Trabecular Bone Parameters, TIMP-2, MMP-8, MMP-13, VEGF Expression and Immunolocalization in Bone and Cartilage in Newborn Offspring Prenatally Exposed to Fumonisins
Abstract
:1. Introduction
2. Results
2.1. Histomorphometry
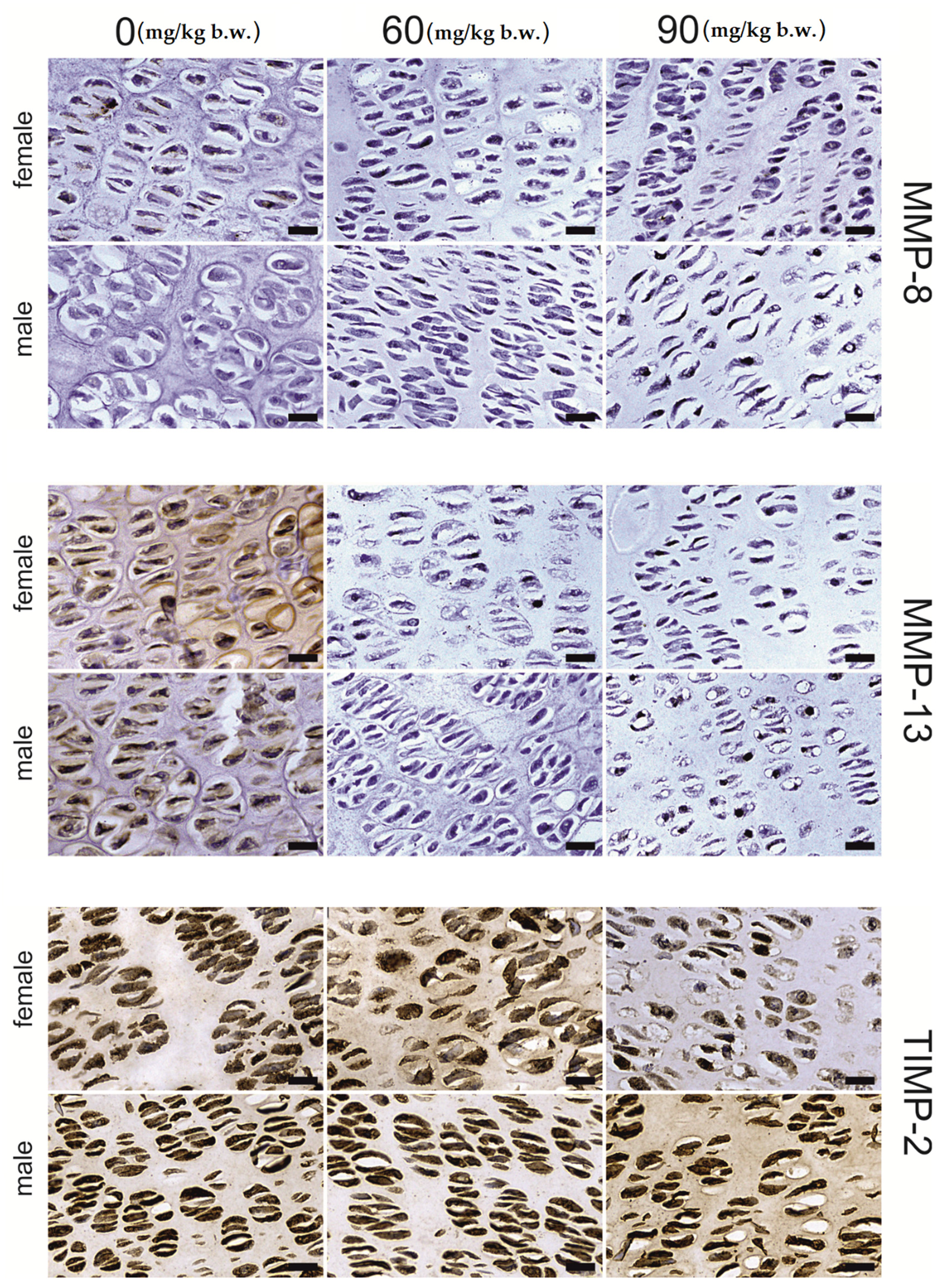
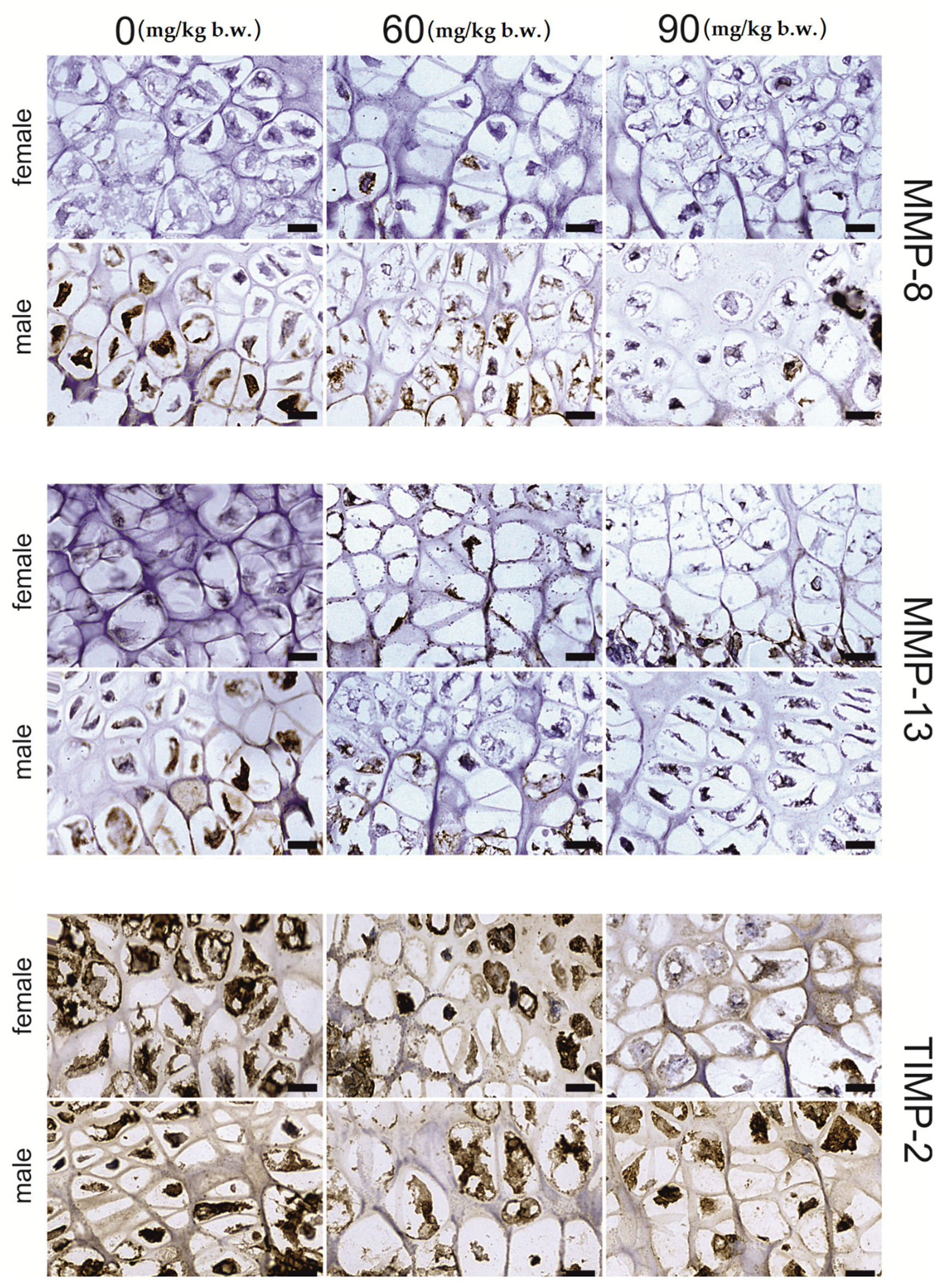
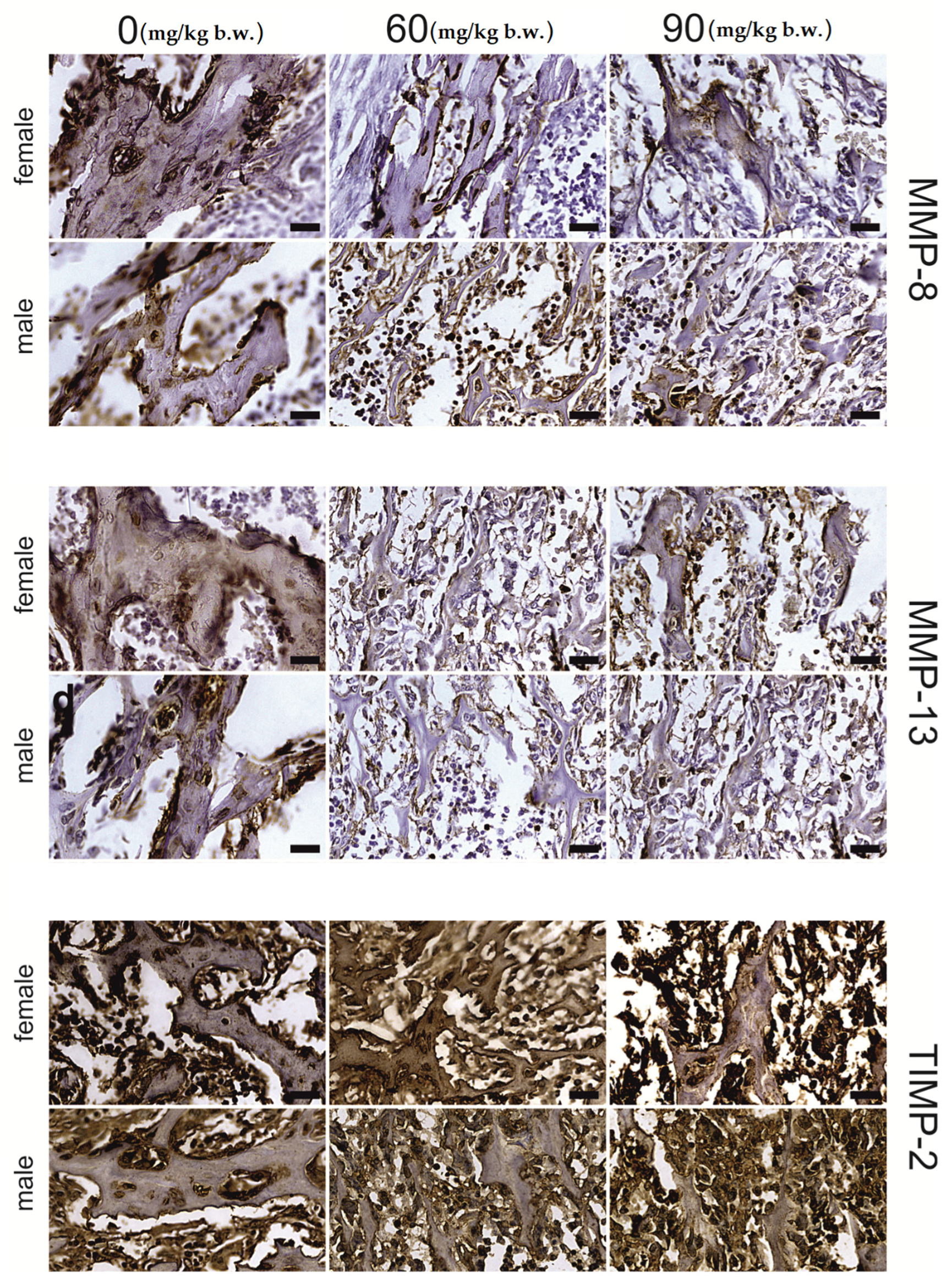
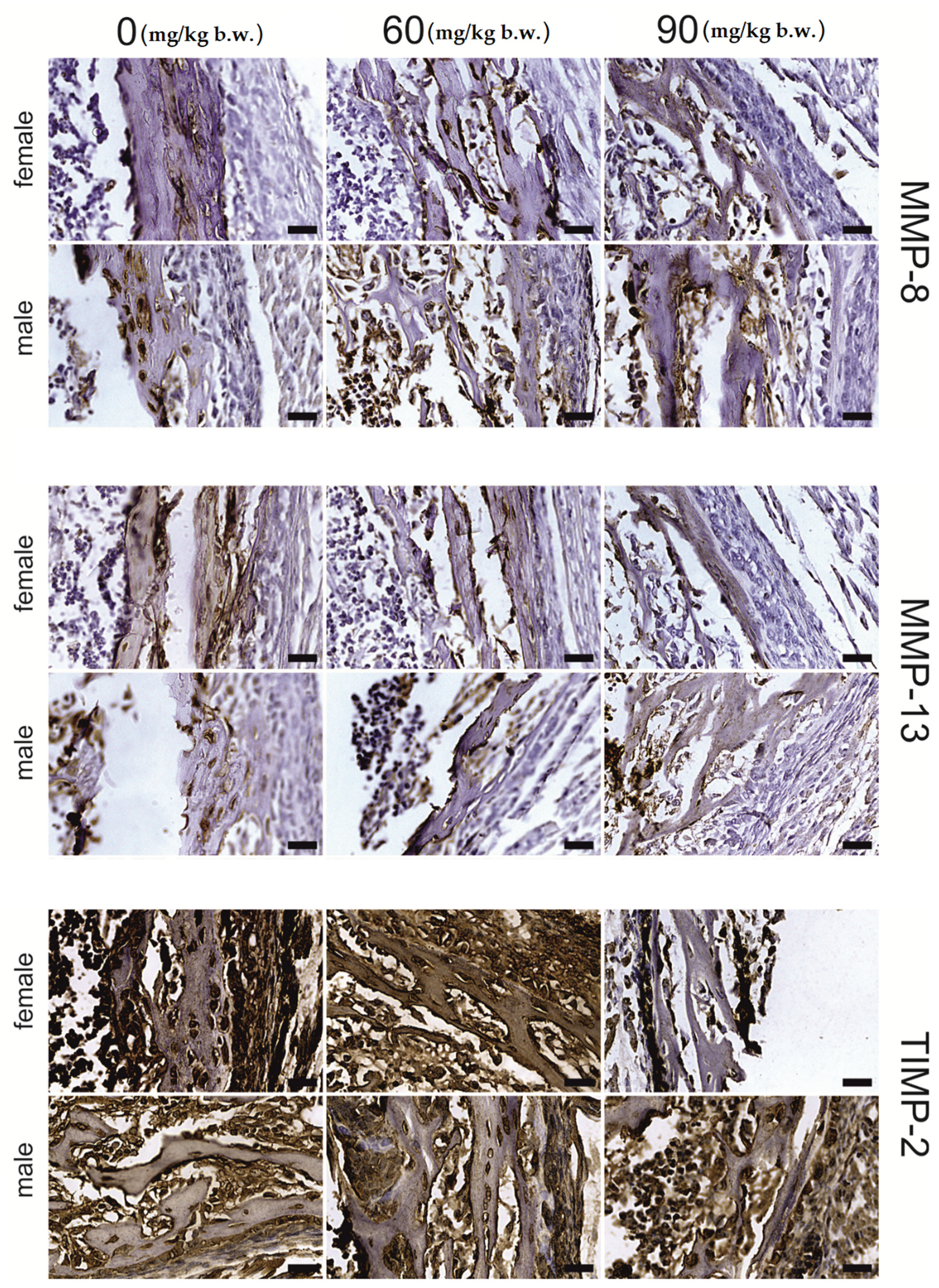
2.2. Immunostaining
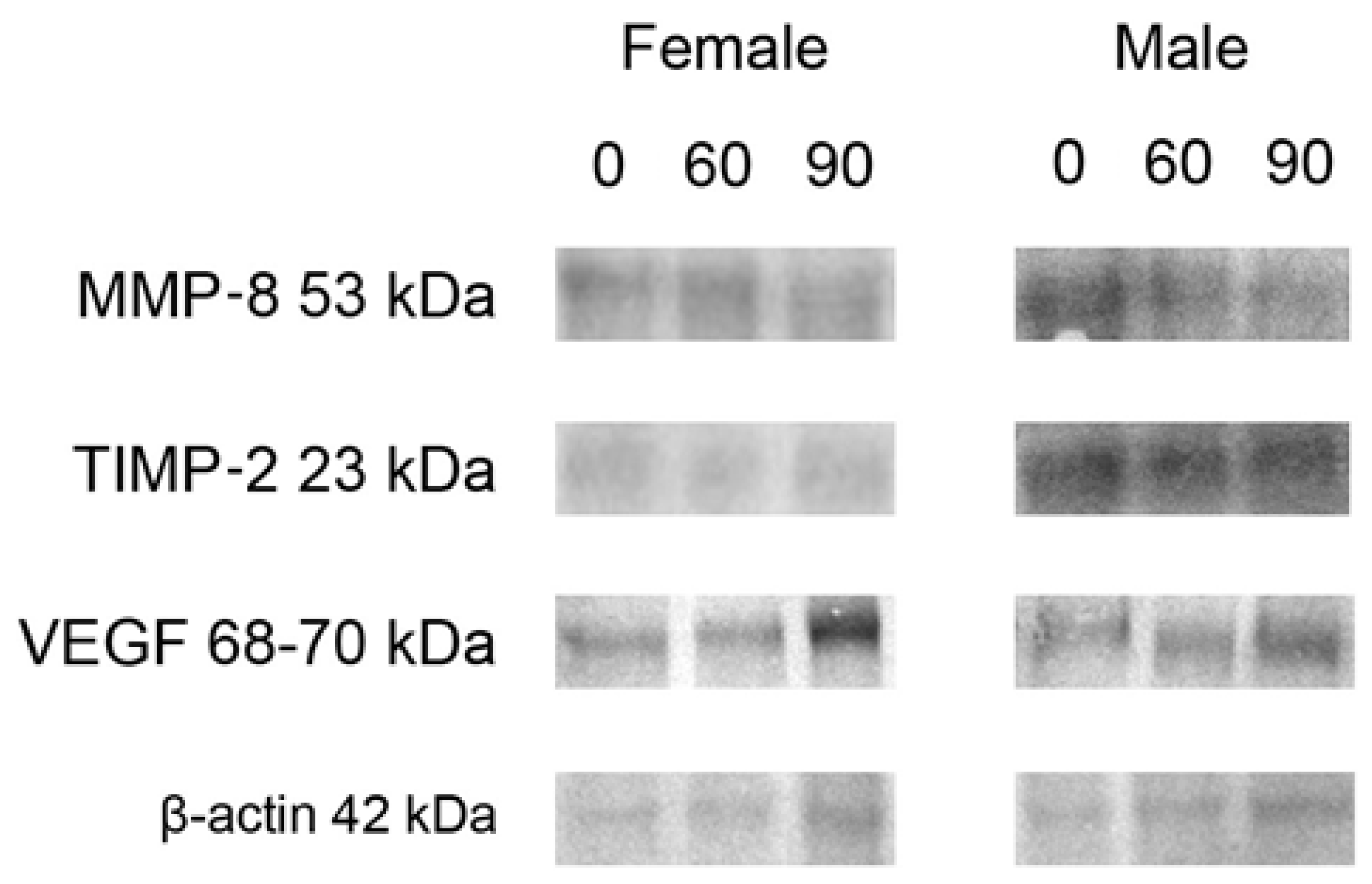
2.3. Western Blot Protein Expression
3. Discussion
4. Materials and Methods
4.1. Animals and Experimental Design
4.2. Histomorphometry
4.3. Immunohistochemistry
4.4. Western Blot
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stockmann-Juvala, H.; Savolainen, K. A review of the toxic effects and mechanisms of action of fumonisin B1. Hum. Exp. Toxicol. 2008, 27, 799–809. [Google Scholar] [CrossRef]
- Placinta, C.M.; D’Mello, J.P.F.; Macdonald, A.M.C. A review of worldwide contamination of cereal grains and animal feed with Fusarium mycotoxins. Anim. Feed Sci. Technol. 1999, 78, 21–37. [Google Scholar] [CrossRef]
- Streit, E.; Naehrer, K.; Rodrigues, I.; Schatzmayr, G. Mycotoxin occurrence in feed and feed raw materials worldwide: Long-term analysis with special focus on Europe and Asia. J. Sci. Food Agric. 2013, 93, 2892–2899. [Google Scholar] [CrossRef]
- Wilson, D.A. Fumonisin Toxicosis. In Clinical Veterinary Advisor: The Horse, 1st ed.; Elsevier: St. Louis, MO, USA, 2012; pp. 214–215. [Google Scholar]
- European Commision (EC). Commission Regulation (EU) 2005/856 amending Regulation 2001/466/EC as regards Fusarium toxins. Off. J. Eur. Union L. 2005, 143, 3–8. [Google Scholar]
- European Commission (EC). Commission Recommendation 2006/576/EC on the presence of deoxynivalenol, zearalenone, ochratoxin A, T-2 and HT-2 and fumonisins in products intended for animal feeding. Off. J. Eur. Union L. 2006, 229, 7–9. [Google Scholar]
- Ledoux, D.R.; Brown, T.P.; Weibking, T.S.; Rottinghaus, G.E. Fumonisin toxicity in broiler chicks. J. Vet. Diagn. Invest. 1992, 4, 330–333. [Google Scholar] [CrossRef] [Green Version]
- European Food Safety Authority (EFSA). Panel on Contaminants in the Food Chain (CONTAM). Risks for animal health related to the presence of fumonisins, their modified forms and hidden forms in feed. EFSA J. 2018, 16, 5242. [Google Scholar] [CrossRef]
- Riley, R.T.; Voss, K.A. Differential sensitivity of rat kidney and liver to fumonisin toxicity: Organ-specific differences in toxin accumulation and sphingoid base metabolism. Toxicol. Sci. 2006, 92, 335–345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rudyk, H.; Tomaszewska, E.; Kotsyumbas, I.; Muszyński, S.; Tomczyk-Warunek, A.; Szymańczyk, S.; Dobrowolski, P.; Wiącek, D.; Kamiński, D.; Brezvyn, O. Bone homeostasis in experimental fumonisins intoxication of rats. Ann. Anim. Sci. 2019, 19, 403–419. [Google Scholar] [CrossRef] [Green Version]
- Antonissen, G.; Devreese, M.; Van Immerseel, F.; De Baere, S.; Hessenberger, S.; Martel, A.; Croubels, S. Chronic exposure to deoxynivalenol has no influence on the oral bioavailability of fumonisin B1 in broiler chickens. Toxins 2015, 7, 560–571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Devreese, M.; De Backer, P.; Croubels, S. Overview of the most important mycotoxins for the pig and poultry husbandry. Vlaams Diergen. Tijds. 2013, 82, 171–180. [Google Scholar] [CrossRef]
- Tomaszewska, E.; Rudyk, H.; Dobrowolski, P.; Donaldson, J.; Świetlicka, I.; Puzio, I.; Kamiński, D.; Wiącek, D.; Kushnir, V.; Brezvyn, O.; et al. Changes in the intestinal histomorphometry, the expression of intestinal tight junction proteins, and the bone structure and liver of pre-laying hens following oral administration of fumonisins for 21 days. Toxins 2021, 13, 375. [Google Scholar] [CrossRef] [PubMed]
- Howard, P.C.; Warbritton, A.; Voss, K.A.; Lorentzen, R.J.; Thurman, J.D.; Kovach, R.M.; Bucci, T.J. Compensatory regeneration as a mechanism for renal tubule carcinogenesis of fumonisin B1 in the F344/N/Nctr BR rat. Environ. Health Perspect. 2001, 109 (Suppl. 2), 309–314. [Google Scholar] [CrossRef] [Green Version]
- Gelderblom, W.C.A.; Cawood, M.E.; Snyman, S.D.; Marasas, W.F.O. Fumonisin B1 dosimetry in relation to cancer initiation in rat liver. Carcinogenesis 1994, 15, 790–790. [Google Scholar] [CrossRef] [Green Version]
- Kosicki, R.; Twarużek, M.; Kannenberg, K.; Grajewski, J. Contamination of acorns of pedunculate oak (Quercus robur L.), as feed material, by moulds and mycotoxins. Ann. Anim. Sci. 2021, 21, 977–990. [Google Scholar] [CrossRef]
- Coppock, R.W.; Jacobsen, B.J. Mycotoxins in animal and human patients. Toxicol. Ind. Health 2009, 25, 637–655. [Google Scholar] [CrossRef]
- Gelderblom, W.; Marasas, W. Controversies in fumonisin mycotoxicology and risk assessment. Hum. Exp. Toxicol. 2012, 31, 215–235. [Google Scholar] [CrossRef] [PubMed]
- Scott, P.M. Recent research on fumonisins: A review. Food Addit. Contam. Chem. Anal. Control. Expo. Risk Assess. 2012, 29, 242–248. [Google Scholar] [CrossRef]
- FDA. Food and Drug Administration Guidance for Industry: Fumonisin Levels in Human Foods and Animal Feeds; FDA: Rome, Italy, 2001; Volume 66, pp. 56688–56689. [Google Scholar]
- Voss, K.A.; Smith, G.W.; Haschek, W.M. Fumonisins: Toxicokinetics, mechanism of action and toxicity. Anim. Feed Sci. Technol. 2007, 137, 299–325. [Google Scholar] [CrossRef]
- Didwania, N.; Joshi, M. Mycotoxins: A critical review on occurrence and significance. Int. J. Pharm. Sci. 2013, 5, 1014–1019. [Google Scholar]
- Muller, S.; Dekant, W.; Mally, A. Fumonisin B1 and the kidney: Modes of action for renal tumor formation by fumonisin B1 in rodents. Food Chem. Toxicol. 2012, 50, 3833–3846. [Google Scholar] [CrossRef]
- Antonissen, G.; Croubels, S.; Pasmans, F.; Ducatelle, R.; Eeckhaut, V.; Devreese, M.; Verlinden, M.; Haesebrouck, F.; Eeckhout, M.; De Saeger, S.; et al. Fumonisins affect the intestinal microbial homeostasis in broiler chickens, predisposing to necrotic enteritis. Vet. Res. 2015, 46, 98. [Google Scholar] [CrossRef] [Green Version]
- Dauncey, M.J.; Bicknell, R.J. Nutrition and neurodevelopment: Mechanisms of developmental dysfunction and disease in later life. Nutr. Res. Rev. 1999, 12, 231–253. [Google Scholar] [CrossRef] [Green Version]
- Tomaszewska, E.; Dobrowolski, P.; Puzio, I.; Donaldson, J.; Muszyński, S. Acrylamide-induced prenatal programming of bone structure in mammal model. Ann. Anim. Sci. 2020, 20, 1257–1287. [Google Scholar] [CrossRef]
- Chen, Q.; Bao, N.; Yao, Q.; Li, Z.Y. Fractal dimension: A complementary diagnostic indicator of osteoporosis to bone mineral density. Med. Hypotheses 2018, 116, 136–138. [Google Scholar] [CrossRef] [PubMed]
- Rudyk, H.; Tomaszewska, E.; Arciszewski, M.B.; Muszyński, S.; Tomczyk-Warunek, A.; Dobrowolski, P.; Donaldson, J.; Brezvyn, O.; Kotsyumbas, I. Histomorphometrical changes in intestine structure and innervation following experimental fumonisins intoxication in male Wistar rats. Pol. J. Vet. Sci. 2020, 23, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Voss, K.A.; Riley, R.T.; Gelineau-van Waes, J. Fumonisin B1 induced neural tube defects were not increased in LM/Bc mice fed folate-deficient diet. Mol. Nutr. Food Res. 2014, 58, 1190–1198. [Google Scholar] [CrossRef]
- Reddy, R.V.; Johnson, G.; Rottinghaus, G.E.; Casteel, S.W.; Reddy, C.S. Developmental effects of fumonisin B1 in mice. Mycopathologia 1996, 134, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Voss, K.A.; Bacon, C.W.; Norred, W.P.; Chapin, R.E.; Chamberlain, W.J.; Plattner, R.D.; Meredith, F.I. Studies on the reproductive effects of Fusarium moniliforme culture material in rats and the biodistribution of [14C] fumonisin B1 in pregnant rats. Nat. Toxins 1996, 4, 24–33. [Google Scholar] [CrossRef]
- Abdel-Wahhab, M.A.; Hassan, A.M.; Amer, H.A.; Naguib, K.M. Prevention of fumonisin-induced maternal and developmental toxicity in rats by certain plant extracts. J. Appl. Toxicol. 2004, 24, 469–474. [Google Scholar] [CrossRef]
- Tomaszewska, E.; Muszyński, S.; Dobrowolski, P.; Kostro, K.; Taszkun, I.; Żmuda, A.; Blicharski, T.; Kędzia, P. Bentonite diminishes DON-induced changes in bone development in mink dams. J. Vet. Res. 2016, 60, 349–355. [Google Scholar] [CrossRef] [Green Version]
- Tomaszewska, E.; Dobrowolski, P.; Muszyński, S.; Kostro, K.; Taszkun, I.; Żmuda, A.; Blicharski, T.; Hułas-Stasiak, M. DON-induced changes in bone homeostasis in mink dams. J. Vet. Res. 2017, 61, 357–362. [Google Scholar] [CrossRef] [Green Version]
- Khavandgar, Z.; Murshed, M. Sphingolipid metabolism and its role in the skeletal tissues. Cell Mol. Life Sci. 2015, 72, 959–969. [Google Scholar] [CrossRef]
- Ma, H.; Liu, C.M.; Shao, S.Q.; Song, Y.Y.; Zhou, J.; Cao, L.N.; Yin, H.Q.; Yin, S.L. Myriocin alleviates Oleic/Palmitate induced chondrocyte degeneration via the suppression of ceramide. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 12938–12947. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Garcia, O.R.; Rogers, N.H.; Smith, R.G.; Lotz, M.K. Palmitate has proapoptotic and proinflammatory effects on articular cartilage and synergizes with interleukin-1. Arthritis Rheumatol. 2014, 66, 1779–1788. [Google Scholar] [CrossRef] [Green Version]
- Matsubara, S.; Onodera, T.; Maeda, E.; Momma, D.; Matsuoka, M.; Homan, K.; Ohashi, T.; Iwasaki, N. Depletion of glycosphingolipids induces excessive response of chondrocytes under mechanical stress. J. Biomech. 2019, 94, 22–30. [Google Scholar] [CrossRef]
- Sasaki, K.; Yoshida, H. Golgi stress response and organelle zones. FEBS Lett. 2019, 593, 2330–2340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kakoi, H.; Maeda, S.; Shinohara, N.; Matsuyama, K.; Imamura, K.; Kawamura, I.; Nagano, S.; Setoguchi, T.; Yokouchi, M.; Ishidou, Y.; et al. Bone morphogenic protein (BMP) signaling up-regulates neutral sphingomyelinase 2 to suppress chondrocyte maturation via the Akt protein signaling pathway as a negative feedback mechanism. J. Biol. Chem. 2014, 289, 8135–8150. [Google Scholar] [CrossRef] [Green Version]
- Simonaro, C.M.; Sachot, S.; Ge, Y.; He, X.; Deangelis, V.A.; Eliyahu, E.; Leong, D.J.; Sun, H.B.; Mason, J.B.; Haskins, M.E.; et al. Acid ceramidase maintains the chondrogenic phenotype of expanded primary chondrocytes and improves the chondrogenic differentiation of bone marrow-derived mesenchymal stem cells. PLoS ONE 2013, 8, e62715. [Google Scholar] [CrossRef] [PubMed]
- Seito, N.; Yamashita, T.; Tsukuda, Y.; Matsui, Y.; Urita, A.; Onodera, T.; Mizutani, T.; Haga, H.; Fujitani, N.; Shinohara, Y.; et al. Interruption of glycosphingolipid synthesis enhances osteoarthritis development in mice. Arthritis Rheum. 2012, 64, 2579–2588. [Google Scholar] [CrossRef] [Green Version]
- Gilbert, S.J.; Blain, E.J.; Duance, V.C.; Mason, D.J. Sphingomyelinase decreases type II collagen expression in bovine articular cartilage chondrocytes via the ERK signaling pathway. Arthritis Rheum. 2008, 58, 209–220. [Google Scholar] [CrossRef]
- Simonaro, C.M.; D’Angelo, M.; He, X.; Eliyahu, E.; Shtraizent, N.; Haskins, M.E.; Schuchman, E.H. Mechanism of glycosaminoglycan-mediated bone and joint disease: Implications for the mucopolysaccharidoses and other connective tissue diseases. Am. J. Pathol. 2008, 172, 112–122. [Google Scholar] [CrossRef] [Green Version]
- Masuko, K.; Murata, M.; Nakamura, H.; Yudoh, K.; Nishioka, K.; Kato, T. Sphingosine-1-phosphate attenuates proteoglycan aggrecan expression via production of prostaglandin E2 from human articular chondrocytes. BMC Musculoskelet. Disord. 2007, 8, 29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gilbert, S.J.; Blain, E.J.; Jones, P.; Duance, V.C.; Mason, D.J. Exogenous sphingomyelinase increases collagen and sulphated glycosaminoglycan production by primary articular chondrocytes: An in vitro study. Arthritis Res. Ther. 2006, 8, R89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sabatini, M.; Rolland, G.; Leonce, S.; Thomas, M.; Lesur, C.; Perez, V.; de Nanteuil, G.; Bonnet, J. Effects of ceramide on apoptosis, proteoglycan degradation, and matrix metalloproteinase expression in rabbit articular cartilage. Biochem. Biophys. Res. Commun. 2000, 267, 438–444. [Google Scholar] [CrossRef] [PubMed]
- Shastak, Y.; Rodehuyscord, M. A review of the role of magnesium in poultry nutrition. Worlds Poult. Sci. J. 2015, 71, 125–138. [Google Scholar] [CrossRef]
- Torres, C.A.; Korver, D.R. Influences of trace mineral nutrition and maternal flock age on broiler embryo bone development. Poult. Sci. 2018, 97, 2996–3003. [Google Scholar] [CrossRef]
- Muszynski, S.; Tomaszewska, E.; Kwiecien, M.; Dobrowolski, P.; Tomczyk, A. Effect of dietary phytase supplementation on bone and hyaline cartilage development of broilers fed with organically complexed copper in a Cu-deficient diet. Biol. Trace Elem. Res. 2018, 182, 339–353. [Google Scholar] [CrossRef] [Green Version]
- Rath, N.C.; Huff, G.R.; Huff, W.E.; Balog, J.M. Factors regulating bone maturity and strength in poultry. Poult. Sci. 2000, 79, 1024–1032. [Google Scholar] [CrossRef]
- Lambert, E.; Dassé, E.; Haye, B.; Petitfrère, E. TIMPs as multifacial proteins. Crit. Rev. Oncol. Hematol. 2004, 49, 187–198. [Google Scholar] [CrossRef]
- Ries, C.; Egea, V.; Karow, M.; Kolb, H.; Jochum, M.; Neth, P. MMP-2, MT1-MMP, and TIMP-2 are essential for the invasive capacity of human mesenchymal stem cells: Differential regulation by inflammatory cytokines. Blood 2007, 109, 4055–4063. [Google Scholar] [CrossRef]
- Teitelbaum, S.L. Bone resorption by osteoclasts. Science 2000, 289, 1504–1508. [Google Scholar] [CrossRef]
- Prideaux, M.; Staines, K.A.; Jones, E.R.; Riley, G.P.; Pitsillides, A.A.; Farquharson, C. MMP and TIMP temporal gene expression during osteocytogenesis. Gene Expr. Patterns 2015, 18, 29–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.; Kim, D.H.; Keum, M.C.; Han, E.; An, B.K.; Chang, H.H.; Choi, Y.H.; Moon, B.H.; Lee, K.W. Effects of fumonisin B1 and mycotoxin binders on growth performance, tibia characteristics, gut physiology, and stress indicators in broiler chickens raised in different stocking densities. Poult. Sci. 2018, 97, 845–854. [Google Scholar] [CrossRef]
- Stetler-Stevenson, W.G.; Seo, D.W. TIMP-2: An endogenous inhibitor of angiogenesis. Trends Mol. Med. 2005, 11, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Fujio, Y.; Walsh, K. Akt mediates cytoprotection of endothelial cells by vascular endothelial growth factor in an anchorage-dependent manner. J. Biol. Chem. 1999, 274, 16349–16354. [Google Scholar] [CrossRef] [Green Version]
- Serra, R. Matrix Metalloproteinases in Health and Disease. Biomolecules 2020, 10, 1138. [Google Scholar] [CrossRef]
- Tokuhara, C.K.; Santesso, M.R.; Oliveira, G.S.N.; Ventura, T.; Doyama, J.T.; Zambuzzi, W.F.; Oliveira, R.C. Updating the role of matrix metalloproteinases in mineralized tissue and related diseases. J. Appl. Oral Sci. 2019, 27, e20180596. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, Y.; Zhao, X.; Meng, C.; Ma, L.; Kong, Y. MicroRNA-411 inhibited matrix metalloproteinase 13 expression in human chondrocytes. Am. J. Transl. Res. 2015, 7, 2000–2006. [Google Scholar]
- Sasano, Y.; Zhu, J.X.; Tsubota, M.; Takahashi, I.; Onodera, K.; Mizoguchi, I.; Kagayama, M. Gene expression of MMP8 and MMP13 during embryonic development of bone and cartilage in the rat mandible and hind limb. J. Histochem. Cytochem. 2002, 50, 325–332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, X.; He, G.; Levine, A.; Cao, Y.; Mullins, C. Adenovirus-mediated expression of TIMP-1 and TIMP-2 in bone inhibits osteolytic degradation by human prostate cancer. Int. J. Cancer 2008, 122, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Voss, K.A.; Gelineau-van Waes, J.B.; Riley, R.T. Fumonisins: Current research trends in developmental toxicology. Mycotoxin Res. 2006, 22, 61–69. [Google Scholar] [CrossRef]
- Dobrowolski, P.; Tomaszewska, E.; Kurlak, P.; Pierzynowski, S.G. Dietary 2-oxoglutarate mitigates gastrectomy-evoked structural changes in cartilage of female rats. Exp. Biol. Med. 2016, 241, 14–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Puzio, I.; Muszyński, S.; Dobrowolski, P.; Kapica, M.; Pawłowska-Olszewska, M.; Donaldson, J.; Tomaszewska, E. Alterations in small intestine and liver morphology, immunolocalization of leptin, ghrelin and Nesfatin-1 as well as immunoexpression of tight junction proteins in intestinal mucosa after gastrectomy in rat model. J. Clin. Med. 2021, 10, 272. [Google Scholar] [CrossRef] [PubMed]
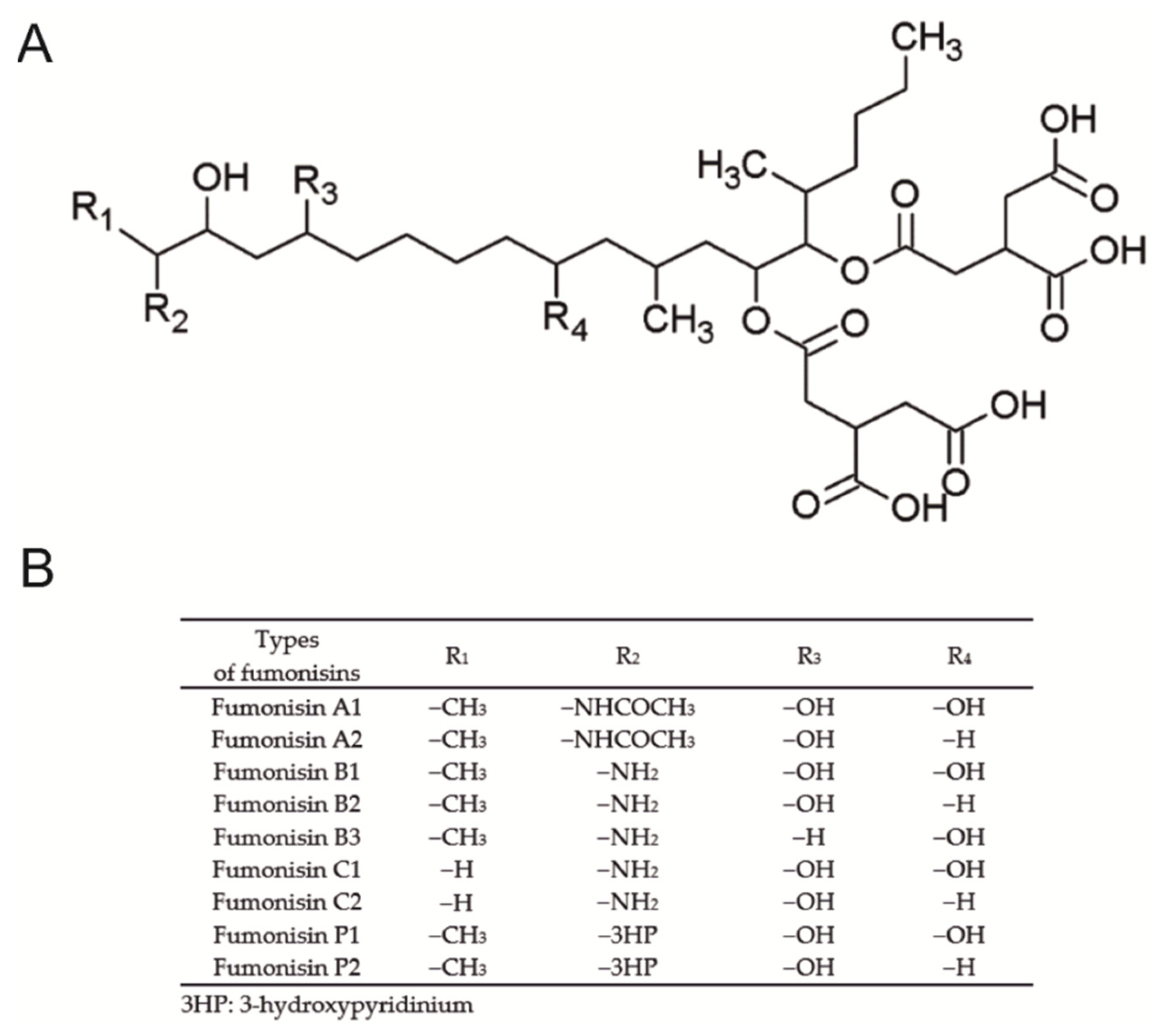

| Dependent Variable | Sex | FB (mg/kg b.w.) | p-Value | p-Level | |||
|---|---|---|---|---|---|---|---|
| 0 | 60 | 90 | Linear | Quadratic | |||
| BV/TV | F | 20.14 ± 1.32 a | 13.52 ± 0.49 b | 15.85 ± 0.99 b | 0.001 | 0.002 | <0.001 |
| Tb.Th (mm) | 17.14 ± 0.74 a | 14.92 ± 0.43 b | 13.11 ± 0.55 b | 0.001 | <0.001 | 0.002 | |
| Tb.Thmax (mm) | 44.42 ± 2.95 a | 31.96 ± 0.87 b | 29.80 ± 1.92 b | <0.001 | <0.001 | <0.001 | |
| Tb.Sp (mm) | 74.97 ± 5.07 a | 99.67 ± 7.63 b | 85.73 ± 3.54 a,b | 0.025 | 0.085 | 0.011 | |
| Tb.Spmax (mm) | 213.83 ± 22.11 | 206.33 ± 15.25 | 174.17 ± 13.39 | 0.259 | 0.159 | 0.457 | |
| Tb.N | 11.73 ± 0.43 a | 9.06 ± 0.15 b | 12.12 ± 0.71 a | 0.001 | 0.735 | 0.011 | |
| Length (mm) | 10.97 ± 0.07 a | 9.73 ± 0.19 b | 9.68 ± 0.15 b | <0.001 | <0.001 | <0.001 | |
| BV/TV | M | 17.53 ± 1.03 | 17.20 ± 0.70 | 18.24 ± 0.48 | 0.634 | 0.626 | 0.972 |
| Tb.Th (mm) | 13.06 ± 0.26 | 13.67 ± 0.62 | 14.70 ± 0.72 | 0.156 | 0.071 | 0.223 | |
| Tb.Thmax (mm) | 29.70 ± 2.93 | 35.84 ± 3.08 | 34.24 ± 1.55 | 0.257 | 0.171 | 0.105 | |
| Tb.Sp (mm) | 68.76 ± 3.85 a | 87.67 ± 6.85 b | 75.00 ± 2.85 a,b | 0.041 | 0.181 | 0.024 | |
| Tb.Spmax (mm) | 151.17 ± 11.91 a | 242.67 ± 16.52 b | 179.50 ± 14.01 a | 0.001 | 0.062 | <0.001 | |
| Tb.N | 13.45 ± 0.84 | 12.68 ± 0.69 | 12.49 ± 0.36 | 0.567 | 0.299 | 0.339 | |
| Length (mm) | 11.54 ± 0.09 a | 10.76 ± 0.28 b | 10.04 ± 0.25 b | 0.001 | <0.001 | 0.003 | |
| Dependent Variable | Region | FB (mg/kg b.w.) | p-Value | p-Level | ||||
|---|---|---|---|---|---|---|---|---|
| 0 | 60 | 90 | Linear | Quadratic | ||||
| MMP-13 | growing plate | proliferative | 116.66 ± 7.79 a | 78.09 ± 1.87 b | 98.92 ± 8.76 a,b | <0.001 | 0.026 | 0.002 |
| hypertrophic | 86.66 ± 1.86 a | 111.41 ± 7.38 b | 119.13 ± 7.43 b | 0.005 | 0.002 | 0.003 | ||
| articular | 150.76 ± 12.45 a | 101.29 ± 3.27 b | 86.97 ± 2.61 b | <0.001 | <0.001 | <0.001 | ||
| trabecular | osteocyte | 190.59 ± 9.27 a | 158.14 ± 7.42 b | 139.62 ± 6.72 b | 0.001 | <0.001 | 0.002 | |
| matrix | 158.23 ± 7.04 a | 112.42 ± 4.86 b | 92.23 ± 7.72 b | <0.001 | <0.001 | <0.001 | ||
| compact | osteocyte | 184.21 ± 10.01 a | 210.61 ± 5.77 a | 116.11 ± 5.56 b | <0.001 | <0.001 | 0.065 | |
| matrix | 134.83 ± 8.40 | 152.05 ± 4.21 | 120.60 ± 1.42 | 0.054 | 0.282 | 0.253 | ||
| MMP-8 | growing plate | proliferative | 93.27 ± 1.05 a | 80.76 ± 3.91 b | 77.28 ± 2.31 b | 0.002 | 0.001 | 0.001 |
| hypertrophic | 125.69 ± 11.56 | 105.63 ± 4.74 | 104.15 ± 9.98 | 0.188 | 0.078 | 0.092 | ||
| articular | 191.28 ± 11.99 a | 117.59 ± 8.28 b | 111.88 ± 5.04 b | <0.001 | <0.001 | <0.001 | ||
| trabecular | osteocyte | 220.02 ± 12.91 a | 178.91 ± 4.73 b | 105.58 ± 9.40 c | <0.001 | <0.001 | <0.001 | |
| matrix | 139.81 ± 3.09 a | 99.48 ± 10.87 b | 151.86 ± 14.32 a | 0.009 | 0.912 | 0.002 | ||
| compact | osteocyte | 174.57 ± 9.16 a | 220.66 ± 3.99 b | 128.16 ± 7.34 c | <0.001 | 0.009 | 0.045 | |
| matrix | 125.95 ± 6.99 | 110.64 ± 4.06 | 118.98 ± 3.38 | 0.154 | 0.230 | 0.070 | ||
| TIMP-2 | growing plate | proliferative | 242.35 ± 3.18 a | 217.17 ± 5.67 b | 88.21 ± 5.64 c | <0.001 | <0.001 | <0.001 |
| hypertrophic | 226.13 ± 3.68 a | 185.12 ± 6.18 b | 132.12 ± 5.08 c | 0.001 | <0.001 | <0.001 | ||
| articular | 166.55 ± 6.25 | 182.75 ± 2.57 | 193.23 ± 15.33 | 0.180 | 0.069 | 0.137 | ||
| trabecular | osteocyte | 221.86 ± 5.93 | 205.72 ± 3.31 | 225.94 ± 1.84 | 0.003 | 0.994 | 0.039 | |
| matrix | 151.75 ± 3.68 | 136.24 ± 0.97 | 139.78 ± 7.48 | 0.092 | 0.062 | 0.061 | ||
| compact | osteocyte | 237.74 ± 3.68 a | 180.51 ± 1.86 b | 198.87 ± 3.99 c | <0.001 | <0.001 | <0.001 | |
| matrix | 156.21 ± 6.70 | 140.79 ± 2.21 | 141.25 ± 5.25 | 0.081 | 0.055 | 0.221 | ||
| Dependent Variable | Region | FB (mg/kg b.w.) | p-Value | p-Level | ||||
|---|---|---|---|---|---|---|---|---|
| 0 | 60 | 90 | Linear | Quadratic | ||||
| MMP-13 | growing plate | proliferative | 89.99 ± 3.51 | 87.71 ± 1.93 | 94.59 ± 11.12 | 0.771 | 0.719 | 0.972 |
| hypertrophic | 155.85 ± 13.50 | 116.79 ± 10.17 | 141.59 ± 9.09 | 0.071 | 0.201 | 0.057 | ||
| articular | 124.54 ± 8.22 a | 81.79 ± 1.59 b | 83.79 ± 3.25 b | <0.001 | <0.001 | <0.001 | ||
| trabecular | osteocyte | 196.98 ± 4.73 a | 156.76 ± 4.04 b | 117.00 ± 1.33 c | <0.001 | <0.001 | 0.960 | |
| matrix | 168.63 ± 8.26 a | 106.15 ± 9.95 b | 111.16 ± 4.21 b | <0.001 | <0.001 | <0.001 | ||
| compact | osteocyte | 202.95 ± 8.29 a | 130.40 ± 12.72 b | 128.04 ± 3.67 b | <0.001 | <0.001 | <0.001 | |
| matrix | 121.41 ± 5.59 a | 158.13 ± 4.11 b | 110.34 ± 2.70 a | <0.001 | 0.789 | 0.001 | ||
| MMP-8 | growing plate | proliferative | 113.24 ± 4.13 | 123.66 ± 1.33 | 126.96 ± 8.85 | 0.237 | 0.097 | 0.134 |
| hypertrophic | 201.27 ± 12.25 | 208.31 ± 5.99 | 172.15 ± 19.43 | 0.176 | 0.237 | 0.852 | ||
| articular | 181.91 ± 9.48 a | 118.87 ± 8.88 b | 133.31 ± 6.81 b | <0.001 | <0.001 | <0.001 | ||
| trabecular | osteocyte | 222.45 ± 3.89 a | 175.96 ± 8.45 b | 149.31 ± 1.68 c | <0.001 | <0.001 | <0.001 | |
| matrix | 104.90 ± 5.67 | 123.29 ± 9.80 | 131.28 ± 9.49 | 0.114 | 0.048 | 0.074 | ||
| compact | osteocyte | 206.23 ± 5.28 a | 117.86 ± 7.97 b | 208.96 ± 7.25 a | <0.001 | 0.105 | <0.001 | |
| matrix | 130.26 ± 7.47 a | 88.12 ± 5.28 b | 180.73 ± 6.57 c | <0.001 | 0.002 | 0.075 | ||
| TIMP-2 | growing plate | proliferative | 246.78 ± 2.59 a | 168.37 ± 10.77 b | 169.25 ± 3.73 b | <0.001 | <0.001 | <0.001 |
| hypertrophic | 243.00 ± 2.21 a | 94.88 ± 6.88 b | 146.66 ± 2.10 c | <0.001 | <0.001 | <0.001 | ||
| articular | 196.23 ± 2.65 a | 147.51 ± 11.60 b | 175.32 ± 10.45 a,b | 0.007 | 0.041 | 0.003 | ||
| trabecular | osteocyte | 243.27 ± 1.94 a | 222.82 ± 5.58 b | 158.03 ± 4.52 c | <0.001 | <0.001 | <0.001 | |
| matrix | 153.18 ± 3.38 a | 136.16 ± 1.62 b | 110.27 ± 2.42 c | <0.001 | <0.001 | <0.001 | ||
| compact | osteocyte | 193.98 ± 4.30 | 189.69 ± 5.23 | 193.03 ± 10.20 | 0.904 | 0.862 | 0.714 | |
| matrix | 124.61 ± 5.50 | 125.30 ± 2.99 | 131.35 ± 8.04 | 0.679 | 0.480 | 0.749 | ||
| Dependent Variable | Sex | FB (mg/kg b.w.) | p-Value | p-Level | |||
|---|---|---|---|---|---|---|---|
| 0 | 60 | 90 | Linear | Quadratic | |||
| MMP-8 | F | 1.096 ± 0.022 a | 1.303 ± 0.041 a | 0.886 ± 0.086 b | <0.001 | 0.103 | 0.235 |
| TIMP-2 | 0.655 ± 0.012 a,b | 0.803 ± 0.055 a | 0.486 ± 0.063 b | 0.001 | 0.116 | 0.366 | |
| VEGF | 0.992 ± 0.058 a | 1.077 ± 0.019 a,b | 1.148 ± 0.036 b | 0.005 | 0.002 | 0.010 | |
| MMP-8 | M | 1.070 ± 0.078 a | 1.110 ± 0.009 a | 0.783 ± 0.055 b | <0.001 | 0.067 | 0.455 |
| TIMP-2 | 1.053 ± 0.140 | 1.223 ± 0.085 | 0.860 ± 0.073 | 0.076 | 0.384 | 0.619 | |
| VEGF | 0.804 ± 0.028 a | 1.067 ± 0.049 b | 0.889 ± 0.051 a | 0.002 | 0.051 | 0.002 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomaszewska, E.; Rudyk, H.; Świetlicka, I.; Hułas-Stasiak, M.; Donaldson, J.; Arczewska, M.; Muszyński, S.; Dobrowolski, P.; Mielnik-Błaszczak, M.; Arciszewski, M.B.; et al. Trabecular Bone Parameters, TIMP-2, MMP-8, MMP-13, VEGF Expression and Immunolocalization in Bone and Cartilage in Newborn Offspring Prenatally Exposed to Fumonisins. Int. J. Mol. Sci. 2021, 22, 12528. https://doi.org/10.3390/ijms222212528
Tomaszewska E, Rudyk H, Świetlicka I, Hułas-Stasiak M, Donaldson J, Arczewska M, Muszyński S, Dobrowolski P, Mielnik-Błaszczak M, Arciszewski MB, et al. Trabecular Bone Parameters, TIMP-2, MMP-8, MMP-13, VEGF Expression and Immunolocalization in Bone and Cartilage in Newborn Offspring Prenatally Exposed to Fumonisins. International Journal of Molecular Sciences. 2021; 22(22):12528. https://doi.org/10.3390/ijms222212528
Chicago/Turabian StyleTomaszewska, Ewa, Halyna Rudyk, Izabela Świetlicka, Monika Hułas-Stasiak, Janine Donaldson, Marta Arczewska, Siemowit Muszyński, Piotr Dobrowolski, Maria Mielnik-Błaszczak, Marcin Bartłomiej Arciszewski, and et al. 2021. "Trabecular Bone Parameters, TIMP-2, MMP-8, MMP-13, VEGF Expression and Immunolocalization in Bone and Cartilage in Newborn Offspring Prenatally Exposed to Fumonisins" International Journal of Molecular Sciences 22, no. 22: 12528. https://doi.org/10.3390/ijms222212528
APA StyleTomaszewska, E., Rudyk, H., Świetlicka, I., Hułas-Stasiak, M., Donaldson, J., Arczewska, M., Muszyński, S., Dobrowolski, P., Mielnik-Błaszczak, M., Arciszewski, M. B., Kushnir, V., Brezvyn, O., Muzyka, V., & Kotsyumbas, I. (2021). Trabecular Bone Parameters, TIMP-2, MMP-8, MMP-13, VEGF Expression and Immunolocalization in Bone and Cartilage in Newborn Offspring Prenatally Exposed to Fumonisins. International Journal of Molecular Sciences, 22(22), 12528. https://doi.org/10.3390/ijms222212528







