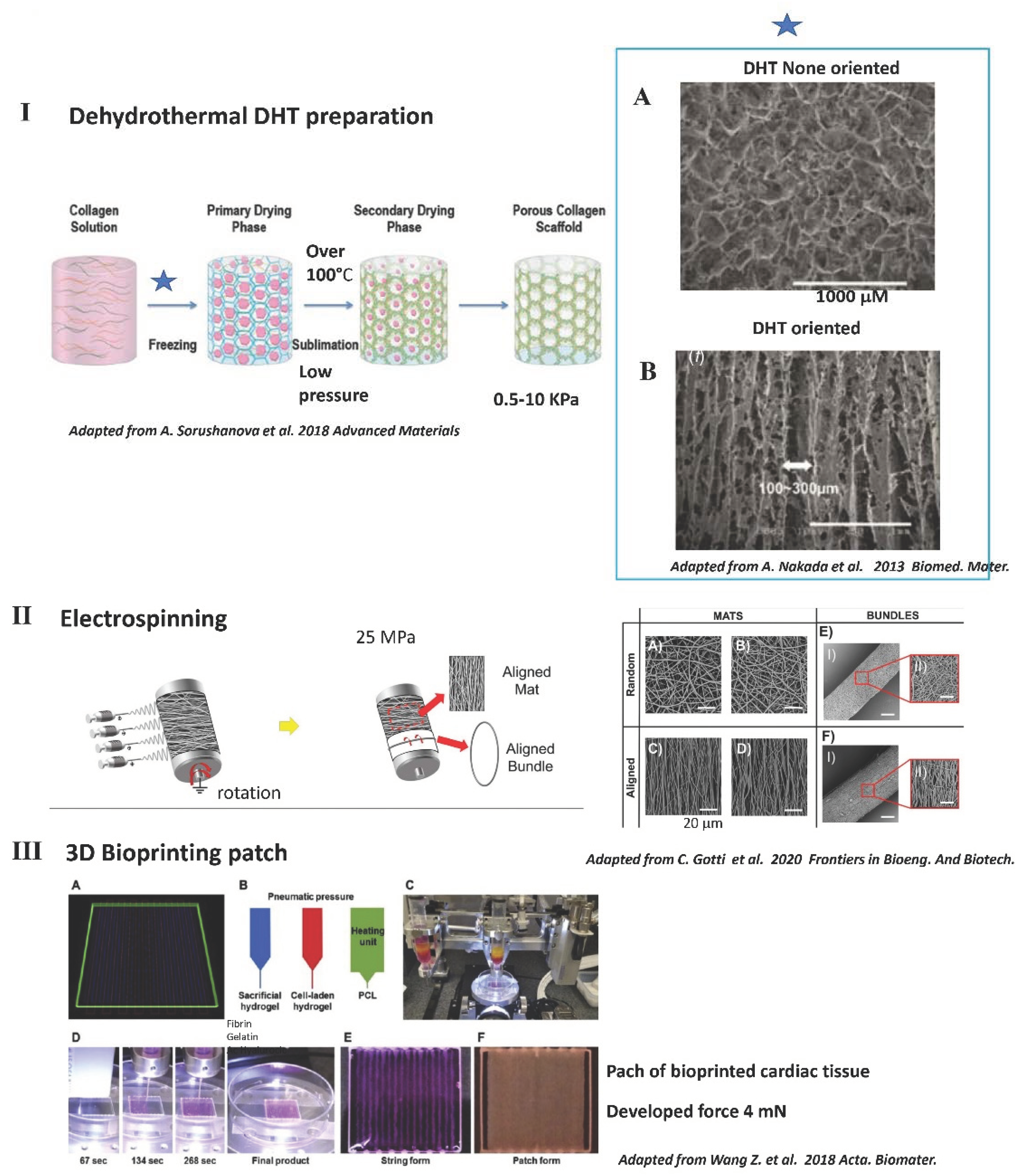
3.3. Common Methods Used to Fabricate Solid 3D Scaffolds including Those Used for Contractility
4. Contractility Reported for Different 3D Materials (Depending on Polymer Composition) or with Decellularized Tissue
4.1. No Contractility in 3D Scaffolds Made of Polysaccharides Such as Alginate or Chitosan, Even with an RGD Peptide
4.1.1. No Contractility in Soft Hydrogel of Alginate, Even with the RGD Peptide
4.1.2. In a Solid Porous Alginate Scaffold, Functionalization with RGD Increased Contractile Differentiation, but with No Contractility
4.1.3. Possible Use of a Solid 3D Alginate Scaffold for Non-Contractile Paracrine MSC Delivery after MI
4.2. Best Contractility Achieved with Preparations Containing Natural Polymers Such as Collagen, Gelatin, Fibrin or Matrigel™
4.2.1. The Different Natural Polymers: Collagen and Gelatin
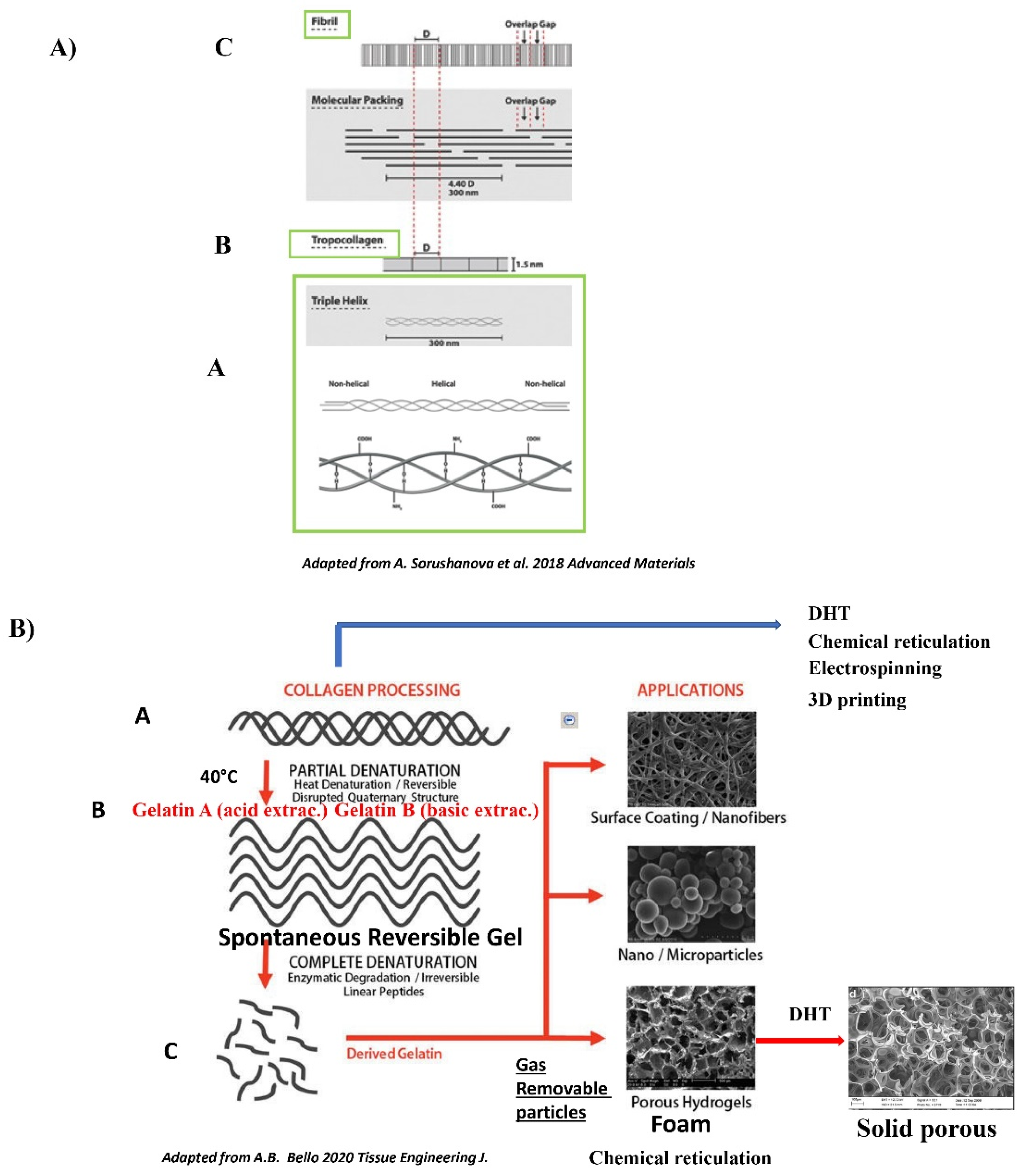
The Collagen Polymer: Generalities
The Gelatin Polymer: Generalities
4.2.2. Contractility in Gelatin Hydrogel Associated with Fibrin or PMDS and Neonatal Rat Cardiomyocytes
4.2.3. Contractility in 3D Gels of Collagen, Fibrin or Matrigel™ with Neonatal Rat Cardiomyocytes or Human Cells
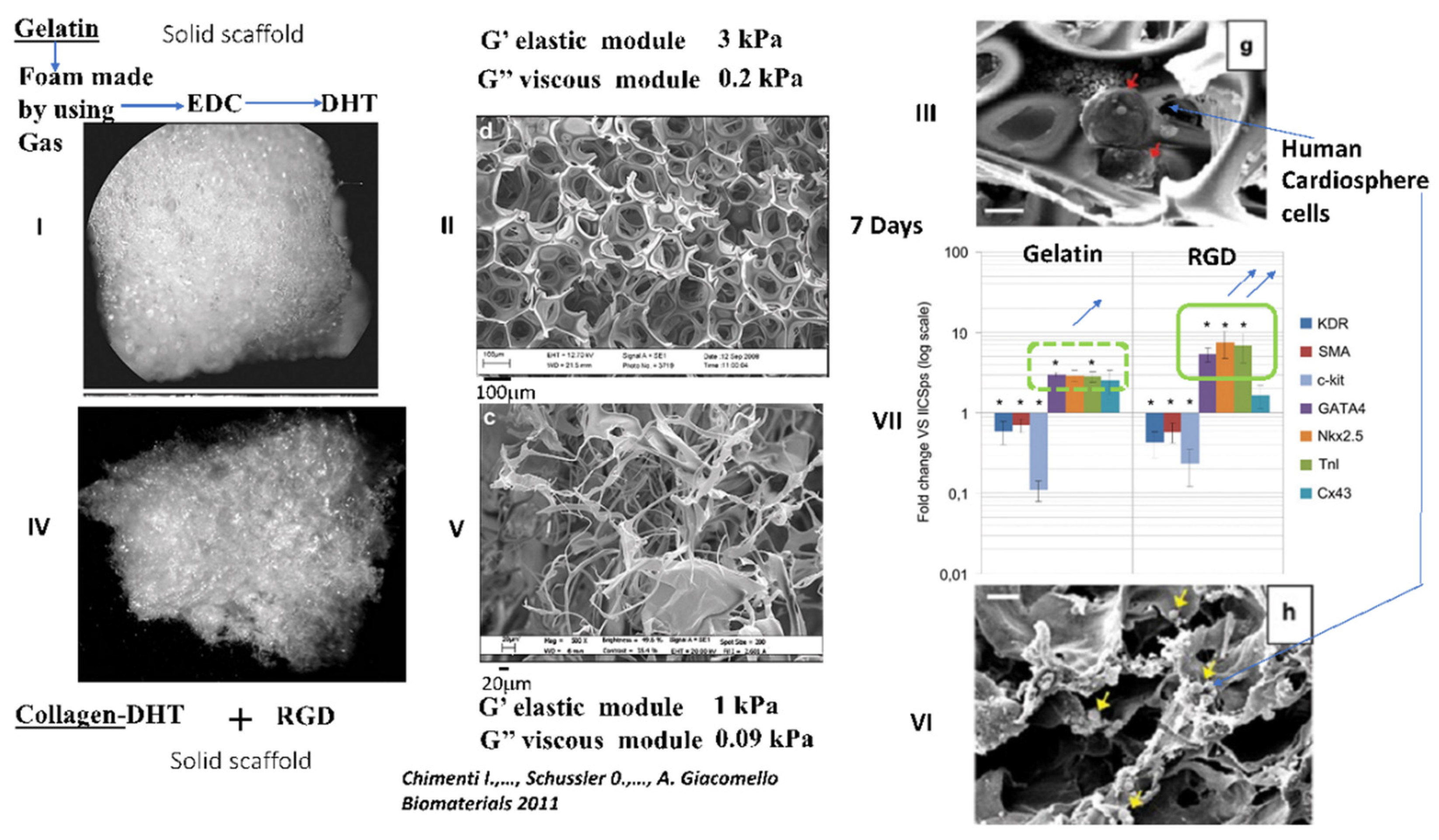
4.2.4. Interesting Long-Term Contractility and Thick Tissue Obtained In Vitro in Solid Porous DHT Collagen Scaffolds
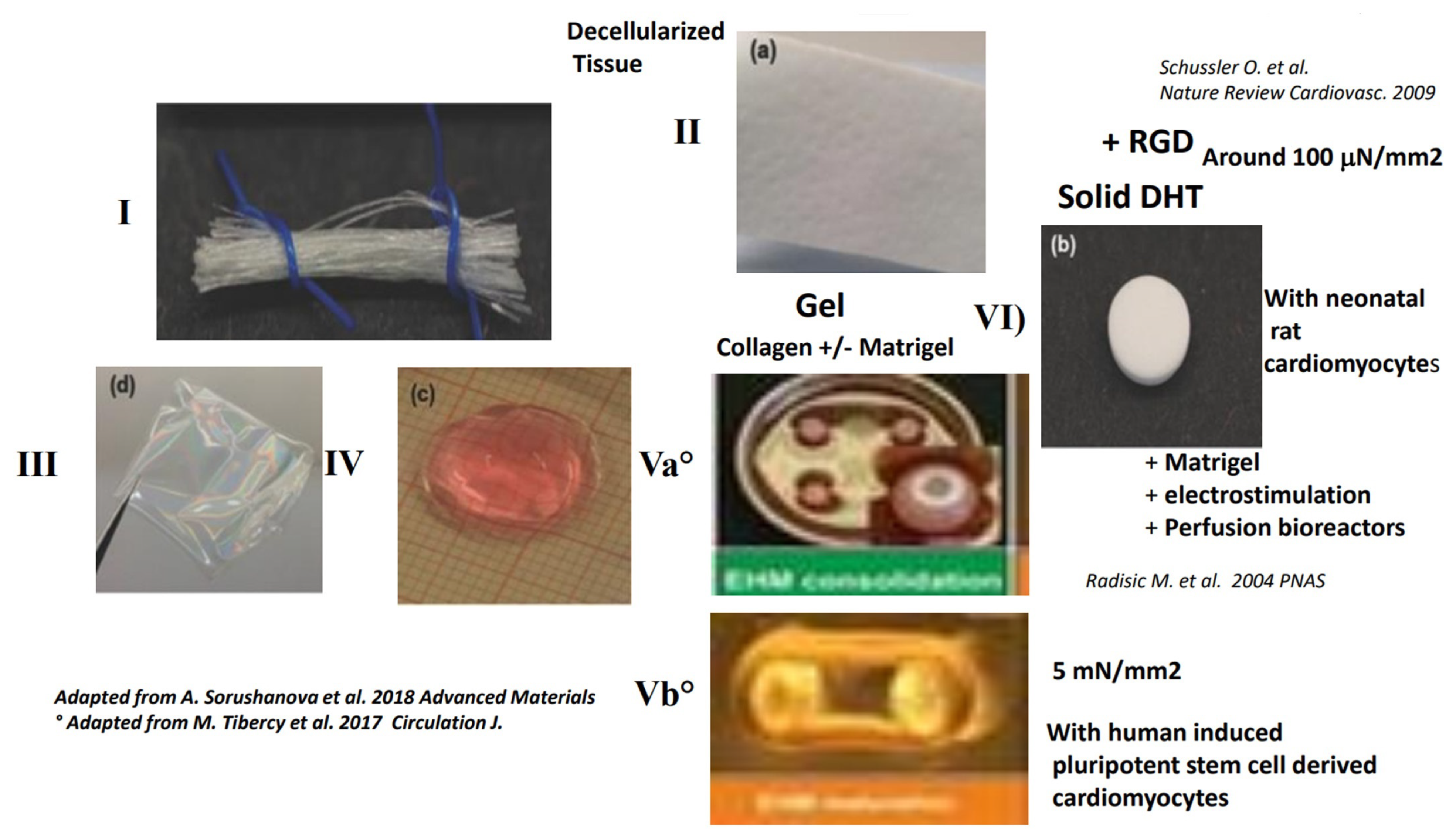
4.3. Contractility with Decellularized Tissue Containing Collagen
4.3.1. Classical Methods for Obtaining a Decellularized Matrix Tissue and the Limits of This Matrix
4.3.2. True Contractility with Shortening and Developed Forces Have Been Demonstrated Only on Thin Sections of Decellularized Tissue
5. Possible Engineering of a Large Contractile Patch with Human Cells (hESC-CM) in Gels of Matrigel™, Fibrin or Collagen
6. Rationale for Using Collagen or Gelatin as a Polymer Backbone for Tissue Engineering 3D Scaffolds and the Limits of Hydrogels and Gels
6.1. Rationale for Using Collagen Type I or III as a Polymer Backbone
6.2. Limits of Gelatin as a Natural Polymer Backbone
6.3. Structural Limits of Collagen or Gelatin Hydrogels and Gels for Contractile Tissue Engineering and the Necessity for Reinforcement by Reticulation or Association with Structural Polymers
6.3.1. Limits of Contractility Reported in Hydrogels of Gelatin or Collagen
6.3.2. Limits of Contractility Reported in Gels of Gelatin or Collagen
7. Rationale for Having a Solid 3D Scaffold Obtained by Physical Reticulation Instead of Chemical Reticulation
8. Functionalization of Biomaterials with Peptides Such as RGD
9. Functionalization of Solid Collagen Scaffolds with the RGD Peptide
10. Positive Effects of RGD on Most Biomaterials for Limiting MI Size and for Heart Cell Therapy
11. Possible Use of an Empty Solid Collagen Sponge and Application to an Infarct Area without a Cellular Component, but with Growth Factors or Exosome
11.1. Possible Use of a Solid, Empty Reconstituted Collagen Scaffold Alone
11.2. Possible Use of a Collagen Patch with Growth Factors or Exosomes Containing Growth Factors
11.2.1. Reconstituted Solid DHT Collagen Associated with Periostin
11.2.2. Association of Solid Collagen Sponge “Ultrafoam™” with VEGF or Angiopoetin1
11.2.3. Association of Collagen Patch with FSLT1 (Follistatin –Like1)
11.2.4. Association of Collagen Patch with Exosome
12. Possible Use of a Solid Collagen Scaffold for Epicardial Cell Delivery in the Context of MI
13. Current Challenges for Cellular Therapy after MI and the Possible Use of Collagen Scaffolds Functionalized with the RGD Peptide for the Delivery of Paracrine Human Mscs or Human Cardiopshere Cells
14. Future Directions and Conclusions
Author Contributions
Funding
Institutional review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wilmot, K.A.; O’Flaherty, M.; Capewell, S.; Ford, E.S.; Vaccarino, V. Coronary Heart Disease Mortality Declines in the United States From 1979 Through 2011: Evidence for Stagnation in Young Adults, Especially Women. Circulation 2015, 132, 997–1002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bergmann, O.; Bhardwaj, R.D.; Bernard, S.; Zdunek, S.; Barnabe-Heider, F.; Walsh, S.; Zupicich, J.; Alkass, K.; Buchholz, B.A.; Druid, H.; et al. Evidence for cardiomyocyte renewal in humans. Science 2009, 324, 98–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frangogiannis, N.G. The extracellular matrix in myocardial injury, repair, and remodeling. J. Clin. Investig. 2017, 127, 1600–1612. [Google Scholar] [CrossRef] [Green Version]
- Prabhu, S.D.; Frangogiannis, N.G. The Biological Basis for Cardiac Repair After Myocardial Infarction: From Inflammation to Fibrosis. Circ. Res. 2016, 119, 91–112. [Google Scholar] [CrossRef]
- Ryan, T.D.; Rothstein, E.C.; Aban, I.; Tallaj, J.A.; Husain, A.; Lucchesi, P.A.; Dell’Italia, L.J. Left ventricular eccentric remodeling and matrix loss are mediated by bradykinin and precede cardiomyocyte elongation in rats with volume overload. J. Am. Coll. Cardiol. 2007, 49, 811–821. [Google Scholar] [CrossRef] [Green Version]
- Bruggink, A.H.; van Oosterhout, M.F.; de Jonge, N.; Cleutjens, J.P.; van Wichen, D.F.; van Kuik, J.; Tilanus, M.G.; Gmelig-Meyling, F.H.; van den Tweel, J.G.; de Weger, R.A. Type IV collagen degradation in the myocardial basement membrane after unloading of the failing heart by a left ventricular assist device. Lab. Investig. 2007, 87, 1125–1137. [Google Scholar] [CrossRef] [PubMed]
- Yanamandala, M.; Zhu, W.; Garry, D.J.; Kamp, T.J.; Hare, J.M.; Jun, H.W.; Yoon, Y.S.; Bursac, N.; Prabhu, S.D.; Dorn, G.W., 2nd; et al. Overcoming the Roadblocks to Cardiac Cell Therapy Using Tissue Engineering. J. Am. Coll. Cardiol. 2017, 70, 766–775. [Google Scholar] [CrossRef] [PubMed]
- Menasche, P.; Vanneaux, V.; Fabreguettes, J.R.; Bel, A.; Tosca, L.; Garcia, S.; Bellamy, V.; Farouz, Y.; Pouly, J.; Damour, O.; et al. Towards a clinical use of human embryonic stem cell-derived cardiac progenitors: A translational experience. Eur. Heart J. 2014, 36, 743–750. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, P.K.; Rhee, J.W.; Wu, J.C. Adult Stem Cell Therapy and Heart Failure, 2000 to 2016: A Systematic Review. JAMA Cardiol. 2016, 1, 831–841. [Google Scholar] [CrossRef] [Green Version]
- Blackburn, N.J.; Sofrenovic, T.; Kuraitis, D.; Ahmadi, A.; McNeill, B.; Deng, C.; Rayner, K.J.; Zhong, Z.; Ruel, M.; Suuronen, E.J. Timing underpins the benefits associated with injectable collagen biomaterial therapy for the treatment of myocardial infarction. Biomaterials 2014, 39, 182–192. [Google Scholar] [CrossRef]
- Liu, J.; Narsinh, K.H.; Lan, F.; Wang, L.; Nguyen, P.K.; Hu, S.; Lee, A.; Han, L.; Gong, Y.; Huang, M.; et al. Early stem cell engraftment predicts late cardiac functional recovery: Preclinical insights from molecular imaging. Circ. Cardiovasc. Imaging 2012, 5, 481–490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bachmann, M.; Kukkurainen, S.; Hytonen, V.P.; Wehrle-Haller, B. Cell Adhesion by Integrins. Physiol. Rev. 2019, 99, 1655–1699. [Google Scholar] [CrossRef]
- Patil, V.A.; Masters, K.S. Engineered Collagen Matrices. Bioengineering 2020, 7, 163. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Hoop, C.L.; Case, D.A.; Baum, J. Cryptic binding sites become accessible through surface reconstruction of the type I collagen fibril. Sci. Rep. 2018, 8, 16646. [Google Scholar] [CrossRef]
- Bello, A.B.; Kim, D.; Park, H.; Lee, S.H. Engineering and Functionalization of Gelatin Biomaterials: From Cell Culture to Medical Applications. Tissue Eng. Part. B Rev. 2020, 26, 164–180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sorushanova, A.; Delgado, L.M.; Wu, Z.; Shologu, N.; Kshirsagar, A.; Raghunath, R.; Mullen, A.M.; Bayon, Y.; Pandit, A.; Raghunath, M.; et al. The Collagen Suprafamily: From Biosynthesis to Advanced Biomaterial Development. Adv. Mater. 2018, 31, e1801651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, H.; Borg, T.K.; Liu, H.; Gao, B.Z. Interactive relationship between basement-membrane development and sarcomerogenesis in single cardiomyocytes. Exp. Cell Res. 2014, 330, 222–232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manso, A.M.; Kang, S.M.; Ross, R.S. Integrins, focal adhesions, and cardiac fibroblasts. J. Investig. Med. 2009, 57, 856–860. [Google Scholar] [CrossRef]
- Burgess, M.L.; Terracio, L.; Hirozane, T.; Borg, T.K. Differential integrin expression by cardiac fibroblasts from hypertensive and exercise-trained rat hearts. Cardiovasc. Pathol. 2002, 11, 78–87. [Google Scholar] [CrossRef]
- Higuchi, T.; Bengel, F.M.; Seidl, S.; Watzlowik, P.; Kessler, H.; Hegenloh, R.; Reder, S.; Nekolla, S.G.; Wester, H.J.; Schwaiger, M. Assessment of alphavbeta3 integrin expression after myocardial infarction by positron emission tomography. Cardiovasc. Res. 2008, 78, 395–403. [Google Scholar] [CrossRef] [Green Version]
- Konstandin, M.H.; Toko, H.; Gastelum, G.M.; Quijada, P.; De La Torre, A.; Quintana, M.; Collins, B.; Din, S.; Avitabile, D.; Volkers, M.; et al. Fibronectin is essential for reparative cardiac progenitor cell response after myocardial infarction. Circ. Res. 2013, 113, 115–125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, M.; Opavsky, M.A.; Stewart, D.J.; Rabinovitch, M.; Dawood, F.; Wen, W.H.; Liu, P.P. Temporal response and localization of integrins beta1 and beta3 in the heart after myocardial infarction: Regulation by cytokines. Circulation 2003, 107, 1046–1052. [Google Scholar] [CrossRef] [Green Version]
- Schussler, O.; Chachques, J.C.; Alifano, M.; Lecarpentier, Y. Key Roles RGD-recognizing integrins During Cardiac Development, on Cardiac Cells and after Myocardial Infarction. J. Cardiovasc. Transl. Res. 2021, in press. [Google Scholar] [CrossRef]
- Van Wijk, B.; Gunst, Q.D.; Moorman, A.F.; van den Hoff, M.J. Cardiac regeneration from activated epicardium. PLoS ONE 2012, 7, e44692. [Google Scholar] [CrossRef] [Green Version]
- Pomeroy, J.E.; Helfer, A.; Bursac, N. Biomaterializing the promise of cardiac tissue engineering. Biotechnol. Adv. 2019, 42, 107353. [Google Scholar] [CrossRef]
- Jang, Y.; Park, Y.; Kim, K. Engineering biomaterials to guide heart cells for matured cardiac tissue. Coatings 2020, 10, 925. [Google Scholar] [CrossRef]
- Nguyen, A.H.; Marsh, P.; Schmiess-Heine, L.; Burke, P.J.; Lee, A.; Lee, J.; Cao, H. Cardiac tissue engineering: State-of-the-art methods and outlook. J. Biol. Eng. 2019, 13, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Zhu, W.; Radisic, M.; Vunjak-Novakovic, G. Can We Engineer a Human Cardiac Patch for Therapy? Circ. Res. 2018, 123, 244–265. [Google Scholar] [CrossRef] [PubMed]
- Schussler, O.; Chachques, J.C.; Mesana, T.G.; Suuronen, E.J.; Lecarpentier, Y.; Ruel, M. 3-dimensional structures to enhance cell therapy and engineer contractile tissue. Asian Cardiovasc. Thorac. Ann. 2010, 18, 188–198. [Google Scholar] [CrossRef]
- Gao, L.; Gregorich, Z.R.; Zhu, W.; Mattapally, S.; Oduk, Y.; Lou, X.; Kannappan, R.; Borovjagin, A.V.; Walcott, G.P.; Pollard, A.E.; et al. Large Cardiac Muscle Patches Engineered From Human Induced-Pluripotent Stem Cell-Derived Cardiac Cells Improve Recovery From Myocardial Infarction in Swine. Circulation 2017, 137, 1712–1730. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Chang, Y.H.; Xiong, Q.; Zhang, P.; Zhang, L.; Somasundaram, P.; Lepley, M.; Swingen, C.; Su, L.; Wendel, J.S.; et al. Cardiac repair in a porcine model of acute myocardial infarction with human induced pluripotent stem cell-derived cardiovascular cells. Cell Stem Cell 2014, 15, 750–761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmadi, A.; McNeill, B.; Vulesevic, B.; Kordos, M.; Mesana, L.; Thorn, S.; Renaud, J.M.; Manthorp, E.; Kuraitis, D.; Toeg, H.; et al. The role of integrin alpha2 in cell and matrix therapy that improves perfusion, viability and function of infarcted myocardium. Biomaterials 2014, 35, 4749–4758. [Google Scholar] [CrossRef]
- Liu, S.; Jiang, Z.; Qiao, L.; Guo, B.; Xiao, W.; Zhang, X.; Chang, L.; Li, Y. Integrin beta-3 is required for the attachment, retention and therapeutic benefits of human cardiospheres in myocardial infarction. J. Cell. Mol. Med. 2017, 22, 382–389. [Google Scholar] [CrossRef] [Green Version]
- Desai, V.D.; Hsia, H.C.; Schwarzbauer, J.E. Reversible modulation of myofibroblast differentiation in adipose-derived mesenchymal stem cells. PLoS ONE 2014, 9, e86865. [Google Scholar] [CrossRef] [Green Version]
- Reis, L.A.; Chiu, L.L.; Feric, N.; Fu, L.; Radisic, M. Biomaterials in myocardial tissue engineering. J. Tissue Eng. Eng. Med. 2014, 10, 11–28. [Google Scholar] [CrossRef] [Green Version]
- Feric, N.T.; Radisic, M. Strategies and Challenges to Myocardial Replacement Therapy. Stem Cells Transl. Med. 2016, 5, 410–416. [Google Scholar] [CrossRef]
- Matsuo, T.; Masumoto, H.; Tajima, S.; Ikuno, T.; Katayama, S.; Minakata, K.; Ikeda, T.; Yamamizu, K.; Tabata, Y.; Sakata, R.; et al. Efficient long-term survival of cell grafts after myocardial infarction with thick viable cardiac tissue entirely from pluripotent stem cells. Sci. Rep. 2015, 5, 16842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jackman, C.P.; Shadrin, I.Y.; Carlson, A.L.; Bursac, N. Human Cardiac Tissue Engineering: From Pluripotent Stem Cells to Heart Repair. Curr. Opin. Chem. Eng. 2015, 7, 57–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruvinov, E.; Cohen, S. Alginate biomaterial for the treatment of myocardial infarction: Progress, translational strategies, and clinical outlook: From ocean algae to patient bedside. Adv. Drug Deliv. Rev. 2015, 96, 54–76. [Google Scholar] [CrossRef]
- Weinberger, F.; Mannhardt, I.; Eschenhagen, T. Engineering Cardiac Muscle Tissue: A Maturating Field of Research. Circ. Res. 2017, 120, 1487–1500. [Google Scholar] [CrossRef] [PubMed]
- Radisic, M.; Park, H.; Shing, H.; Consi, T.; Schoen, F.J.; Langer, R.; Freed, L.E.; Vunjak-Novakovic, G. Functional assembly of engineered myocardium by electrical stimulation of cardiac myocytes cultured on scaffolds. Proc. Natl. Acad. Sci. USA 2004, 101, 18129–18134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Radisic, M.; Malda, J.; Epping, E.; Geng, W.; Langer, R.; Vunjak-Novakovic, G. Oxygen gradients correlate with cell density and cell viability in engineered cardiac tissue. Biotechnol. Bioeng. 2006, 93, 332–343. [Google Scholar] [CrossRef] [PubMed]
- Radisic, M.; Deen, W.; Langer, R.; Vunjak-Novakovic, G. Mathematical model of oxygen distribution in engineered cardiac tissue with parallel channel array perfused with culture medium containing oxygen carriers. Am. J. Physiol. Heart Circ. Physiol. 2005, 288, H1278–H1289. [Google Scholar] [CrossRef]
- Wallace, D.G.; Rosenblatt, J. Collagen gel systems for sustained delivery and tissue engineering. Adv. Drug Deliv. Rev. 2003, 55, 1631–1649. [Google Scholar] [CrossRef]
- Cortes-Morichetti, M.; Frati, G.; Schussler, O.; Duong Van Huyen, J.P.; Lauret, E.; Genovese, J.A.; Carpentier, A.F.; Chachques, J.C. Association between a cell-seeded collagen matrix and cellular cardiomyoplasty for myocardial support and regeneration. Tissue Eng. 2007, 13, 2681–2687. [Google Scholar] [CrossRef]
- Chachques, J.C.; Trainini, J.C.; Lago, N.; Cortes-Morichetti, M.; Schussler, O.; Carpentier, A. Myocardial Assistance by Grafting a New Bioartificial Upgraded Myocardium (MAGNUM trial): Clinical feasibility study. Ann. Thorac. Surg. 2008, 85, 901–908. [Google Scholar] [CrossRef] [PubMed]
- Chachques, J.C.; Trainini, J.C.; Lago, N.; Masoli, O.H.; Barisani, J.L.; Cortes-Morichetti, M.; Schussler, O.; Carpentier, A. Myocardial assistance by grafting a new bioartificial upgraded myocardium (MAGNUM clinical trial): One year follow-up. Cell Transplant. 2007, 16, 927–934. [Google Scholar] [CrossRef]
- Schussler, O.; Coirault, C.; Louis-Tisserand, M.; Al-Chare, W.; Oliviero, P.; Menard, C.; Michelot, R.; Bochet, P.; Salomon, D.R.; Chachques, J.C.; et al. Use of arginine-glycine-aspartic acid adhesion peptides coupled with a new collagen scaffold to engineer a myocardium-like tissue graft. Nat. Clin. Pract. Cardiovasc. Med. 2009, 6, 240–249. [Google Scholar] [CrossRef]
- Radisic, M.; Marsano, A.; Maidhof, R.; Wang, Y.; Vunjak-Novakovic, G. Cardiac tissue engineering using perfusion bioreactor systems. Nat. Protoc. 2008, 3, 719–738. [Google Scholar] [CrossRef] [Green Version]
- Paez-Mayorga, J.; Hernandez-Vargas, G.; Ruiz-Esparza, G.U.; Iqbal, H.M.N.; Wang, X.; Zhang, Y.S.; Parra-Saldivar, R.; Khademhosseini, A. Bioreactors for Cardiac Tissue Engineering. Adv. Healthc. Mater. 2018, 8, e1701504. [Google Scholar] [CrossRef]
- Lecarpentier, Y.; Schussler, O.; Sakic, A.; Rincon-Garriz, J.M.; Soulie, P.; Bochaton-Piallat, M.L.; Kindler, V. Human Bone Marrow Contains Mesenchymal Stromal Stem Cells That Differentiate In Vitro into Contractile Myofibroblasts Controlling T Lymphocyte Proliferation. Stem. Cells Int. 2018, 2018, 6134787. [Google Scholar] [CrossRef] [Green Version]
- Lecarpentier, Y.; Kindler, V.; Bochaton-Piallat, M.L.; Sakic, A.; Claes, V.; Hebert, J.L.; Vallee, A.; Schussler, O. Tripeptide Arg-Gly-Asp (RGD) modifies the molecular mechanical properties of the non-muscle myosin IIA in human bone marrow-derived myofibroblasts seeded in a collagen scaffold. PLoS ONE 2019, 14, e0222683. [Google Scholar] [CrossRef]
- Lecarpentier, Y.; Kindler, V.; Krokidis, X.; Bochaton-Piallat, M.L.; Claes, V.; Hebert, J.L.; Vallee, A.; Schussler, O. Statistical Mechanics of Non-Muscle Myosin IIA in Human Bone Marrow-Derived Mesenchymal Stromal Cells Seeded in a Collagen Scaffold: A Thermodynamic Near-Equilibrium Linear System Modified by the Tripeptide Arg-Gly-Asp (RGD). Cells 2020, 9, 1510. [Google Scholar] [CrossRef] [PubMed]
- Rashedi, I.; Talele, N.; Wang, X.H.; Hinz, B.; Radisic, M.; Keating, A. Collagen scaffold enhances the regenerative properties of mesenchymal stromal cells. PLoS ONE 2017, 12, e0187348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaetani, R.; Zizzi, E.A.; Deriu, M.A.; Morbiducci, U.; Pesce, M.; Messina, E. When Stiffness Matters: Mechanosensing in Heart Development and Disease. Front. Cell Dev. Biol. 2020, 8, 334. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, O.; Cooper-White, J.; Janmey, P.A.; Mooney, D.J.; Shenoy, V.B. Effects of extracellular matrix viscoelasticity on cellular behaviour. Nature 2020, 584, 535–546. [Google Scholar] [CrossRef]
- Darnell, M.; O’Neil, A.; Mao, A.; Gu, L.; Rubin, L.L.; Mooney, D.J. Material microenvironmental properties couple to induce distinct transcriptional programs in mammalian stem cells. Proc. Natl. Acad. Sci. USA 2018, 115, E8368–E8377. [Google Scholar] [CrossRef] [Green Version]
- Seo, B.R.; Chen, X.; Ling, L.; Song, Y.H.; Shimpi, A.A.; Choi, S.; Gonzalez, J.; Sapudom, J.; Wang, K.; Andresen Eguiluz, R.C.; et al. Collagen microarchitecture mechanically controls myofibroblast differentiation. Proc. Natl. Acad. Sci. USA 2020, 117, 11387–11398. [Google Scholar] [CrossRef]
- Carson, D.; Hnilova, M.; Yang, X.; Nemeth, C.L.; Tsui, J.H.; Smith, A.S.; Jiao, A.; Regnier, M.; Murry, C.E.; Tamerler, C.; et al. Nanotopography-Induced Structural Anisotropy and Sarcomere Development in Human Cardiomyocytes Derived from Induced Pluripotent Stem Cells. ACS Appl. Mater. Interfaces 2016, 8, 21923–21932. [Google Scholar] [CrossRef] [Green Version]
- Nicolas, J.; Magli, S.; Rabbachin, L.; Sampaolesi, S.; Nicotra, F.; Russo, L. 3D Extracellular Matrix Mimics: Fundamental Concepts and Role of Materials Chemistry to Influence Stem Cell Fate. Biomacromolecules 2020, 21, 1968–1994. [Google Scholar] [CrossRef]
- Hersel, U.; Dahmen, C.; Kessler, H. RGD modified polymers: Biomaterials for stimulated cell adhesion and beyond. Biomaterials 2003, 24, 4385–4415. [Google Scholar] [CrossRef]
- Kapp, T.G.; Rechenmacher, F.; Neubauer, S.; Maltsev, O.V.; Cavalcanti-Adam, E.A.; Zarka, R.; Reuning, U.; Notni, J.; Wester, H.J.; Mas-Moruno, C.; et al. Comprehensive Evaluation of the Activity and Selectivity Profile of Ligands for RGD-binding Integrins. Sci. Rep. 2017, 7, 39805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spicer, C.D.; Pashuck, E.T.; Stevens, M.M. Achieving Controlled Biomolecule-Biomaterial Conjugation. Chem. Rev. 2018, 118, 7702–7743. [Google Scholar] [CrossRef]
- Israeli-Rosenberg, S.; Manso, A.M.; Okada, H.; Ross, R.S. Integrins and integrin-associated proteins in the cardiac myocyte. Circ. Res. 2014, 114, 572–586. [Google Scholar] [CrossRef] [Green Version]
- Dhavalikar, P.; Robinson, A.; Lan, Z.; Jenkins, D.; Chwatko, M.; Salhadar, K.; Jose, A.; Kar, R.; Shoga, E.; Kannapiran, A.; et al. Review of Integrin-Targeting Biomaterials in Tissue Engineering. Adv. Healthc. Mater. 2020, 9, e2000795. [Google Scholar] [CrossRef]
- Engelmayr, G.C., Jr.; Cheng, M.; Bettinger, C.J.; Borenstein, J.T.; Langer, R.; Freed, L.E. Accordion-like honeycombs for tissue engineering of cardiac anisotropy. Nat. Mater. 2008, 7, 1003–1010. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Lee, S.J.; Cheng, H.J.; Yoo, J.J.; Atala, A. 3D bioprinted functional and contractile cardiac tissue constructs. Acta Biomater. 2018, 70, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Miller, K.; Ma, X.; Dewan, S.; Lawrence, N.; Whang, G.; Chung, P.; McCulloch, A.D.; Chen, S. Direct 3D bioprinting of cardiac micro-tissues mimicking native myocardium. Biomaterials 2020, 256, 120204. [Google Scholar] [CrossRef]
- Chimenti, I.; Massai, D.; Morbiducci, U.; Beltrami, A.P.; Pesce, M.; Messina, E. Stem Cell Spheroids and Ex Vivo Niche Modeling: Rationalization and Scaling-Up. J. Cardiovasc. Transl. Res. 2017, 10, 150–166. [Google Scholar] [CrossRef]
- Ashur, C.; Frishman, W.H. Cardiosphere-Derived Cells and Ischemic Heart Failure. Cardiol. Rev. 2017, 26, 8–21. [Google Scholar] [CrossRef]
- Chakravarty, T.; Henry, T.D.; Kittleson, M.; Lima, J.; Siegel, R.J.; Slipczuk, L.; Pogoda, J.M.; Smith, R.R.; Malliaras, K.; Marban, L.; et al. Allogeneic cardiosphere-derived cells for the treatment of heart failure with reduced ejection fraction: The Dilated cardiomYopathy iNtervention with Allogeneic MyocardIally-regenerative Cells (DYNAMIC) trial. EuroIntervention 2020, 16, e293–e300. [Google Scholar] [CrossRef] [PubMed]
- Zuppinger, C. Measurement of Contractility and Calcium Release in Cardiac Spheroids. Methods Mol. Biol. 2019, 1929, 41–52. [Google Scholar]
- Smith, R.R.; Marban, E.; Marban, L. Enhancing retention and efficacy of cardiosphere-derived cells administered after myocardial infarction using a hyaluronan-gelatin hydrogel. Biomatter 2013, 3, e24490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sasaki, D.; Matsuura, K.; Seta, H.; Haraguchi, Y.; Okano, T.; Shimizu, T. Contractile force measurement of human induced pluripotent stem cell-derived cardiac cell sheet-tissue. PLoS ONE 2018, 13, e0198026. [Google Scholar] [CrossRef]
- Takahashi, H.; Okano, T. Thermally-triggered fabrication of cell sheets for tissue engineering and regenerative medicine. Adv. Drug Deliv. Rev. 2019, 138, 276–292. [Google Scholar] [CrossRef] [PubMed]
- Tong, Z.; Jin, L.; Oliveira, J.M.; Reis, R.L.; Zhong, Q.; Mao, Z.; Gao, C. Adaptable hydrogel with reversible linkages for regenerative medicine: Dynamic mechanical microenvironment for cells. Bioact. Mater. 2021, 6, 1375–1387. [Google Scholar] [CrossRef]
- Husseini, G.A.; Mjalli, F.S.; Pitt, W.G.; Abdel-Jabbar, N. Using artificial neural networks and model predictive control to optimize acoustically assisted Doxorubicin release from polymeric micelles. Technol. Cancer Res. Treat. 2009, 8, 479–488. [Google Scholar] [CrossRef]
- Hapach, L.A.; VanderBurgh, J.A.; Miller, J.P.; Reinhart-King, C.A. Manipulation of in vitro collagen matrix architecture for scaffolds of improved physiological relevance. Phys. Biol. 2015, 12, 061002. [Google Scholar] [CrossRef]
- Tiburcy, M.; Hudson, J.E.; Balfanz, P.; Schlick, S.; Meyer, T.; Chang Liao, M.L.; Levent, E.; Raad, F.; Zeidler, S.; Wingender, E.; et al. Defined Engineered Human Myocardium With Advanced Maturation for Applications in Heart Failure Modeling and Repair. Circulation 2017, 135, 1832–1847. [Google Scholar] [CrossRef]
- Afewerki, S.; Sheikhi, A.; Kannan, S.; Ahadian, S.; Khademhosseini, A. Gelatin-polysaccharide composite scaffolds for 3D cell culture and tissue engineering: Towards natural therapeutics. Bioeng. Transl. Med. 2018, 4, 96–115. [Google Scholar] [CrossRef] [PubMed]
- Andriakopoulou, C.E.; Zadpoor, A.A.; Grant, M.H.; Riches, P.E. Development and mechanical characterisation of self-compressed collagen gels. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 84, 243–247. [Google Scholar] [CrossRef] [Green Version]
- Chai, Q.; Jiao, Y.; Yu, X. Hydrogels for Biomedical Applications: Their Characteristics and the Mechanisms behind Them. Gels 2017, 3, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Huang, Y. Rational Design of Smart Hydrogels for Biomedical Applications. Front. Chem. 2021, 8, 615665. [Google Scholar] [CrossRef]
- Pena, B.; Laughter, M.; Jett, S.; Rowland, T.J.; Taylor, M.R.G.; Mestroni, L.; Park, D. Injectable Hydrogels for Cardiac Tissue Engineering. Macromol. Biosci. 2018, 18, e1800079. [Google Scholar] [CrossRef]
- Wang, L.L.; Liu, Y.; Chung, J.J.; Wang, T.; Gaffey, A.C.; Lu, M.; Cavanaugh, C.A.; Zhou, S.; Kanade, R.; Atluri, P.; et al. Local and sustained miRNA delivery from an injectable hydrogel promotes cardiomyocyte proliferation and functional regeneration after ischemic injury. Nat. Biomed. Eng. 2018, 1, 983–992. [Google Scholar] [CrossRef] [Green Version]
- Rogovina, L.Z.; Vasiliev, V.G.; Braudo, E.E. Definition of the concept of polymer gel. Polym. Sci. Ser. C 2008, 50, 85–92. [Google Scholar] [CrossRef]
- Zhao, Y.; Rafatian, N.; Wang, E.Y.; Feric, N.T.; Lai, B.F.L.; Knee-Walden, E.J.; Backx, P.H.; Radisic, M. Engineering microenvironment for human cardiac tissue assembly in heart-on-a-chip platform. Matrix Biol. 2019, 85, 189–204. [Google Scholar] [CrossRef] [PubMed]
- Engler, A.J.; Sen, S.; Sweeney, H.L.; Discher, D.E. Matrix elasticity directs stem cell lineage specification. Cell 2006, 126, 677–689. [Google Scholar] [CrossRef] [Green Version]
- Heras-Bautista, C.O.; Mikhael, N.; Lam, J.; Shinde, V.; Katsen-Globa, A.; Dieluweit, S.; Molcanyi, M.; Uvarov, V.; Jutten, P.; Sahito, R.G.A.; et al. Cardiomyocytes facing fibrotic conditions re-express extracellular matrix transcripts. Acta Biomater. 2019, 89, 180–192. [Google Scholar] [CrossRef]
- Dugan, J.M.; Collins, R.F.; Gough, J.E.; Eichhorn, S.J. Oriented surfaces of adsorbed cellulose nanowhiskers promote skeletal muscle myogenesis. Acta Biomater. 2012, 9, 4707–4715. [Google Scholar] [CrossRef]
- Luo, H.; Cha, R.; Li, J.; Hao, W.; Zhang, Y.; Zhou, F. Advances in tissue engineering of nanocellulose-based scaffolds: A review. Carbohydr. Polym. 2019, 224, 115144. [Google Scholar] [CrossRef]
- Leon-Lopez, A.; Morales-Penaloza, A.; Martinez-Juarez, V.M.; Vargas-Torres, A.; Zeugolis, D.I.; Aguirre-Alvarez, G. Hydrolyzed Collagen-Sources and Applications. Molecules 2019, 24, 4031. [Google Scholar] [CrossRef] [Green Version]
- Nakada, A.; Shigeno, K.; Sato, T.; Kobayashi, T.; Wakatsuki, M.; Uji, M.; Nakamura, T. Manufacture of a weakly denatured collagen fiber scaffold with excellent biocompatibility and space maintenance ability. Biomed. Mater. 2013, 8, 045010. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and Electrospun Nanofibers: Methods, Materials, and Applications. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef] [PubMed]
- Gotti, C.; Sensini, A.; Fornaia, G.; Gualandi, C.; Zucchelli, A.; Focarete, M.L. Biomimetic Hierarchically Arranged Nanofibrous Structures Resembling the Architecture and the Passive Mechanical Properties of Skeletal Muscles: A Step Forward toward Artificial Muscle. Front. Bioeng. Biotechnol. 2020, 8, 767. [Google Scholar] [CrossRef] [PubMed]
- Fereshteh, Z.; Fathi, M.; Bagri, A.; Boccaccini, A.R. Preparation and characterization of aligned porous PCL/zein scaffolds as drug delivery systems via improved unidirectional freeze-drying method. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 68, 613–622. [Google Scholar] [CrossRef] [PubMed]
- Grenier, J.; Duval, H.; Barou, F.; Lv, P.; David, B.; Letourneur, D. Mechanisms of pore formation in hydrogel scaffolds textured by freeze-drying. Acta Biomater. 2019, 94, 195–203. [Google Scholar] [CrossRef]
- Happe, C.L.; Engler, A.J. Mechanical Forces Reshape Differentiation Cues That Guide Cardiomyogenesis. Circ. Res. 2016, 118, 296–310. [Google Scholar] [CrossRef] [Green Version]
- Van Putten, S.; Shafieyan, Y.; Hinz, B. Mechanical control of cardiac myofibroblasts. J. Mol. Cell. Cardiol. 2015, 93, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Davidenko, N.; Schuster, C.F.; Bax, D.V.; Farndale, R.W.; Hamaia, S.; Best, S.M.; Cameron, R.E. Evaluation of cell binding to collagen and gelatin: A study of the effect of 2D and 3D architecture and surface chemistry. J. Mater. Sci. Mater. Med. 2016, 27, 148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malcor, J.D.; Bax, D.; Hamaia, S.W.; Davidenko, N.; Best, S.M.; Cameron, R.E.; Farndale, R.W.; Bihan, D. The synthesis and coupling of photoreactive collagen-based peptides to restore integrin reactivity to an inert substrate, chemically-crosslinked collagen. Biomaterials 2016, 85, 65–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirt, M.N.; Hansen, A.; Eschenhagen, T. Cardiac tissue engineering: State of the art. Circ. Res. 2014, 114, 354–367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, H.; Radisic, M.; Lim, J.O.; Chang, B.H.; Vunjak-Novakovic, G. A novel composite scaffold for cardiac tissue engineering. Vitr. Cell. Dev. Biol. Anim. 2005, 41, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.E.; Motion, J.P.; Narmoneva, D.A.; Takahashi, T.; Hakuno, D.; Kamm, R.D.; Zhang, S.; Lee, R.T. Injectable self-assembling peptide nanofibers create intramyocardial microenvironments for endothelial cells. Circulation 2005, 111, 442–450. [Google Scholar] [CrossRef] [Green Version]
- Eschenhagen, T.; Eder, A.; Vollert, I.; Hansen, A. Physiological aspects of cardiac tissue engineering. Am. J. Physiol. Heart Circ. Physiol. 2012, 303, H133–H143. [Google Scholar] [CrossRef] [Green Version]
- Shadrin, I.Y.; Allen, B.W.; Qian, Y.; Jackman, C.P.; Carlson, A.L.; Juhas, M.E.; Bursac, N. Cardiopatch platform enables maturation and scale-up of human pluripotent stem cell-derived engineered heart tissues. Nat. Commun. 2017, 8, 1825. [Google Scholar] [CrossRef] [Green Version]
- Ronaldson-Bouchard, K.; Ma, S.P.; Yeager, K.; Chen, T.; Song, L.; Sirabella, D.; Morikawa, K.; Teles, D.; Yazawa, M.; Vunjak-Novakovic, G. Advanced maturation of human cardiac tissue grown from pluripotent stem cells. Nature 2018, 556, 239–243. [Google Scholar] [CrossRef]
- Riegler, J.; Tiburcy, M.; Ebert, A.; Tzatzalos, E.; Raaz, U.; Abilez, O.J.; Shen, Q.; Kooreman, N.G.; Neofytou, E.; Chen, V.C.; et al. Human Engineered Heart Muscles Engraft and Survive Long Term in a Rodent Myocardial Infarction Model. Circ. Res. 2015, 117, 720–730. [Google Scholar] [CrossRef] [Green Version]
- Chong, J.J.; Yang, X.; Don, C.W.; Minami, E.; Liu, Y.W.; Weyers, J.J.; Mahoney, W.M.; Van Biber, B.; Cook, S.M.; Palpant, N.J.; et al. Human embryonic-stem-cell-derived cardiomyocytes regenerate non-human primate hearts. Nature 2014, 510, 273–277. [Google Scholar] [CrossRef]
- Sapir, Y.; Kryukov, O.; Cohen, S. Integration of multiple cell-matrix interactions into alginate scaffolds for promoting cardiac tissue regeneration. Biomaterials 2010, 32, 1838–1847. [Google Scholar] [CrossRef]
- Shachar, M.; Tsur-Gang, O.; Dvir, T.; Leor, J.; Cohen, S. The effect of immobilized RGD peptide in alginate scaffolds on cardiac tissue engineering. Acta Biomater. 2010, 7, 152–162. [Google Scholar] [CrossRef]
- Bai, X.P.; Zheng, H.X.; Fang, R.; Wang, T.R.; Hou, X.L.; Li, Y.; Chen, X.B.; Tian, W.M. Fabrication of engineered heart tissue grafts from alginate/collagen barium composite microbeads. Biomed. Mater. 2011, 6, 045002. [Google Scholar] [CrossRef]
- Ceccaldi, C.; Bushkalova, R.; Alfarano, C.; Lairez, O.; Calise, D.; Bourin, P.; Frugier, C.; Rouzaud-Laborde, C.; Cussac, D.; Parini, A.; et al. Evaluation of polyelectrolyte complex-based scaffolds for mesenchymal stem cell therapy in cardiac ischemia treatment. Acta Biomater. 2014, 10, 901–911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meng, D.; Tanaka, H.; Kobayashi, T.; Hatayama, H.; Zhang, X.; Ura, K.; Yunoki, S.; Takagi, Y. The effect of alkaline pretreatment on the biochemical characteristics and fibril-forming abilities of types I and II collagen extracted from bester sturgeon by-products. Int. J. Biol. Macromol. 2019, 131, 572–580. [Google Scholar] [CrossRef]
- Busby, G.A.; Grant, M.H.; Mackay, S.P.; Riches, P.E. Confined compression of collagen hydrogels. J. Biomech. 2012, 46, 837–840. [Google Scholar] [CrossRef] [Green Version]
- Haugh, M.G.; Jaasma, M.J.; O’Brien, F.J. The effect of dehydrothermal treatment on the mechanical and structural properties of collagen-GAG scaffolds. J. Biomed. Mater. Res. A 2009, 89, 363–369. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, F.J.; Harley, B.A.; Yannas, I.V.; Gibson, L. Influence of freezing rate on pore structure in freeze-dried collagen-GAG scaffolds. Biomaterials 2004, 25, 1077–1086. [Google Scholar] [CrossRef]
- Nakada, A.; Shigeno, K.; Sato, T.; Hatayama, T.; Wakatsuki, M.; Nakamura, T. Optimal dehydrothermal processing conditions to improve biocompatibility and durability of a weakly denatured collagen scaffold. J. Biomed. Mater. Res. B Appl. Biomater. 2016, 105, 2301–2307. [Google Scholar] [CrossRef] [PubMed]
- Byrne, E.M.; Farrell, E.; McMahon, L.A.; Haugh, M.G.; O’Brien, F.J.; Campbell, V.A.; Prendergast, P.J.; O’Connell, B.C. Gene expression by marrow stromal cells in a porous collagen-glycosaminoglycan scaffold is affected by pore size and mechanical stimulation. J. Mater. Sci. Mater. Med. 2008, 19, 3455–3463. [Google Scholar] [CrossRef]
- Murphy, C.M.; O’Brien, F.J. Understanding the effect of mean pore size on cell activity in collagen-glycosaminoglycan scaffolds. Cell Adhes. Migr. 2010, 4, 377–381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corin, K.A.; Gibson, L.J. Cell contraction forces in scaffolds with varying pore size and cell density. Biomaterials 2010, 31, 4835–4845. [Google Scholar] [CrossRef]
- Soller, E.C.; Tzeranis, D.S.; Miu, K.; So, P.T.; Yannas, I.V. Common features of optimal collagen scaffolds that disrupt wound contraction and enhance regeneration both in peripheral nerves and in skin. Biomaterials 2012, 33, 4783–4791. [Google Scholar] [CrossRef]
- Madaghiele, M.; Calo, E.; Salvatore, L.; Bonfrate, V.; Pedone, D.; Frigione, M.; Sannino, A. Assessment of collagen crosslinking and denaturation for the design of regenerative scaffolds. J. Biomed. Mater. Res. A 2015, 104, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Weadock, K.S.; Miller, E.J.; Bellincampi, L.D.; Zawadsky, J.P.; Dunn, M.G. Physical crosslinking of collagen fibers: Comparison of ultraviolet irradiation and dehydrothermal treatment. J. Biomed. Mater. Res. 1995, 29, 1373–1379. [Google Scholar] [CrossRef]
- Grover, C.N.; Gwynne, J.H.; Pugh, N.; Hamaia, S.; Farndale, R.W.; Best, S.M.; Cameron, R.E. Crosslinking and composition influence the surface properties, mechanical stiffness and cell reactivity of collagen-based films. Acta Biomater. 2012, 8, 3080–3090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, K.; Serpooshan, V.; Hurtado, C.; Diez-Cunado, M.; Zhao, M.; Maruyama, S.; Zhu, W.; Fajardo, G.; Noseda, M.; Nakamura, K.; et al. Epicardial FSTL1 reconstitution regenerates the adult mammalian heart. Nature 2015, 525, 479–485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbetta, A.; Barigelli, E.; Dentini, M. Porous alginate hydrogels: Synthetic methods for tailoring the porous texture. Biomacromolecules 2009, 10, 2328–2337. [Google Scholar] [CrossRef]
- Chimenti, I.; Rizzitelli, G.; Gaetani, R.; Angelini, F.; Ionta, V.; Forte, E.; Frati, G.; Schussler, O.; Barbetta, A.; Messina, E.; et al. Human cardiosphere-seeded gelatin and collagen scaffolds as cardiogenic engineered bioconstructs. Biomaterials 2011, 32, 9271–9281. [Google Scholar] [CrossRef]
- Gaetani, R.; Feyen, D.A.; Verhage, V.; Slaats, R.; Messina, E.; Christman, K.L.; Giacomello, A.; Doevendans, P.A.; Sluijter, J.P. Epicardial application of cardiac progenitor cells in a 3D-printed gelatin/hyaluronic acid patch preserves cardiac function after myocardial infarction. Biomaterials 2015, 61, 339–348. [Google Scholar] [CrossRef]
- Naito, H.; Melnychenko, I.; Didie, M.; Schneiderbanger, K.; Schubert, P.; Rosenkranz, S.; Eschenhagen, T.; Zimmermann, W.H. Optimizing engineered heart tissue for therapeutic applications as surrogate heart muscle. Circulation 2006, 114 (Suppl. 1), I72–I78. [Google Scholar] [CrossRef] [Green Version]
- Jackman, C.; Li, H.; Bursac, N. Long-term contractile activity and thyroid hormone supplementation produce engineered rat myocardium with adult-like structure and function. Acta Biomater. 2018, 78, 98–110. [Google Scholar] [CrossRef] [PubMed]
- Hirt, M.N.; Boeddinghaus, J.; Mitchell, A.; Schaaf, S.; Bornchen, C.; Muller, C.; Schulz, H.; Hubner, N.; Stenzig, J.; Stoehr, A.; et al. Functional improvement and maturation of rat and human engineered heart tissue by chronic electrical stimulation. J. Mol. Cell. Cardiol. 2014, 74, 151–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Radisic, M.; Fast, V.G.; Sharifov, O.F.; Iyer, R.K.; Park, H.; Vunjak-Novakovic, G. Optical mapping of impulse propagation in engineered cardiac tissue. Tissue Eng. Part A 2009, 15, 851–860. [Google Scholar] [CrossRef]
- Park, H.; Bhalla, R.; Saigal, R.; Radisic, M.; Watson, N.; Langer, R.; Vunjak-Novakovic, G. Effects of electrical stimulation in C2C12 muscle constructs. J. Tissue Eng. Regen. Med. 2008, 2, 279–287. [Google Scholar] [CrossRef]
- Dawson, J.; Schussler, O.; Al-Madhoun, A.; Menard, C.; Ruel, M.; Skerjanc, I.S. Collagen scaffolds with or without the addition of RGD peptides support cardiomyogenesis after aggregation of mouse embryonic stem cells. In Vitro Cell. Dev. Biol. Anim. 2011, 47, 653–664. [Google Scholar] [CrossRef]
- Miyagi, Y.; Chiu, L.L.; Cimini, M.; Weisel, R.D.; Radisic, M.; Li, R.K. Biodegradable collagen patch with covalently immobilized VEGF for myocardial repair. Biomaterials 2010, 32, 1280–1290. [Google Scholar] [CrossRef]
- Chiu, L.L.; Radisic, M. Scaffolds with covalently immobilized VEGF and Angiopoietin-1 for vascularization of engineered tissues. Biomaterials 2009, 31, 226–241. [Google Scholar] [CrossRef]
- Hall, M.L.; Ogle, B.M. Cardiac Extracellular Matrix Modification as a Therapeutic Approach. Adv. Exp. Med. Biol 2018, 1098, 131–150. [Google Scholar]
- Taylor, D.A.; Sampaio, L.C.; Ferdous, Z.; Gobin, A.S.; Taite, L.J. Decellularized matrices in regenerative medicine. Acta Biomater. 2018, 74, 74–89. [Google Scholar] [CrossRef]
- Fong, A.H.; Romero-Lopez, M.; Heylman, C.M.; Keating, M.; Tran, D.; Sobrino, A.; Tran, A.Q.; Pham, H.H.; Fimbres, C.; Gershon, P.D.; et al. Three-Dimensional Adult Cardiac Extracellular Matrix Promotes Maturation of Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes. Tissue Eng. Part A 2016, 22, 1016–1025. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guyette, J.P.; Charest, J.M.; Mills, R.W.; Jank, B.J.; Moser, P.T.; Gilpin, S.E.; Gershlak, J.R.; Okamoto, T.; Gonzalez, G.; Milan, D.J.; et al. Bioengineering Human Myocardium on Native Extracellular Matrix. Circ. Res. 2015, 118, 56–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asgari, M.; Latifi, N.; Heris, H.K.; Vali, H.; Mongeau, L. In vitro fibrillogenesis of tropocollagen type III in collagen type I affects its relative fibrillar topology and mechanics. Sci. Rep. 2017, 7, 1392. [Google Scholar] [CrossRef] [Green Version]
- Callegari, A.; Bollini, S.; Iop, L.; Chiavegato, A.; Torregrossa, G.; Pozzobon, M.; Gerosa, G.; De Coppi, P.; Elvassore, N.; Sartore, S. Neovascularization induced by porous collagen scaffold implanted on intact and cryoinjured rat hearts. Biomaterials 2007, 28, 5449–5461. [Google Scholar] [CrossRef]
- Chiu, L.L.; Weisel, R.D.; Li, R.K.; Radisic, M. Defining conditions for covalent immobilization of angiogenic growth factors onto scaffolds for tissue engineering. J. Tissue Eng. Regen. Med. 2010, 5, 69–84. [Google Scholar] [CrossRef]
- Hein, S.; Schaper, J. The extracellular matrix in normal and diseased myocardium. J. Nucl. Cardiol. 2001, 8, 188–196. [Google Scholar] [CrossRef]
- Telemeco, T.A.; Ayres, C.; Bowlin, G.L.; Wnek, G.E.; Boland, E.D.; Cohen, N.; Baumgarten, C.M.; Mathews, J.; Simpson, D.G. Regulation of cellular infiltration into tissue engineering scaffolds composed of submicron diameter fibrils produced by electrospinning. Acta Biomater. 2005, 1, 377–385. [Google Scholar] [CrossRef]
- Grover, C.N.; Cameron, R.E.; Best, S.M. Investigating the morphological, mechanical and degradation properties of scaffolds comprising collagen, gelatin and elastin for use in soft tissue engineering. J. Mech. Behav. Biomed. Mater. 2012, 10, 62–74. [Google Scholar] [CrossRef] [PubMed]
- Bil’diug, N.B.; Iudintseva, N.M.; Pinaev, G.P. Contractile apparatus organization of cardiomyocytes upon their cultivation in collagen gels. Tsitologiia 2015, 56, 822–827. [Google Scholar]
- Bil’diug, N.B.; Pinaev, G.P. Extracellular matrix dependence of the cardiomyocyte contractile apparatus organization. Tsitologiia 2013, 55, 713–724. [Google Scholar]
- Zhang, W.; Kong, C.W.; Tong, M.H.; Chooi, W.H.; Huang, N.; Li, R.A.; Chan, B.P. Maturation of human embryonic stem cell-derived cardiomyocytes (hESC-CMs) in 3D collagen matrix: Effects of niche cell supplementation and mechanical stimulation. Acta Biomater. 2016, 49, 204–217. [Google Scholar] [CrossRef]
- Davidenko, N.; Schuster, C.F.; Bax, D.V.; Raynal, N.; Farndale, R.W.; Best, S.M.; Cameron, R.E. Control of crosslinking for tailoring collagen-based scaffolds stability and mechanics. Acta Biomater. 2015, 25, 131–142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pieper, J.S.; Oosterhof, A.; Dijkstra, P.J.; Veerkamp, J.H.; van Kuppevelt, T.H. Preparation and characterization of porous crosslinked collagenous matrices containing bioavailable chondroitin sulphate. Biomaterials 1999, 20, 847–858. [Google Scholar] [CrossRef]
- Zhao, J.; Santino, F.; Giacomini, D.; Gentilucci, L. Integrin-Targeting Peptides for the Design of Functional Cell-Responsive Biomaterials. Biomedicines 2020, 8, 307. [Google Scholar] [CrossRef]
- Tallawi, M.; Rosellini, E.; Barbani, N.; Cascone, M.G.; Rai, R.; Saint-Pierre, G.; Boccaccini, A.R. Strategies for the chemical and biological functionalization of scaffolds for cardiac tissue engineering: A review. J. R. Soc. Interface 2015, 12, 20150254. [Google Scholar] [CrossRef]
- Xiao, Y.; Reis, L.A.; Zhao, Y.; Radisic, M. Modifications of collagen-based biomaterials with immobilized growth factors or peptides. Methods 2015, 84, 44–52. [Google Scholar] [CrossRef]
- Pierschbacher, M.D.; Ruoslahti, E. Influence of stereochemistry of the sequence Arg-Gly-Asp-Xaa on binding specificity in cell adhesion. J. Biol. Chem. 1987, 262, 17294–17298. [Google Scholar] [CrossRef]
- Gallet, R.; Dawkins, J.; Valle, J.; Simsolo, E.; de Couto, G.; Middleton, R.; Tseliou, E.; Luthringer, D.; Kreke, M.; Smith, R.R.; et al. Exosomes secreted by cardiosphere-derived cells reduce scarring, attenuate adverse remodelling, and improve function in acute and chronic porcine myocardial infarction. Eur. Heart J. 2017, 38, 201–211. [Google Scholar] [CrossRef] [Green Version]
- Tsur-Gang, O.; Ruvinov, E.; Landa, N.; Holbova, R.; Feinberg, M.S.; Leor, J.; Cohen, S. The effects of peptide-based modification of alginate on left ventricular remodeling and function after myocardial infarction. Biomaterials 2009, 30, 189–195. [Google Scholar] [CrossRef]
- Kamata, S.; Miyagawa, S.; Fukushima, S.; Nakatani, S.; Kawamoto, A.; Saito, A.; Harada, A.; Shimizu, T.; Daimon, T.; Okano, T.; et al. Improvement of cardiac stem cell sheet therapy for chronic ischemic injury by adding endothelial progenitor cell transplantation: Analysis of layer-specific regional cardiac function. Cell Transplant. 2013, 23, 1305–1319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, F.; Sadrzadeh Rafie, A.H.; Abilez, O.J.; Wang, H.; Blundo, J.T.; Pruitt, B.; Zarins, C.; Wu, J.C. In vivo imaging and evaluation of different biomatrices for improvement of stem cell survival. J. Tissue Eng. Regen. Med. 2007, 1, 465–468. [Google Scholar] [CrossRef] [Green Version]
- Freeman, B.T.; Kouris, N.A.; Ogle, B.M. Tracking fusion of human mesenchymal stem cells after transplantation to the heart. Stem Cells Transl. Med. 2015, 4, 685–694. [Google Scholar] [CrossRef] [Green Version]
- Kutschka, I.; Chen, I.Y.; Kofidis, T.; Arai, T.; von Degenfeld, G.; Sheikh, A.Y.; Hendry, S.L.; Pearl, J.; Hoyt, G.; Sista, R.; et al. Collagen matrices enhance survival of transplanted cardiomyoblasts and contribute to functional improvement of ischemic rat hearts. Circulation 2006, 114 (Suppl. 1), I167–I173. [Google Scholar] [CrossRef] [Green Version]
- Tongers, J.; Webber, M.J.; Vaughan, E.E.; Sleep, E.; Renault, M.A.; Roncalli, J.G.; Klyachko, E.; Thorne, T.; Yu, Y.; Marquardt, K.T.; et al. Enhanced potency of cell-based therapy for ischemic tissue repair using an injectable bioactive epitope presenting nanofiber support matrix. J. Mol. Cell. Cardiol. 2014, 74, 231–239. [Google Scholar] [CrossRef] [Green Version]
- Rocha, A.L.; Shirasu, B.K.; Hayacibara, R.M.; Magro-Filho, O.; Zanoni, J.N.; Araujo, M.G. Clinical and histological evaluation of subepithelial connective tissue after collagen sponge implantation in the human palate. J. Periodontal Res. 2012, 47, 758–765. [Google Scholar] [CrossRef] [PubMed]
- Brandao, R.A.; Costa, B.S.; Dellaretti, M.A.; de Carvalho, G.T.; Faria, M.P.; de Sousa, A.A. Efficacy and safety of a porcine collagen sponge for cranial neurosurgery: A prospective case-control study. World Neurosurg. 2011, 79, 544–550. [Google Scholar] [CrossRef]
- Formanek, M.B.; Herwaldt, L.A.; Perencevich, E.N.; Schweizer, M.L. Gentamicin/collagen sponge use may reduce the risk of surgical site infections for patients undergoing cardiac operations: A meta-analysis. Surg. Infect. 2014, 15, 244–255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaballa, M.A.; Sunkomat, J.N.; Thai, H.; Morkin, E.; Ewy, G.; Goldman, S. Grafting an acellular 3-dimensional collagen scaffold onto a non-transmural infarcted myocardium induces neo-angiogenesis and reduces cardiac remodeling. J. Heart Lung Transplant. 2006, 25, 946–954. [Google Scholar] [CrossRef]
- Xiang, Z.; Liao, R.; Kelly, M.S.; Spector, M. Collagen-GAG scaffolds grafted onto myocardial infarcts in a rat model: A delivery vehicle for mesenchymal stem cells. Tissue Eng. 2006, 12, 2467–2478. [Google Scholar] [CrossRef]
- Ladage, D.; Yaniz-Galende, E.; Rapti, K.; Ishikawa, K.; Tilemann, L.; Shapiro, S.; Takewa, Y.; Muller-Ehmsen, J.; Schwarz, M.; Garcia, M.J.; et al. Stimulating myocardial regeneration with periostin Peptide in large mammals improves function post-myocardial infarction but increases myocardial fibrosis. PLoS ONE 2013, 8, e59656. [Google Scholar] [CrossRef] [PubMed]
- Shimazaki, M.; Nakamura, K.; Kii, I.; Kashima, T.; Amizuka, N.; Li, M.; Saito, M.; Fukuda, K.; Nishiyama, T.; Kitajima, S.; et al. Periostin is essential for cardiac healing after acute myocardial infarction. J. Exp. Med. 2008, 205, 295–303. [Google Scholar] [CrossRef]
- Zakharova, L.; Mastroeni, D.; Mutlu, N.; Molina, M.; Goldman, S.; Diethrich, E.; Gaballa, M.A. Transplantation of cardiac progenitor cell sheet onto infarcted heart promotes cardiogenesis and improves function. Cardiovasc. Res. 2010, 87, 40–49. [Google Scholar] [CrossRef]
- Saucerman, J.J.; Tan, P.M.; Buchholz, K.S.; McCulloch, A.D.; Omens, J.H. Mechanical regulation of gene expression in cardiac myocytes and fibroblasts. Nat. Rev. Cardiol. 2019, 16, 361–378. [Google Scholar] [CrossRef] [PubMed]
- Kechagia, J.Z.; Ivaska, J.; Roca-Cusachs, P. Integrins as biomechanical sensors of the microenvironment. Nat. Rev. Mol. Cell Biol. 2019, 20, 457–473. [Google Scholar] [CrossRef] [PubMed]
- Dergilev, K.V.; Shevchenko, E.K.; Tsokolaeva, Z.I.; Beloglazova, I.B.; Zubkova, E.S.; Boldyreva, M.A.; Menshikov, M.Y.; Ratner, E.I.; Penkov, D.; Parfyonova, Y.V. Cell Sheet Comprised of Mesenchymal Stromal Cells Overexpressing Stem Cell Factor Promotes Epicardium Activation and Heart Function Improvement in a Rat Model of Myocardium Infarction. Int. J. Mol. Sci. 2020, 21, 9603. [Google Scholar] [CrossRef] [PubMed]
- Qu, H.; Xie, B.D.; Wu, J.; Lv, B.; Chuai, J.B.; Li, J.Z.; Cai, J.; Wu, H.; Jiang, S.L.; Leng, X.P.; et al. Improved Left Ventricular Aneurysm Repair with Cell- and Cytokine-Seeded Collagen Patches. Stem Cells Int. 2018, 2018, 4717802. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.L.; Wang, H.J.; Li, Z.H.; Wang, Y.L.; Wu, X.P.; Tan, Y.Z. Mesenchymal stem cell-loaded cardiac patch promotes epicardial activation and repair of the infarcted myocardium. J. Cell. Mol. Med. 2017, 21, 1751–1766. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schussler, O.; Falcoz, P.E.; Chachques, J.C.; Alifano, M.; Lecarpentier, Y. Possible Treatment of Myocardial Infarct Based on Tissue Engineering Using a Cellularized Solid Collagen Scaffold Functionalized with Arg-Glyc-Asp (RGD) Peptide. Int. J. Mol. Sci. 2021, 22, 12563. https://doi.org/10.3390/ijms222212563
Schussler O, Falcoz PE, Chachques JC, Alifano M, Lecarpentier Y. Possible Treatment of Myocardial Infarct Based on Tissue Engineering Using a Cellularized Solid Collagen Scaffold Functionalized with Arg-Glyc-Asp (RGD) Peptide. International Journal of Molecular Sciences. 2021; 22(22):12563. https://doi.org/10.3390/ijms222212563
Chicago/Turabian StyleSchussler, Olivier, Pierre E. Falcoz, Juan C. Chachques, Marco Alifano, and Yves Lecarpentier. 2021. "Possible Treatment of Myocardial Infarct Based on Tissue Engineering Using a Cellularized Solid Collagen Scaffold Functionalized with Arg-Glyc-Asp (RGD) Peptide" International Journal of Molecular Sciences 22, no. 22: 12563. https://doi.org/10.3390/ijms222212563





