Human Pluripotent Stem Cell-Derived Neural Progenitor Cells Promote Retinal Ganglion Cell Survival and Axon Recovery in an Optic Nerve Compression Animal Model
Abstract
:1. Introduction
2. Results
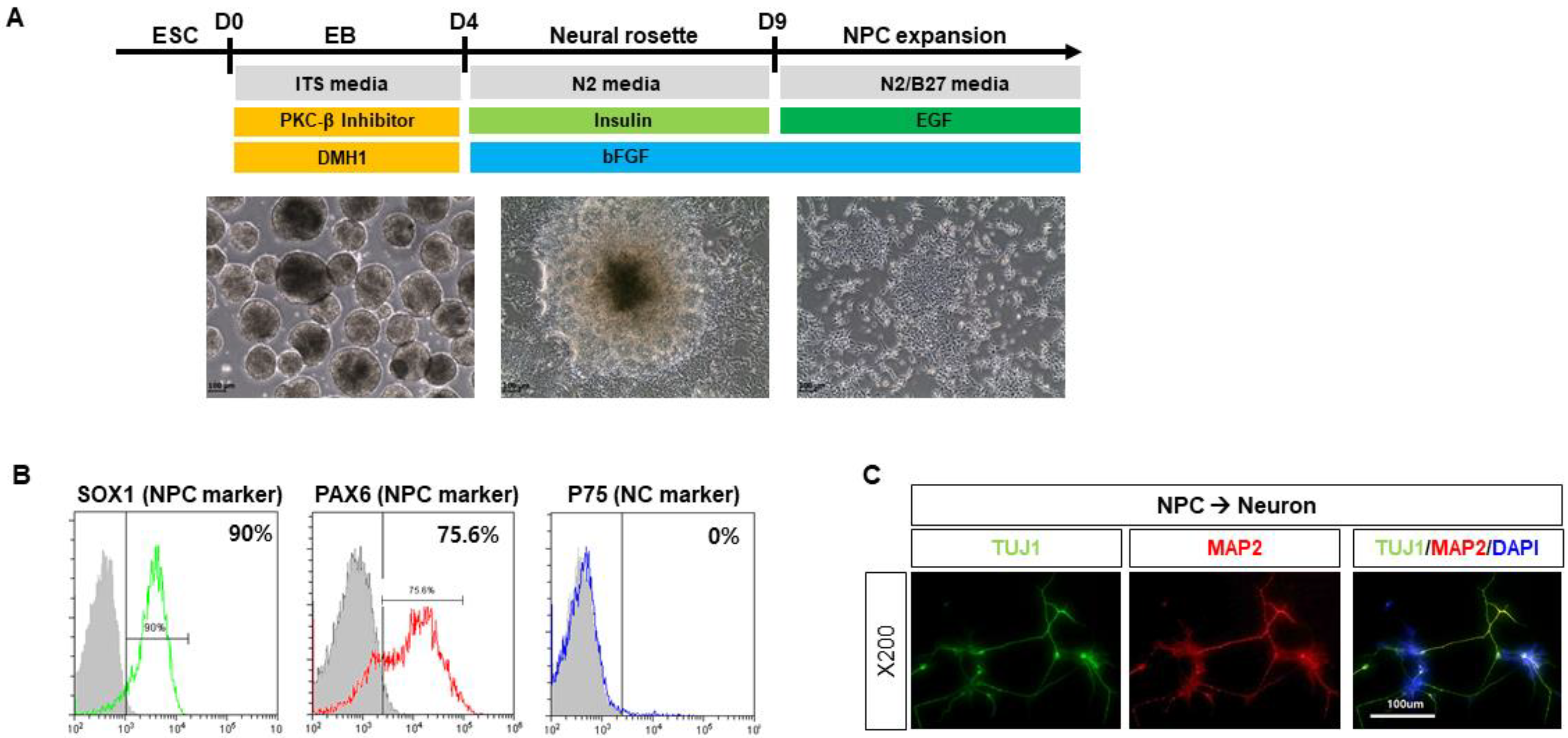
2.1. Characterization of Human Pluripotent Stem Cell-Derived Neuronal Progenitor Cells (NPCs)
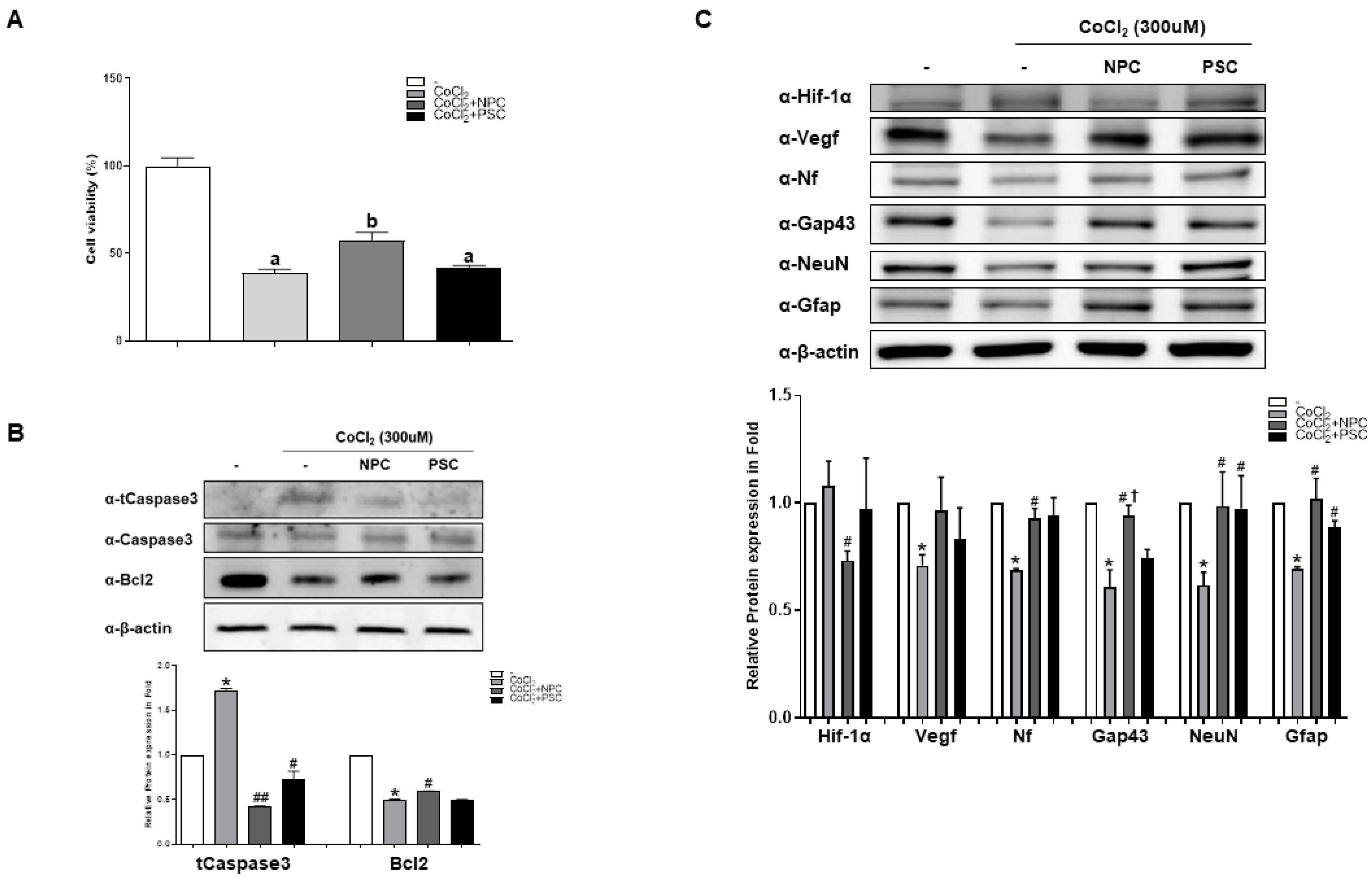
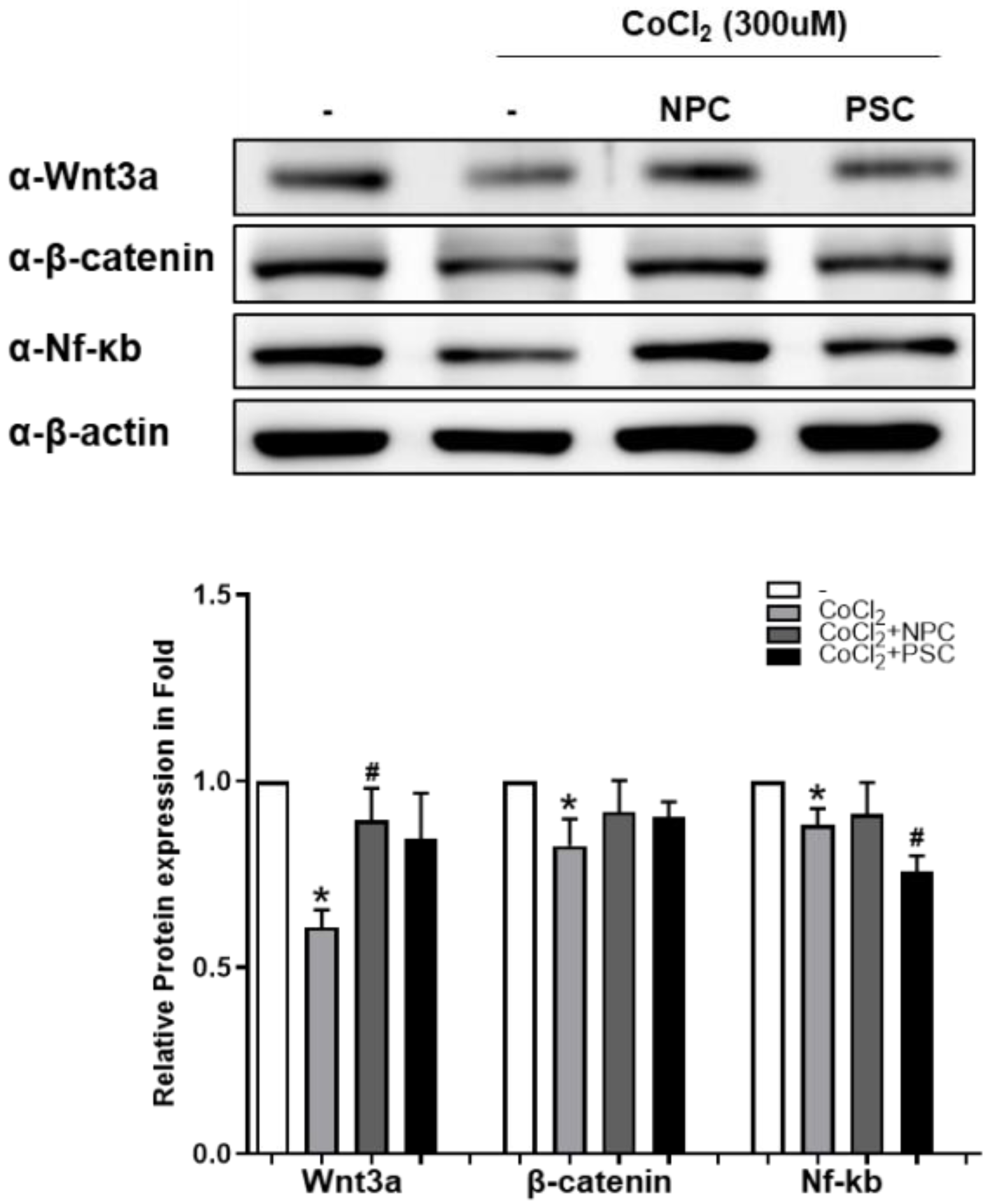
2.2. NPCs Reduce Cell Apoptosis and Regulate Target Proteins
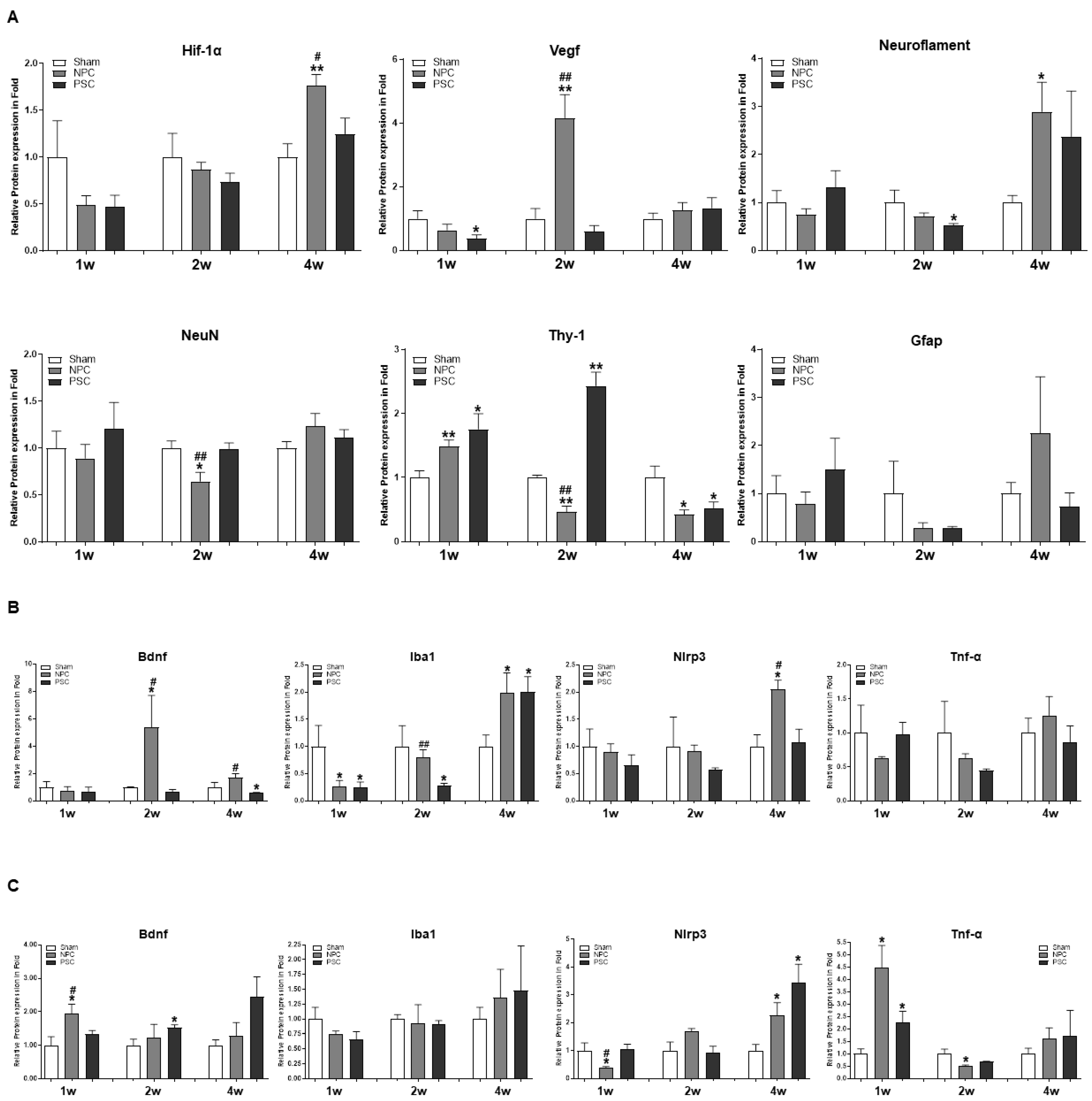
2.3. Changes in Neurogenic Marker Expression in the Retina after Injection of NPCs or PSCs in the Optic Nerve Compression Animal Model
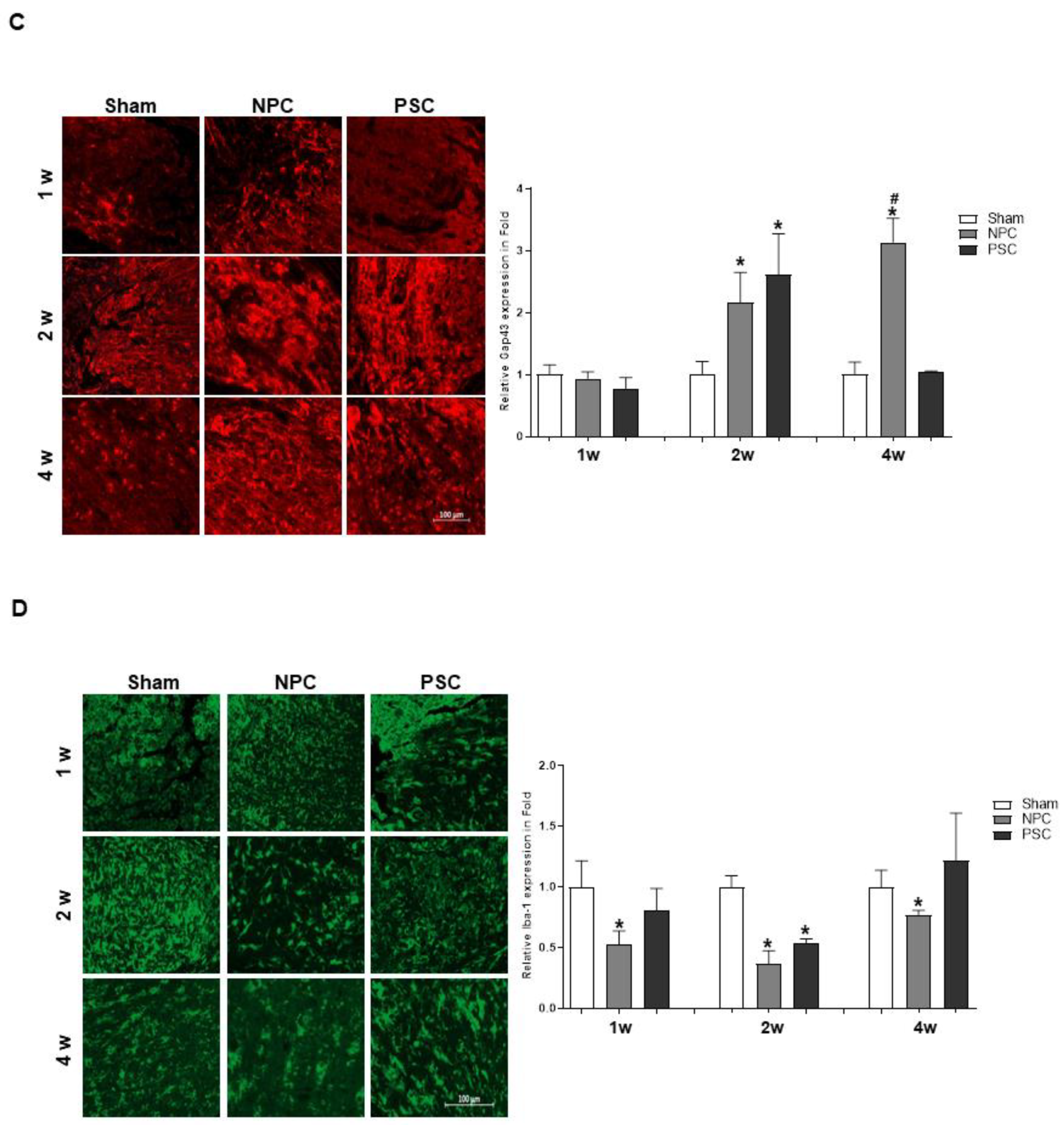
2.4. Comparison of the Target Protein Expression between the Retina and the Optic Nerve Tissue in the Optic Nerve Compression Model
2.5. Protective Effects of NPCs and PSCs on the Retinal RGCs in the Optic Nerve Compression Animal Model
2.6. Effects of NPCs and PSCs on Optic Nerve Axon Damage in the Optic Nerve Injury Animal Model
2.7. Wnt/β-Catenin Signal Is Involved during Recovery of Retinal Ganglion Cells by NPCs
3. Discussion
4. Materials and Methods
4.1. In Vitro Study
4.1.1. Human Pluripotent Stem Cell-Derived Neural Progenitor Cells (NPCs) Preparation
Culture of Human Pluripotent Stem Cells
Embryoid Body (EB) Formation and Induction of NPCs
Flow Cytometry Analysis
Immunocytochemistry
4.1.2. Human Placenta-Derived Mesenchymal Stem Cells (PSC) Preparation
4.1.3. Mammalian Cell Culture and Treatment
4.1.4. Cell Viability Assay
4.1.5. Immunoblot Analyses
4.2. In Vivo Study
4.2.1. Animals and the Study Group
4.2.2. The Optic Nerve Compression Model and Subtenon Cell Injection
4.2.3. Assessment of Axon Regeneration Factors in the Optic Nerve of ONC Model
4.2.4. Flat-Mounted Retinas and RGC Survival Analyses
4.3. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kwon, H.; Park, M.; Nepali, S.; Lew, H. Hypoxia-Preconditioned Placenta-Derived Mesenchymal Stem Cells Rescue Optic Nerve Axons via Differential Roles of Vascular Endothelial Growth Factor in an Optic Nerve Compression Animal Model. Mol. Neurobiol. 2020, 57, 3362–3375. [Google Scholar] [CrossRef]
- Huang, Y.; Li, Z.; van Rooijen, N.; Wang, N.; Pang, C.P.; Cui, Q. Different responses of macrophages in retinal ganglion cell survival after acute ocular hypertension in rats with different autoimmune backgrounds. Exp. Eye Res. 2007, 85, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Muller, A.; Hauk, T.G.; Fischer, D. Astrocyte-derived CNTF switches mature RGCs to a regenerative state following inflammatory stimulation. Brain J. Neurol. 2007, 130 Pt 12, 3308–3320. [Google Scholar] [CrossRef] [Green Version]
- Park, K.K.; Hu, Y.; Muhling, J.; Pollett, M.A.; Dallimore, E.J.; Turnley, A.M.; Cui, Q.; Harvey, A.R. Cytokine-induced SOCS expression is inhibited by cAMP analogue: Impact on regeneration in injured retina. Mol. Cell. Neurosci. 2009, 41, 313–324. [Google Scholar] [CrossRef]
- Yin, Y.; Cui, Q.; Gilbert, H.Y.; Yang, Y.; Yang, Z.; Berlinicke, C.; Li, Z.; Zaverucha-do-Valle, C.; He, H.; Petkova, V.; et al. Oncomodulin links inflammation to optic nerve regeneration. Proc. Natl. Acad. Sci. USA 2009, 106, 19587–19592. [Google Scholar] [CrossRef] [Green Version]
- Park, K.K.; Liu, K.; Hu, Y.; Smith, P.D.; Wang, C.; Cai, B.; Xu, B.; Connolly, L.; Kramvis, I.; Sahin, M.; et al. Promoting axon regeneration in the adult CNS by modulation of the PTEN/mTOR pathway. Science 2008, 322, 963–966. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.; He, Q.; Wang, H.; Tang, X.; Ho, K.W.; Gao, X.; Zhang, Q.; Shen, Y.; Cheung, A.; Wong, F.; et al. Injured adult retinal axons with Pten and Socs3 co-deletion reform active synapses with suprachiasmatic neurons. Neurobiol. Dis. 2015, 73, 366–376. [Google Scholar] [CrossRef] [PubMed]
- De Lima, S.; Koriyama, Y.; Kurimoto, T.; Oliveira, J.T.; Yin, Y.; Li, Y.; Gilbert, H.Y.; Fagiolini, M.; Martinez, A.M.; Benowitz, L. Full-length axon regeneration in the adult mouse optic nerve and partial recovery of simple visual behaviors. Proc. Natl. Acad. Sci. USA 2012, 109, 9149–9154. [Google Scholar] [CrossRef] [Green Version]
- Kurimoto, T.; Yin, Y.; Omura, K.; Gilbert, H.Y.; Kim, D.; Cen, L.P.; Moko, L.; Kugler, S.; Benowitz, L.I. Long-distance axon regeneration in the mature optic nerve: Contributions of oncomodulin, cAMP, and pten gene deletion. J. Neurosci. Off. J. Soc. Neurosci. 2010, 30, 15654–15663. [Google Scholar] [CrossRef] [Green Version]
- Cunningham, J.J.; Ulbright, T.M.; Pera, M.F.; Looijenga, L.H. Lessons from human teratomas to guide development of safe stem cell therapies. Nat. Biotechnol. 2012, 30, 849–857. [Google Scholar] [CrossRef]
- Mesentier-Louro, L.A.; Zaverucha-do-Valle, C.; Rosado-de-Castro, P.H.; Silva-Junior, A.J.; Pimentel-Coelho, P.M.; Mendez-Otero, R.; Santiago, M.F. Bone Marrow-Derived Cells as a Therapeutic Approach to Optic Nerve Diseases. Stem Cells Int. 2016, 2016, 5078619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, T.V.; DeKorver, N.W.; Levasseur, V.A.; Osborne, A.; Tassoni, A.; Lorber, B.; Heller, J.P.; Villasmil, R.; Bull, N.D.; Martin, K.R.; et al. Identification of retinal ganglion cell neuroprotection conferred by platelet-derived growth factor through analysis of the mesenchymal stem cell secretome. Brain J. Neurol. 2014, 137 Pt 2, 503–519. [Google Scholar] [CrossRef]
- Yu, S.; Tanabe, T.; Dezawa, M.; Ishikawa, H.; Yoshimura, N. Effects of bone marrow stromal cell injection in an experimental glaucoma model. Biochem. Biophys. Res. Commun. 2006, 344, 1071–1079. [Google Scholar] [CrossRef]
- Johnson, T.V.; Bull, N.D.; Hunt, D.P.; Marina, N.; Tomarev, S.I.; Martin, K.R. Neuroprotective effects of intravitreal mesenchymal stem cell transplantation in experimental glaucoma. Investig. Ophthalmol. Vis. Sci. 2010, 51, 2051–2059. [Google Scholar] [CrossRef]
- Manuguerra-Gagne, R.; Boulos, P.R.; Ammar, A.; Leblond, F.A.; Krosl, G.; Pichette, V.; Lesk, M.R.; Roy, D.C. Transplantation of mesenchymal stem cells promotes tissue regeneration in a glaucoma model through laser-induced paracrine factor secretion and progenitor cell recruitment. Stem Cells 2013, 31, 1136–1148. [Google Scholar] [CrossRef]
- Emre, E.; Yuksel, N.; Duruksu, G.; Pirhan, D.; Subasi, C.; Erman, G.; Karaoz, E. Neuroprotective effects of intravitreally transplanted adipose tissue and bone marrow-derived mesenchymal stem cells in an experimental ocular hypertension model. Cytotherapy 2015, 17, 543–559. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Li, X.R.; Yuan, J.Q. Effects of bone-marrow mesenchymal stem cells transplanted into vitreous cavity of rat injured by ischemia/reperfusion. Graefe’s Arch. Clin. Exp. Ophthalmol. Albrecht Von Graefes Arch. Fur Klin. Und Exp. Ophthalmol. 2009, 247, 503–514. [Google Scholar]
- Zaverucha-do-Valle, C.; Gubert, F.; Bargas-Rega, M.; Coronel, J.L.; Mesentier-Louro, L.A.; Mencalha, A.; Abdelhay, E.; Santiago, M.F.; Mendez-Otero, R. Bone marrow mononuclear cells increase retinal ganglion cell survival and axon regeneration in the adult rat. Cell Transplant. 2011, 20, 391–406. [Google Scholar] [CrossRef]
- Rosado-de-Castro, P.H.; Pimentel-Coelho, P.M.; da Fonseca, L.M.; de Freitas, G.R.; Mendez-Otero, R. The rise of cell therapy trials for stroke: Review of published and registered studies. Stem Cells Dev. 2013, 22, 2095–2111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mesentier-Louro, L.A.; Zaverucha-do-Valle, C.; da Silva-Junior, A.J.; Nascimento-Dos-Santos, G.; Gubert, F.; de Figueiredo, A.B.; Torres, A.L.; Paredes, B.D.; Teixeira, C.; Tovar-Moll, F.; et al. Distribution of mesenchymal stem cells and effects on neuronal survival and axon regeneration after optic nerve crush and cell therapy. PLoS ONE 2014, 9, e110722. [Google Scholar]
- Labrador-Velandia, S.; Alonso-Alonso, M.L.; Alvarez-Sanchez, S.; Gonzalez-Zamora, J.; Carretero-Barrio, I.; Pastor, J.C.; Fernandez-Bueno, I.; Srivastava, G.K. Mesenchymal stem cell therapy in retinal and optic nerve diseases: An update of clinical trials. World J. Stem Cells 2016, 8, 376–383. [Google Scholar] [CrossRef] [Green Version]
- Chung, S.; Rho, S.; Kim, G.; Kim, S.R.; Baek, K.H.; Kang, M.; Lew, H. Human umbilical cord blood mononuclear cells and chorionic plate-derived mesenchymal stem cells promote axon survival in a rat model of optic nerve crush injury. Int. J. Mol. Med. 2016, 37, 1170–1180. [Google Scholar] [CrossRef] [Green Version]
- Park, M.; Kim, H.C.; Kim, O.; Lew, H. Human placenta mesenchymal stem cells promote axon survival following optic nerve compression through activation of NF-kappaB pathway. J. Tissue Eng. Regen. Med. 2018, 12, e1441–e1449. [Google Scholar] [CrossRef]
- Garcia, A.L.; Udeh, A.; Kalahasty, K.; Hackam, A.S. A growing field: The regulation of axonal regeneration by Wnt signaling. Neural Regen. Res. 2018, 13, 43–52. [Google Scholar] [PubMed]
- Patel, A.K.; Park, K.K.; Hackam, A.S. Wnt signaling promotes axonal regeneration following optic nerve injury in the mouse. Neuroscience 2017, 343, 372–383. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.M.; Noh, H.B.; Lee, S.H.; Lee, K.G.; Chang, B.; Cheong, E.; Lee, C.J.; Hwang, D.Y. Fine-tuning of dual-SMAD inhibition to differentiate human pluripotent stem cells into neural crest stem cells. Cell Prolif. 2021, 54, e13103. [Google Scholar] [CrossRef] [PubMed]
- Dvoriantchikova, G.; Pappas, S.; Luo, X.; Ribeiro, M.; Danek, D.; Pelaez, D.; Park, K.K.; Ivanov, D. Virally delivered, constitutively active NFkappaB improves survival of injured retinal ganglion cells. Eur. J. Neurosci. 2016, 44, 2935–2943. [Google Scholar] [CrossRef]
- Liu, X.; Wang, K.; Wei, X.; Xie, T.; Lv, B.; Zhou, Q.; Wang, X. Interaction of NF-kappaB and Wnt/beta-catenin Signaling Pathways in Alzheimer’s Disease and Potential Active Drug Treatments. Neurochem. Res. 2021, 46, 711–731. [Google Scholar] [CrossRef]
- Koh, K.; Park, M.; Bae, E.S.; Duong, V.A.; Park, J.M.; Lee, H.; Lew, H. UBA2 activates Wnt/beta-catenin signaling pathway during protection of R28 retinal precursor cells from hypoxia by extracellular vesicles derived from placental mesenchymal stem cells. Stem Cell Res. Ther. 2020, 11, 428. [Google Scholar] [CrossRef] [PubMed]
- Heuss, N.D.; Pierson, M.J.; Roehrich, H.; McPherson, S.W.; Gram, A.L.; Li, L.; Gregerson, D.S. Optic nerve as a source of activated retinal microglia post-injury. Acta Neuropathol. Commun. 2018, 6, 66. [Google Scholar] [CrossRef] [Green Version]
- Cui, W.; Sun, C.; Ma, Y.; Wang, S.; Wang, X.; Zhang, Y. Inhibition of TLR4 Induces M2 Microglial Polarization and Provides Neuroprotection via the NLRP3 Inflammasome in Alzheimer’s Disease. Front. Neurosci. 2020, 14, 444. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Liu, Y.; Zhang, N.; Li, C.; Sandhu, A.F.; Williams, G., III; Shen, Y.; Li, H.; Wu, Q.; Yu, S. Electroacupuncture Improves M2 Microglia Polarization and Glia Anti-inflammation of Hippocampus in Alzheimer’s Disease. Front. Neurosci. 2021, 15, 689629. [Google Scholar] [CrossRef] [PubMed]
- Sung, Y.; Lee, S.M.; Park, M.; Choi, H.J.; Kang, S.; Choi, B.I.; Lew, H. Treatment of traumatic optic neuropathy using human placenta-derived mesenchymal stem cells in Asian patients. Regen. Med. 2020, 15, 2163–2179. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, M.; Kim, H.-M.; Shin, H.-A.; Lee, S.-H.; Hwang, D.-Y.; Lew, H. Human Pluripotent Stem Cell-Derived Neural Progenitor Cells Promote Retinal Ganglion Cell Survival and Axon Recovery in an Optic Nerve Compression Animal Model. Int. J. Mol. Sci. 2021, 22, 12529. https://doi.org/10.3390/ijms222212529
Park M, Kim H-M, Shin H-A, Lee S-H, Hwang D-Y, Lew H. Human Pluripotent Stem Cell-Derived Neural Progenitor Cells Promote Retinal Ganglion Cell Survival and Axon Recovery in an Optic Nerve Compression Animal Model. International Journal of Molecular Sciences. 2021; 22(22):12529. https://doi.org/10.3390/ijms222212529
Chicago/Turabian StylePark, Mira, Hyun-Mun Kim, Hyun-Ah Shin, Seung-Hyun Lee, Dong-Youn Hwang, and Helen Lew. 2021. "Human Pluripotent Stem Cell-Derived Neural Progenitor Cells Promote Retinal Ganglion Cell Survival and Axon Recovery in an Optic Nerve Compression Animal Model" International Journal of Molecular Sciences 22, no. 22: 12529. https://doi.org/10.3390/ijms222212529
APA StylePark, M., Kim, H.-M., Shin, H.-A., Lee, S.-H., Hwang, D.-Y., & Lew, H. (2021). Human Pluripotent Stem Cell-Derived Neural Progenitor Cells Promote Retinal Ganglion Cell Survival and Axon Recovery in an Optic Nerve Compression Animal Model. International Journal of Molecular Sciences, 22(22), 12529. https://doi.org/10.3390/ijms222212529





