Anti-Müllerian Hormone in Pathogenesis, Diagnostic and Treatment of PCOS
Abstract
1. Introduction
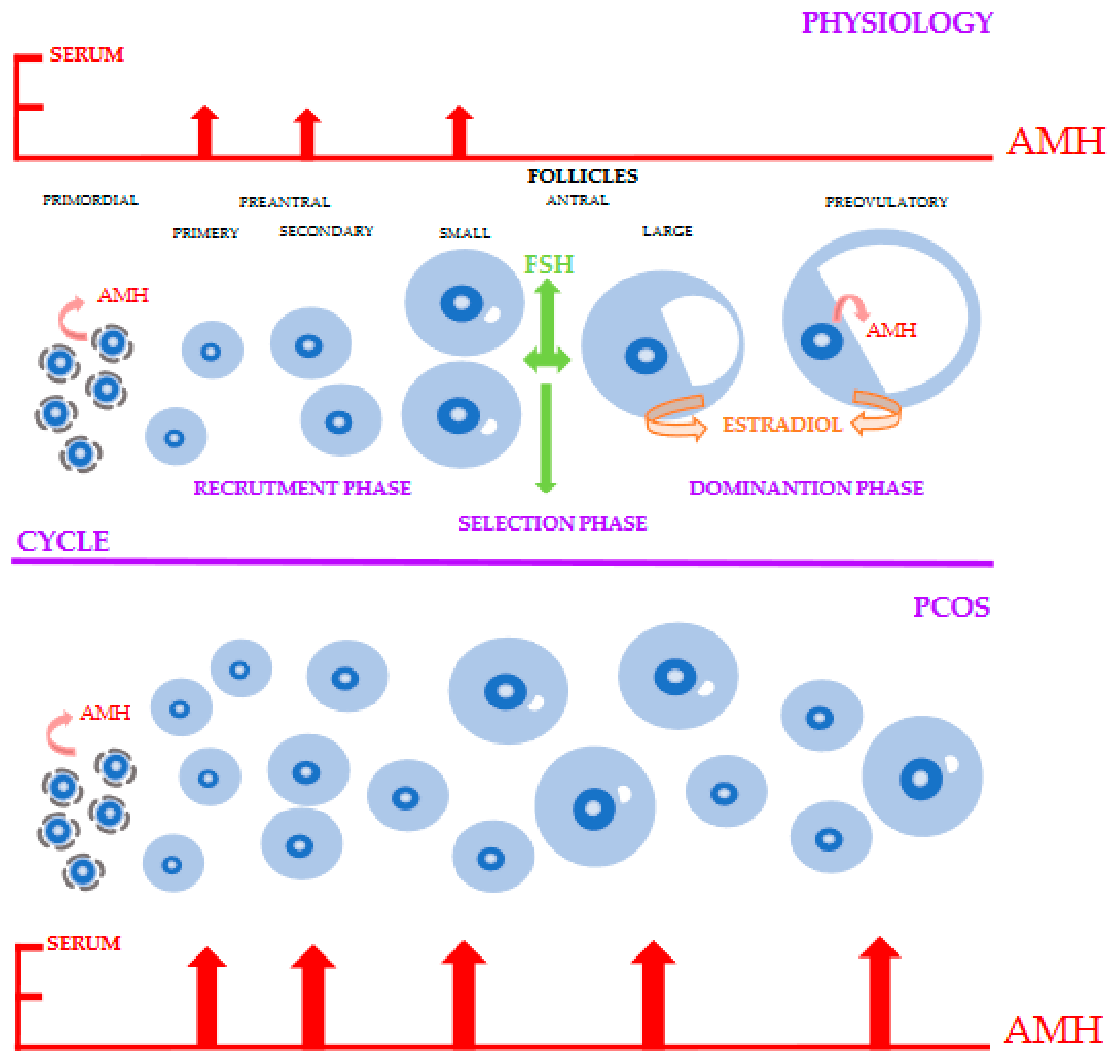
2. Physiology of AMH
3. AMH Assay
4. AMH as an Indicator of Ovarian Reserve and Follicle Growth
5. AMH in Pathogenesis and Treatment of PCOS
5.1. Pathophysiology
5.2. AMH in Diagnosis and Treatment of PCOS
5.3. AMH and Ovarian Response in Women with PCOS
6. Conclusions
Review Criteria
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AFC | Antral follicle count |
| AMH | Anti-Müllerian Hormone |
| AMH-EIA | AMH enzyme immunoassay |
| AMHR2 | AMH type II receptor |
| BMI | body mass index |
| BMP15 | bone morphogenetic factor 15 |
| CC | clomifen citrate |
| CPA | cyproterone acetate |
| CPR | clinical pregnancy rate |
| EE | ethinylestardiol |
| FOR | functional ovarian reserve |
| FSH | Follicle stimulating hormone |
| GDF9 | Growth differentiation factor 9 |
| HOMA-IR | Homeostatic Model Assessment for Insulin Resistance |
| HPG | Hypothalamic–Pituitary–Gonadal |
| IOT | Immunotech assay |
| IR | implantation rate |
| IVF | in vitro fertilization |
| LOD | laparoscopic ovarian drilling |
| MIS | Müllerian inhibiting substance |
| PCOM | polycystic ovaries morphology |
| ROC | receiver operation curve |
| SMAD | proteins |
| TGF-B | transforming growth factor-B |
| VNO | vomeronasal organ |
References
- Neven, A.C.H.; Laven, J.; Teede, H.J.; Boyle, J.A. A summary on polycystic ovary syndrome: Diagnostic criteria, prevalence, clinical manifestations, and management according to the latest international guidelines. Semin. Reprod. Med. 2018, 36, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Bozdag, G.; Mumusoglu, S.; Zengin, D.; Karabulut, E.; Yildiz, B.O. The prevalence and phenotypic features of polycystic ovary syndrome: A systematic review and meta-analysis. Hum. Reprod. 2016, 31, 2841–2855. [Google Scholar] [CrossRef] [PubMed]
- Rotterdam EA-SPCWG. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil. Steril. 2004, 81, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Lv, P.P.; Jin, M.; Rao, J.P.; Chen, J.; Wang, L.Q.; Huang, C.C. Role of anti-Müllerian hormone and testosterone in follicular growth: A cross-sectional study. BMC Endocr. Disord. 2020, 20, 101. [Google Scholar] [CrossRef]
- Alebic, M.S.; Stojanovic, N.; Dewailly, D. Discordance between serum anti-Mullerian hormone concentrations and antral follicle counts: Not only technical issues. Hum. Reprod. 2018, 3, 1141–1148. [Google Scholar] [CrossRef]
- Dewailly, D.; Andersen, C.Y.; Balen, A.; Broekmans, F.; Dilaver, N.; Fanchin, R.; Griesinger, G.; Kelsey, T.; La Marca, A.; Lambalk, C.; et al. The physiology and clinicalutility of anti-Mullerian hormone in women. Hum. Reprod. Update 2014, 20, 370–385. [Google Scholar] [CrossRef]
- Balen, A.H.; Laven, J.S.; Tan, S.L.; Dewailly, D. Ultrasound assessment of the polycystic ovary: International consensus definitions. Hum. Reprod. Update 2003, 9, 505–514. [Google Scholar] [CrossRef]
- Eilertsen, T.B.; Vanky, E.; Carlsen, S.M. Anti-Mullerian hormone in the diagnosis of polycystic ovary syndrome: Can morphologic description be replaced? Hum. Reprod. 2012, 27, 2494–2502. [Google Scholar] [CrossRef]
- Silva, M.S.B.; Giacobini, P. New insights into anti-Müllerian hormone role in the hypothalamic-pituitary-gonadal axis and neuroendocrine development. Cell. Mol. Life Sci. 2021, 78, 1–16. [Google Scholar] [CrossRef]
- Dąbkowska-Huc, A.; Cygal, A.; Skałba, P. The influence of anti-Mullerian hormone on folliculogenesis. Ginekol. Pol. 2008, 79, 137–140. [Google Scholar]
- Anderson, R.A.; Su, H.I. The Clinical Value and Interpretation of Anti-Müllerian Hormone in Women with Cancer. Front. Endocrinol. 2020, 11, 574263. [Google Scholar] [CrossRef]
- Moolhuijsen, L.M.E.; Visser, J.A. AMH in PCOS: Controlling the ovary, placenta, or brain? Curr. Opin. Endocr. Metab. Res. 2020, 12, 91–97. [Google Scholar] [CrossRef]
- Kostrzewa, M.; Głowacka, E.; Stetkiewicz, T.; Grzesiak, M.; Szyłło, K.; Stachowiak, G.; Wilczynski, J. Is serum anti-Müllerian hormone (AMH) assay a satisfactory measure for ovarian reserve estimation? A comparison of serum and peritoneal fluid AMH levels. Adv. Clin. Exp. Med.-Wroc. Med Univ. 2020, 29, 853–856. [Google Scholar] [CrossRef]
- Dumont, A.; Robin, G.; Dewailly, D. Anti-müllerian hormone in the pathophysiology and diagnosis of polycystic ovarian syndrome. Curr. Opin. Endocrinol. Diabetes Obes. 2018, 25, 377–384. [Google Scholar] [CrossRef]
- Pigny, P. Anti-Mullerian hormone assay: What’s up in 2013? Médecine de lareproduction. Gynécologie Endocrinol. 2014, 16, 16–20. [Google Scholar]
- Pellatt, L.; Hanna, L.; Brincat, M.; Galea, R.; Brain, H.; Whitehead, S. Granulosa cell production of anti-Müllerian hormone is increased in polycystic ovaries. J. Clin. Endocrinol. Metab. 2007, 92, 240–245. [Google Scholar] [CrossRef]
- van Helden, J.; Weiskirchen, R. Performance of the two new fully automatedanti-Mullerian hormone immunoassays compared with the clinical standardassay. Hum. Reprod. 2015, 30, 1918–1926. [Google Scholar] [CrossRef]
- Han, X.; McShane, M.; Sahertian, R.; White, C.; Ledger, W. Pre-mixing serumsamples with assay buffer is a prerequisite for reproducible anti-Mullerianhormone measurement using the Beckman Coulter Gen II assay. Hum. Reprod. 2014, 29, 1042–1048. [Google Scholar] [CrossRef]
- Dewailly, D.; Gronier, H.; Poncelet, E.; Robin, G.; Leroy, M.; Pigny, P.; Duhamel, A.; Catteau-Jonard, A. Diagnosis of polycystic ovary syndrome (PCOS): Revisiting the threshold values of follicle count on ultrasound and of the serum AMH level for the definition of polycystic ovaries. Hum. Reprod. 2011, 26, 3123–3319. [Google Scholar] [CrossRef]
- Gleicher, N.; Weghofer, A.; Barad, D.H. Defining ovarian reserve to better understand ovarian aging. Reprod. Biol. Endocrinol. 2011, 9, 23. [Google Scholar] [CrossRef]
- Findlay, J.K.; Hutt, K.J.; Hickey, M.; Anderson, R.A. What is the “ovarian reserve”? Fertil. Steril. 2015, 103, 628–630. [Google Scholar] [CrossRef]
- Hansen, K.R.; Hodnett, G.M.; Knowlton, N.; Craig, L.B. Correlation of ovarian reserve tests with histologically determined primordial follicle number. Fertil. Steril. 2011, 95, 170–175. [Google Scholar] [CrossRef]
- von Wolff, M.; Roumet, M.; Stute, P.; Liebenthron, J. Serum anti-Mullerian hormone (AMH) concentration has limited prognostic value for density of primordial and primary follicles, questioning it as an accurate parameter for the ovarian reserve. Maturitas 2020, 134, 34–40. [Google Scholar] [CrossRef]
- Liebenthron, J.; Reinsberg, J.; van der Ven, K.; Saenger, N.; Kruessel, J.S.; von Wolff, M. Serum anti-Müllerian hormone concentration and follicle density throughout reproductive life and in different diseases-implications in fertility preservation. Hum. Reprod. 2019, 34, 2513–2522. [Google Scholar] [CrossRef]
- Sermondade, N.; Sonigo, C.; Sifer, C.; Valtat, S.; Ziol, M.; Eustache, F.; Grynberg, M. Serum antimüllerian hormone is associated with the number of oocytes matured in vitro and with primordial follicle density in candidates for fertility preservation. Fertil. Steril. 2019, 111, 357–362. [Google Scholar] [CrossRef]
- Gorkem, U.; Togrul, C. Is There a Need to Alter the Timing of Anti-Müllerian Hormone Measurement During the Menstrual Cycle? Geburtshilfe Frauenheilkd. 2019, 79, 731–737. [Google Scholar] [CrossRef]
- Lambert-Messerlian, G.; Plante, B.; Eklund, E.E.; Raker, C.; Moore, R.G. Levels of antimüllerian hormone in serum during the normal menstrual cycle. Fertil. Steril. 2016, 105, 208–213. [Google Scholar] [CrossRef]
- Melado, L.; Lawrenz, B.; Sibal, J.; Abu, E.; Coughlan, C.; Navarro, A.T.; Mousavi Fatemi, H. Anti-müllerian Hormone During Natural Cycle Presents Significant Intra and Intercycle Variations When Measured with Fully Automated Assay. Front. Endocrinol. 2018, 9, 686. [Google Scholar] [CrossRef] [PubMed]
- Bungum, L.; Tagevi, J.; Jokubkiene, L.; Bungum, M.; Giwercman, A.; Macklon, N.; Andersen, C.; Wirenfeldt Klausen, T.; Tørring, N.; Kumar, A.; et al. The Impact of the Biological Variability or Assay Performance on AMH Measurements: A Prospective Cohort Study with AMH Tested on Three Analytical Assay-Platforms. Front. Endocrinol. 2018, 9, 603. [Google Scholar] [CrossRef] [PubMed]
- Steiner, A.Z.; Herring, A.H.; Kesner, J.S.; Meadows, J.W.; Stanczyk, F.Z.; Hoberman, S.; Baird, D. Antimüllerian hormone as a predictor of natural fecundability in women aged 30–42 years. Obstet. Gynecol. 2011, 117, 798–804. [Google Scholar] [CrossRef] [PubMed]
- Steiner, A.Z.; Pritchard, D.; Stanczyk, F.Z.; Kesner, J.S.; Meadows, J.W.; Herring, A.H.; Baird, D.D. Association Between Biomarkers of Ovarian Reserve and Infertility Among Older Women of Reproductive Age. JAMA 2017, 318, 1367–1376. [Google Scholar] [CrossRef]
- Dólleman, M.; Depmann, M.; Eijkemans, M.J.C.; Heimensem, J.; Broer, S.L.; van der Stroom, E.M.; Laven, J.S.E.; Van Rooij, I.A.J.; Scheffer, G.J.; Peeters, P.H.M.; et al. Anti-Müllerian hormone is a more accurate predictor of individual time to menopause than mother’s age at menopause. Hum. Reprod. 2014, 29, 584–591. [Google Scholar] [CrossRef]
- Amer, S.A.K.S.; James, C.; Al-Hussaini, T.K.; Mohamed, A.A. Assessment of Circulating Anti-Müllerian Hormone in Women Using Hormonal Contraception: A Systematic Review. J. Women’s Health (Larchmt) 2020, 29, 100–110. [Google Scholar] [CrossRef]
- Landersoe, S.K.; Forman, J.L.; Birch, P.K.; Larsen, E.C.; Nøhr, B.; Hvidman, H.W.; Nielsen, H.; Andersen, A.N. Ovarian reserve markers in women using various hormonal contraceptives. Eur. J. Contracept. Reprod. Health Care 2020, 25, 65–71. [Google Scholar] [CrossRef]
- Moslehi, N.; Shab-Bidar, S.; Ramezani Tehrani, F.; Mirmiran, P.; Azizi, F. Is ovarian reserve associated with body mass index and obesity in reproductive aged women? A meta-analysis. Menopause 2018, 25, 1046–1055. [Google Scholar] [CrossRef]
- Dennis, N.A.; Houghton, L.A.; Jones, G.T.; van Rij, A.M.; Morgan, K.; McLennan, I.S. The level of serum anti-Müllerian hormone correlates with vitamin D status in men and women but not in boys. J. Clin. Endocrinol. Metab. 2012, 97, 2450–2455. [Google Scholar] [CrossRef]
- Dennis, N.A.; Houghton, L.A.; Pankhurst, M.W.; Harper, M.J.; McLennan, I.S. Acute Supplementation with High Dose Vitamin D3 Increases Serum Anti-Müllerian Hormone in Young Women. Nutrients 2017, 9, 719. [Google Scholar] [CrossRef]
- Xu, J.; Hennebold, J.D.; Seifer, D.B. Direct vitamin D3 actions on rhesus macaque follicles in three-dimensional culture: Assessment of follicle survival, growth, steroid, and antimüllerian hormone production. Fertil. Steril. 2016, 106, 1815–1820. [Google Scholar] [CrossRef]
- Weenen, C.; Laven, J.S.; Von Bergh, A.R.; Cranfield, M.; Groome, N.P.; Visser, J.A.; Kramer, P.; Fauser, B.C.J.M.; Themmen, A.P.N. Anti-Müllerian hormone expression pattern in the human ovary: Potential implications for initial and cyclic follicle recruitment. Mol. Hum. Reprod. 2004, 10, 77–83. [Google Scholar] [CrossRef]
- Bhide, P.; Dilgil, M.; Gudi, A.; Shah, A.; Akwaa, C.; Homburg, R. Each small antral follicle in ovaries of women with polycystic ovary syndrome produces more antimüllerian hormone than its counterpart in a normal ovary: An observational cross-sectional study. Fertil. Steril. 2015, 103, 537–541. [Google Scholar] [CrossRef]
- Azziz, R.; Carmina, E.; Dewailly, D.; Diamanti-Kandarakis, E.; Escobar-Morreale, H.F.; Futterweit, W.; Janssen, O.E.; Legro, R.S.; Norman, R.J.; Taylor, A.E.; et al. The Androgen Excess and PCOS Society criteria for the polycystic ovary syndrome: The complete task force report. Fertil. Steril. 2009, 91, 456–488. [Google Scholar] [CrossRef]
- Laven, J.S.; Mulders, A.G.; Visser, J.A.; Themmen, A.P.; De Jong, F.H.; Fauser, B.C. Anti-Müllerian hormone serum concentrations in normoovulatory and anovulatory women of reproductive age. J. Clin. Endocrinol. Metab. 2004, 89, 318–323. [Google Scholar] [CrossRef]
- Dewailly, D.; Robin, G.; Peigne, M.; Decanter, C.; Pigny, P.; Catteau-Jonard, S. Interactions between androgens, FSH, anti-Müllerian hormone and estradiol during folliculogenesis in the human normal and polycystic ovary. Hum. Reprod. Update 2016, 22, 709–724. [Google Scholar] [CrossRef]
- Jonard, S.; Dewailly, D. The follicular excess in polycystic ovaries, due to intra-ovarian hyperandrogenism, may be the main culprit for the follicular arrest. Hum. Reprod. Update 2004, 10, 107–117. [Google Scholar] [CrossRef]
- Catteau-Jonard, S.; Jamin, S.P.; Leclerc, A.; Gonzalès, J.; Dewailly, D.; di Clemente, N. Anti-Mullerian hormone, its receptor, FSH receptor, and androgen receptor genes are overexpressed by granulosa cells from stimulated follicles in women with polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 2008, 93, 4456–4461. [Google Scholar] [CrossRef]
- Pierre, A.; Taieb, J.; Giton, F.; Grynberg, M.; Touleimat, S.; El Hachem, H.; Fanchin, R.; Monniaux, D.; Cohen-Tannoudji, J.; di Clemente, N.; et al. Dysregulation of the Anti-Müllerian Hormone System by Steroids in Women With Polycystic Ovary Syndrome. J. Clin. Endocrinol. Metab. 2017, 102, 3970–3980. [Google Scholar] [CrossRef]
- Alebić, M.; Stojanović, N.; Duhamel, A.; Dewailly, D. The phenotypic diversity in per-follicle anti-Müllerian hormone production in polycystic ovary syndrome. Hum. Reprod. 2015, 30, 1927–1933. [Google Scholar] [CrossRef]
- Panidis, D.; Katsikis, I.; Karkanaki, A.; Piouka, A.; Armeni, A.K.; Georgopoulos, N.A. Serum anti-Müllerian hormone (AMH) levels are differentially modulated by both serum gonadotropins and not only by serum follicle stimulating hormone (FSH) levels. Med. Hypotheses 2011, 77, 649–653. [Google Scholar] [CrossRef]
- Grynberg, M.; Pierre, A.; Rey, R.; Leclerc, A.; Arouche, N.; Hesters, L.; Catteau-Jonard, S.; Frydman, R.; Picard, Y.-E.; Fanchin, R.; et al. Differential regulation of ovarian anti-müllerian hormone (AMH) by estradiol through α- and β-estrogen receptors. J. Clin. Endocrinol. Metab. 2012, 97, E1649–E1657. [Google Scholar] [CrossRef]
- Kristensen, S.G.; Kumar, A.; Kalra, B.; Pors, S.E.; Botkjaer, J.A.; Mamsen, L.S.; Colmorn, L.B.; Fedder, J.; Ernst, E.; Owens, L.; et al. Quantitative differences in TGF-beta family members measured in small antral follicle fluids from women with or without PCO. J. Clin. Endocrinol. Metab. 2019, 104, 6371–6384. [Google Scholar] [CrossRef]
- Willis, D.S.; Watson, H.; Mason, H.D.; Galea, R.; Brincat, M.; Franks, S. Premature response to luteinizing hormone of granulosa cells from anovulatory women with polycystic ovary syndrome: Relevance to mechanism of anovulation. J. Clin. Endocrinol. Metab. 1998, 83, 3984–3991. [Google Scholar] [CrossRef] [PubMed]
- Jakimiuk, A.J.; Weitsman, S.R.; Navab, A.; Magoffin, D.A. Luteinizing hormone receptor, steroidogenesis acute regulatory protein, and steroidogenic enzyme messenger ribonucleic acids are overexpressed in thecal and granulosa cells from polycystic ovaries. J. Clin. Endocrinol. Metab. 2001, 86, 1318–1323. [Google Scholar] [PubMed]
- Malone, S.A.; Papadakis, G.E.; Messina, A.; Mimouni, N.E.H.; Trova, S.; Imbernon, M.C.; Allet, I.; Cimino, I.; Acierno, D.; Cassatella, C.; et al. Defective AMH. signaling disrupts GnRH neuron development and function and contributes to hypogonadotropic hypogonadism. Elife 2019, 8, e47198. [Google Scholar] [CrossRef] [PubMed]
- Cimino, I.; Casoni, F.; Liu, X.; Messina, A.; Parkash, J.; Jamin, S.P.; Catteau-Jonard, S.; Collier, F.; Baroncini, M.; Dewailly, D.; et al. Novel role for anti-Mullerian hormone in the regulation of GnRH neuron excitability and hormone secretion. Nat. Commun. 2016, 7, 10055. [Google Scholar] [CrossRef] [PubMed]
- Pierre, A.; Peigne, M.; Grynberg, M.; Arouche, N.; Taieb, J.; Hesters, L.; Gonzales, J.; Picard, J.Y.; Dewailly, D.; Fanchin, R.; et al. Loss of LH-induced down-regulation of anti-Mullerian hormone receptor expression may contribute toanovulation in women with polycystic ovary syndrome. Hum. Reprod. 2013, 28, 762–769. [Google Scholar] [CrossRef] [PubMed]
- Stojsin-Carter, A.; Costa, N.N.; De Morais, R.; De Bem, T.H.; Costa, M.P.; Carter, T.F.; Gillis, D.J.; Neal, M.S.; Ohashi, O.M.; Miranda, M.S.; et al. Fetal sex alters maternal anti-Mullerian hormone during pregnancy in cattle. Anim. Reprod. Sci. 2017, 186, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Tata, B.; Mimouni, N.E.H.; Barbotin, A.L.; Malone, S.A.; Loyens, A.; Pigny, P.; Dewailly, D.; Catteau-Jonard, S.; Sundstrom-Poromaa, I.; Piltonen, T.T.; et al. Elevated prenatal anti-Mullerian hormone reprograms the fetus and induces polycystic ovary syndrome in adulthoo. Nat. Med. 2018, 24, 834–846. [Google Scholar] [CrossRef]
- Novembri, R.; Funghi, L.; Voltolini, C.; Belmonte, G.; Vannuccini, S.; Torricelli, M.; Petraglia, F. Placenta expresses anti-Mullerian hormone and its receptor: Sex-related difference in fetal membranes. Placenta 2015, 36, 731–737. [Google Scholar] [CrossRef]
- Carmina, E.; Campagna, A.M.; Fruzzetti, F.; Lobo, R.A. AMH measurement versus ovarian ultrasound in the diagnosis of polycystic ovary syndrome in different phenotypes. Endocr. Pract. 2016, 22, 287–293. [Google Scholar] [CrossRef]
- Dewailly, D.; Alebić, M.; Duhamel, A.; Stojanović, N. Using cluster analysis to identify a homogeneous subpopulation of women with polycystic ovarian morphology in a population of non-hyperandrogenic women with regular menstrual cycles. Hum. Reprod. 2014, 29, 2536–2543. [Google Scholar] [CrossRef]
- Dewailly, D.; Lujan, M.E.; Carmina, E.; Cedars, M.I.; Laven, J.; Norman, R.J.; Escobar-Morreal, H.F. Definition and significance of polycystic ovarian morphology: A task force report from the Androgen Excess and Polycystic Ovary Syndrome Society. Hum. Reprod. Update 2014, 20, 334–352. [Google Scholar] [CrossRef]
- Lujan, M.E.; Jarrett, B.Y.; Brooks, E.D.; Reines, J.K.; Peppin, A.K.; Muhn, N.; Haider, E.; Pierson, R.A.; Chizen, D.R. Updated ultrasound criteria for polycystic ovary syndrome: Reliable thresholds for elevated follicle population and ovarian volume. Hum. Reprod. 2013, 28, 1361–1368. [Google Scholar] [CrossRef]
- Dewailly, D.; Pigny, P.; Soudan, B.; Catteau-Jonard, S.; Decanter, C.; Poncelet, E.; Duhamel, A. Reconciling the definitions of polycystic ovary syndrome: The ovarian follicle number and serum anti-Müllerian hormone concentrations aggregate with the markers of hyperandrogenism. J. Clin. Endocrinol. Metab. 2010, 95, 4399–4405. [Google Scholar] [CrossRef]
- Wiweko, B.; Indra, I.; Susanto, C.; Natadisastra, M.; Hestiantoro, A. The correlation between serum AMH and HOMA-IR among PCOS phenotypes. BMC Res. Notes 2018, 11, 114. [Google Scholar] [CrossRef]
- Skałba, P.; Cygal, A.; Madej, P.; Dąbkowska-Huć, A.; Sikora, J.; Martirosian, G.; Romanik, M.; Olszanecka-Glinianowicz, M. Is the plasma anti-Müllerian hormone (AMH) level associated with body weight and metabolic, and hormonal disturbances in women with and without polycystic ovary syndrome? Eur. J. Obstet. Gynecol. Reprod. Biol. 2011, 158, 254–259. [Google Scholar] [CrossRef]
- Tomova, A.; Deepinder, F.; Robeva, R.; Kirilov, G.; Mechandjiev, Z.; Kumanov, P. Anti-Müllerian hormone in women with polycystic ovary syndrome before and after therapy with metformin. Horm. Metab. Res. 2011, 43, 723–727. [Google Scholar] [CrossRef]
- Panidis, D.; Georgopoulos, N.A.; Piouka, A.; Katsikis, I.; Saltamavros, A.D.; Decavalas, G.; Diamanti-Kandarakis, E. The impact of oral contraceptives and metformin on anti-Müllerian hormone serum levels in women with polycystic ovary syndrome and biochemical hyperandrogenemia. Gynecol. Endocrinol. 2011, 27, 587–592. [Google Scholar] [CrossRef]
- Moran, L.J.; Noakes, M.; Clifton, P.M.; Norman, R.J. The use of anti-mullerian hormone in predicting menstrual response after weight loss in overweight women with polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 2007, 92, 3796–3802. [Google Scholar] [CrossRef][Green Version]
- Nybacka, Å.; Carlström, K.; Fabri, F.; Hellström, P.M.; Hirschberg, A.L. Serum antimüllerian hormone in response to dietary management and/or physical exercise in overweight/obese women with polycystic ovary syndrome: Secondary analysis of a randomized controlled trial. Fertil. Steril. 2013, 100, 1096–1102. [Google Scholar] [CrossRef]
- Hart, R.; Doherty, D.A.; Norman, R.J.; Franks, S.; Dickinson, J.E.; Hickey, M.; Sloboda, D.M. Serum antimullerian hormone (AMH) levels are elevated in adolescent girls with polycystic ovaries and the polycystic ovarian syndrome (PCOS). Fertil. Steril. 2010, 94, 1118–1121. [Google Scholar] [CrossRef]
- Sopher, A.B.; Grigoriev, G.; Laura, D.; Cameo, T.; Lerner, J.P.; Chang, R.J.; McMahon, D.J.; Oberfield, S.E. Anti-Mullerian hormone may be a useful adjunct in the diagnosis of polycystic ovary syndrome in nonobese adolescents. J. Pediatr. Endocrinol. Metab. 2014, 27, 1175–1179. [Google Scholar] [CrossRef]
- Goodman, N.F.; Cobin, R.H.; Futterweit, W.; Glueck, J.S.; Legro, R.S.; Carmina, E. American Association Of Clinical Endocrinologists, American College Of Endocrinology, And Androgen Excess And PCOS Society Disease State Clinical Review: Guide To The Best Practices In The Evaluation And Treatment Of Polycystic Ovary Syndrome—PART 1. Endocr. Pract. 2015, 21, 1291–1300. [Google Scholar] [CrossRef]
- Amer, S.A.; Mahran, A.; Abdelmaged, A.; El-Adawy, A.R.; Eissa, M.K.; Shaw, R.W. The influence of circulating anti-Müllerian hormone on ovarian responsiveness to ovulation induction with gonadotrophins in women with polycystic ovarian syndrome: A pilot study. Reprod. Biol. Endocrinol. 2013, 11, 115. [Google Scholar] [CrossRef]
- Mahran, A.; Abdelmeged, A.; El-Adawy, A.R.; Eissa, M.K.; Shaw, R.W.; Amer, S.A. The predictive value of circulating anti-Müllerian hormone in women with polycystic ovarian syndrome receiving clomiphene citrate: A prospective observational study. J. Clin. Endocrinol. Metab. 2013, 98, 4170–4175. [Google Scholar] [CrossRef] [PubMed]
- Sachdeva, G.; Gainder, S.; Suri, V.; Sachdeva, N.; Chopra, S. Prediction of Responsiveness to Clomiphene Citrate in Infertile Women with PCOS. J. Reprod. Infertil. 2019, 20, 143–150. [Google Scholar] [PubMed]
- Ellakwa, H.E.; Sanad, Z.F.; Hamza, H.A.; Emara, M.A.; Elsayed, M.A. Predictors of patient responses to ovulation induction with clomiphene citrate in patients with polycystic ovary syndrome experiencing infertility. Int. J. Gynaecol. Obstet. 2016, 133, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Gülşen, M.S.; Ulu, İ.; Yıldırım Köpük, Ş.; Kıran, G. The role of anti-Müllerian hormone in predicting clomiphene citrate resistance in women with polycystic ovarian syndrome. Gynecol. Endocrinol. 2019, 35, 86–89. [Google Scholar] [CrossRef]
- Xi, W.; Yang, Y.; Mao, H.; Zhao, X.; Liu, M.; Fu, S. Circulating anti-mullerian hormone as predictor of ovarian response to clomiphene citrate in women with polycystic ovary syndrome. J. Ovarian Res. 2016, 9, 3. [Google Scholar] [CrossRef]
- El-Mazny, A.; Abou-Salem, N. Anti-Müllerian hormone and antral follicle count for prediction of ovarian stimulation response in polycystic ovary syndrome. Gynecol. Endocrinol. 2013, 29, 826–829. [Google Scholar] [CrossRef]
- Vaiarelli, A.; Drakopoulos, P.; Blockeel, C.; De Vos, M.; van de Vijver, A.; Camus, M.; Cosyns, S.; Tournaye, H.; Polyzos, N. Limited ability of circulating anti-Müllerian hormone to predict dominant follicular recruitment in PCOS women treated with clomiphene citrate: A comparison of two different assays. Gynecol. Endocrinol. Off. J. Int. Soc. Gynecol. Endocrinol. 2016, 32, 227–230. [Google Scholar] [CrossRef]
- El-Halawaty, S.; Rizk, A.; Kamal, M.; Aboulhassan, M.; Al-Sawah, H.; Noah, O.; Al-Inany, H. Clinical significance of serum concentration of anti-Müllerian hormone in obese women with polycystic ovary syndrome. Reprod. Biomed. Online 2007, 15, 495–499. [Google Scholar] [CrossRef]
- Farquhar, C.; Lilford, R.J.; Marjoribanks, J.; Vandekerckhove, P. Laparoscopic ‘drilling’ by diathermy or laser for ovulation induction in anovulatory polycystic ovary syndrome. Cochrane Database Syst. Rev. 2007, 3, Cd001122. [Google Scholar]
- Amer, S.A.; Li, T.C.; Ledger, W.L. The value of measuring anti-Mullerian hormone in women with anovulatory polycystic ovary syndrome undergoing laparoscopic ovarian diathermy. Hum. Reprod. 2009, 24, 2760–2766. [Google Scholar] [CrossRef]
- Rezk, M.; Emarh, M.; Alhalaby, A. Anti-Müllerian hormone and luteinizing hormone for prediction of spontaneous ovulation after laparoscopic ovarian drilling in clomiphene-resistant polycystic ovary syndrome. Middle East Fertil. Soc. J. 2016, 21, 91–95. [Google Scholar] [CrossRef]
- Bosch, E.; Broer, S.; Griesinger, G.; Grynberg, M.; Humaidan, P.; Kolibianakis, E.; Kunicki, M.; La Marca, A.; Lainas, G.; Le Clef, N.; et al. ESHRE guideline: Ovarian stimulation for IVF/ICSI. Hum. Reprod. Open 2020, 2. [Google Scholar] [CrossRef]
- Lie Fong, S.; Baart, E.B.; Martini, E.; Schipper, I.; Visser, J.A.; Themmen, A.P.; de Jong, F.H.; Fauser, B.J.C.M.; Laven, J.S.E. Anti-Müllerian hormone: A marker for oocyte quantity, oocyte quality and embryo quality? Reprod. Biomed. Online 2008, 16, 664–670. [Google Scholar] [CrossRef]
- Ebner, T.; Sommergruber, M.; Moser, M.; Shebl, O.; Schreier-Lechner, E.; Tews, G. Basal level of anti-Müllerian hormone is associated with oocyte quality in stimulated cycles. Hum. Reprod. 2006, 21, 2022–2026. [Google Scholar] [CrossRef]
- Silberstein, T.; MacLaughlin, D.T.; Shai, I.; Trimarchi, J.R.; Lambert-Messerlian, G.; Seifer, D.B.; Keefe, D.L.; Blazar, A.S.al. Mullerian inhibiting substance levels at the time of HCG administration in IVF cycles predict both ovarian reserve and embryo morphology. Hum. Reprod. 2006, 21, 159–163. [Google Scholar] [CrossRef]
- Kaya, C.; Pabuccu, R.; Satıroglu, H. Serum antimüllerian hormone concentrations on day 3 of the in vitro fertilization stimulation cycle are predictive of the fertilization, implantation, and pregnancy in polycystic ovary syndrome patients undergoing assisted reproduction. Fertil. Steril. 2010, 94, 2202–2207. [Google Scholar] [CrossRef]
- Plachot, M.; Belaisch-Allart, J.; Mayenga, J.M.; Chouraqui, A.; Tesquier, A.; Serkine, A.M.; Boujenah, A.; Abirached, F. Oocyte and embryo quality in polycystic ovary syndrome. Gynecol. Obstet. Fertil. 2003, 31, 350–354. [Google Scholar] [CrossRef]
- Heijnen, E.M.; Eijkemans, M.J.; Hughes, E.G.; Laven, J.S.; Macklon, N.S.; Fauser, B.C. A meta-analysis of outcomes of conventional IVF in women with polycystic ovary syndrome. Hum. Reprod. Update 2006, 12, 13–21. [Google Scholar] [CrossRef]
- Ludwig, M.; Finas, D.F.; al-Hasani, S.; Diedrich, K.; Ortmann, O. Oocyte quality and treatment outcome in intracytoplasmic sperm injection cycles of polycystic ovarian syndrome patients. Hum. Reprod. 1999, 14, 354–358. [Google Scholar] [CrossRef]
- Zhang, F.; Liu, X.L.; Rong, N.; Huang, X.W. Clinical value of serum anti-mullerian hormone and inhibin B in prediction of ovarian response in patients with polycystic ovary syndrome. J. Huazhong Univ. Sci. Technol. Med. Sci. 2017, 37, 70–73. [Google Scholar] [CrossRef]
- Vembu, R.; Reddy, N.S.; Serum, A.M.H. Level to Predict the Hyper Response in Women with PCOS and Nn-PCOS Undergoing Controlled Ovarian Stimulation in ART. J. Hum. Reprod. Sci. 2017, 10, 91–94. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rudnicka, E.; Kunicki, M.; Calik-Ksepka, A.; Suchta, K.; Duszewska, A.; Smolarczyk, K.; Smolarczyk, R. Anti-Müllerian Hormone in Pathogenesis, Diagnostic and Treatment of PCOS. Int. J. Mol. Sci. 2021, 22, 12507. https://doi.org/10.3390/ijms222212507
Rudnicka E, Kunicki M, Calik-Ksepka A, Suchta K, Duszewska A, Smolarczyk K, Smolarczyk R. Anti-Müllerian Hormone in Pathogenesis, Diagnostic and Treatment of PCOS. International Journal of Molecular Sciences. 2021; 22(22):12507. https://doi.org/10.3390/ijms222212507
Chicago/Turabian StyleRudnicka, Ewa, Michał Kunicki, Anna Calik-Ksepka, Katarzyna Suchta, Anna Duszewska, Katarzyna Smolarczyk, and Roman Smolarczyk. 2021. "Anti-Müllerian Hormone in Pathogenesis, Diagnostic and Treatment of PCOS" International Journal of Molecular Sciences 22, no. 22: 12507. https://doi.org/10.3390/ijms222212507
APA StyleRudnicka, E., Kunicki, M., Calik-Ksepka, A., Suchta, K., Duszewska, A., Smolarczyk, K., & Smolarczyk, R. (2021). Anti-Müllerian Hormone in Pathogenesis, Diagnostic and Treatment of PCOS. International Journal of Molecular Sciences, 22(22), 12507. https://doi.org/10.3390/ijms222212507






