Optimized Silica-Binding Peptide-Mediated Delivery of Bactericidal Lysin Efficiently Prevents Staphylococcus aureus from Adhering to Device Surfaces
Abstract
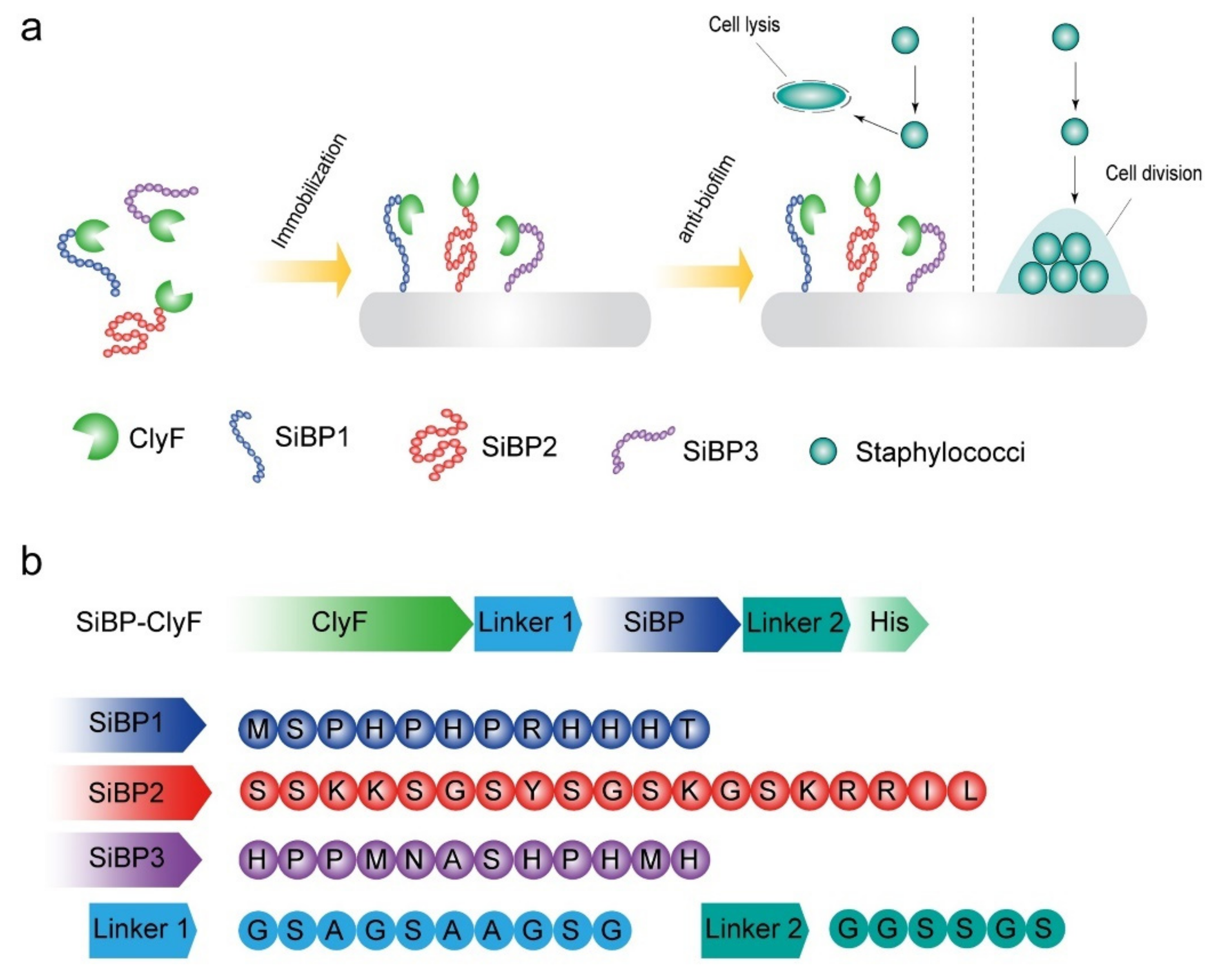
:1. Introduction
2. Results
2.1. Construction and Biochemical Characterization of ClyF Variants
2.2. Bactericidal Activities of Free ClyF Variants
2.3. Bactericidal Capacities of Immobilized ClyF Variants against Planktonic S. aureus
2.4. Antibiofilm Capacity of Immobilized SiBP1-ClyF against Static S. aureus Biofilms
2.5. Antibiofilm Capacity of Immobilized SiBP1-ClyF against Dynamic S. aureus Biofilms
2.6. SiBP1-ClyF Immobilized Surface Supports Normal Growth of Mammalian Cells
3. Discussion
4. Materials and Methods
4.1. Construction of SiBP-ClyF Fusions
4.2. Protein Expression and Purification
4.3. Structure Prediction of ClyF and Its Variants
4.4. Nano Differential Scanning Fluorimetry
4.5. Circular Dichroism
4.6. Bactericidal Activity of Free ClyF and Its SiBP-Fused Variants
4.7. Antibacterial Activity of Lysin-Functionalized Surfaces
4.8. Recycle Capacity and Stability of Lysin-Functionalized Surface
4.9. Prevention of Biofilm Formation on Lysin-Functionalized Surfaces
4.10. Cytotoxicity of Lysin-Functionalized Surface
4.11. Statistic Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saint, S.; Savel, R.H.; Matthay, M.A. Enhancing the safety of critically ill patients by reducing urinary and central venous catheter-related infections. Am. J. Respir Crit. Care Med. 2002, 165, 1475–1479. [Google Scholar] [CrossRef] [PubMed]
- Brisbois, E.J.; Davis, R.P.; Jones, A.M.; Major, T.C.; Bartlett, R.H.; Meyerhoff, M.E.; Handa, H. Reduction in Thrombosis and Bacterial Adhesion with 7 Day Implantation of S-Nitroso-N-acetylpenicillamine (SNAP)-Doped Elast-eon E2As Catheters in Sheep. J. Mater. Chem. B 2015, 3, 1639–1645. [Google Scholar] [CrossRef] [Green Version]
- Parienti, J.J.; Mongardon, N.; Megarbane, B.; Mira, J.P.; Kalfon, P.; Gros, A.; Marque, S.; Thuong, M.; Pottier, V.; Ramakers, M.; et al. Intravascular Complications of Central Venous Catheterization by Insertion Site. N. Engl. J. Med. 2015, 373, 1220–1229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schumm, K.; Lam, T.B. Types of urethral catheters for management of short-term voiding problems in hospitalised adults. Cochrane Database Syst. Rev. 2008, 16, 2:CD004013. [Google Scholar]
- Andersen, M.J.; Flores-Mireles, A.L. Urinary Catheter Coating Modifications: The Race against Catheter-Associated Infections. Coatings 2020, 10, 23. [Google Scholar] [CrossRef] [Green Version]
- Haddadin, Y.; Annamaraju, P.; Regunath, H. Central Line Associated Blood Stream Infections; StatPearls: Treasure Island, FL, USA, 2020. [Google Scholar]
- Neoh, K.G.; Li, M.; Kang, E.T.; Chiong, E.; Tambyah, P.A. Surface modification strategies for combating catheter-related complications: Recent advances and challenges. J. Mater. Chem. B 2017, 5, 2045–2067. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Larsen, L.H.; Lorenzen, J.; Hall-Stoodley, L.; Kikhney, J.; Moter, A.; Thomsen, T.R. Microbiological diagnosis of device-related biofilm infections. APMIS 2017, 125, 289–303. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Y.; He, L.; Asiamah, T.K.; Otto, M. Colonization of medical devices by staphylococci. Environ. Microbiol. 2018, 20, 3141–3153. [Google Scholar] [CrossRef] [Green Version]
- Durant, D.J. Nurse-driven protocols and the prevention of catheter-associated urinary tract infections: A systematic review. Am. J. Infect. Control 2017, 45, 1331–1341. [Google Scholar] [CrossRef]
- Singh, S.; Singh, S.K.; Chowdhury, I.; Singh, R. Understanding the Mechanism of Bacterial Biofilms Resistance to Antimicrobial Agents. Open Microbiol. J. 2017, 11, 53–62. [Google Scholar] [CrossRef] [Green Version]
- Schmelcher, M.; Loessner, M.J. Bacteriophage endolysins—Extending their application to tissues and the bloodstream. Curr. Opin. Biotechnol. 2021, 68, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Linden, S.B.; Alreja, A.B.; Nelson, D.C. Application of bacteriophage-derived endolysins to combat streptococcal disease: Current state and perspectives. Curr. Opin. Biotechnol. 2021, 68, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Zhang, H.; Wang, J.; Yu, J.; Wei, H. A novel chimeric lysin with robust antibacterial activity against planktonic and biofilm methicillin-resistant Staphylococcus aureus. Sci. Rep. 2017, 7, 40182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gondil, V.S.; Harjai, K.; Chhibber, S. Endolysins as emerging alternative therapeutic agents to counter drug-resistant infections. Int. J. Antimicrob. Agents 2020, 55, 105844. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Linden, S.B.; Wang, J.; Yu, J.; Nelson, D.C.; Wei, H. A chimeolysin with extended-spectrum streptococcal host range found by an induced lysis-based rapid screening method. Sci. Rep. 2015, 5, 17257. [Google Scholar] [CrossRef] [Green Version]
- Huang, L.; Luo, D.; Gondil, V.S.; Gong, Y.; Jia, M.; Yan, D.; He, J.; Hu, S.; Yang, H.; Wei, H. Construction and characterization of a chimeric lysin ClyV with improved bactericidal activity against Streptococcus agalactiae in vitro and in vivo. Appl. Microbiol. Biotechnol. 2020, 104, 1609–1619. [Google Scholar] [CrossRef]
- Loeffler, J.M.; Djurkovic, S.; Fischetti, V.A. Phage lytic enzyme Cpl-1 as a novel antimicrobial for pneumococcal bacteremia. Infect. Immun. 2003, 71, 6199–6204. [Google Scholar] [CrossRef] [Green Version]
- Gondil, V.S.; Dube, T.; Panda, J.J.; Yennamalli, R.M.; Harjai, K.; Chhibber, S. Comprehensive evaluation of chitosan nanoparticle based phage lysin delivery system; a novel approach to counter, S. pneumoniae infections. Int. J. Pharm. 2020, 573, 118850. [Google Scholar] [CrossRef]
- Guo, M.; Feng, C.; Ren, J.; Zhuang, X.; Zhang, Y.; Zhu, Y.; Dong, K.; He, P.; Guo, X.; Qin, J. A Novel Antimicrobial Endolysin, LysPA26, against Pseudomonas aeruginosa. Front. Microbiol. 2017, 8, 293. [Google Scholar] [CrossRef] [Green Version]
- Ghose, C.; Euler, C.W. Gram-Negative Bacterial Lysins. Antibiotics 2020, 9, 74. [Google Scholar] [CrossRef] [Green Version]
- Gerstmans, H.; Criel, B.; Briers, Y. Synthetic biology of modular endolysins. Biotechnol. Adv. 2018, 36, 624–640. [Google Scholar] [CrossRef] [PubMed]
- Sao-Jose, C. Engineering of Phage-Derived Lytic Enzymes: Improving Their Potential as Antimicrobials. Antibiotics 2018, 7, 29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Maesschalck, V.; Gutierrez, D.; Paeshuyse, J.; Lavigne, R.; Briers, Y. Advanced engineering of third-generation lysins and formulation strategies for clinical applications. Crit. Rev. Microbiol. 2020, 46, 548–564. [Google Scholar] [CrossRef]
- Filatova, L.Y.; Donovan, D.M.; Ishnazarova, N.T.; Foster-Frey, J.A.; Becker, S.C.; Pugachev, V.G.; Balabushevich, N.G.; Dmitrieva, N.F.; Klyachko, N.L. A Chimeric LysK-Lysostaphin Fusion Enzyme Lysing Staphylococcus aureus Cells: A Study of Both Kinetics of Inactivation and Specifics of Interaction with Anionic Polymers. Appl. Biochem. Biotechnol. 2016, 180, 544–557. [Google Scholar] [CrossRef] [PubMed]
- Abouhmad, A.; Mamo, G.; Dishisha, T.; Amin, M.A.; Hatti-Kaul, R. T4 lysozyme fused with cellulose-binding module for antimicrobial cellulosic wound dressing materials. J. Appl. Microbiol. 2016, 121, 115–125. [Google Scholar] [CrossRef] [Green Version]
- Johnson, A.K.; Zawadzka, A.M.; Deobald, L.A.; Crawford, R.L.; Paszczynski, A.J. Novel method for immobilization of enzymes to magnetic nanoparticles. J. Nanopart. Res. 2008, 10, 1009–1025. [Google Scholar] [CrossRef]
- Care, A.; Bergquist, P.L.; Sunna, A. Solid-binding peptides: Smart tools for nanobiotechnology. Trends Biotechnol. 2015, 33, 259–268. [Google Scholar] [CrossRef]
- Cetinel, S.; Caliskan, H.B.; Yucesoy, D.T.; Donatan, A.S.; Yuca, E.; Urgen, M.; Karaguler, N.G.; Tamerler, C. Addressable self-immobilization of lactate dehydrogenase across multiple length scales. Biotechnol. J. 2013, 8, 262–272. [Google Scholar] [CrossRef]
- Naik, R.R.; Brott, L.L.; Clarson, S.J.; Stone, M.O. Silica-precipitating peptides isolated from a combinatorial phage display peptide library. J. Nanosci. Nanotechnol. 2002, 2, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Kroger, N.; Deutzmann, R.; Sumper, M. Polycationic peptides from diatom biosilica that direct silica nanosphere formation. Science 1999, 286, 1129–1132. [Google Scholar] [CrossRef] [Green Version]
- Eteshola, E.; Brillson, L.J.; Lee, S.C. Selection and characteristics of peptides that bind thermally grown silicon dioxide films. Biomol. Eng. 2005, 22, 201–204. [Google Scholar] [CrossRef] [PubMed]
- Arciola, C.R.; Campoccia, D.; Montanaro, L. Implant infections: Adhesion, biofilm formation and immune evasion. Nat. Rev. Microbiol. 2018, 16, 397–409. [Google Scholar] [CrossRef] [PubMed]
- Moormeier, D.E.; Bayles, K.W. Staphylococcus aureus biofilm: A complex developmental organism. Mol. Microbiol. 2017, 104, 365–376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Talebi Bezmin Abadi, A.; Rizvanov, A.A.; Haertlé, T.; Blatt, N.L. World Health Organization Report: Current Crisis of Antibiotic Resistance. BioNanoScience 2019, 9, 778–788. [Google Scholar] [CrossRef]
- Romero-Calle, D.; Guimarães Benevides, R.; Góes-Neto, A.; Billington, C. Bacteriophages as Alternatives to Antibiotics in Clinical Care. Antibiotics 2019, 8, 138. [Google Scholar] [CrossRef] [Green Version]
- Gondil, V.S.; Kalaiyarasan, T.; Bharti, V.K.; Chhibber, S. Antibiofilm potential of Seabuckthorn silver nanoparticles (SBT@AgNPs) against Pseudomonas aeruginosa. 3 Biotech 2019, 9, 402. [Google Scholar] [CrossRef]
- Simoes, M.; Bennett, R.N.; Rosa, E.A. Understanding antimicrobial activities of phytochemicals against multidrug resistant bacteria and biofilms. Nat. Prod. Rep. 2009, 26, 746–757. [Google Scholar] [CrossRef]
- Gondil, V.S.; Asif, M.; Bhalla, T.C. Optimization of physicochemical parameters influencing the production of prodigiosin from Serratia nematodiphila RL2 and exploring its antibacterial activity. 3 Biotech 2017, 7, 338. [Google Scholar] [CrossRef]
- Mahlapuu, M.; Håkansson, J.; Ringstad, L.; Björn, C. Antimicrobial Peptides: An Emerging Category of Therapeutic Agents. Front. Cell. Infect. Microbiol. 2016, 6, 194. [Google Scholar] [CrossRef] [Green Version]
- Douglass, M.; Hopkins, S.; Pandey, R.; Singha, P.; Norman, M.; Handa, H. S-Nitrosoglutathione-Based Nitric Oxide-Releasing Nanofibers Exhibit Dual Antimicrobial and Antithrombotic Activity for Biomedical Applications. Macromol. Biosci. 2021, 21, e2000248. [Google Scholar] [CrossRef]
- Brena, B.; Gonzalez-Pombo, P.; Batista-Viera, F. Immobilization of enzymes: A literature survey. Methods Mol. Biol. 2013, 1051, 15–31. [Google Scholar]
- Rodriguez-Rubio, L.; Chang, W.L.; Gutierrez, D.; Lavigne, R.; Martinez, B.; Rodriguez, A.; Govers, S.K.; Aertsen, A.; Hirl, C.; Biebl, M.; et al. ’Artilysation’ of endolysin lambdaSa2lys strongly improves its enzymatic and antibacterial activity against streptococci. Sci. Rep. 2016, 6, 35382. [Google Scholar] [CrossRef]
- Diez-Martinez, R.; de Paz, H.D.; Bustamante, N.; Garcia, E.; Menendez, M.; Garcia, P. Improving the lethal effect of cpl-7, a pneumococcal phage lysozyme with broad bactericidal activity, by inverting the net charge of its cell wall-binding module. Antimicrob. Agents Chemother. 2013, 57, 5355–5365. [Google Scholar] [CrossRef] [Green Version]
- Talbert, J.N.; Goddard, J.M. Enzymes on material surfaces. Colloids Surf. B Biointerfaces 2012, 93, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ogorzalek, T.L.; Yang, P.; Schroeder, M.M.; Marsh, E.N.; Chen, Z. Molecular orientation of enzymes attached to surfaces through defined chemical linkages at the solid-liquid interface. J. Am. Chem. Soc. 2013, 135, 12660–12669. [Google Scholar] [CrossRef] [PubMed]
- Pangule, R.C.; Brooks, S.J.; Dinu, C.Z.; Bale, S.S.; Salmon, S.L.; Zhu, G.; Metzger, D.W.; Kane, R.S.; Dordick, J.S. Antistaphylococcal nanocomposite films based on enzyme-nanotube conjugates. ACS Nano 2010, 4, 3993–4000. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nileback, L.; Widhe, M.; Seijsing, J.; Bysell, H.; Sharma, P.K.; Hedhammar, M. Bioactive Silk Coatings Reduce the Adhesion of Staphylococcus aureus while Supporting Growth of Osteoblast-like Cells. ACS Appl. Mater. Interfaces 2019, 11, 24999–25007. [Google Scholar] [CrossRef]
- Nguyen, P.Q.; Botyanszki, Z.; Tay, P.K.; Joshi, N.S. Programmable biofilm-based materials from engineered curli nanofibres. Nat. Commun. 2014, 5, 4945. [Google Scholar] [CrossRef] [Green Version]
- Trautner, B.W.; Darouiche, R.O. Role of biofilm in catheter-associated urinary tract infection. Am. J. Infect. Control 2004, 32, 177–183. [Google Scholar] [CrossRef]
- Costerton, J.W.; Stewart, P.S.; Greenberg, E.P. Bacterial biofilms: A common cause of persistent infections. Science 1999, 284, 1318–1322. [Google Scholar] [CrossRef] [Green Version]
- Stewart, P.S.; Costerton, J.W. Antibiotic resistance of bacteria in biofilms. Lancet 2001, 358, 135–138. [Google Scholar] [CrossRef]
- Baek, M.; DiMaio, F.; Anishchenko, I.; Dauparas, J.; Ovchinnikov, S.; Lee, G.R.; Wang, J.; Cong, Q.; Kinch, L.N.; Schaeffer, R.D.; et al. Accurate prediction of protein structures and interactions using a three-track neural network. Science 2021, 373, 871–876. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Zhang, Y.; Huang, Y.; Yu, J.; Wei, H. Degradation of methicillin-resistant Staphylococcus aureus biofilms using a chimeric lysin. Biofouling 2014, 30, 667–674. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Bi, Y.; Shang, X.; Wang, M.; Linden, S.B.; Li, Y.; Li, Y.; Nelson, D.C.; Wei, H. Antibiofilm Activities of a Novel Chimeolysin against Streptococcus mutans under Physiological and Cariogenic Conditions. Antimicrob. Agents Chemother. 2016, 60, 7436–7443. [Google Scholar] [CrossRef] [PubMed] [Green Version]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, W.; Gondil, V.S.; Luo, D.; He, J.; Wei, H.; Yang, H. Optimized Silica-Binding Peptide-Mediated Delivery of Bactericidal Lysin Efficiently Prevents Staphylococcus aureus from Adhering to Device Surfaces. Int. J. Mol. Sci. 2021, 22, 12544. https://doi.org/10.3390/ijms222212544
Yang W, Gondil VS, Luo D, He J, Wei H, Yang H. Optimized Silica-Binding Peptide-Mediated Delivery of Bactericidal Lysin Efficiently Prevents Staphylococcus aureus from Adhering to Device Surfaces. International Journal of Molecular Sciences. 2021; 22(22):12544. https://doi.org/10.3390/ijms222212544
Chicago/Turabian StyleYang, Wan, Vijay Singh Gondil, Dehua Luo, Jin He, Hongping Wei, and Hang Yang. 2021. "Optimized Silica-Binding Peptide-Mediated Delivery of Bactericidal Lysin Efficiently Prevents Staphylococcus aureus from Adhering to Device Surfaces" International Journal of Molecular Sciences 22, no. 22: 12544. https://doi.org/10.3390/ijms222212544
APA StyleYang, W., Gondil, V. S., Luo, D., He, J., Wei, H., & Yang, H. (2021). Optimized Silica-Binding Peptide-Mediated Delivery of Bactericidal Lysin Efficiently Prevents Staphylococcus aureus from Adhering to Device Surfaces. International Journal of Molecular Sciences, 22(22), 12544. https://doi.org/10.3390/ijms222212544





