Bone Regeneration of a 3D-Printed Alloplastic and Particulate Xenogenic Graft with rhBMP-2
Abstract
:1. Introduction
2. Results
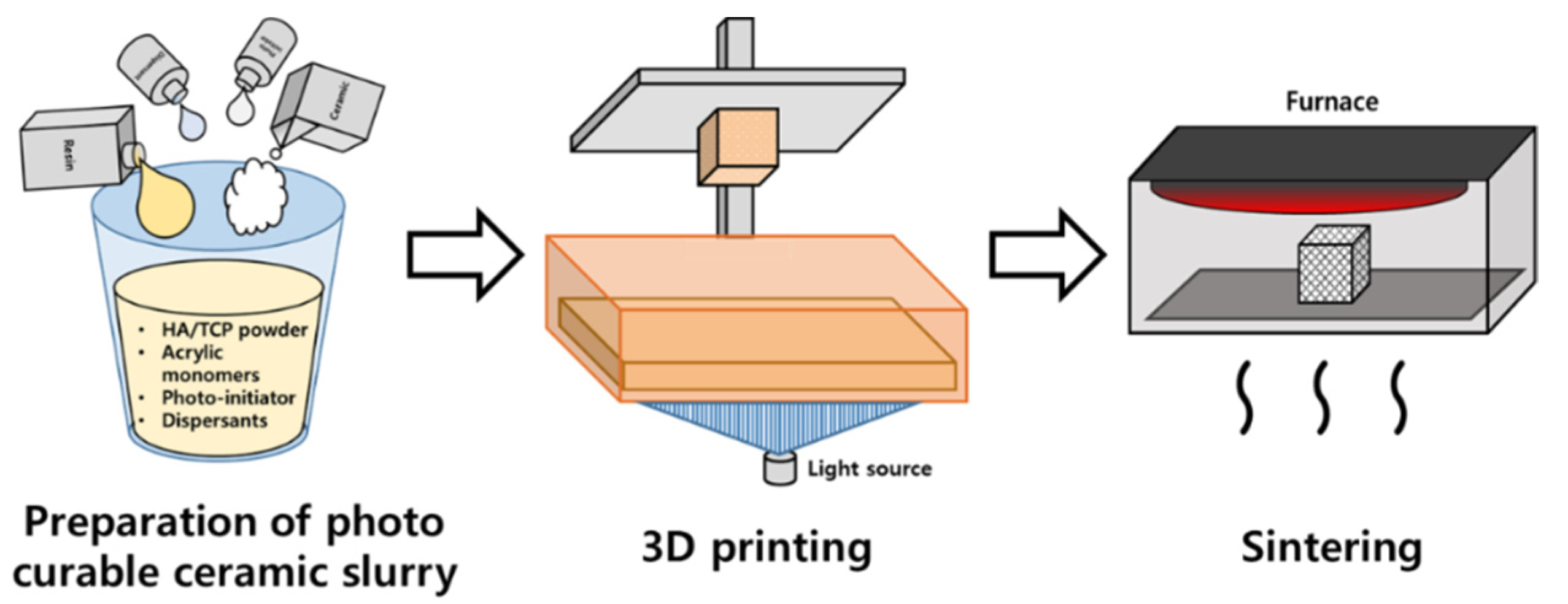
2.1. Architecture of Scaffold
2.2. Cytotoxicity Test
2.3. Clinical Findings
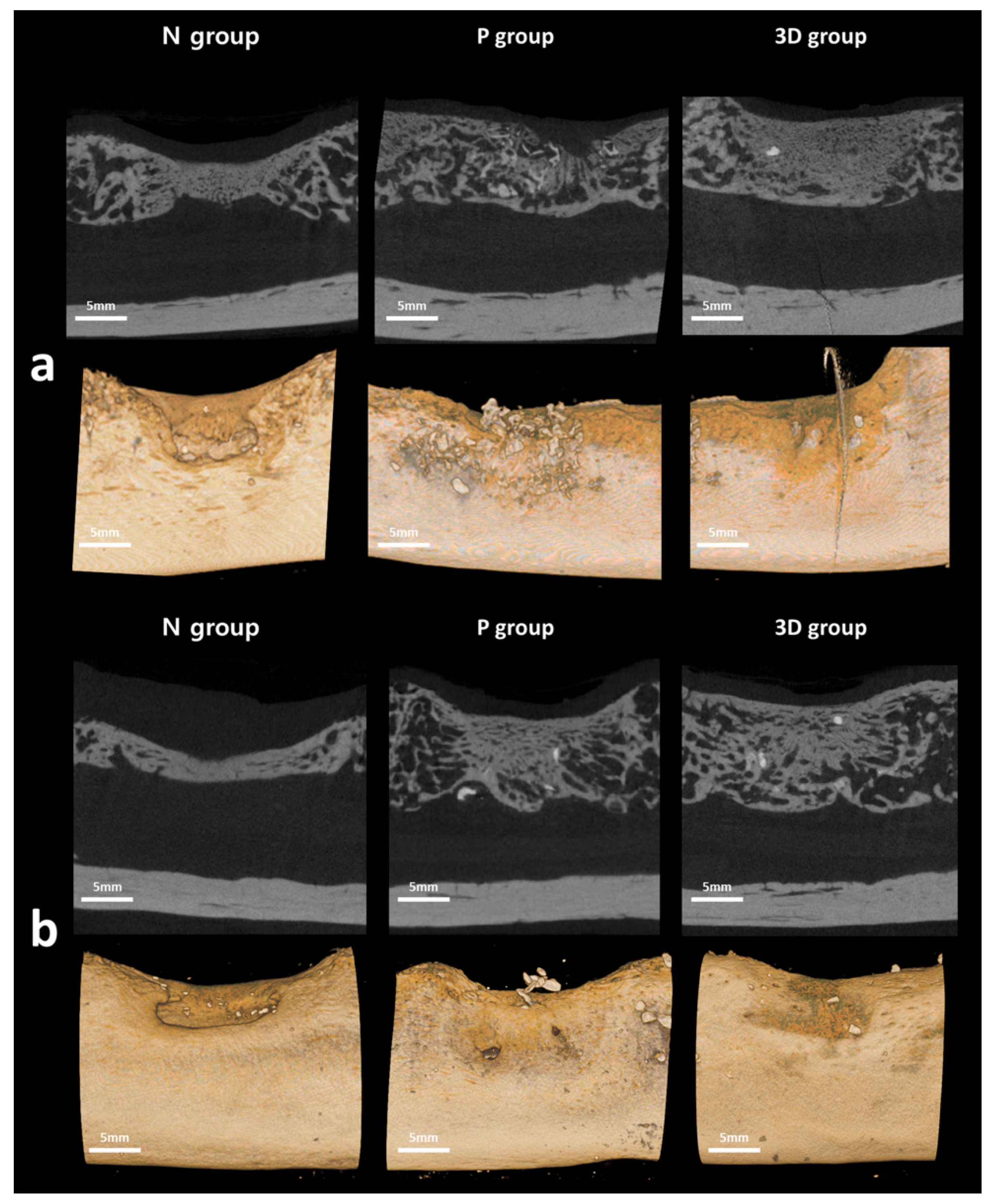
2.4. Radiological Evaluation
2.5. Histological Evaluation
3. Discussion
4. Materials and Methods
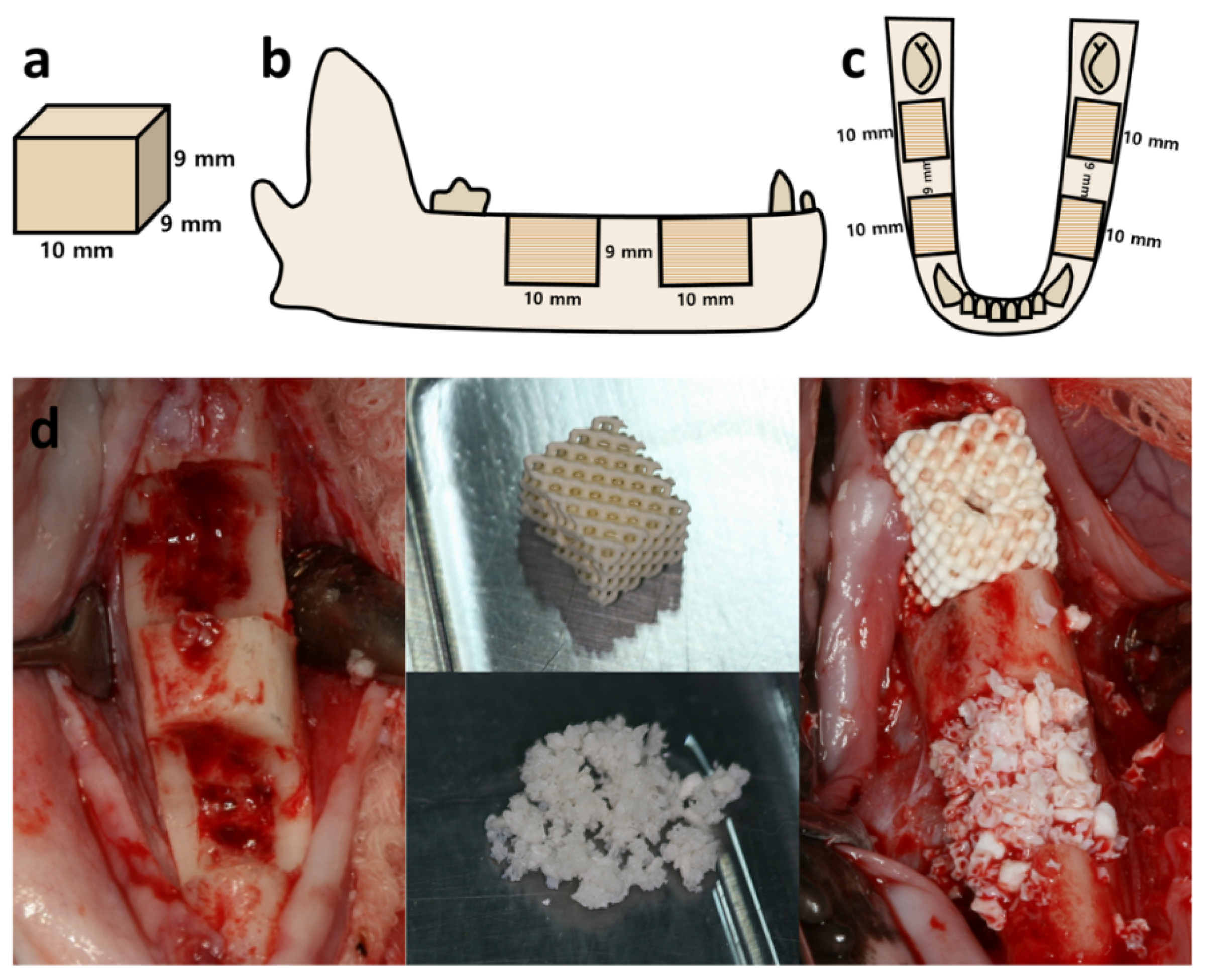
4.1. Subject
4.2. Preparation of Scaffolds
4.3. Cytotoxic Tests
4.4. Surgical Procedures
4.5. Analysis
4.5.1. Radiological Examination
4.5.2. Histological Examination
4.5.3. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Johnson, K. A study of the dimensional changes occurring in the maxilla following tooth extraction. Aust. Dent. J. 1969, 14, 241–244. [Google Scholar] [CrossRef]
- Levi, I.; Halperin-Sternfeld, M.; Horwitz, J.; Zigdon-Giladi, H.; Machtei, E.E. Dimensional changes of the maxillary sinus following tooth extraction in the posterior maxilla with and without socket preservation. Clin. Implant. Dent. Relat. Res. 2017, 19, 952–958. [Google Scholar] [CrossRef]
- Hansson, S.; Halldin, A. Alveolar ridge resorption after tooth extraction: A consequence of a fundamental principle of bone physiology. J. Dent. Biomech. 2012, 3, 1758736012456543. [Google Scholar] [CrossRef] [PubMed]
- Schropp, L.; Wenzel, A.; Kostopoulos, L.; Karring, T. Bone healing and soft tissue contour changes following single-tooth extraction: A clinical and radiographic 12-month prospective study. Int. J. Periodontics Restor. Dent. 2003, 23, 313–323. [Google Scholar]
- Jensen, S.S.; Terheyden, H. Bone augmentation procedures in localized defects in the alveolar ridge: Clinical results with different bone grafts and bone-substitute materials. Int. J. Oral Maxillofac. Implant. 2009, 24, 218–236. [Google Scholar]
- Khojasteh, A.; Behnia, H.; Shayesteh, Y.S.; Morad, G.; Alikhasi, M. Localized bone augmentation with cortical bone blocks tented over different particulate bone substitutes: A retrospective study. Int. J. Oral Maxillofac. Implant. 2012, 27, 1481–1493. [Google Scholar]
- Brener, D. The mandibular ramus donor site. Aust. Dent. J. 2006, 51, 187–190. [Google Scholar] [CrossRef]
- Hjorting-Hansen, E. Bone grafting to the jaws with special reference to reconstructive preprosthetic surgery. A historical review. Mund Kiefer. Gesichtschir. 2002, 6, 6–14. [Google Scholar] [CrossRef]
- Swan, M.C.; Goodacre, T.E. Morbidity at the iliac crest donor site following bone grafting of the cleft alveolus. Br. J. Oral Maxillofac. Surg. 2006, 44, 129–133. [Google Scholar] [CrossRef]
- Ku, J.K.; Kim, Y.K.; Yun, P.Y. Influence of biodegradable polymer membrane on new bone formation and biodegradation of biphasic bone substitutes: An animal mandibular defect model study. Maxillofac. Plast. Reconstr. Surg. 2020, 42, 34. [Google Scholar] [CrossRef]
- Lim, H.K.; Kwon, Y.J.; Hong, S.J.; Choi, H.G.; Chung, S.M.; Yang, B.E.; Lee, J.H.; Byun, S.H. Bone regeneration in ceramic scaffolds with variable concentrations of PDRN and rhBMP-2. Sci. Rep. 2021, 11, 11470. [Google Scholar] [CrossRef]
- Kim, H.S.; Park, J.C.; Yun, P.Y.; Kim, Y.K. Evaluation of bone healing using rhBMP-2 soaked hydroxyapatite in ridge augmentation: A prospective observational study. Maxillofac. Plast. Reconstr. Surg. 2017, 39, 40. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.J.; Kim, S.K.; Lee, J.H. Bone regeneration of demineralized dentin matrix with platelet-rich fibrin and recombinant human bone morphogenetic protein-2 on the bone defects in rabbit calvaria. Maxillofac. Plast. Reconstr. Surg. 2021, 43, 34. [Google Scholar] [CrossRef]
- Kim, S.K.; Huh, C.K.; Lee, J.H.; Kim, K.W.; Kim, M.Y. Histologic study of bone-forming capacity on polydeoxyribonucleotide combined with demineralized dentin matrix. Maxillofac. Plast. Reconstr. Surg. 2016, 38, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Urist, M.R.; Strates, B.S. Bone morphogenetic protein. J. Dent. Res. 1971, 50, 1392–1406. [Google Scholar] [CrossRef]
- Wozney, J.M.; Rosen, V.; Celeste, A.J.; Mitsock, L.M.; Whitters, M.J.; Kriz, R.W.; Hewick, R.M.; Wang, E.A. Novel regulators of bone formation: Molecular clones and activities. Science 1988, 242, 1528–1534. [Google Scholar] [CrossRef] [PubMed]
- Francisco, I.; Paula, A.B.; Oliveiros, B.; Fernandes, M.H.; Carrilho, E.; Marto, C.M.; Vale, F. Regenerative Strategies in Cleft Palate: An Umbrella Review. Bioengineering 2021, 8, 76. [Google Scholar] [CrossRef] [PubMed]
- Francis, C.S.; Mobin, S.S.; Lypka, M.A.; Rommer, E.; Yen, S.; Urata, M.M.; Hammoudeh, J.A. rhBMP-2 with a demineralized bone matrix scaffold versus autologous iliac crest bone graft for alveolar cleft reconstruction. Plast. Reconstr. Surg. 2013, 131, 1107–1115. [Google Scholar] [CrossRef] [Green Version]
- Herford, A.S.; Boyne, P.J.; Rawson, R.; Williams, R.P. Bone morphogenetic protein-induced repair of the premaxillary cleft. J. Oral Maxillofac. Surg. 2007, 65, 2136–2141. [Google Scholar] [CrossRef] [PubMed]
- Chin, M.; Ng, T.; Tom, W.K.; Carstens, M. Repair of alveolar clefts with recombinant human bone morphogenetic protein (rhBMP-2) in patients with clefts. J. Craniofac. Surg. 2005, 16, 778–789. [Google Scholar] [CrossRef]
- Hwang, D.Y.; On, S.W.; Song, S.I. Bone regenerative effect of recombinant human bone morphogenetic protein-2 after cyst enucleation. Maxillofac. Plast. Reconstr. Surg. 2016, 38, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tonello, G.; Daglio, M.; Zaccarelli, N.; Sottofattori, E.; Mazzei, M.; Balbi, A. Characterization and quantitation of the active polynucleotide fraction (PDRN) from human placenta, a tissue repair stimulating agent. J. Pharm. Biomed. Anal. 1996, 14, 1555–1560. [Google Scholar] [CrossRef]
- Bianchini, P.; Tellini, N.; Morani, A.M.; Folloni, M.G. Pharmacological data on polydeoxyribonucleotide of human placenta. Int. J. Tissue React. 1981, 3, 151–154. [Google Scholar] [PubMed]
- Guizzardi, S.; Galli, C.; Govoni, P.; Boratto, R.; Cattarini, G.; Martini, D.; Belletti, S.; Scandroglio, R. Polydeoxyribonucleotide (PDRN) promotes human osteoblast proliferation: A new proposal for bone tissue repair. Life Sci. 2003, 73, 1973–1983. [Google Scholar] [CrossRef]
- Tamimi, F.; Torres, J.; Al-Abedalla, K.; Lopez-Cabarcos, E.; Alkhraisat, M.H.; Bassett, D.C.; Gbureck, U.; Barralet, J.E. Osseointegration of dental implants in 3D-printed synthetic onlay grafts customized according to bone metabolic activity in recipient site. Biomaterials 2014, 35, 5436–5445. [Google Scholar] [CrossRef]
- Luongo, F.; Mangano, F.G.; Macchi, A.; Luongo, G.; Mangano, C. Custom-Made Synthetic Scaffolds for Bone Reconstruction: A Retrospective, Multicenter Clinical Study on 15 Patients. Biomed. Res. Int. 2016, 2016, 5862586. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.H. Recent advances in the reconstruction of cranio-maxillofacial defects using computer-aided design/computer-aided manufacturing. Maxillofac. Plast. Reconstr. Surg. 2018, 40, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alasseri, N.; Alasraj, A. Patient-specific implants for maxillofacial defects: Challenges and solutions. Maxillofac. Plast. Reconstr. Surg. 2020, 42, 15. [Google Scholar] [CrossRef] [PubMed]
- Salah, M.; Tayebi, L.; Moharamzadeh, K.; Naini, F.B. Three-dimensional bio-printing and bone tissue engineering: Technical innovations and potential applications in maxillofacial reconstructive surgery. Maxillofac. Plast. Reconstr. Surg. 2020, 42, 18. [Google Scholar] [CrossRef]
- Garagiola, U.; Grigolato, R.; Soldo, R.; Bacchini, M.; Bassi, G.; Roncucci, R.; De Nardi, S. Computer-aided design/computer-aided manufacturing of hydroxyapatite scaffolds for bone reconstruction in jawbone atrophy: A systematic review and case report. Maxillofac. Plast. Reconstr. Surg. 2016, 38, 2. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.W.; Yang, B.E.; Hong, S.J.; Choi, H.G.; Byeon, S.J.; Lim, H.K.; Chung, S.M.; Lee, J.H.; Byun, S.H. Bone Regeneration Capability of 3D Printed Ceramic Scaffolds. Int. J. Mol. Sci. 2020, 21, 4837. [Google Scholar] [CrossRef]
- Lim, H.K.; Hong, S.J.; Byeon, S.J.; Chung, S.M.; On, S.W.; Yang, B.E.; Lee, J.H.; Byun, S.H. 3D-Printed Ceramic Bone Scaffolds with Variable Pore Architectures. Int. J. Mol. Sci. 2020, 21, 6942. [Google Scholar] [CrossRef]
- Rogers, G.F.; Greene, A.K. Autogenous bone graft: Basic science and clinical implications. J. Craniofac. Surg. 2012, 23, 323–327. [Google Scholar] [CrossRef] [PubMed]
- Garrett, M.P.; Kakarla, U.K.; Porter, R.W.; Sonntag, V.K. Formation of painful seroma and edema after the use of recombinant human bone morphogenetic protein-2 in posterolateral lumbar spine fusions. Neurosurgery 2010, 66, 1044–1049, discussion 1049. [Google Scholar] [CrossRef] [PubMed]
- Smucker, J.D.; Rhee, J.M.; Singh, K.; Yoon, S.T.; Heller, J.G. Increased swelling complications associated with off-label usage of rhBMP-2 in the anterior cervical spine. Spine 2006, 31, 2813–2819. [Google Scholar] [CrossRef]
- Latzman, J.M.; Kong, L.; Liu, C.; Samadani, U. Administration of human recombinant bone morphogenetic protein-2 for spine fusion may be associated with transient postoperative renal insufficiency. Spine 2010, 35, E231–E237. [Google Scholar] [CrossRef]
- Carragee, E.J.; Chu, G.; Rohatgi, R.; Hurwitz, E.L.; Weiner, B.K.; Yoon, S.T.; Comer, G.; Kopjar, B. Cancer risk after use of recombinant bone morphogenetic protein-2 for spinal arthrodesis. J. Bone Joint. Surg. Am. 2013, 95, 1537–1545. [Google Scholar] [CrossRef] [Green Version]
- Lad, S.P.; Bagley, J.H.; Karikari, I.O.; Babu, R.; Ugiliweneza, B.; Kong, M.; Isaacs, R.E.; Bagley, C.A.; Gottfried, O.N.; Patil, C.G.; et al. Cancer after spinal fusion: The role of bone morphogenetic protein. Neurosurgery 2013, 73, 440–449. [Google Scholar] [CrossRef] [Green Version]
- Tannoury, C.A.; An, H.S. Complications with the use of bone morphogenetic protein 2 (BMP-2) in spine surgery. Spine J. 2014, 14, 552–559. [Google Scholar] [CrossRef]
- James, A.W.; LaChaud, G.; Shen, J.; Asatrian, G.; Nguyen, V.; Zhang, X.; Ting, K.; Soo, C. A Review of the Clinical Side Effects of Bone Morphogenetic Protein-2. Tissue Eng. Part B Rev. 2016, 22, 284–297. [Google Scholar] [CrossRef] [PubMed]
- Pinholt, E.M.; Bang, G.; Haanaes, H.R. Alveolar ridge augmentation in rats by Bio-Oss. Scand. J. Dent. Res. 1991, 99, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Wenz, B.; Oesch, B.; Horst, M. Analysis of the risk of transmitting bovine spongiform encephalopathy through bone grafts derived from bovine bone. Biomaterials 2001, 22, 1599–1606. [Google Scholar] [CrossRef]
- Berglundh, T.; Lindhe, J. Healing around implants placed in bone defects treated with Bio-Oss. An experimental study in the dog. Clin. Oral Implants. Res. 1997, 8, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Araujo, M.G.; Lindhe, J. Ridge preservation with the use of Bio-Oss collagen: A 6-month study in the dog. Clin. Oral Implants. Res. 2009, 20, 433–440. [Google Scholar] [CrossRef]
- Carmagnola, D.; Adriaens, P.; Berglundh, T. Healing of human extraction sockets filled with Bio-Oss. Clin. Oral Implants. Res. 2003, 14, 137–143. [Google Scholar] [CrossRef]
- Skoglund, A.; Hising, P.; Young, C. A clinical and histologic examination in humans of the osseous response to implanted natural bone mineral. Int. J. Oral Maxillofac. Implants 1997, 12, 194–199. [Google Scholar]
- Klinge, B.; Alberius, P.; Isaksson, S.; Jonsson, J. Osseous response to implanted natural bone mineral and synthetic hydroxylapatite ceramic in the repair of experimental skull bone defects. J. Oral Maxillofac. Surg. 1992, 50, 241–249. [Google Scholar] [CrossRef]
- Jeong, Y.G.; Lee, W.S.; Lee, K.B. Accuracy evaluation of dental models manufactured by CAD/CAM milling method and 3D printing method. J. Adv. Prosthodont 2018, 10, 245–251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, W.S.; Lee, D.H.; Lee, K.B. Evaluation of internal fit of interim crown fabricated with CAD/CAM milling and 3D printing system. J. Adv. Prosthodont 2017, 9, 265–270. [Google Scholar] [CrossRef] [Green Version]
- Govindaraj, S.; Costantino, P.D.; Friedman, C.D. Current use of bone substitutes in maxillofacial surgery. Facial Plast. Surg. 1999, 15, 73–81. [Google Scholar] [CrossRef]
- Kolan, K.C.R.; Huang, Y.W.; Semon, J.A.; Leu, M.C. 3D-printed Biomimetic Bioactive Glass Scaffolds for Bone Regeneration in Rat Calvarial Defects. Int. J. Bioprint. 2020, 6, 274. [Google Scholar] [CrossRef] [PubMed]
- Dasmah, A.; Thor, A.; Ekestubbe, A.; Sennerby, L.; Rasmusson, L. Particulate vs. block bone grafts: Three-dimensional changes in graft volume after reconstruction of the atrophic maxilla, a 2-year radiographic follow-up. J. Craniomaxillofac. Surg. 2012, 40, 654–659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benic, G.I.; Eisner, B.M.; Jung, R.E.; Basler, T.; Schneider, D.; Hammerle, C.H.F. Hard tissue changes after guided bone regeneration of peri-implant defects comparing block versus particulate bone substitutes: 6-month results of a randomized controlled clinical trial. Clin. Oral Implants. Res. 2019, 30, 1016–1026. [Google Scholar] [CrossRef] [PubMed]
- Hockers, T.; Abensur, D.; Valentini, P.; Legrand, R.; Hammerle, C.H. The combined use of bioresorbable membranes and xenografts or autografts in the treatment of bone defects around implants. A study in beagle dogs. Clin. Oral Implants. Res. 1999, 10, 487–498. [Google Scholar] [CrossRef] [PubMed]


| N Group | P Group | 3D Group | Difference (p) | ||
|---|---|---|---|---|---|
| 6 weeks | New bone (%) | 21.57 ± 1.86 | 32.63 ± 7.06 | 29.57 ± 9.41 | 0.041 * |
| Total bone (%) | 21.57 ± 1.86 | 34.89 ± 6.25 | 30.50 ± 9.81 | 0.033 * | |
| 12 weeks | New bone (%) | 27.51 ± 14.22 | 45.49 ± 12.09 | 43.79 ± 19.35 | 0.073 |
| Total bone (%) | 27.51 ± 14.22 | 49.22 ± 14.33 | 45.70 ± 19.39 | 0.039 * | |
| N Group | P Group | 3D Group | Difference (p) | ||
|---|---|---|---|---|---|
| 6 weeks | New bone (%) | 21.56 ± 1.84 | 25.25 ± 6.49 | 23.90 ± 9.57 | 0.365 |
| Total bone (%) | 21.56 ± 1.84 | 32.38 ± 7.68 | 27.81 ± 6.73 | 0.013 * | |
| 12 weeks | New bone (%) | 25.42 ± 14.27 | 30.54 ± 14.92 | 39.54 ± 7.83 | 0.065 |
| Total bone (%) | 25.42 ± 14.27 | 39.32 ± 12.16 | 43.01 ± 8.23 | 0.026 * | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ryu, J.-I.; Yang, B.-E.; Yi, S.-M.; Choi, H.G.; On, S.-W.; Hong, S.J.; Lim, H.-K.; Byun, S.-H. Bone Regeneration of a 3D-Printed Alloplastic and Particulate Xenogenic Graft with rhBMP-2. Int. J. Mol. Sci. 2021, 22, 12518. https://doi.org/10.3390/ijms222212518
Ryu J-I, Yang B-E, Yi S-M, Choi HG, On S-W, Hong SJ, Lim H-K, Byun S-H. Bone Regeneration of a 3D-Printed Alloplastic and Particulate Xenogenic Graft with rhBMP-2. International Journal of Molecular Sciences. 2021; 22(22):12518. https://doi.org/10.3390/ijms222212518
Chicago/Turabian StyleRyu, Ji-In, Byoung-Eun Yang, Sang-Min Yi, Hyo Geun Choi, Sung-Woon On, Seok Jin Hong, Ho-Kyung Lim, and Soo-Hwan Byun. 2021. "Bone Regeneration of a 3D-Printed Alloplastic and Particulate Xenogenic Graft with rhBMP-2" International Journal of Molecular Sciences 22, no. 22: 12518. https://doi.org/10.3390/ijms222212518
APA StyleRyu, J.-I., Yang, B.-E., Yi, S.-M., Choi, H. G., On, S.-W., Hong, S. J., Lim, H.-K., & Byun, S.-H. (2021). Bone Regeneration of a 3D-Printed Alloplastic and Particulate Xenogenic Graft with rhBMP-2. International Journal of Molecular Sciences, 22(22), 12518. https://doi.org/10.3390/ijms222212518







