Synbiotics Alleviate Hepatic Damage, Intestinal Injury and Muscular Beclin-1 Elevation in Rats after Chronic Ethanol Administration
Abstract
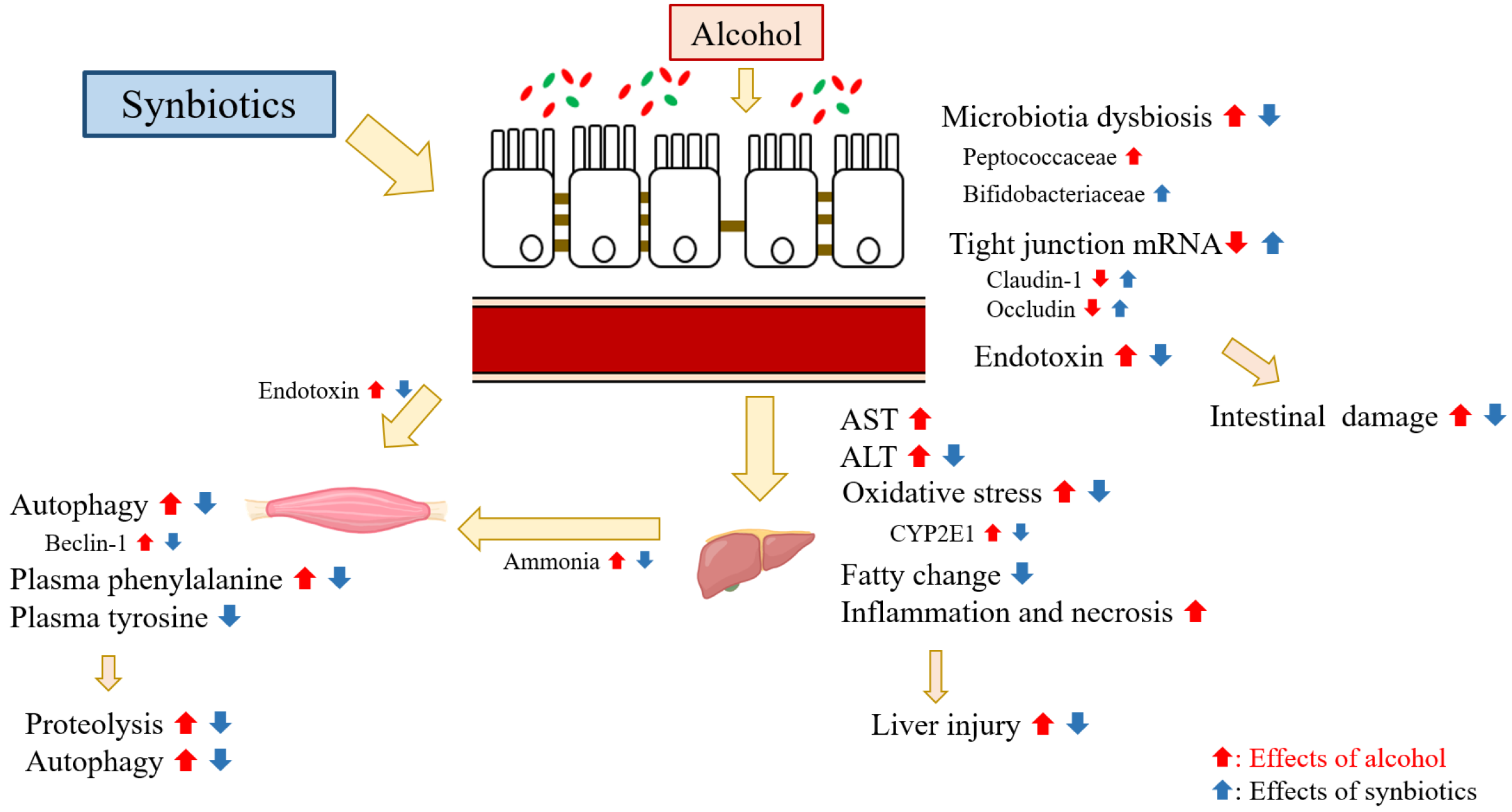
:1. Introduction
2. Results
2.1. Food Intake and Food Efficiency
2.2. Final BWs and Relative Liver Weights
2.3. Liver Damage
2.3.1. Plasma AST and ALT Activities, Ammonia Level, and Hepatic Histopathology Scores
2.3.2. Hepatic Cytokines
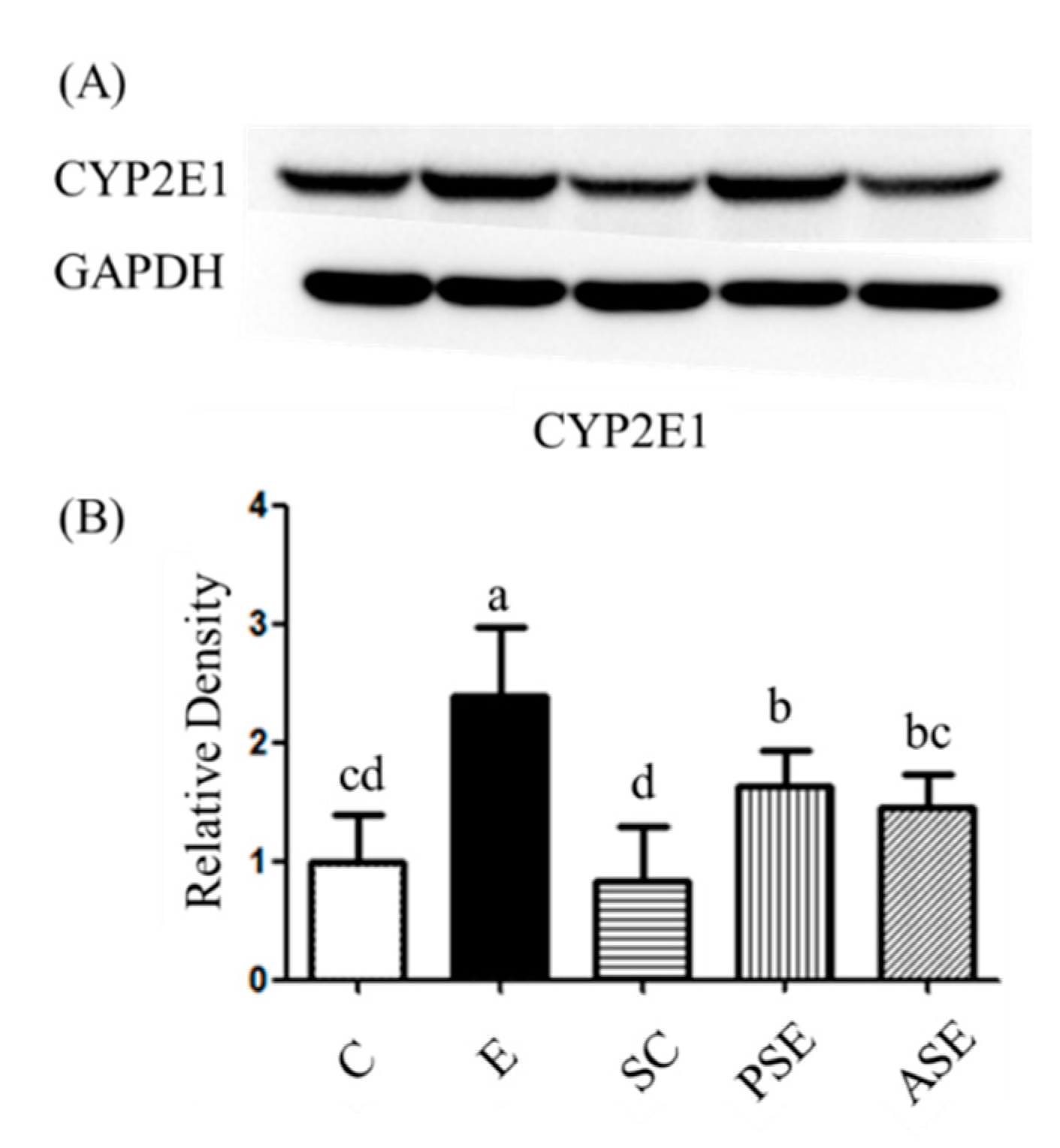
2.3.3. Oxidative Stress
2.4. Intestinal Damage
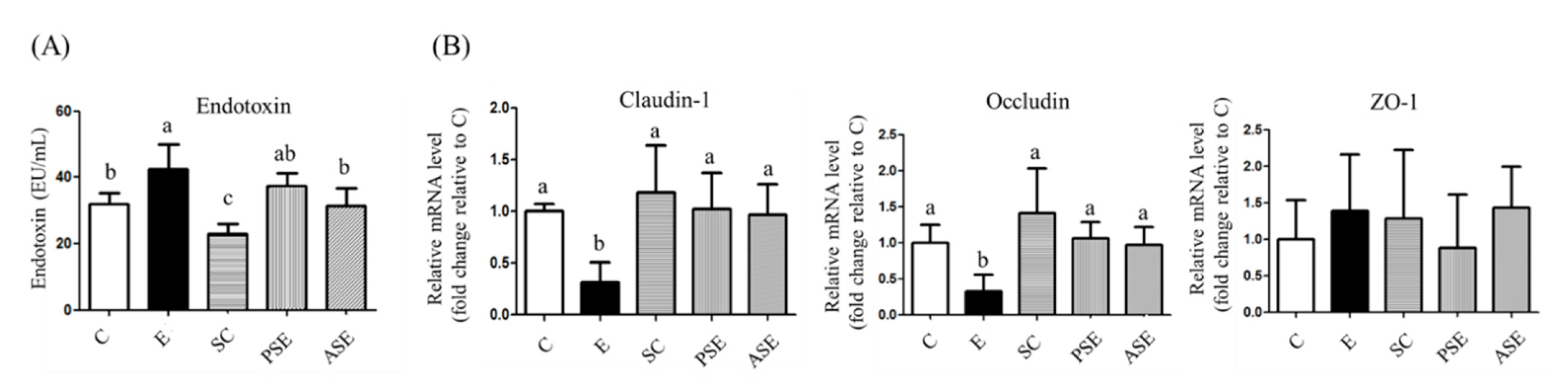
2.4.1. Serum Endotoxin Level
2.4.2. Intestinal Tight Junction Protein mRNA Expressions
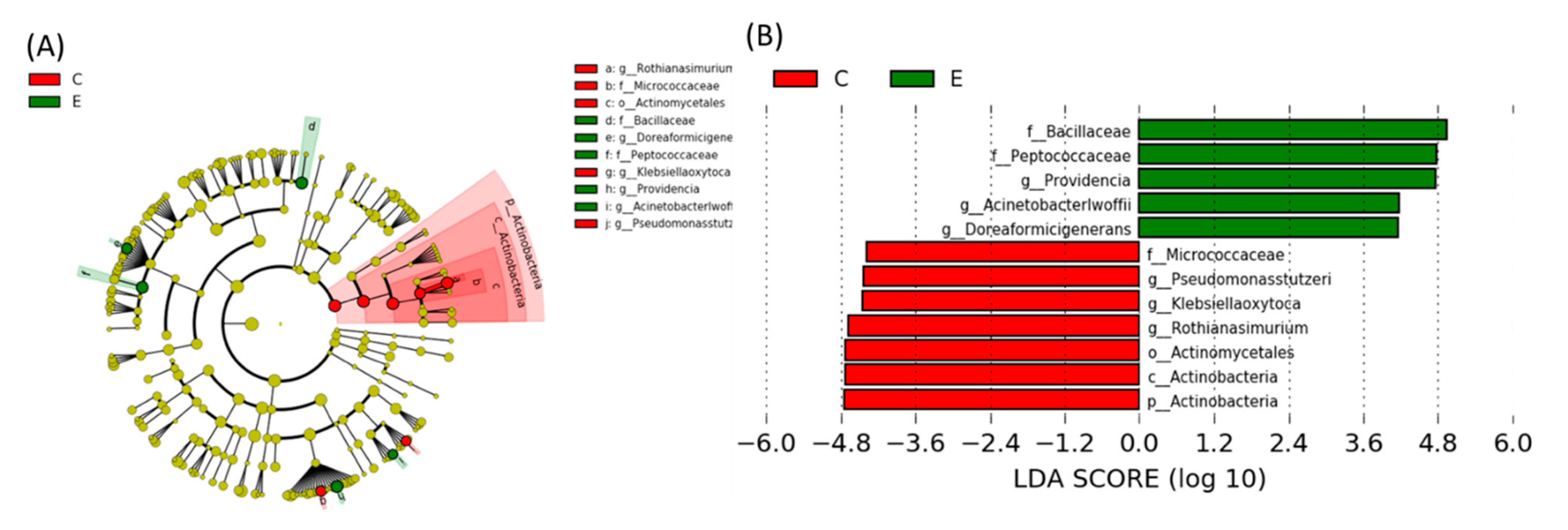
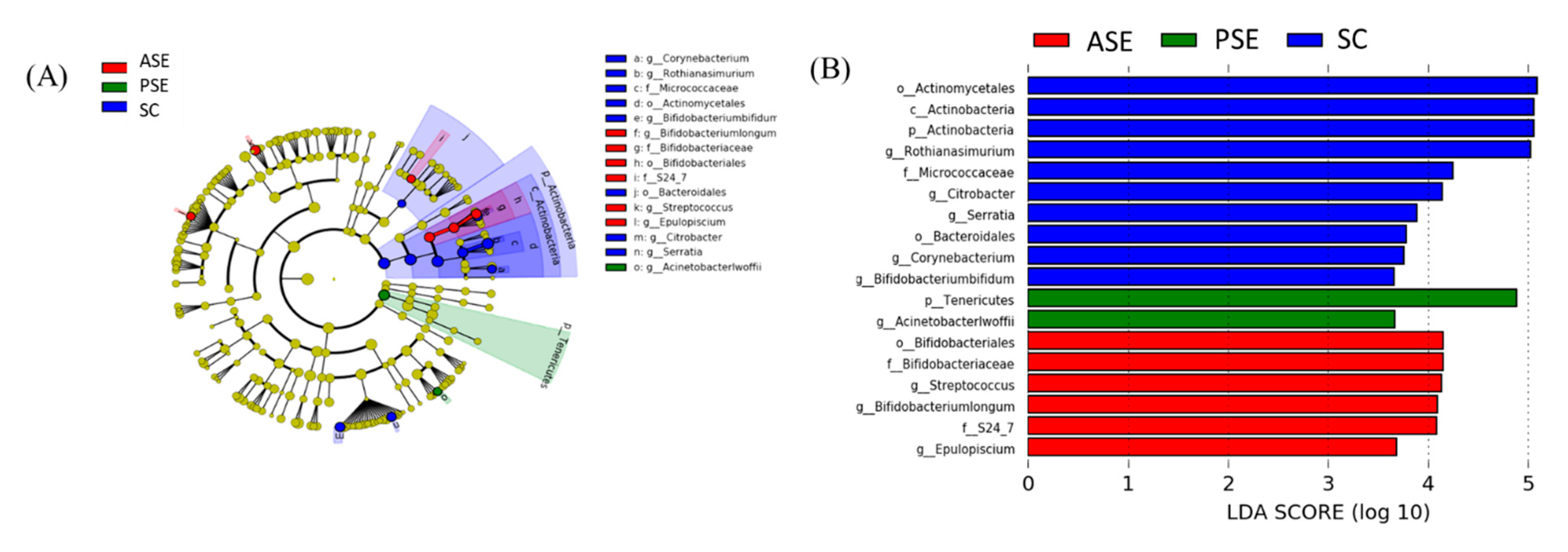
2.4.3. Fecal Microbiotic Composition
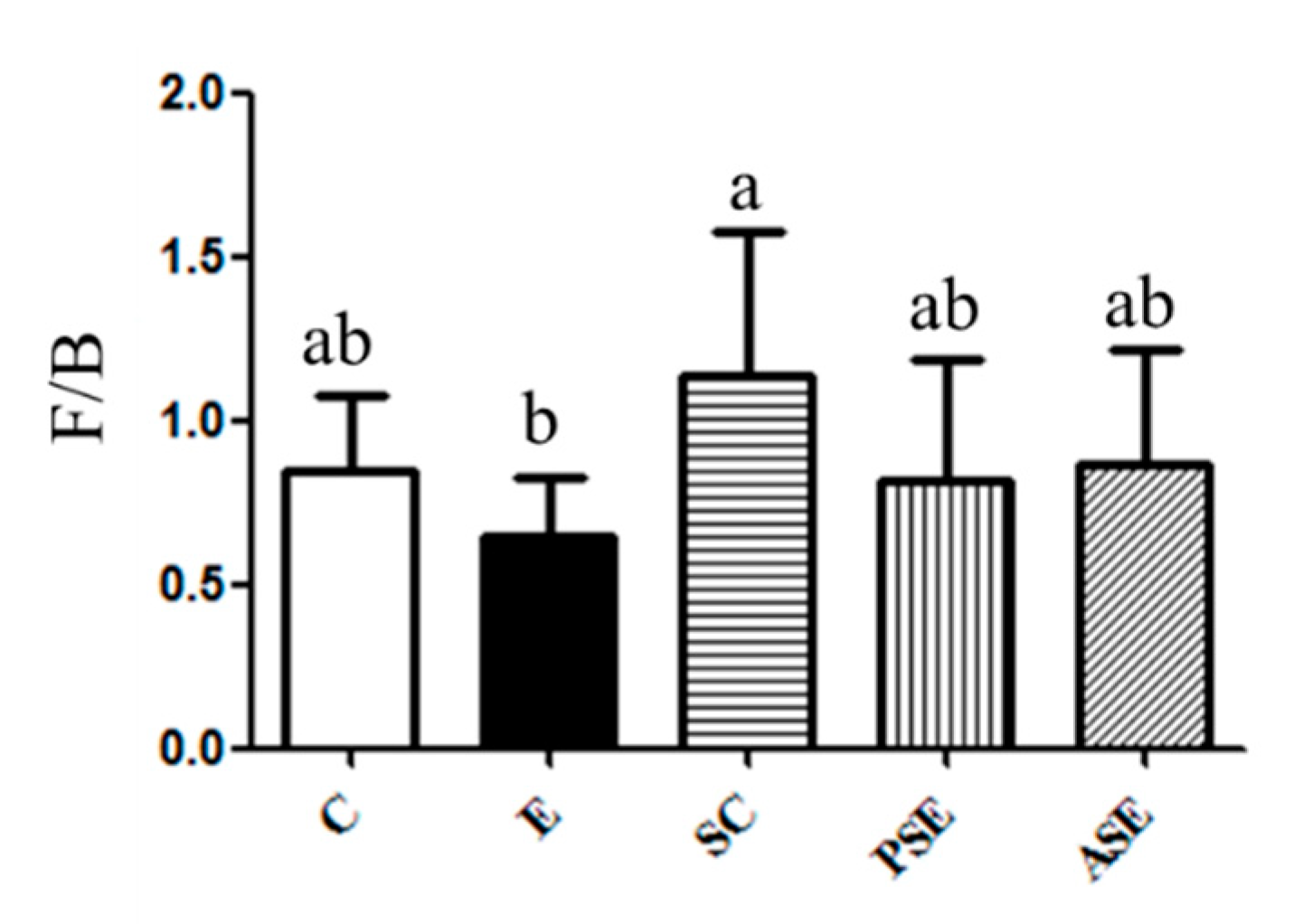
Firmicutes (F) to Bacteroidetes (B) Ratio
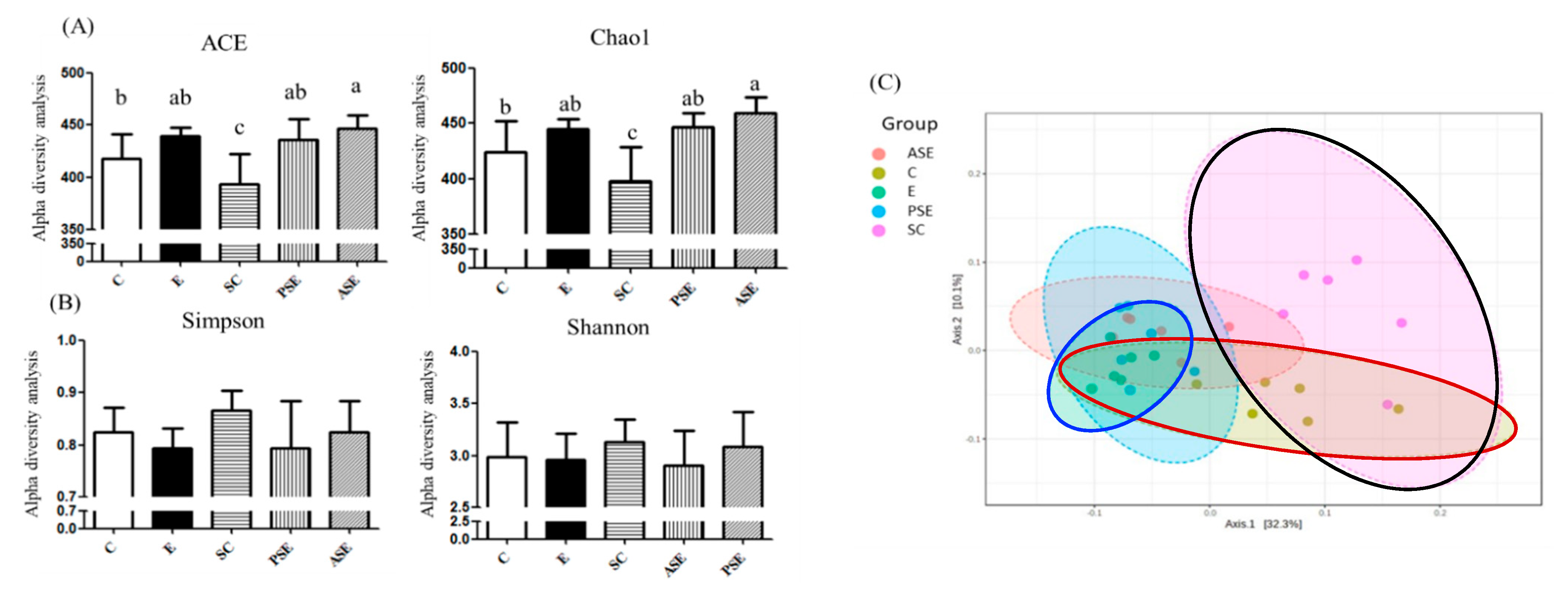
The α-Diversity and β-Diversity Indices
Linear Discriminant Analysis of the Effect Size (LEfSe)
2.5. Muscle Loss
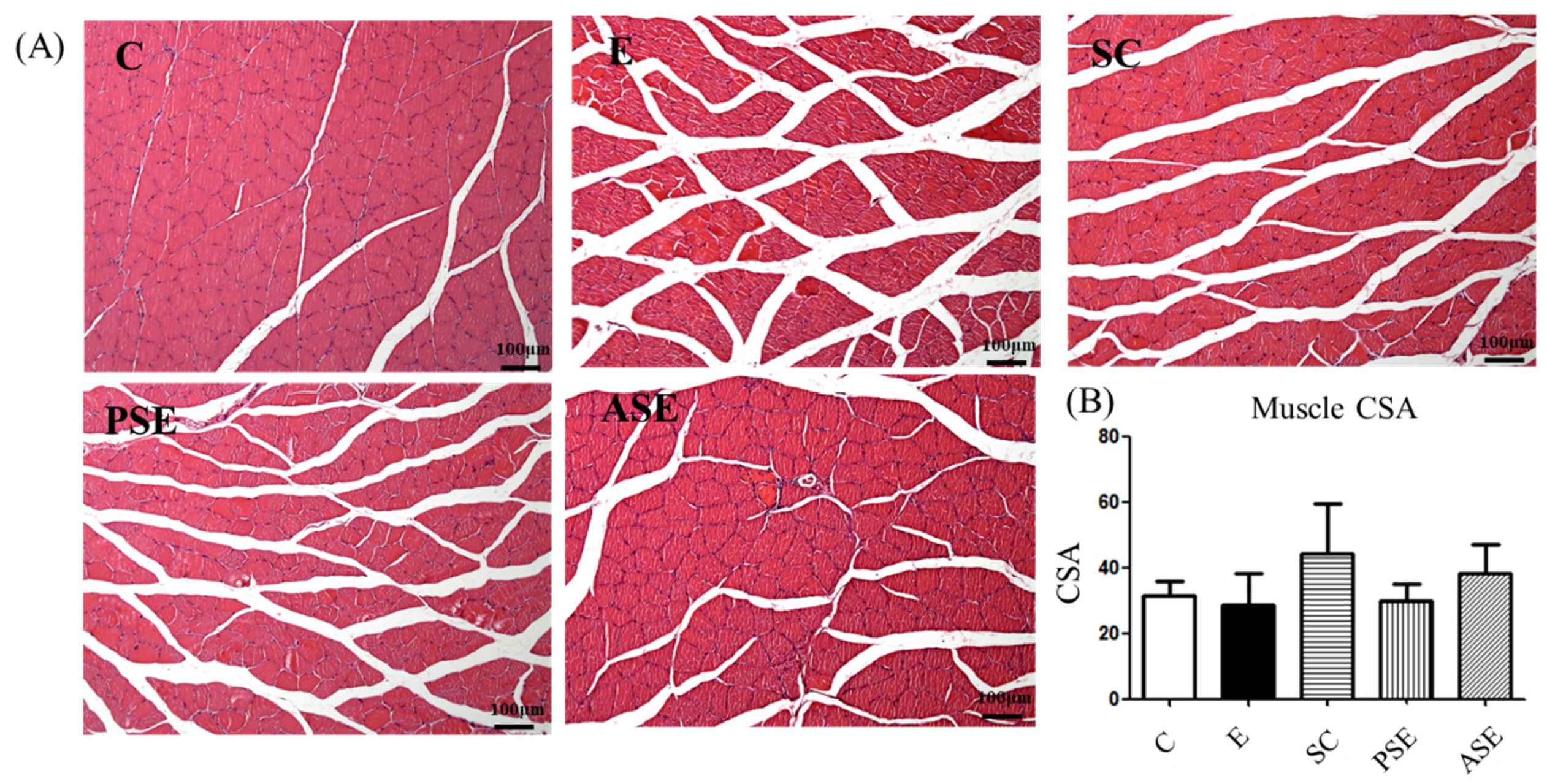
2.5.1. Grip Strength and Muscle Histopathology
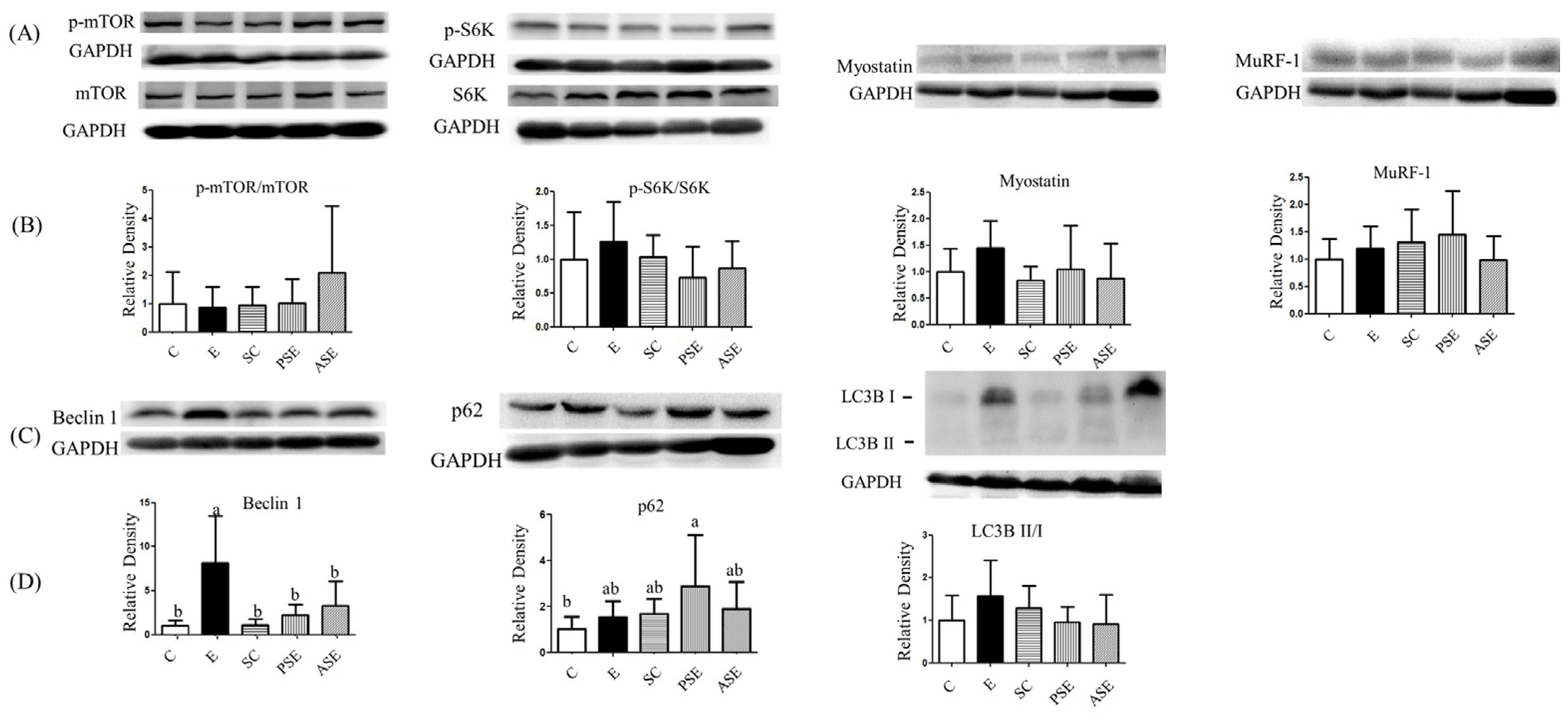
2.5.2. Muscle Protein Synthesis and Degradation
2.6. Amino Acid Composition
2.6.1. Amino Acid Levels in Plasma
2.6.2. Amino Acid LEVELS in Liver
2.6.3. Amino Acid Levels in Muscles
3. Discussion
3.1. Food Intake, Alcohol Intake, and Synbiotics Intake
3.2. BWs and Relative Liver Weights
3.3. Liver Damage
3.4. Intestinal Damage
3.5. Muscle Protein Metabolism and Liver-Gut-Muscle Axis
3.6. Amino Acid Composition
3.7. The Study Limitation
4. Materials and Methods
4.1. Animals
4.2. Study Protocol
4.3. Measurements and Analytical Procedures
4.3.1. Indicators of Liver Injury
Liver Function Index and Ammonia Level
Histological Examinations
Hepatic Cytokines
Plasma and Hepatic Lipid Peroxidation
Protein Expression of Hepatic Cytochrome P450 2E1 (CYP2E1)
4.3.2. Assessment of Intestinal Damage
Serum Endotoxin Level
The mRNA Expressions of Intestinal Tight Junction
Fecal Microbiotic Composition
4.3.3. Assessment of Muscle Loss
Grip Strength
Histological Examination
Protein Expressions of Protein Synthesis and Degradation
4.3.4. Amino Acid Composition of Plasma, Liver, and Muscle Tissues
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Global Status Report on Alcohol and Health 2018; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Dasarathy, S. Nutrition and alcoholic liver disease: Effects of alcoholism on nutrition, effects of nutrition on alcoholic liver disease, and nutritional therapies for alcoholic liver disease. Clin. Liver Dis. 2016, 20, 535–550. [Google Scholar] [CrossRef] [Green Version]
- Giusto, M.; Lattanzi, B.; Albanese, C.; Galtieri, A.; Farcomeni, A.; Giannelli, V.; Lucidi, C.; Di Martino, M.; Catalano, C.; Merli, M. Sarcopenia in liver cirrhosis: The role of computed tomography scan for the assessment of muscle mass compared with dual-energy X-ray absorptiometry and anthropometry. Eur. J. Gastroenterol. Hepatol. 2015, 27, 328–334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thapaliya, S.; Runkana, A.; McMullen, M.R.; Nagy, L.E.; McDonald, C.; Naga Prasad, S.V.; Dasarathy, S. Alcohol-induced autophagy contributes to loss in skeletal muscle mass. Autophagy 2014, 10, 677–690. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Barakat, M.; Chen, D.; Chen, L. Bicellular Tight Junctions and Wound Healing. Int. J. Mol. Sci. 2018, 19, 3862. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, B.K.; Lee, I.O.; Tan, P.L.; Eor, J.Y.; Hwang, J.K.; Kim, S.H. Protective Effect of Lactobacillus fermentum LA12 in an Alcohol-Induced Rat Model of Alcoholic Steatohepatitis. Korean J. Food Sci. Anim. Resour. 2017, 37, 931–939. [Google Scholar] [CrossRef] [PubMed]
- Rao, R.K. Acetaldehyde-induced increase in paracellular permeability in Caco-2 cell monolayer. Alcohol. Clin. Exp. Res. 1998, 22, 1724–1730. [Google Scholar] [CrossRef]
- Mutlu, E.A.; Gillevet, P.M.; Rangwala, H.; Sikaroodi, M.; Naqvi, A.; Engen, P.A.; Kwasny, M.; Lau, C.K.; Keshavarzian, A. Colonic microbiome is altered in alcoholism. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 302, G966–G978. [Google Scholar] [CrossRef]
- Bull-Otterson, L.; Feng, W.; Kirpich, I.; Wang, Y.; Qin, X.; Liu, Y.; Gobejishvili, L.; Joshi-Barve, S.; Ayvaz, T.; Petrosino, J.; et al. Metagenomic analyses of alcohol induced pathogenic alterations in the intestinal microbiome and the effect of Lactobacillus rhamnosus GG treatment. PLoS ONE 2013, 8, e530282013. [Google Scholar] [CrossRef]
- Cassard, A.M.; Ciocan, D. Microbiota, a key player in alcoholic liver disease. Clin. Mol. Hepatol. 2018, 24, 100–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiu, J.; Thapaliya, S.; Runkana, A.; Yang, Y.; Tsien, C.; Mohan, M.L.; Narayanan, A.; Eghtesad, B.; Mozdziak, P.E.; McDonald, C.; et al. Hyperammonemia in cirrhosis induces transcriptional regulation of myostatin by an NF-κB-mediated mechanism. Proc. Natl. Acad. Sci. USA 2013, 110, 18162–18167. [Google Scholar] [CrossRef] [Green Version]
- Dasarathy, J.; McCullough, A.J.; Dasarathy, S. Sarcopenia in Alcoholic Liver Disease: Clinical and Molecular Advances. Alcohol. Clin. Exp. Res. 2017, 41, 1419–1431. [Google Scholar] [CrossRef]
- Gumucio, J.P.; Mendias, C.L. Atrogin-1, MuRF-1, and sarcopenia. Endocrine 2013, 43, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.T.; Yang, Y.J.; Huang, R.H.; Zhang, Z.H.; Lin, X. Myostatin activates the ubiquitin-proteasome and autophagy-lysosome systems contributing to muscle wasting in chronic kidney disease. Oxidative Med. Cell. Longev. 2015, 2015, 684965. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.Y.; Hopkinson, N.S.; Kemp, P.R. Myostatin induces autophagy in skeletal muscle in vitro. Biochem. Biophys. Res. Commun. 2011, 415, 632–636. [Google Scholar] [CrossRef]
- Steiner, J.L.; Lang, C.H. Dysregulation of skeletal muscle protein metabolism by alcohol. Am. J. Physiol. Endocrinol. Metab. 2015, 308, E699–E712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roychowdhury, S.; Glueck, B.; Han, Y.; Mohammad, M.A.; Cresci, G.A.M. A Designer Synbiotic Attenuates Chronic-Binge Ethanol-Induced Gut-Liver Injury in Mice. Nutrients 2019, 11, 97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gibson, G.R.; Roberfroid, M.B. Dietary modulation of the human colonic microbiota: Introducing the concept of prebiotics. J. Nutr. 1995, 125, 1401–1412. [Google Scholar] [CrossRef]
- Chiu, W.C.; Huang, Y.L.; Chen, Y.L.; Peng, H.C.; Liao, W.H.; Chuang, H.L.; Chen, J.R.; Yang, S.C. Synbiotics reduce ethanol-induced hepatic steatosis and inflammation by improving intestinal permeability and microbiota in rats. Food Funct. 2015, 6, 1692–1700. [Google Scholar] [CrossRef]
- Hézode, C.; Lonjon, I.; Roudot-Thoraval, F.; Pawlotsky, J.M.; Zafrani, E.S.; Dhumeaux, D. Impact of moderate alcohol consumption on histological activity and fibrosis in patients with chronic hepatitis C, and specific influence of steatosis: A prospective study. Aliment. Pharmacol. Ther. 2003, 17, 1031–1037. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.Y.; Peng, H.C.; Chien, Y.W.; Chen, Y.L.; Lu, N.S.; Yang, S.C. Effects of Fish Oil on Lipid Metabolism and Its Molecular Biological Regulators in Chronic Ethanol-Fed Rats. Nutrients 2018, 10, 802. [Google Scholar] [CrossRef] [Green Version]
- Yuan, Y.; Guo, H.; Zhang, Y.; Zhou, D.; Gan, P.; Liang, D.M.; Chen, J.Y. Protective effects of L-carnitine on intestinal ischemia/reperfusion injury in a rat model. J. Clin. Med. Res. 2011, 3, 78–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Israel, Y.; Britton, R.S.; Orrego, H. Liver cell enlargement induced by chronic alcohol consumption: Studies on its causes and consequences. Clin. Biochem. 1982, 15, 189–192. [Google Scholar] [CrossRef]
- Fang, T.J.; Guo, J.T.; Lin, M.K.; Lee, M.S.; Chen, Y.L.; Lin, W.H. Protective effects of Lactobacillus plantarum against chronic alcohol-induced liver injury in the murine model. Appl. Microbiol. Biotechnol. 2019, 103, 8597–8608. [Google Scholar] [CrossRef]
- Lu, Y.; Cederbaum, A.I. Cytochrome P450s and alcoholic liver disease. Curr. Pharm. Des. 2018, 24, 1502–1517. [Google Scholar] [CrossRef] [PubMed]
- Szabo, G.; Petrasek, J. Gut-liver axis and sterile signals in the development of alcoholic liver disease. Alcohol Alcohol. 2017, 52, 414–424. [Google Scholar] [CrossRef]
- Wang, Y.; Kirpich, I.; Liu, Y.; Ma, Z.; Barve, S.; McClain, C.J.; Feng, W. Lactobacillus rhamnosus GG treatment potentiates intestinal hypoxia-inducible factor, promotes intestinal integrity and ameliorates alcohol-induced liver injury. Am. J. Pathol. 2011, 179, 2866–2875. [Google Scholar] [CrossRef]
- Yang, X.; He, F.; Zhang, Y.; Xue, J.; Li, K.; Zhang, X.; Zhu, L.; Wang, Z.; Wang, H.; Yang, S. Inulin ameliorates alcoholic liver disease via suppressing LPS-TLR4-Mψ axis and modulating gut microbiota in mice. Alcohol. Clin. Exp. Res. 2019, 43, 411–424. [Google Scholar] [CrossRef]
- Anderson, C.; Andersson, T.; Molander, M. Ethanol absorption across human skin measured by in vivo microdialysis technique. Acta Derm. Venereol. 1991, 71, 389–393. [Google Scholar]
- Chaudhry, K.K.; Samak, G.; Shukla, P.K.; Mir, H.; Gangwar, R.; Manda, B.; Isse, T.; Kawamoto, T.; Salaspuro, M.; Kaihovaara, P.; et al. ALDH2 Deficiency promotes ethanol-induced gut barrier dysfunction and fatty liver in mice. Alcohol. Clin. Exp. Res. 2015, 39, 1465–1475. [Google Scholar] [CrossRef] [Green Version]
- Bishehsari, F.; Magno, E.; Swanson, G.; Desai, V.; Voigt, R.M.; Forsyth, C.B.; Keshavarzian, A. Alcohol and Gut-Derived Inflammation. Alcohol Res. Curr. Rev. 2017, 38, 163–171. [Google Scholar]
- Ghosh, A.R. Probiotics in the Rescue of Gut Inflammation. In Therapeutic, Probiotic, and Unconventional Foods; Elsevier: Amsterdam, The Netherlands, 2018; pp. 101–116. [Google Scholar]
- Chen, Y.L.; Shirakawa, H.; Lu, N.S.; Peng, H.C.; Xiao, Q.; Yang, S.C. Impacts of fish oil on the gut microbiota of rats with alcoholic liver damage. J. Nutr. Biochem. 2020, 86, 108491. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yan, Y.; Yi, X.; Duan, Y.; Wang, J.; Li, S.; Luo, L.; Huang, T.; Inglis, B.; Li, X.; et al. Histopathological Features and Composition of Gut Microbiota in Rhesus Monkey of Alcoholic Liver Disease. Front. Microbiol. 2019, 10, 165. [Google Scholar] [CrossRef] [Green Version]
- Hartmann, P.; Seebauer, C.T.; Schnabl, B. Alcoholic liver disease: The gut microbiome and liver cross talk. Alcohol. Clin. Exp. Res. 2015, 39, 763–775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ponziani, F.; Pecere, S.; Sterbini, F.P.; Petito, V.; Siciliano, M.; Di Rienzo, T.; Palladini, A.; Zambrano, D.; Franceschi, F.; Gaetani, E. First steps towards understanding the dynamic evolution of gut microbiota in different stages of liver disease. Dig. Liver Dis. 2015, 47, e53. [Google Scholar] [CrossRef]
- Przewłócka, K.; Folwarski, M.; Kaźmierczak-Siedlecka, K.; Skonieczna-Żydecka, K.; Kaczor, J.J. Gut-Muscle AxisExists and May Affect Skeletal Muscle Adaptation to Training. Nutrients 2020, 12, 1451. [Google Scholar] [CrossRef] [PubMed]
- Ehling-Schulz, M.; Lereclus, D.; Koehler, T.M. The Bacillus cereus Group: Bacillus Species with Pathogenic Potential. Microbiol. Spectr. 2019, 7, 10. [Google Scholar] [CrossRef]
- Shah, M.M.; Odoyo, E.; Ichinose, Y. Epidemiology and Pathogenesis of Providencia alcalifaciens Infections. Am. J. Trop. Med. Hyg. 2019, 101, 290–293. [Google Scholar] [CrossRef]
- Regalado, N.G.; Martin, G.; Antony, S.J. Acinetobacter lwoffii: Bacteremia associated with acute gastroenteritis. Travel Med. Infect. Dis. 2009, 7, 316–317. [Google Scholar] [CrossRef]
- Dasarathy, S. Cause and management of muscle wasting in chronic liver disease. Curr. Opin. Gastroenterol. 2016, 32, 159–165. [Google Scholar] [CrossRef] [Green Version]
- Lang, C.H.; Frost, R.A.; Svanberg, E.; Vary, T.C. IGF-I/IGFBP-3 ameliorates alterations in protein synthesis, eIF4E availability, and myostatin in alcohol-fed rats. Am. J. Physiol. Endocrinol. Metab. 2004, 286, E916–E926. [Google Scholar] [CrossRef] [Green Version]
- Korzick, D.H.; Sharda, D.R.; Pruznak, A.M.; Lang, C.H. Aging accentuates alcohol-induced decrease in protein synthesis in gastrocnemius. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 304, R887–R898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong-Brown, L.Q.; Brown, C.R.; Navaratnarajah, M.; Lang, C.H. FoxO1-AMPK-ULK1 Regulates Ethanol-Induced Autophagy in Muscle by Enhanced ATG14 Association with the BECN1-PIK3C3 Complex. Alcohol. Clin. Exp. Res. 2017, 41, 895–910. [Google Scholar] [CrossRef] [Green Version]
- Kant, S.; Davuluri, G.; Alchirazi, K.A.; Welch, N.; Heit, C.; Kumar, A.; Gangadhariah, M.; Kim, A.; McMullen, M.R.; Willard, B.; et al. Ethanol sensitizes skeletal muscle to ammonia-induced molecular perturbations. J. Biol. Chem. 2019, 294, 7231–7244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jindal, A.; Jagdish, R.K. Sarcopenia: Ammonia metabolism and hepatic encephalopathy. Clin. Mol. Hepatol. 2019, 25, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Shaw, S.; Lieber, C.S. Plasma amino acid abnormalities in the alcoholic: Respective role of alcohol, nutrition, and liver injury. Gastroenterology 1978, 74, 677–682. [Google Scholar] [CrossRef]
- Dam, G.; Sørensen, M.; Buhl, M.; Sandahl, T.D.; Møller, N.; Ott, P.; Vilstrup, H. Muscle metabolism and whole blood amino acid profile in patients with liver disease. Scand. J. Clin. Lab. Investig. 2015, 75, 674–680. [Google Scholar]
- Morgan, M.Y.; Marshall, A.W.; Milsom, J.P.; Sherlock, S. Plasma amino-acid patterns in liver disease. Gut 1982, 23, 362–370. [Google Scholar] [CrossRef] [Green Version]
- Dam, G.; Keiding, S.; Munk, O.L.; Ott, P.; Buhl, M.; Vilstrup, H.; Bak, L.K.; Waagepetersen, H.S.; Schousboe, A.; Møller, N.; et al. Branched-chain amino acids increase arterial blood ammonia in spite of enhanced intrinsic muscle ammonia metabolism in patients with cirrhosis and healthy subjects. Am. J. Physiology. Gastrointest. Liver Physiol. 2011, 301, G269–G277. [Google Scholar] [CrossRef] [Green Version]
- Mazi, T.A.; Sarode, G.V.; Czlonkowska, A.; Litwin, T.; Kim, K.; Shibata, N.M.; Medici, V. Dysregulated Choline, Methionine, and Aromatic Amino Acid Metabolism in Patients with Wilson Disease: Exploratory Metabolomic Profiling and Implications for Hepatic and Neurologic Phenotypes. Int. J. Mol. Sci. 2019, 20, 5937. [Google Scholar] [CrossRef] [Green Version]
- Mata, J.M.; Kershenobich, D.; Villarreal, E.; Rojkind, M. Serum free proline and free hydroxyproline in patients with chronic liver disease. Gastroenterology 1975, 68 Pt 1, 1265–1269. [Google Scholar] [CrossRef]
- Uesugi, T.; Froh, M.; Arteel, G.E.; Bradford, B.U.; Wheeler, M.D.; Gäbele, E.; Isayama, F.; Thurman, R.G. Role of lipopolysaccharide-binding protein in early alcohol-induced liver injury in mice. J. Immunol. 2002, 168, 2963–2969. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.L.; Peng, H.C.; Tan, S.W.; Tsai, C.Y.; Huang, Y.H.; Wu, H.Y.; Yang, S.C. Amelioration of ethanol-induced liver injury in rats by nanogold flakes. Alcohol 2013, 47, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; Breitkopf, S.B.; Yang, X.; Asara, J.M. A positive/negative ion-switching, targeted mass spectrometry-based metabolomics platform for bodily fluids, cells, and fresh and fixed tissue. Nat. Protoc. 2012, 7, 872–881. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Groups | Food Intake (g) 2 | Ethanol Intake (g) | Synbiotics Intake (g/kg BW) | Food Efficiency 3 (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C | 81.3 | ± | 0.8 | - | - | 4.3 | ± | 0.2 | ab | ||||
| E | 80.7 | ± | 2.7 | 4.0 | ± | 0.1 | - | 3.8 | ± | 0.4 | c | ||
| SC | 81.2 | ± | 0.8 | - | 1.4 | ± | 0.1 | 4.5 | ± | 0.3 | a | ||
| PSE | 78.6 | ± | 1.9 | 3.9 | ± | 0.1 | 1.4 | ± | 0.7 | 4.0 | ± | 0.4 | bc |
| ASE | 80.1 | ± | 1.1 | 4.0 | ± | 0.1 | 1.6 | ± | 0.1 | 3.9 | ± | 0.5 | bc |
| Groups | Final Body Weight (g) | Liver Weight (g) | Relative Liver Weight (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C | 418.2 | ± | 14.1 | ab | 10.5 | ± | 1.3 | b | 2.5 | ± | 0.3 | cd |
| E | 389.8 | ± | 21.4 | c | 12.9 | ± | 1.5 | a | 3.3 | ± | 0.3 | a |
| SC | 424.7 | ± | 13.0 | a | 10.4 | ± | 0.5 | b | 2.5 | ± | 0.1 | d |
| PSE | 398.4 | ± | 26.3 | bc | 10.8 | ± | 0.9 | b | 2.7 | ± | 0.1 | bc |
| ASE | 397.6 | ± | 18.8 | bc | 10.9 | ± | 0.4 | b | 2.7 | ± | 0.1 | b |
| Groups | AST (U/L) | ALT (U/L) | AMM (μg/dL) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C | 70.0 | ± | 8.6 | b | 45.0 | ± | 8.5 | c | 63.4 | ± | 12.5 | bc |
| E | 139.5 | ± | 32.4 | a | 120.3 | ± | 24.9 | a | 104.4 | ± | 52.1 | a |
| SC | 82.0 | ± | 8.9 | b | 46.7 | ± | 4.3 | c | 37.0 | ± | 11.0 | bc |
| PSE | 122.8 | ± | 48.5 | a | 80.5 | ± | 15.3 | b | 20.5 | ± | 5.3 | c |
| ASE | 134.8 | ± | 25.5 | a | 76.8 | ± | 6.2 | b | 26.5 | ± | 13.9 | bc |
| Groups | TNF-α (pg/μg Protein) | IL-1β (pg/μg Protein) | IL-6 (pg/μg Protein) | IL-10 (pg/μg Protein) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C | 62.0 | ± | 11.8 | ab | 72.0 | ± | 11.0 | ab | 199.6 | ± | 41.3 | ab | 24.1 | ± | 5.4 | ab |
| E | 67.1 | ± | 18.2 | a | 74.5 | ± | 26.4 | a | 235.6 | ± | 62.8 | a | 27.3 | ± | 7.7 | a |
| SC | 71.0 | ± | 13.0 | a | 70.0 | ± | 13.7 | ab | 238.8 | ± | 40.7 | a | 23.4 | ± | 4.8 | ab |
| PSE | 46.7 | ± | 7.5 | b | 44.9 | ± | 9.8 | c | 166.6 | ± | 24.9 | b | 18.9 | ± | 2.0 | b |
| ASE | 61.2 | ± | 12.0 | ab | 53.2 | ± | 10.6 | bc | 207.4 | ± | 40.3 | ab | 24.7 | ± | 4.8 | ab |
| Groups | Plasma (μM/mL) | Liver (μM/mg Protein) | |||||
|---|---|---|---|---|---|---|---|
| C | 13.3 | ± | 7.0 | 0.09 | ± | 0.04 | b |
| E | 15.5 | ± | 5.5 | 0.18 | ± | 0.10 | a |
| SC | 11.3 | ± | 3.9 | 0.16 | ± | 0.08 | ab |
| PSE | 13.8 | ± | 6.6 | 0.11 | ± | 0.01 | b |
| ASE | 12.8 | ± | 6.2 | 0.10 | ± | 0.04 | b |
| Groups | Initial | Final | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Grip Strength (g) | Grip Strength/kg BW | Grip Strength (g) | Grip strength/kg BW | |||||||||
| C | 932.2 | ± | 128.1 | 2.8 | ± | 0.4 | 1135.9 | ± | 349.6 | 2.7 | ± | 0.7 |
| E | 954.4 | ± | 252.9 | 3.0 | ± | 0.8 | 1031.8 | ± | 197.1 | 2.6 | ± | 0.5 |
| SC | 996.0 | ± | 128.1 | 3.1 | ± | 0.4 | 1108.7 | ± | 263.2 | 2.7 | ± | 0.7 |
| PSE | 1095.1 | ± | 135.9 | 3.3 | ± | 0.3 | 994.2 | ± | 177.4 | 2.5 | ± | 0.5 |
| ASE | 1081.9 | ± | 122.1 | 3.4 | ± | 0.3 | 1148.0 | ± | 130.4 | 2.9 | ± | 0.3 |
| Component | Amount/1.5 g |
|---|---|
| Calories | 4 kcal |
| Protein | 1 g |
| Fat | 0 g |
| Carbohydrate | 80 mg |
| Sodium | 14 mg |
| Inulin from chicory, powdered extract (root) | 50 mg |
| Vitamin B1 | 75 mg |
| Vitamin B2 | 0.85 μg |
| Niacinamide | 1 mg |
| Vitamin B6 | 100 μg |
| Vitamin B12 | 0.3 μg |
| Biotin | 15 μg |
| Folate | 20 μg |
| Pantothenic acid | 0.5 mg |
| Proprietary blend culture count | 2 × 109 CFU |
| Lactobacillus acidophilus and Lactobacillus bulgaricus | 5.4 × 108 CFU |
| Bifidobacterium bifidum and Bifidobacterium longum | 1.3 × 109 CFU |
| Streptococcus thermophilus | 1.2 × 108 CFU |
| Sample | Antibody | Source |
|---|---|---|
| Liver | Rabbit polyclonal CYP2E1 | Millipore, Burlington, MA, USA |
| Muscle | Rabbit polyclonal mTOR | Cell Signaling, Danvers, MA, USA |
| Muscle | Rabbit polyclonal p-mTOR Ser2448 | Cell Signaling, Danvers, MA, USA |
| Muscle | Rabbit polyclonal S6K | Affinity Bioscience, Cincinnati, OH, USA |
| Muscle | Rabbit polyclonal p-S6K T389 | ABclonal Technology, Woburn, MA, USA |
| Muscle | Rabbit polyclonal Myostatin | Proteintech Group, Inc., Rosemont, IL, USA |
| Muscle | Rabbit monoclonal MuRF-1 | Abcam, Cambridge, UK |
| Muscle | Rabbit monoclonal Beclin1 | Genetex, Irvine, CA, USA |
| Muscle | Rabbit monoclonal p62 | Cell Signaling, Danvers, MA, USA |
| Muscle | Rabbit monoclonal LC3B II/I | Cell Signaling, Danvers, MA, USA |
| Internal control | Mouse monoclonal GAPDH | Millipore, Burlington, MA, USA |
| Forward 5′→3′ | Reverse 5′→3′ | |
|---|---|---|
| ZO-1 | CTTGCCACACTGTGACCCTA | ACAGTTGGCTCCAACAAGGT |
| Occludin | CTGTCTATGCTCGTCATCG | CATTCCCGATCTAATGACGC |
| Claudin-1 | AAACTCCGCTTTCTGCACCT | TTTGCGAAACGCAGGACATC |
| β-actin | CACCAGTTCGCCATGGATGACGA | CCATCACACCCTGGTGCCTAGGGC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.-H.; Chiu, W.-C.; Xiao, Q.; Chen, Y.-L.; Shirakawa, H.; Yang, S.-C. Synbiotics Alleviate Hepatic Damage, Intestinal Injury and Muscular Beclin-1 Elevation in Rats after Chronic Ethanol Administration. Int. J. Mol. Sci. 2021, 22, 12547. https://doi.org/10.3390/ijms222212547
Chen Y-H, Chiu W-C, Xiao Q, Chen Y-L, Shirakawa H, Yang S-C. Synbiotics Alleviate Hepatic Damage, Intestinal Injury and Muscular Beclin-1 Elevation in Rats after Chronic Ethanol Administration. International Journal of Molecular Sciences. 2021; 22(22):12547. https://doi.org/10.3390/ijms222212547
Chicago/Turabian StyleChen, Yi-Hsiu, Wan-Chun Chiu, Qian Xiao, Ya-Ling Chen, Hitoshi Shirakawa, and Suh-Ching Yang. 2021. "Synbiotics Alleviate Hepatic Damage, Intestinal Injury and Muscular Beclin-1 Elevation in Rats after Chronic Ethanol Administration" International Journal of Molecular Sciences 22, no. 22: 12547. https://doi.org/10.3390/ijms222212547
APA StyleChen, Y.-H., Chiu, W.-C., Xiao, Q., Chen, Y.-L., Shirakawa, H., & Yang, S.-C. (2021). Synbiotics Alleviate Hepatic Damage, Intestinal Injury and Muscular Beclin-1 Elevation in Rats after Chronic Ethanol Administration. International Journal of Molecular Sciences, 22(22), 12547. https://doi.org/10.3390/ijms222212547






