The Association between Gut Microbiota and Osteoarthritis: Does the Disease Begin in the Gut?
Abstract
:1. Introduction
2. The Microbes Within: Good versus Evil
2.1. The Good
2.2. The Evil
3. GBM-Derived Metabolites and Osteoarthritis (OA) Progression
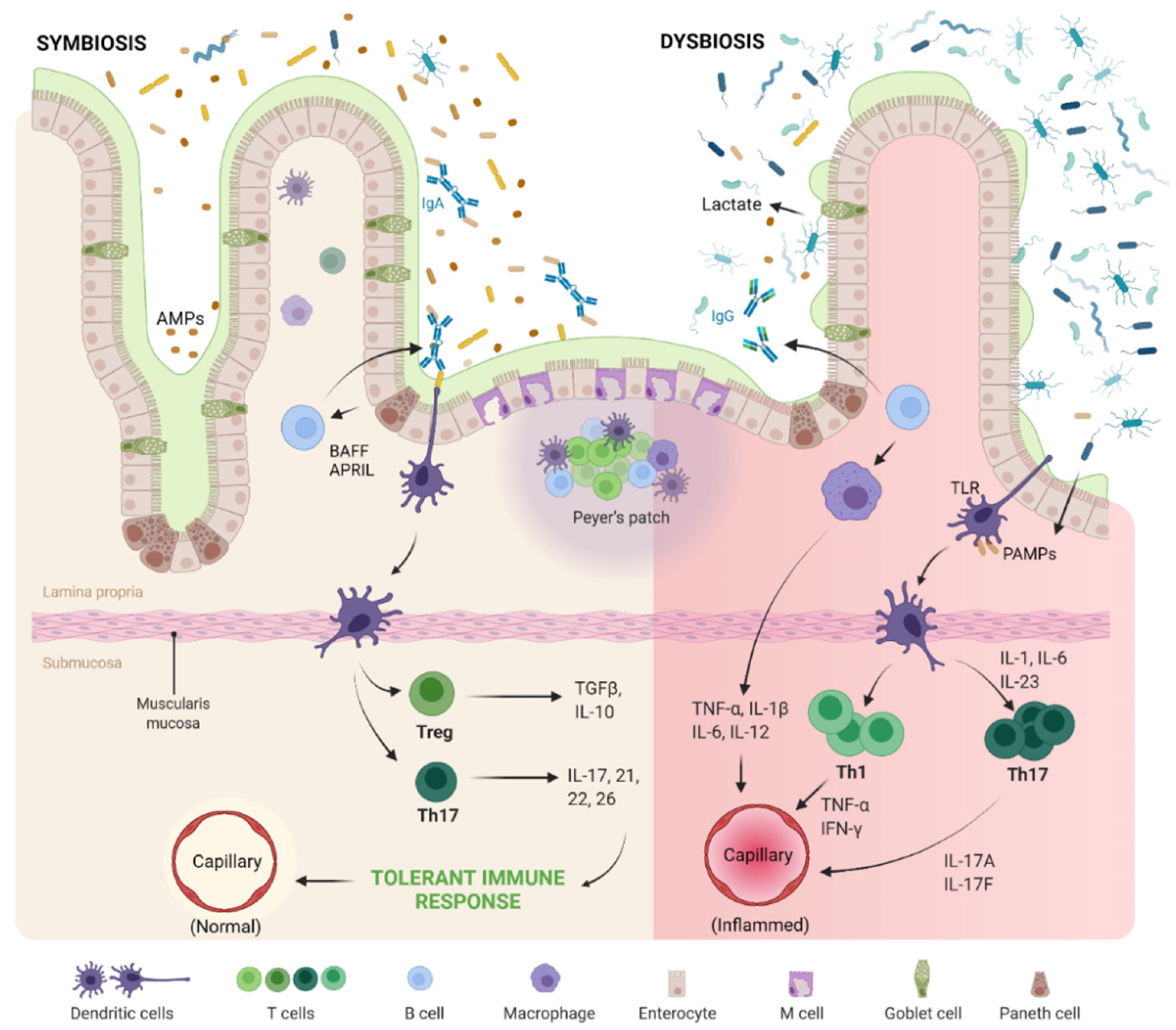
3.1. Gut–Joint Axis Distortion
3.2. Gut–Joint–Brain Axis Distortion
3.3. Evidences on Pathogenesis of OA
4. Interventional Strategies: Fixing Dysbiosis
4.1. Prebiotics
4.2. Probiotics
4.3. Physical Modulation
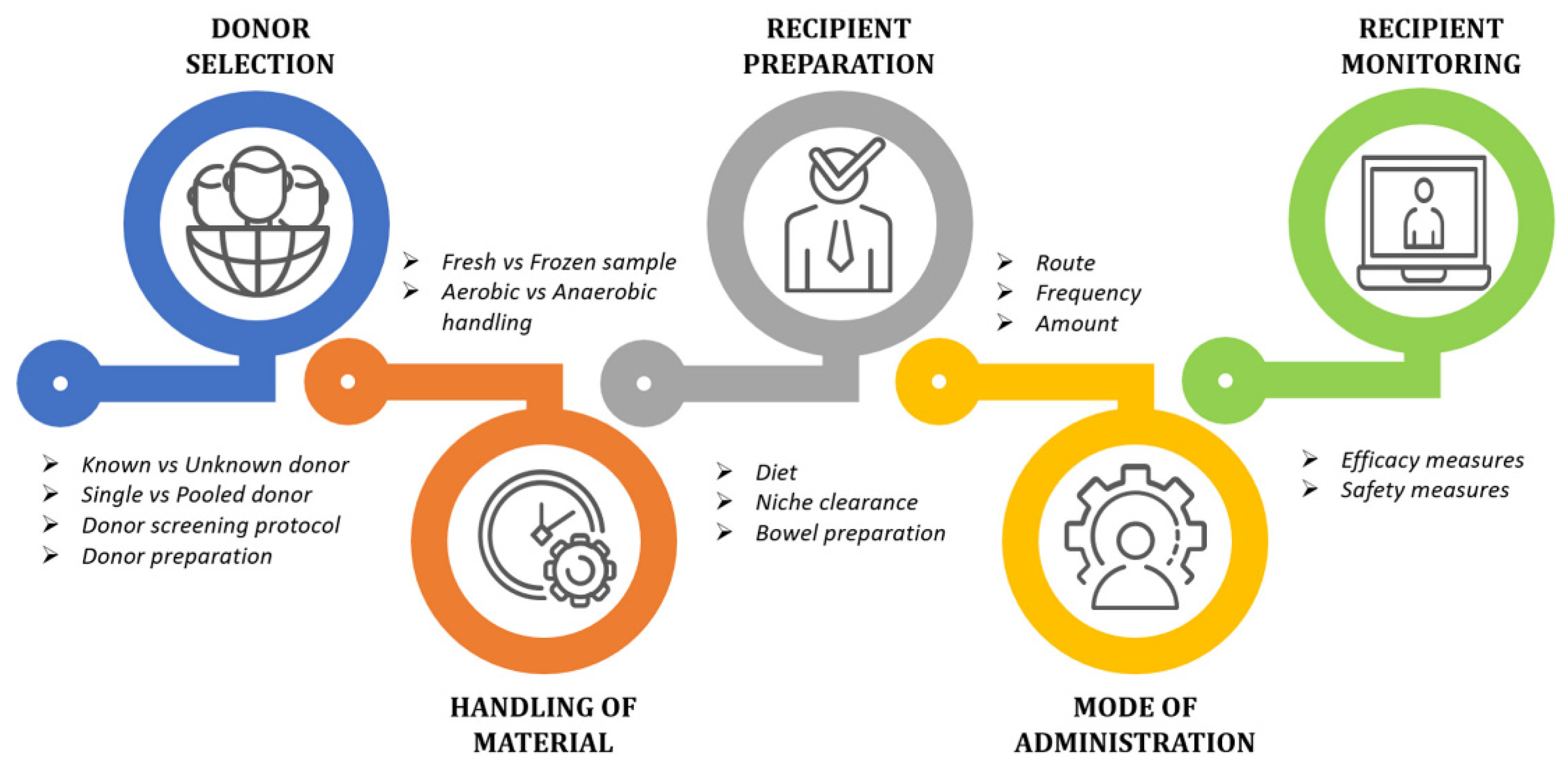
4.4. Fecal Microbiota Transplantation
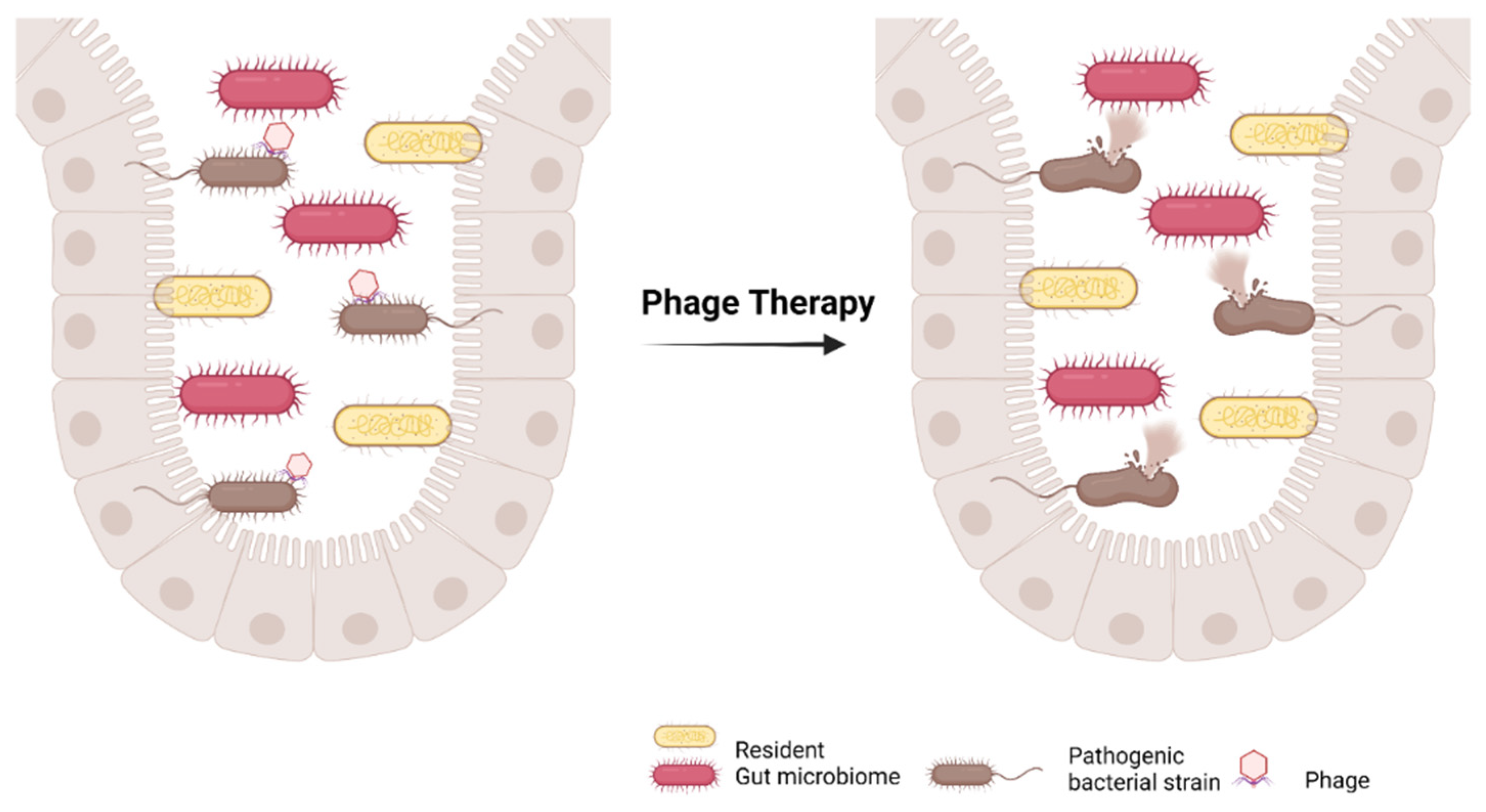
4.5. Bacteriophage Therapy
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hunter, D.J.; March, L.; Chew, M. Osteoarthritis in 2020 and beyond: A Lancet Commission. Lancet 2020, 396, 1711–1712. [Google Scholar] [CrossRef]
- Cui, A.; Li, H.; Wang, D.; Zhong, J.; Chen, Y.; Lu, H. Global, Regional Prevalence, Incidence and Risk Factors of Knee Osteoarthritis in Population-Based Studies. EClinicalMedicine 2020, 29, 100587. [Google Scholar] [CrossRef] [PubMed]
- Azzini, G.O.M.; Santos, G.S.; Visoni, S.B.C.; Azzini, V.O.M.; Dos Santos, R.G.; Huber, S.C.; Lana, J.F. Metabolic Syndrome and Subchondral Bone Alterations: The Rise of Osteoarthritis—A Review. J. Clin. Orthop. Trauma 2020, 11, S849–S855. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Shen, J.; Zhao, W.; Wang, T.; Han, L.; Hamilton, J.L.; Im, H.-J. Osteoarthritis: Toward a Comprehensive Understanding of Pathological Mechanism. Bone Res. 2017, 5, 16044. [Google Scholar] [CrossRef]
- Zhang, Y.; Jordan, J.M. Epidemiology of Osteoarthritis. Clin. Geriatr. Med. 2010, 26, 355–369. [Google Scholar] [CrossRef] [Green Version]
- Lana, J.F.; Macedo, A.; Ingrao, I.L.G.; Huber, S.C.; Santos, G.S.; Santana, M.H.A. Leukocyte-Rich PRP for Knee Osteoarthritis: Current Concepts. J. Clin. Orthop. Trauma 2019, 10, S179–S182. [Google Scholar] [CrossRef]
- Setti, T.; Arab, M.G.L.; Santos, G.S.; Alkass, N.; Andrade, M.A.P.; Lana, J.F.S.D. The Protective Role of Glutathione in Osteoarthritis. J. Clin. Orthop. Trauma 2021, 15, 145–151. [Google Scholar] [CrossRef]
- Alberti, K.G.M.M.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.-C.; James, W.P.T.; Loria, C.M.; Smith, S.C.; et al. Harmonizing the Metabolic Syndrome: A Joint Interim Statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009, 120, 1640–1645. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Xu, M.M.; Wang, K.; Adler, A.J.; Vella, A.T.; Zhou, B. Macrophage Polarization and Meta-Inflammation. Transl. Res. J. Lab. Clin. Med. 2018, 191, 29–44. [Google Scholar] [CrossRef]
- Robinson, W.H.; Lepus, C.M.; Wang, Q.; Raghu, H.; Mao, R.; Lindstrom, T.M.; Sokolove, J. Low-Grade Inflammation as a Key Mediator of the Pathogenesis of Osteoarthritis. Nat. Rev. Rheumatol. 2016, 12, 580–592. [Google Scholar] [CrossRef]
- Kapoor, M.; Martel-Pelletier, J.; Lajeunesse, D.; Pelletier, J.-P.; Fahmi, H. Role of Proinflammatory Cytokines in the Pathophysiology of Osteoarthritis. Nat. Rev. Rheumatol. 2011, 7, 33–42. [Google Scholar] [CrossRef]
- Sellam, J.; Berenbaum, F. The Role of Synovitis in Pathophysiology and Clinical Symptoms of Osteoarthritis. Nat. Rev. Rheumatol. 2010, 6, 625–635. [Google Scholar] [CrossRef] [PubMed]
- Mora, J.C.; Przkora, R.; Cruz-Almeida, Y. Knee Osteoarthritis: Pathophysiology and Current Treatment Modalities. J. Pain Res. 2018, 11, 2189–2196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hafsi, K.; McKay, J.; Li, J.; Lana, J.F.; Macedo, A.; Santos, G.S.; Murrell, W.D. Nutritional, Metabolic and Genetic Considerations to Optimise Regenerative Medicine Outcome for Knee Osteoarthritis. J. Clin. Orthop. Trauma 2019, 10, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Vitetta, L.; Coulson, S.; Linnane, A.W.; Butt, H. The Gastrointestinal Microbiome and Musculoskeletal Diseases: A Beneficial Role for Probiotics and Prebiotics. Pathogens 2013, 2, 606–626. [Google Scholar] [CrossRef] [PubMed]
- Marcum, Z.A.; Hanlon, J.T. Recognizing the Risks of Chronic Nonsteroidal Anti-Inflammatory Drug Use in Older Adults. Ann. Long-Term Care Off. J. Am. Med. Dir. Assoc. 2010, 18, 24. [Google Scholar]
- Kundu, P.; Blacher, E.; Elinav, E.; Pettersson, S. Our Gut Microbiome: The Evolving Inner Self. Cell 2017, 171, 1481–1493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van de Guchte, M.; Blottière, H.M.; Doré, J. Humans as Holobionts: Implications for Prevention and Therapy. Microbiome 2018, 6, 81. [Google Scholar] [CrossRef]
- Lazar, V.; Ditu, L.-M.; Pircalabioru, G.G.; Gheorghe, I.; Curutiu, C.; Holban, A.M.; Picu, A.; Petcu, L.; Chifiriuc, M.C. Aspects of Gut Microbiota and Immune System Interactions in Infectious Diseases, Immunopathology, and Cancer. Front. Immunol. 2018, 9, 1830. [Google Scholar] [CrossRef] [Green Version]
- Favazzo, L.J.; Hendesi, H.; Villani, D.A.; Soniwala, S.; Dar, Q.-A.; Schott, E.M.; Gill, S.R.; Zuscik, M.J. The Gut Microbiome-Joint Connection: Implications in Osteoarthritis. Curr. Opin. Rheumatol. 2020, 32, 92–101. [Google Scholar] [CrossRef]
- Griffin, T.M.; Huebner, J.L.; Kraus, V.B.; Yan, Z.; Guilak, F. Induction of Osteoarthritis and Metabolic Inflammation by a Very High-Fat Diet in Mice: Effects of Short-Term Exercise. Arthritis Rheum. 2012, 64, 443–453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yusuf, E.; Nelissen, R.G.; Ioan-Facsinay, A.; Stojanovic-Susulic, V.; DeGroot, J.; van Osch, G.; Middeldorp, S.; Huizinga, T.W.J.; Kloppenburg, M. Association between Weight or Body Mass Index and Hand Osteoarthritis: A Systematic Review. Ann. Rheum. Dis. 2010, 69, 761–765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thursby, E.; Juge, N. Introduction to the Human Gut Microbiota. Biochem. J. 2017, 474, 1823–1836. [Google Scholar] [CrossRef] [PubMed]
- Ley, R.E.; Turnbaugh, P.J.; Klein, S.; Gordon, J.I. Microbial Ecology: Human Gut Microbes Associated with Obesity. Nature 2006, 444, 1022–1023. [Google Scholar] [CrossRef] [PubMed]
- Jandhyala, S.M.; Talukdar, R.; Subramanyam, C.; Vuyyuru, H.; Sasikala, M.; Reddy, D.N. Role of the Normal Gut Microbiota. World J. Gastroenterol. 2015, 21, 8787. [Google Scholar] [CrossRef]
- Frank, D.N.; Amand, A.L.S.; Feldman, R.A.; Boedeker, E.C.; Harpaz, N.; Pace, N.R. Molecular-Phylogenetic Characterization of Microbial Community Imbalances in Human Inflammatory Bowel Diseases. Proc. Natl. Acad. Sci. USA 2007, 104, 13780–13785. [Google Scholar] [CrossRef] [Green Version]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What Is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef] [Green Version]
- Huttenhower, C.; Gevers, D.; Knight, R.; Abubucker, S.; Badger, J.H.; Chinwalla, A.T.; Creasy, H.H.; Earl, A.M.; Fitzgerald, M.G.; Fulton, R.S.; et al. Structure, Function and Diversity of the Healthy Human Microbiome. Nature 2012, 486, 207–214. [Google Scholar] [CrossRef] [Green Version]
- Khan, R.; Petersen, F.C.; Shekhar, S. Commensal Bacteria: An Emerging Player in Defense against Respiratory Pathogens. Front. Immunol. 2019, 10, 1203. [Google Scholar] [CrossRef] [Green Version]
- Sartor, R.B. Microbial Influences in Inflammatory Bowel Diseases. Gastroenterology 2008, 134, 577–594. [Google Scholar] [CrossRef]
- Delzenne, N.M. Oligosaccharides: State of the Art. Proc. Nutr. Soc. 2003, 62, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Magwira, C.A.; Kullin, B.; Lewandowski, S.; Rodgers, A.; Reid, S.J.; Abratt, V.R. Diversity of Faecal Oxalate-Degrading Bacteria in Black and White South African Study Groups: Insights into Understanding the Rarity of Urolithiasis in the Black Group. J. Appl. Microbiol. 2012, 113, 418–428. [Google Scholar] [CrossRef] [PubMed]
- Siener, R.; Bangen, U.; Sidhu, H.; Hönow, R.; Von Unruh, G.; Hesse, A. The Role of Oxalobacter Formigenes Colonization in Calcium Oxalate Stone Disease. Kidney Int. 2013, 83, 1144–1149. [Google Scholar] [CrossRef] [Green Version]
- Hooper, L.V.; Wong, M.H.; Thelin, A.; Hansson, L.; Falk, P.G.; Gordon, J.I. Molecular Analysis of Commensal Host-Microbial Relationships in the Intestine. Science 2001, 291, 881–884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Devillard, E.; McIntosh, F.M.; Paillard, D.; Thomas, N.A.; Shingfield, K.J.; Wallace, R.J. Differences between Human Subjects in the Composition of the Faecal Bacterial Community and Faecal Metabolism of Linoleic Acid. Microbiology 2009, 155, 513–520. [Google Scholar] [CrossRef] [Green Version]
- Fukiya, S.; Arata, M.; Kawashima, H.; Yoshida, D.; Kaneko, M.; Minamida, K.; Watanabe, J.; Ogura, Y.; Uchida, K.; Itoh, K.; et al. Conversion of Cholic Acid and Chenodeoxycholic Acid into Their 7-Oxo Derivatives by Bacteroides Intestinalis AM-1 Isolated from Human Feces. FEMS Microbiol. Lett. 2009, 293, 263–270. [Google Scholar] [CrossRef] [Green Version]
- Velagapudi, V.R.; Hezaveh, R.; Reigstad, C.S.; Gopalacharyulu, P.; Yetukuri, L.; Islam, S.; Felin, J.; Perkins, R.; Borén, J.; Orešič, M.; et al. The Gut Microbiota Modulates Host Energy and Lipid Metabolism in Mice. J. Lipid Res. 2010, 51, 1101–1112. [Google Scholar] [CrossRef] [Green Version]
- Thomas, C.M.; Hong, T.; van Pijkeren, J.P.; Hemarajata, P.; Trinh, D.V.; Hu, W.; Britton, R.A.; Kalkum, M.; Versalovic, J. Histamine Derived from Probiotic Lactobacillus Reuteri Suppresses Tnf via Modulation of Pka and Erk Signaling. PLoS ONE 2012, 7, e31951. [Google Scholar] [CrossRef] [Green Version]
- Marín, L.; Miguélez, E.M.; Villar, C.J.; Lombó, F. Bioavailability of Dietary Polyphenols and Gut Microbiota Metabolism: Antimicrobial Properties. BioMed Res. Int. 2015, 2015, 905215. [Google Scholar] [CrossRef] [Green Version]
- Macpherson, A.J.; Uhr, T. Induction of Protective IgA by Intestinal Dendritic Cells Carrying Commensal Bacteria. Science 2004, 303, 1662–1665. [Google Scholar] [CrossRef] [Green Version]
- He, B.; Xu, W.; Santini, P.A.; Polydorides, A.D.; Chiu, A.; Estrella, J.; Shan, M.; Chadburn, A.; Villanacci, V.; Plebani, A.; et al. Intestinal Bacteria Trigger T Cell-Independent Immunoglobulin A2 Class Switching by Inducing Epithelial-Cell Secretion of the Cytokine APRIL. Immunity 2007, 26, 812–826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Rashidi, H.E. Gut Microbiota and Immunity Relevance in Eubiosis and Dysbiosis. Saudi J. Biol. Sci. 2021; in press. [Google Scholar] [CrossRef]
- Hooper, L.V. Do symbiotic bacteria subvert host immunity? Nat. Rev. Genet. 2009, 7, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Cash, H.L.; Whitham, C.V.; Behrendt, C.L.; Hooper, L.V. Symbiotic Bacteria Direct Expression of an Intestinal Bactericidal Lectin. Science 2006, 313, 1126–1130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferreira, C.M.; Vieira, A.T.; Vinolo, M.A.R.; Oliveira, F.A.; Curi, R.; Martins, F.D.S. The Central Role of the Gut Microbiota in Chronic Inflammatory Diseases. J. Immunol. Res. 2014, 2014, 689492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kennedy, P.J.; Cryan, J.F.; Dinan, T.G.; Clarke, G. Irritable Bowel Syndrome: A Microbiome-Gut-Brain Axis Disorder? World J. Gastroenterol. 2014, 20, 14105. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, F.; Tremaroli, V.; Nielsen, J.; Bäckhed, F. Assessing the Human Gut Microbiota in Metabolic Diseases. Diabetes 2013, 62, 3341–3349. [Google Scholar] [CrossRef] [Green Version]
- Bisgaard, H.; Li, N.; Bonnelykke, K.; Chawes, B.; Skov, T.; Paludan-Müller, G.; Stokholm, J.; Smith, B.; Krogfelt, K.A. Reduced diversity of the intestinal microbiota during infancy is associated with increased risk of allergic disease at school age. J. Allergy Clin. Immunol. 2011, 128, 646–652.e5. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Li, S.; Gan, R.Y.; Zhou, T.; Xu, D.P.; Li, H.B. Impacts of Gut Bacteria on Human Health and Diseases. Int. J. Mol. Sci. 2015, 16, 7493–7519. [Google Scholar] [CrossRef]
- Abbott, A. Scientists bust myth that our bodies have more bacteria than human cells. Nature 2016. [Google Scholar] [CrossRef]
- Sender, R.; Fuchs, S.; Milo, R. Revised Estimates for the Number of Human and Bacteria Cells in the Body. PLoS Biol. 2016, 14, e1002533. [Google Scholar] [CrossRef] [Green Version]
- Cani, P.D.; Bibiloni, R.; Knauf, C.; Waget, A.; Neyrinck, A.M.; Delzenne, N.M.; Burcelin, R. Changes in Gut Microbiota Control Metabolic Endotoxemia-Induced Inflammation in High-Fat Diet-Induced Obesity and Diabetes in Mice. Diabetes 2008, 57, 1470–1481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Chen, W.-D.; Wang, Y.-D. The Relationship Between Gut Microbiota and Inflammatory Diseases: The Role of Macrophages. Front. Microbiol. 2020, 11, 1065. [Google Scholar] [CrossRef] [PubMed]
- Viola, A.; Munari, F.; Sánchez-Rodríguez, R.; Scolaro, T.; Castegna, A. The Metabolic Signature of Macrophage Responses. Front Immunol. 2019, 10, 1462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hauner, H. Secretory factors from human adipose tissue and their functional role. Proc. Nutr. Soc. 2005, 64, 163–169. [Google Scholar] [CrossRef] [Green Version]
- Weisberg, S.P.; McCann, D.; Desai, M.; Rosenbaum, M.; Leibel, R.L.; Ferrante, A.W., Jr. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Investig. 2003, 112, 1796–1808. [Google Scholar] [CrossRef]
- Ley, R.E.; Bäckhed, F.; Turnbaugh, P.; Lozupone, C.A.; Knight, R.D.; Gordon, J.I. Obesity alters gut microbial ecology. Proc. Natl. Acad. Sci. USA 2005, 102, 11070–11075. [Google Scholar] [CrossRef] [Green Version]
- Cani, P.D.; Possemiers, S.; Van De Wiele, T.; Guiot, Y.; Everard, A.; Rottier, O.; Geurts, L.; Naslain, D.; Neyrinck, A.; Lambert, D.M.; et al. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut 2009, 58, 1091–1103. [Google Scholar] [CrossRef] [Green Version]
- Houser, M.C.; Tansey, M.G. The Gut-Brain Axis: Is Intestinal Inflammation a Silent Driver of Parkinson’s Disease Pathogenesis? NPJ Park. Dis. 2017, 3, 3. [Google Scholar] [CrossRef]
- Harris, K.; Kassis, A.; Major, G.; Chou, C.J. Is the Gut Microbiota a New Factor Contributing to Obesity and Its Metabolic Disorders? J. Obes. 2012, 2012, 879151. [Google Scholar]
- Caesar, R.; Reigstad, C.S.; Bäckhed, H.K.; Reinhardt, C.; Ketonen, M.; Lundén, G.; Cani, P.D.; Bäckhed, F. Gut-derived lipopolysaccharide augments adipose macrophage accumulation but is not essential for impaired glucose or insulin tolerance in mice. Gut 2012, 61, 1701–1707. [Google Scholar] [CrossRef] [Green Version]
- Harford, K.A.; Reynolds, C.; McGillicuddy, F.; Roche, H.M. Fats, inflammation and insulin resistance: Insights to the role of macrophage and T-cell accumulation in adipose tissue. Proc. Nutr. Soc. 2011, 70, 408–417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orr, J.S.; Puglisi, M.J.; Ellacott, K.L.; Lumeng, C.N.; Wasserman, D.H.; Hasty, A.H. Toll-like Receptor 4 Deficiency Promotes the Alternative Activation of Adipose Tissue Macrophages. Diabetes 2012, 61, 2718–2727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamada, N.; Hisamatsu, T.; Okamoto, S.; Chinen, H.; Kobayashi, T.; Sato, T.; Sakuraba, A.; Kitazume, M.T.; Sugita, A.; Koganei, K.; et al. Unique CD14+ intestinal macrophages contribute to the pathogenesis of Crohn disease via IL-23/IFN-γ axis. J. Clin. Investig. 2008, 118, 2269–2280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collins, K.; Paul, H.; Reimer, R.; Seerattan, R.; Hart, D.; Herzog, W. Relationship between inflammation, the gut microbiota, and metabolic osteoarthritis development: Studies in a rat model. Osteoarthr. Cartil. 2015, 23, 1989–1998. [Google Scholar] [CrossRef] [Green Version]
- Huang, Z.; Stabler, T.; Pei, F.; Kraus, V. Both systemic and local lipopolysaccharide (LPS) burden are associated with knee OA severity and inflammation. Osteoarthr. Cartil. 2016, 24, 1769–1775. [Google Scholar] [CrossRef] [Green Version]
- Ulici, V.; Kelley, K.; Azcarate-Peril, M.; Cleveland, R.; Sartor, R.; Schwartz, T.; Loeser, R. Osteoarthritis induced by destabilization of the medial meniscus is reduced in germ-free mice. Osteoarthr. Cartil. 2018, 26, 1098–1109. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.L.A.; Vieira-Silva, S.; Liston, A.; Raes, J. How informative is the mouse for human gut microbiota research? Dis. Model. Mech. 2015, 8, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Wos-Oxley, M.L.; Bleich, A.; Oxley, A.P.; Kahl, S.; Janus, L.M.; Smoczek, A.; Nahrstedt, H.; Pils, M.C.; Taudien, S.; Platzer, M.; et al. Comparative evaluation of establishing a human gut microbial community within rodent models. Gut Microbes 2012, 3, 234–249. [Google Scholar] [CrossRef] [Green Version]
- Szychlinska, M.A.; Di Rosa, M.; Castorina, A.; Mobasheri, A.; Musumeci, G. A correlation between intestinal microbiota dysbiosis and osteoarthritis. Heliyon 2019, 5, e01134. [Google Scholar] [CrossRef] [Green Version]
- Romero, E.S.; Oliva, E.M.; Pérez, J.A.; Pérez, S.M.; Turroni, S.; Marchese, L.; Villafañe, J. Relationship between the Gut Microbiome and Osteoarthritis Pain: Review of the Literature. Nutrients 2021, 13, 716. [Google Scholar] [CrossRef]
- Collins, S.M.; Surette, M.; Bercik, P. The interplay between the intestinal microbiota and the brain. Nat. Rev. Microbiol. 2012, 10, 735–742. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Hunter, D.; Xu, J.; Ding, C. Metabolic triggered inflammation in osteoarthritis. Osteoarthr. Cartil. 2015, 23, 22–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Ding, W.; Wang, H.; Dai, L.; Zong, W.; Wang, Y.; Bi, J.; Han, W.; Dong, G. Gut microbiota and obesity-associated osteoarthritis. Osteoarthr. Cartil. 2019, 27, 1257–1265. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Gu, X.; Yang, J.; Wei, Y.; Zhao, Y. Gut Microbiota Dysbiosis and Increased Plasma LPS and TMAO Levels in Patients with Preeclampsia. Front. Cell. Infect. Microbiol. 2019, 9, 409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hao, X.; Shang, X.; Liu, J.; Chi, R.; Zhang, J.; Xu, T. The gut microbiota in osteoarthritis: Where do we stand and what can we do? Arthritis Res. Ther. 2021, 23, 42. [Google Scholar] [CrossRef] [PubMed]
- Boutagy, N.E.; McMillan, R.P.; Frisard, M.I.; Hulver, M.W. Metabolic endotoxemia with obesity: Is it real and is it relevant? Biochimie 2016, 124, 11–20. [Google Scholar] [CrossRef] [Green Version]
- Berenbaum, F. Deep phenotyping of osteoarthritis: A step forward. Ann. Rheum. Dis. 2019, 78, 3–5. [Google Scholar] [CrossRef] [Green Version]
- Chadha, R. Revealed aspect of metabolic osteoarthritis. J. Orthop. 2016, 13, 347–351. [Google Scholar] [CrossRef] [Green Version]
- Sellam, J.; Berenbaum, F. Is osteoarthritis a metabolic disease? Jt. Bone Spine 2013, 80, 568–573. [Google Scholar] [CrossRef]
- Berenbaum, F.; Griffin, T.M.; Liu-Bryan, R. Review: Metabolic Regulation of Inflammation in Osteoarthritis. Arthritis Rheumatol. Hoboken NJ 2017, 69, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Turroni, S.; Pedersini, P.; Villafañe, J.H. The Human Gut Microbiome and Its Relationship with Osteoarthritis Pain. Pain Med. Malden Mass 2021, 22, 1467–1469. [Google Scholar] [CrossRef] [PubMed]
- de Sire, A.; de Sire, R.; Petito, V.; Masi, L.; Cisari, C.; Gasbarrini, A.; Scaldaferri, F.; Invernizzi, M. Gut–Joint Axis: The Role of Physical Exercise on Gut Microbiota Modulation in Older People with Osteoarthritis. Nutrients 2020, 12, 574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gracey, E.; Vereecke, L.; McGovern, D.; Fröhling, M.; Schett, G.; Danese, S.; De Vos, M.; Bosch, F.V.D.; Elewaut, D. Revisiting the gut–joint axis: Links between gut inflammation and spondyloarthritis. Nat. Rev. Rheumatol. 2020, 16, 415–433. [Google Scholar] [CrossRef]
- Zaiss, M.M.; Wu, H.-J.J.; Mauro, D.; Schett, G.; Ciccia, F. The gut–joint axis in rheumatoid arthritis. Nat. Rev. Rheumatol. 2021, 17, 224–237. [Google Scholar] [CrossRef]
- Qaiyum, Z.; Lim, M.; Inman, R.D. The gut-joint axis in spondyloarthritis: Immunological, microbial, and clinical insights. Semin. Immunopathol. 2021, 43, 173–192. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Kraus, V.B. Does lipopolysaccharide-mediated inflammation have a role in OA? Nat Rev Rheumatol. 2016, 12, 123–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dunn, C.M.; Velasco, C.; Rivas, A.; Andrews, M.; Garman, C.; Jacob, P.B.; Jeffries, M.A. Identification of Cartilage Microbial DNA Signatures and Associations with Knee and Hip Osteoarthritis. Arthritis Rheumatol. 2020, 72, 1111–1122. [Google Scholar] [CrossRef]
- Guss, J.D.; Ziemian, S.N.; Luna, M.; Sandoval, T.N.; Holyoak, D.T.; Guisado, G.G.; Roubert, S.; Callahan, R.L.; Brito, I.L.; van der Meulen, M.C.; et al. The effects of metabolic syndrome, obesity, and the gut microbiome on load-induced osteoarthritis. Osteoarthr. Cartil. 2019, 27, 129–139. [Google Scholar] [CrossRef] [Green Version]
- King, C.; Sibille, K.; Goodin, B.; Cruz-Almeida, Y.; Glover, T.; Bartley, E.; Riley, J.; Herbert, M.; Sotolongo, A.; Schmidt, J.; et al. Experimental pain sensitivity differs as a function of clinical pain severity in symptomatic knee osteoarthritis. Osteoarthr. Cartil. OARS Osteoarthr. Res. Soc. 2013, 21, 1243–1252. [Google Scholar] [CrossRef] [Green Version]
- Boer, C.G.; Radjabzadeh, D.; Medina-Gomez, C.; Garmaeva, S.; Schiphof, D.; Arp, P.; Koet, T.; Kurilshikov, A.; Fu, J.; Ikram, M.A.; et al. Intestinal microbiome composition and its relation to joint pain and inflammation. Nat. Commun. 2019, 10, 4881. [Google Scholar] [CrossRef] [Green Version]
- Mukhtar, K.; Nawaz, H.; Abid, S. Functional gastrointestinal disorders and gut-brain axis: What does the future hold? World J. Gastroenterol. 2019, 25, 552–566. [Google Scholar] [CrossRef]
- Silva, Y.P.; Bernardi, A.; Frozza, R.L. The Role of Short-Chain Fatty Acids from Gut Microbiota in Gut-Brain Communication. Front. Endocrinol. 2020, 11, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boer, C.; Radjabzadeh, D.; Uitterlinden, A.; Kraaij, R.; van Meurs, J. The role of the gut microbiome in osteoarthritis and joint pain. Osteoarthr. Cartil. 2017, 25, S10. [Google Scholar] [CrossRef] [Green Version]
- Coulson, S.; Butt, H.; Vecchio, P.; Gramotnev, H.; Vitetta, L. Green-lipped mussel extract (Perna canaliculus) and glucosamine sulphate in patients with knee osteoarthritis: Therapeutic efficacy and effects on gastrointestinal microbiota profiles. Inflammopharmacology 2013, 21, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Pedersen, O. Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol. 2021, 19, 55–71. [Google Scholar] [CrossRef]
- Gibson, G.R.; Scott, K.P.; Rastall, R.A.; Tuohy, K.M.; Hotchkiss, A.; Dubert-Ferrandon, A.; Gareau, M.; Murphy, E.F.; Saulnier, D.; Loh, G.; et al. Dietary prebiotics: Current status and new definition. Food Sci. Technol. Bull. Funct. Foods 2010, 7, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef] [Green Version]
- Tzounis, X.; Rodriguez-Mateos, A.; Vulevic, J.; Gibson, G.R.; Kwik-Uribe, C.; Spencer, J.P. Prebiotic evaluation of cocoa-derived flavanols in healthy humans by using a randomized, controlled, double-blind, crossover intervention study. Am. J. Clin. Nutr. 2011, 93, 62–72. [Google Scholar] [CrossRef] [Green Version]
- Davani-Davari, D.; Negahdaripour, M.; Karimzadeh, I.; Seifan, M.; Mohkam, M.; Masoumi, S.J.; Berenjian, A.; Ghasemi, Y. Prebiotics: Definition, Types, Sources, Mechanisms, and Clinical Applications. Foods 2019, 8, 92. [Google Scholar] [CrossRef] [Green Version]
- Makki, K.; Deehan, E.C.; Walter, J.; Bäckhed, F. The Impact of Dietary Fiber on Gut Microbiota in Host Health and Disease. Cell Host Microbe 2018, 23, 705–715. [Google Scholar] [CrossRef] [Green Version]
- Kobyliak, N.; Falalyeyeva, T.; Mykhalchyshyn, G.; Kyriienko, D.; Komissarenko, I. Effect of alive probiotic on insulin resistance in type 2 diabetes patients: Randomized clinical trial. Diabetes Metab. Syndr. Clin. Res. Rev. 2018, 12, 617–624. [Google Scholar] [CrossRef] [PubMed]
- Kamada, N.; Seo, S.-U.; Chen, G.Y.; Núñez, G. Role of the gut microbiota in immunity and inflammatory disease. Nat. Rev. Immunol. 2013, 13, 321–335. [Google Scholar] [CrossRef] [PubMed]
- Guinane, C.M.; Cotter, P.D. Role of the gut microbiota in health and chronic gastrointestinal disease: Understanding a hidden metabolic organ. Ther. Adv. Gastroenterol. 2013, 6, 295–308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, B.; Yao, M.; Lv, L.; Ling, Z.; Li, L. The Human Microbiota in Health and Disease. Engineering 2017, 3, 71–82. [Google Scholar] [CrossRef]
- Wu, H.J.; Wu, E. The role of gut microbiota in immune homeostasis and autoimmunity. Gut Microbes 2012, 3, 4–14. [Google Scholar] [CrossRef] [Green Version]
- Soderholm, A.T.; Pedicord, V.A. Intestinal epithelial cells: At the interface of the microbiota and mucosal immunity. Immunology 2019, 158, 267–280. [Google Scholar] [CrossRef] [Green Version]
- Morhardt, T.L.; Hayashi, A.; Ochi, T.; Quirós, M.; Kitamoto, S.; Nagao-Kitamoto, H.; Kuffa, P.; Atarashi, K.; Honda, K.; Kao, J.Y.; et al. IL-10 produced by macrophages regulates epithelial integrity in the small intestine. Sci. Rep. 2019, 9, 1223. [Google Scholar] [CrossRef] [Green Version]
- Thoo, L.; Noti, M.; Krebs, P. Keep calm: The intestinal barrier at the interface of peace and war. Cell Death Dis. 2019, 10, 849. [Google Scholar] [CrossRef] [Green Version]
- Parada Venegas, D.; De La Fuente, M.K.; Landskron, G.; González, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.J.M.; Faber, K.N.; Hermoso, M.A. Short Chain Fatty Acids (SCFAs)-Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Front. Immunol. 2019, 10, 277. [Google Scholar] [CrossRef] [Green Version]
- Blaak, E.E.; Canfora, E.E.; Theis, S.; Frost, G.; Groen, A.K.; Mithieux, G.; Nauta, A.; Scott, K.; Stahl, B.; Van Harsselaar, J.; et al. Short chain fatty acids in human gut and metabolic health. Benef. Microbes 2020, 11, 411–455. [Google Scholar] [CrossRef]
- Li, M.; Van Esch, B.C.A.M.; Henricks, P.A.J.; Folkerts, G.; Garssen, J. The Anti-inflammatory Effects of Short Chain Fatty Acids on Lipopolysaccharide- or Tumor Necrosis Factor α-Stimulated Endothelial Cells via Activation of GPR41/43 and Inhibition of HDACs. Front. Pharmacol. 2018, 9, 533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, M.; Van Esch, B.C.A.M.; Wagenaar, G.T.M.; Garssen, J.; Folkerts, G.; Henricks, P.A.J. Pro- and anti-inflammatory effects of short chain fatty acids on immune and endothelial cells. Eur. J. Pharmacol. 2018, 831, 52–59. [Google Scholar] [CrossRef]
- Park, J.; Wang, Q.; Wu, Q.; Mao-Draayer, Y.; Kim, C.H. Bidirectional regulatory potentials of short-chain fatty acids and their G-protein-coupled receptors in autoimmune neuroinflammation. Sci. Rep. 2019, 9, 8837. [Google Scholar] [CrossRef] [Green Version]
- Camilleri, M. Leaky gut: Mechanisms, measurement and clinical implications in humans. Gut 2019, 68, 1516–1526. [Google Scholar] [CrossRef] [PubMed]
- Mu, Q.; Kirby, J.; Reilly, C.M.; Luo, X.M. Leaky Gut As a Danger Signal for Autoimmune Diseases. Front. Immunol. 2017, 8, 598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thangaraju, M.; Cresci, G.A.; Liu, K.; Ananth, S.; Gnanaprakasam, J.P.; Browning, D.D.; Mellinger, J.D.; Smith, S.B.; Digby, G.J.; Lambert, N.A.; et al. GPR109A Is a G-protein–Coupled Receptor for the Bacterial Fermentation Product Butyrate and Functions as a Tumor Suppressor in Colon. Cancer Res. 2009, 69, 2826–2832. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stagg, A.J. Intestinal Dendritic Cells in Health and Gut Inflammation. Front. Immunol. 2018, 9, 2883. [Google Scholar] [CrossRef] [Green Version]
- Singh, N.; Gurav, A.; Sivaprakasam, S.; Brady, E.; Padia, R.; Shi, H.; Thangaraju, M.; Prasad, P.D.; Manicassamy, S.; Munn, D.H.; et al. Activation of Gpr109a, Receptor for Niacin and the Commensal Metabolite Butyrate, Suppresses Colonic Inflammation and Carcinogenesis. Immunity 2014, 40, 128–139. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Li, X.; Lin, S.; Chen, Y.; Ma, S.; Fu, Y.; Wei, C.; Xu, W. Nicotinic Acid Receptor GPR109A Exerts Anti-Inflammatory Effects Through Inhibiting the Akt/mTOR Signaling Pathway in MIN6 Pancreatic β cells. Ann. Clin. Lab. Sci. 2017, 47, 729–737. [Google Scholar]
- Macia, L.; Tan, J.; Vieira, A.T.; Leach, K.; Stanley, D.; Luong, S.; Maruya, M.; McKenzie, C.l.; Hijikata, A.; Wong, C.; et al. Metabolite-sensing receptors GPR43 and GPR109A facilitate dietary fibre-induced gut homeostasis through regulation of the inflammasome. Nat. Commun. 2015, 6, 6734. [Google Scholar] [CrossRef] [Green Version]
- Mack, D.R. Probiotics: Mixed messages. Can. Fam. Physician 2005, 51, 1455–1457. [Google Scholar] [PubMed]
- So, J.-S.; Kwon, H.-K.; Lee, C.-G.; Yi, H.-J.; Park, J.-A.; Lim, S.-Y.; Hwang, K.-C.; Jeon, Y.H.; Im, S.-H. Lactobacillus casei suppresses experimental arthritis by down-regulating T helper 1 effector functions. Mol. Immunol. 2008, 45, 2690–2699. [Google Scholar] [CrossRef] [PubMed]
- Amdekar, S.; Singh, V.; Singh, R.; Sharma, P.; Keshav, P.; Kumar, A. Lactobacillus casei reduces the Inflammatory Joint Damage Associated with Collagen-Induced Arthritis (CIA) by Reducing the Pro-Inflammatory Cytokines. J. Clin. Immunol. 2011, 31, 147–154. [Google Scholar] [CrossRef] [PubMed]
- de los Angeles Pineda, M.; Thompson, S.F.; Summers, K.; De Leon, F.; Pope, J.; Reid, G. A randomized, double-blinded, placebo-controlled pilot study of probiotics in active rheumatoid arthritis. Med. Sci. Monit. 2011, 17, CR347–CR354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- So, J.-S.; Song, M.-K.; Kwon, H.-K.; Lee, C.-G.; Chae, C.-S.; Sahoo, A.; Jash, A.; Lee, S.H.; Park, Z.Y.; Im, S.-H. Lactobacillus casei enhances type II collagen/glucosamine-mediated suppression of inflammatory responses in experimental osteoarthritis. Life Sci. 2011, 88, 358–366. [Google Scholar] [CrossRef] [PubMed]
- Bermudez-Brito, M.; Plaza-Díaz, J.; Muñoz-Quezada, S.; Gómez-Llorente, C.; Gil, A. Probiotic Mechanisms of Action. Ann. Nutr. Metab. 2012, 61, 160–174. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.S.; Jeraldo, P.R.; Kurti, A.; Miller, M.E.B.; Cook, M.D.; Whitlock, K.; Goldenfeld, N.; Woods, J.A.; White, B.A.; Chia, N.; et al. Diet and exercise orthogonally alter the gut microbiome and reveal independent associations with anxiety and cognition. Mol. Neurodegener. 2014, 9, 36. [Google Scholar] [CrossRef] [Green Version]
- Petriz, B.A.; Castro, A.P.; Almeida, J.A.; Gomes, C.P.; Fernandes, G.R.; Kruger, R.H.; Pereira, R.W.; Franco, O.L. Exercise induction of gut microbiota modifications in obese, non-obese and hypertensive rats. BMC Genom. 2014, 15, 511. [Google Scholar] [CrossRef] [Green Version]
- Lambert, J.E.; Myslicki, J.P.; Bomhof, M.R.; Belke, D.D.; Shearer, J.; Reimer, R.A. Exercise training modifies gut microbiota in normal and diabetic mice. Appl. Physiol. Nutr. Metab. 2015, 40, 749–752. [Google Scholar] [CrossRef]
- Evans, C.C.; LePard, K.J.; Kwak, J.W.; Stancukas, M.C.; Laskowski, S.; Dougherty, J.; Moulton, L.; Glawe, A.; Wang, Y.; Leone, V.; et al. Exercise Prevents Weight Gain and Alters the Gut Microbiota in a Mouse Model of High Fat Diet-Induced Obesity. PLoS ONE 2014, 9, e92193. [Google Scholar] [CrossRef]
- Denou, E.; Marcinko, K.; Surette, M.G.; Steinberg, G.R.; Schertzer, J.D. High-intensity exercise training increases the diversity and metabolic capacity of the mouse distal gut microbiota during diet-induced obesity. Am. J. Physiol. Endocrinol. Metab. 2016, 310, E982–E993. [Google Scholar] [CrossRef] [PubMed]
- Campbell, S.C.; Wisniewski, P.J.; Noji, M.; McGuinness, L.R.; Haggblom, M.M.; Lightfoot, S.A.; Joseph, L.B.; Kerkhof, L. The Effect of Diet and Exercise on Intestinal Integrity and Microbial Diversity in Mice. PLoS ONE 2016, 11, e0150502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamoureux, E.V.; Grandy, S.A.; Langille, M.G.I. Moderate Exercise Has Limited but Distinguishable Effects on the Mouse Microbiome. mSystems 2017, 2, e00006-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsumoto, M.; Inoue, R.; Tsukahara, T.; Ushida, K.; Chiji, H.; Matsubara, N.; Hara, H. Voluntary Running Exercise Alters Microbiota Composition and Increases n-Butyrate Concentration in the Rat Cecum. Biosci. Biotechnol. Biochem. 2008, 72, 572–576. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.; Miller, M.E.B.; Pence, B.D.; Whitlock, K.; Nehra, V.; Gaskins, H.R.; White, B.A.; Fryer, J.D.; Woods, J.A. Voluntary and forced exercise differentially alters the gut microbiome in C57BL/6J mice. J. Appl. Physiol. 2015, 118, 1059–1066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Säemann, M.D.; Böhmig, G.A.; Österreicher, C.H.; Burtscher, H.; Parolini, O.; Diakos, C.; Stöckl, J.; Hörl, W.H.; Zlabinger, G.J. Anti-inflammatory effects of sodium butyrate on human monocytes: Potent inhibition of IL-12 and up-regulation of IL-10 production. FASEB J. 2000, 14, 2380–2382. [Google Scholar] [CrossRef]
- Peng, L.; Li, Z.-R.; Green, R.S.; Holzman, I.R.; Lin, J. Butyrate Enhances the Intestinal Barrier by Facilitating Tight Junction Assembly via Activation of AMP-Activated Protein Kinase in Caco-2 Cell Monolayers. J. Nutr. 2009, 139, 1619–1625. [Google Scholar] [CrossRef]
- Clarke, S.; Murphy, E.F.; O’Sullivan, O.; Lucey, A.; Humphreys, M.; Hogan, A.; Hayes, P.; O’Reilly, M.; Jeffery, I.; Wood-Martin, R.; et al. Exercise and associated dietary extremes impact on gut microbial diversity. Gut 2014, 63, 1913–1920. [Google Scholar] [CrossRef] [Green Version]
- Bressa, C.; Bailén-Andrino, M.; Pérez-Santiago, J.; González-Soltero, R.; Pérez, M.; Montalvo-Lominchar, M.G.; Maté-Muñoz, J.L.; Domínguez, R.; Moreno, D.; Larrosa, M. Differences in gut microbiota profile between women with active lifestyle and sedentary women. PLoS ONE 2017, 12, e0171352. [Google Scholar] [CrossRef] [Green Version]
- Louis, P.; Flint, H.J. Diversity, metabolism and microbial ecology of butyrate-producing bacteria from the human large intestine. FEMS Microbiol. Lett. 2009, 294, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Dao, M.C.; Everard, A.; Aron-Wisnewsky, J.; Sokolovska, N.; Prifti, E.; Verger, E.O.; Kayser, B.D.; Levenez, F.; Chilloux, J.; Hoyles, L.; et al. Akkermansia muciniphila and improved metabolic health during a dietary intervention in obesity: Relationship with gut microbiome richness and ecology. Gut 2016, 65, 426–436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mailing, L.J.; Allen, J.M.; Buford, T.W.; Fields, C.J.; Woods, J.A. Exercise and the Gut Microbiome: A Review of the Evidence, Potential Mechanisms, and Implications for Human Health. Exerc. Sport Sci. Rev. 2019, 47, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Hoffman-Goetz, L.; Pervaiz, N.; Packer, N.; Guan, J. Freewheel training decreases pro- and increases anti-inflammatory cytokine expression in mouse intestinal lymphocytes. Brain Behav. Immun. 2010, 24, 1105–1115. [Google Scholar] [CrossRef]
- Packer, N.; Hoffman-Goetz, L. Exercise Training Reduces Inflammatory Mediators in the Intestinal Tract of Healthy Older Adult Mice. Can. J. Aging/La Rev. Can. Vieil. 2012, 31, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Hoffman-Goetz, L.; Pervaiz, N.; Guan, J. Voluntary exercise training in mice increases the expression of antioxidant enzymes and decreases the expression of TNF-α in intestinal lymphocytes. Brain Behav. Immun. 2009, 23, 498–506. [Google Scholar] [CrossRef]
- Fehrenbach, E.; Niess, A.M.; Schlotz, E.; Passek, F.; Dickhuth, H.-H.; Northoff, H. Transcriptional and translational regulation of heat shock proteins in leukocytes of endurance runners. J. Appl. Physiol. 2000, 89, 704–710. [Google Scholar] [CrossRef] [Green Version]
- Lira, F.S.; Rosa, J.C.; Pimentel, G.D.; Souza, H.A.; Caperuto, E.C.; Carnevali, L.C., Jr.; Seelaender, M.; Damaso, A.R.; Oyama, L.M.; de Mello, M.T.; et al. Endotoxin levels correlate positively with a sedentary lifestyle and negatively with highly trained subjects. Lipids Heal. Dis. 2010, 9, 82. [Google Scholar] [CrossRef] [Green Version]
- Dokladny, K.; Moseley, P.L.; Ma, T.Y. Physiologically relevant increase in temperature causes an increase in intestinal epithelial tight junction permeability. Am. J. Physiol. Liver Physiol. 2006, 290, G204–G212. [Google Scholar] [CrossRef]
- Meng, Y.; Chen, L.; Lin, W.; Wang, H.; Xu, G.; Weng, X. Exercise Reverses the Alterations in Gut Microbiota Upon Cold Exposure and Promotes Cold-Induced Weight Loss. Front. Physiol. 2020, 11, 311. [Google Scholar] [CrossRef]
- Song, N.; Liu, X.; Feng, Q.; Xu, M.; Lan, X.; Li, M.; Liu, R.; Li, C.; Dong, T.; Wang, D.; et al. Whole Body Vibration Triggers a Change in the Mutual Shaping State of Intestinal Microbiota and Body’s Immunity. Front. Bioeng. Biotechnol. 2019, 7, 377. [Google Scholar] [CrossRef]
- Godinez, A.; Liston, D.B.; Ayzenberg, R.; Toscano, W.B.; Cowings, P.A.; Stone, L.S. G-Loading and Vibration Effects on Heart and Respiration Rates. Aviat. Space Environ. Med. 2014, 85, 949–953. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.C.; Hale, V.L.; Khodadadi, H.; Baban, B. Whole Body Vibration-Induced Omental Macrophage Polarization and Fecal Microbiome Modification in a Murine Model. Int. J. Mol. Sci. 2019, 20, 3125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abercromby, A.F.J.; Amonette, W.E.; Layne, C.S.; Mcfarlin, B.K.; Hinman, M.R.; Paloski, W.H. Vibration Exposure and Biodynamic Responses during Whole-Body Vibration Training. Med. Sci. Sports Exerc. 2007, 39, 1794–1800. [Google Scholar] [CrossRef] [PubMed]
- Merriman, H.; Jackson, K. The Effects of Whole-Body Vibration Training in Aging Adults: A Systematic Review. J. Geriatr. Phys. Ther. 2009, 32, 134–145. [Google Scholar] [CrossRef]
- Okumura, R.; Takeda, K. Maintenance of intestinal homeostasis by mucosal barriers. Inflamm. Regen. 2018, 38, 5. [Google Scholar] [CrossRef]
- Maloy, K.J.; Powrie, F. Intestinal homeostasis and its breakdown in inflammatory bowel disease. Nature 2011, 474, 298–306. [Google Scholar] [CrossRef]
- Heeney, D.D.; Gareau, M.G.; Marco, M.L. Intestinal Lactobacillus in health and disease, a driver or just along for the ride? Curr. Opin. Biotechnol. 2018, 49, 140–147. [Google Scholar] [CrossRef] [Green Version]
- Hirao, L.A.; Grishina, I.; Bourry, O.; Hu, W.K.; Somrit, M.; Sankaran-Walters, S.; Gaulke, C.A.; Fenton, A.N.; Li, J.A.; Crawford, R.W.; et al. Early Mucosal Sensing of SIV Infection by Paneth Cells Induces IL-1β Production and Initiates Gut Epithelial Disruption. PLoS Pathog. 2014, 10, e1004311. [Google Scholar] [CrossRef] [Green Version]
- Gupta, S.; Allen-Vercoe, E.; Petrof, E.O. Fecal microbiota transplantation: In perspective. Ther. Adv. Gastroenterol. 2016, 9, 229–239. [Google Scholar] [CrossRef] [Green Version]
- Ser, H.L.; Letchumanan, V.; Goh, B.H.; Wong, S.H.; Lee, L.H. The Use of Fecal Microbiome Transplant in Treating Human Diseases: Too Early for Poop? Front. Microbiol. 2021, 12, 1005. [Google Scholar] [CrossRef]
- Khoruts, A. Fecal microbiota transplantation–early steps on a long journey ahead. Gut Microbes 2017, 8, 199–204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, M.-Q.; Cao, H.-L.; Wang, W.-Q.; Wang, S.; Cao, X.-C.; Yan, F.; Wang, B.-M. Fecal microbiota transplantation broadening its application beyond intestinal disorders. World J. Gastroenterol. 2015, 21, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Borody, T.J.; Khoruts, A. Fecal microbiota transplantation and emerging applications. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 88–96. [Google Scholar] [CrossRef]
- Van Nood, E.; Speelman, P.; Nieuwdorp, M.; Keller, J. Fecal Microbiota Transplantation: Facts and Controversies. Curr. Opin. Gastroenterol. 2014, 30, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Borody, T.J.; Campbell, J. Fecal Microbiota Transplantation. Techniques, Applications, and Issues. Gastroenterol. Clin. North Am. 2012, 41, 781–803. [Google Scholar] [CrossRef]
- Lin, D.M.; Koskella, B.; Lin, H.C. Phage therapy: An alternative to antibiotics in the age of multi-drug resistance. World J. Gastrointest. Pharmacol. Ther. 2017, 8, 162–173. [Google Scholar] [CrossRef]
- Bourdin, G.; Navarro, A.; Sarker, S.A.; Pittet, A.-C.; Qadri, F.; Sultana, S.; Cravioto, A.; Talukder, K.A.; Reuteler, G.; Brüssow, H. Coverage of diarrhoea-associated Escherichia coli isolates from different origins with two types of phage cocktails. Microb. Biotechnol. 2014, 7, 165–176. [Google Scholar] [CrossRef]
- Wittebole, X.; De Roock, S.; Opal, S.M. A historical overview of bacteriophage therapy as an alternative to antibiotics for the treatment of bacterial pathogens. Virulence 2014, 5, 226–235. [Google Scholar] [CrossRef]
- Ackermann, H.-W. The first phage electron micrographs. Bacteriophage 2011, 1, 225–227. [Google Scholar] [CrossRef] [Green Version]
- Ramachandran, G.; Bikard, D. Editing the microbiome the CRISPR way. Philos. Trans. R. Soc. B Biol. Sci. 2019, 374, 20180103. [Google Scholar] [CrossRef] [Green Version]
- Rakhuba, D.V.; Kolomiets, E.I.; Dey, E.S.; Novik, G.I. Bacteriophage Receptors, Mechanisms of Phage Adsorption and Penetration into Host Cell. Pol. J. Microbiol. 2010, 59, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Motlagh, A.M.; Bhattacharjee, A.; Goel, R. Biofilm control with natural and genetically-modified phages. World J. Microbiol. Biotechnol. 2016, 32, 67. [Google Scholar] [CrossRef] [PubMed]
- Paule, A.; Frezza, D.; Edeas, M. Microbiota and Phage Therapy: Future Challenges in Medicine. Med Sci. 2018, 6, 86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reyes, A.; Wu, M.; McNulty, N.P.; Rohwer, F.L.; Gordon, J.I. Gnotobiotic mouse model of phage-bacterial host dynamics in the human gut. Proc. Natl. Acad. Sci. USA 2013, 110, 20236–20241. [Google Scholar] [CrossRef] [Green Version]
- Łusiak-Szelachowska, M.; Weber-Dąbrowska, B.; Jończyk-Matysiak, E.; Wojciechowska, R.; Górski, A. Bacteriophages in the gastrointestinal tract and their implications. Gut Pathog. 2017, 9, 44. [Google Scholar] [CrossRef] [Green Version]
- Ganeshan, S.D.; Hosseinidoust, Z. Phage Therapy with a focus on the Human Microbiota. Antibiotics 2019, 8, 131. [Google Scholar] [CrossRef] [Green Version]

| Prebiotics | Functions |
|---|---|
| Fructans | Selectively stimulates lactic acid bacteria. |
| Galacto-Oligosaccharides | Stimulates Bifidobacteria, Lactobacilli, Enterobacteria, Bacteroidetes, and Firmicutes. |
| Starch and Glucose-Derived Oligosaccharides | Elevates butyrate production and stimulates Bifidobacteria. |
| Other Oligosaccharides (pectin-derived) | Strengthens the mucus layer, enhances epithelial integrity, and activates or inhibits immune cells. |
| Non-Starch Oligosaccharides (flavonoids) | Inhibits the growth of pathogens, increases the number of Bifidobacterium and Lactobacillus, reduces endotoxin production, converts bile acids, maintains gut homeostasis, and promotes nutrient absorption. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramires, L.C.; Santos, G.S.; Ramires, R.P.; da Fonseca, L.F.; Jeyaraman, M.; Muthu, S.; Lana, A.V.; Azzini, G.; Smith, C.S.; Lana, J.F. The Association between Gut Microbiota and Osteoarthritis: Does the Disease Begin in the Gut? Int. J. Mol. Sci. 2022, 23, 1494. https://doi.org/10.3390/ijms23031494
Ramires LC, Santos GS, Ramires RP, da Fonseca LF, Jeyaraman M, Muthu S, Lana AV, Azzini G, Smith CS, Lana JF. The Association between Gut Microbiota and Osteoarthritis: Does the Disease Begin in the Gut? International Journal of Molecular Sciences. 2022; 23(3):1494. https://doi.org/10.3390/ijms23031494
Chicago/Turabian StyleRamires, Luciano C., Gabriel Silva Santos, Rafaela Pereira Ramires, Lucas Furtado da Fonseca, Madhan Jeyaraman, Sathish Muthu, Anna Vitória Lana, Gabriel Azzini, Curtis Scott Smith, and José Fábio Lana. 2022. "The Association between Gut Microbiota and Osteoarthritis: Does the Disease Begin in the Gut?" International Journal of Molecular Sciences 23, no. 3: 1494. https://doi.org/10.3390/ijms23031494
APA StyleRamires, L. C., Santos, G. S., Ramires, R. P., da Fonseca, L. F., Jeyaraman, M., Muthu, S., Lana, A. V., Azzini, G., Smith, C. S., & Lana, J. F. (2022). The Association between Gut Microbiota and Osteoarthritis: Does the Disease Begin in the Gut? International Journal of Molecular Sciences, 23(3), 1494. https://doi.org/10.3390/ijms23031494










