A Phenylfurocoumarin Derivative Reverses ABCG2-Mediated Multidrug Resistance In Vitro and In Vivo
Abstract
:1. Introduction
2. Results
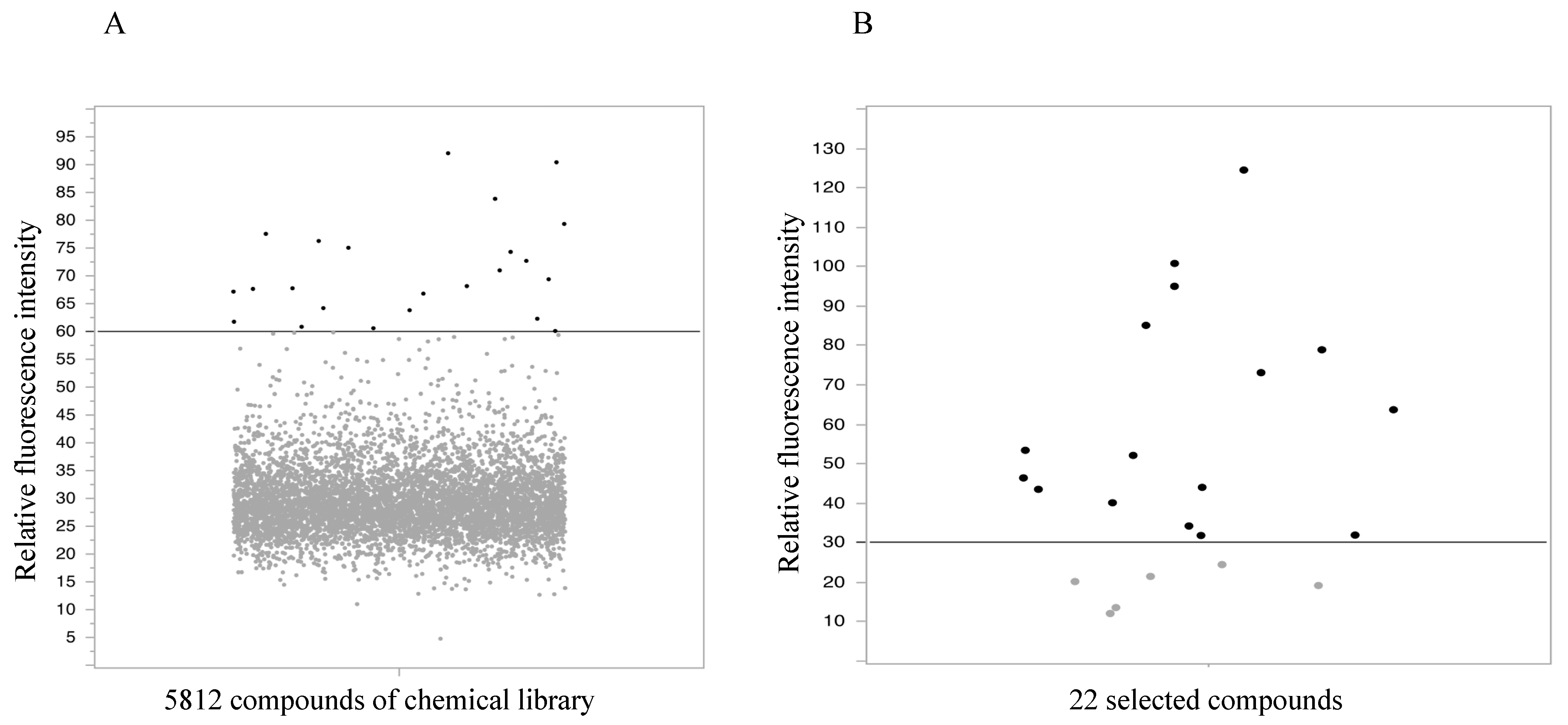
2.1. High-Throughput Screening
2.2. Flow Cytometry Assay
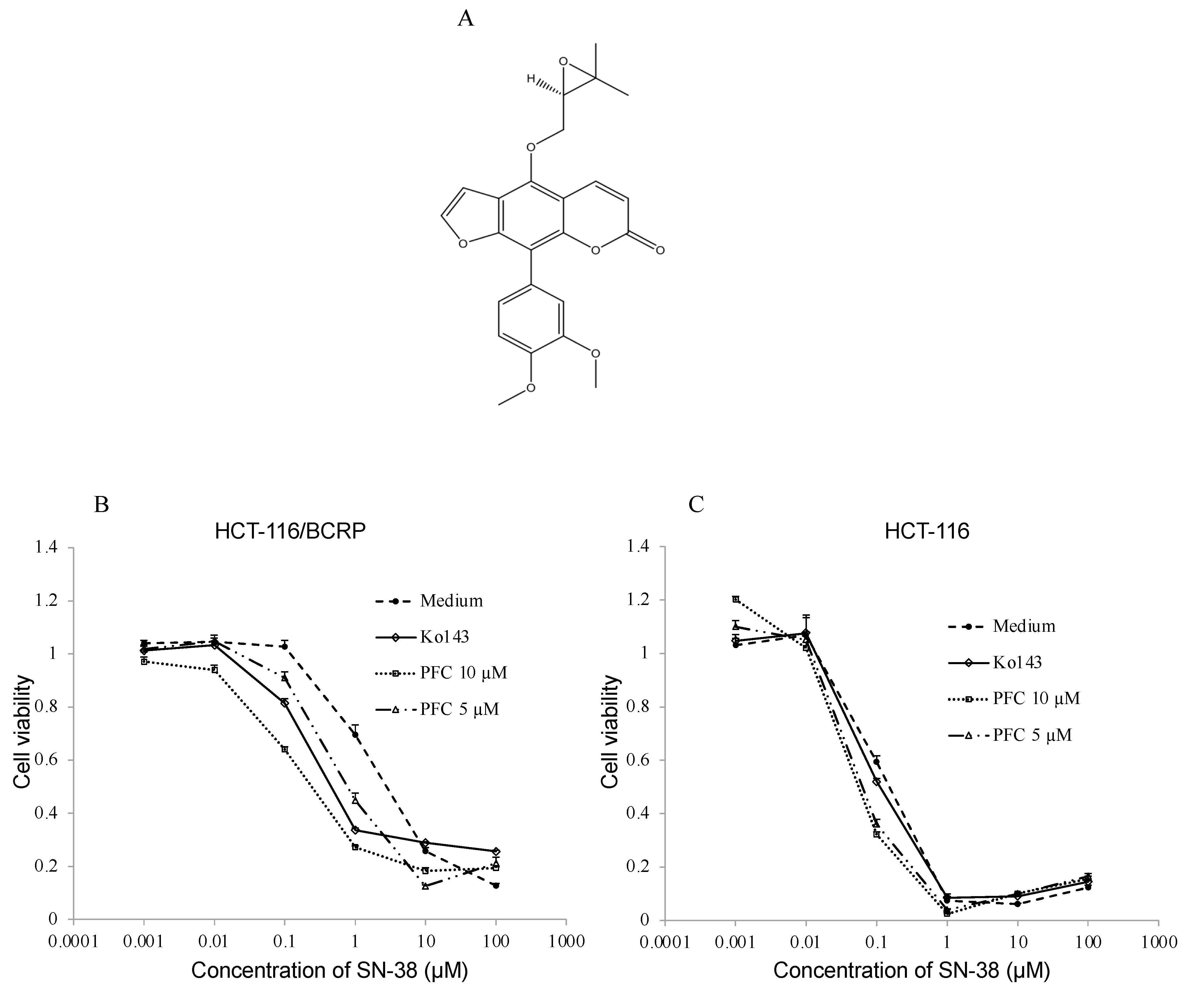
2.3. Cytotoxic Assay
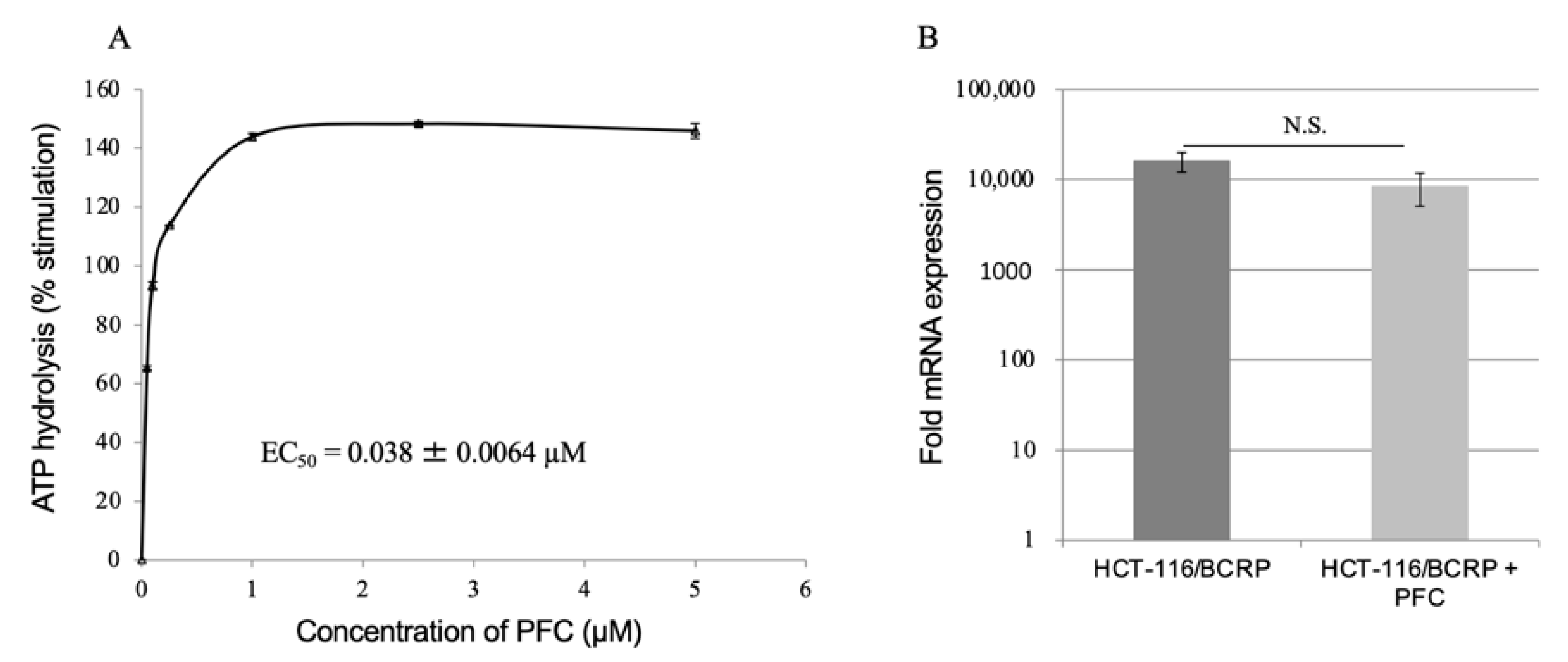
2.4. ATPase Assay
2.5. Reverse Transcription-Quantitative Polymerase Chain Reaction
2.6. Antitumor Activity In Vivo
3. Discussion
4. Materials and Methods
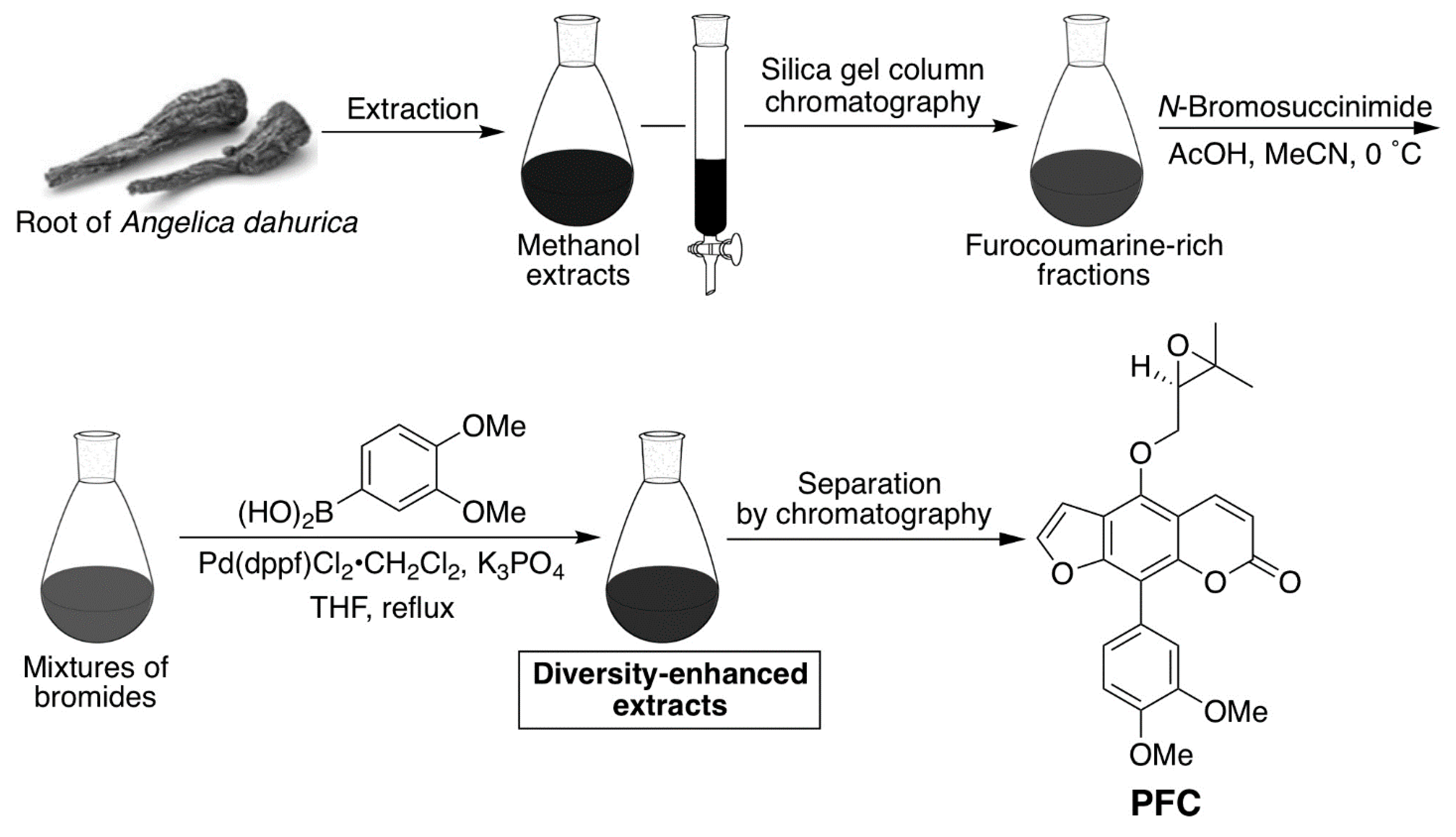
4.1. Screening of Chemical Compounds
4.2. Chemicals
4.3. Cell Lines
4.4. High-Throughput Screening
4.5. Flow Cytometry
4.6. Cytotoxic Assay
4.7. Preparation of the Phenylfurocoumarin Derivative
4.8. ATPase Assay
4.9. Reverse Transcription-Quantitative Polymerase Chain Reaction (RT-qPCR)
4.10. In Vivo Antitumor Activity
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gottesman, M.M. Mechanisms of cancer drug resistance. Annu. Rev. Med. 2002, 53, 615–627. [Google Scholar] [CrossRef] [Green Version]
- Gottesman, M.M.; Fojo, T.; Bates, S.E. Multidrug resistance in cancer: Role of ATP-dependent transporters. Nat. Rev. Cancer 2002, 2, 48–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shukla, S.; Ohnuma, S.; Ambudkar, S.V. Improving cancer chemotherapy with modulators of ABC drug transporters. Curr. Drug Targets 2011, 12, 621–630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dean, M.; Rzhetsky, A.; Allikmets, R. The human ATP-binding cassette (ABC) transporter superfamily. Genome Res. 2001, 11, 1156–1166. [Google Scholar] [CrossRef]
- Allikmets, R.; Schriml, L.; Hutchinson, A.; Romano-Spica, V.; Dean, M. A human placenta-specific ATP-binding cassette gene (ABCP) on chromosome 4q22 that is involved in multidrug resistance. Cancer Res. 1998, 58, 5337–5339. [Google Scholar]
- Doyle, L.A.; Yang, W.; Abruzzo, L.V.; Krogmann, T.; Gao, Y.; Rishi, A.K.; Ross, D.D. A multidrug resistance transporter from human MCF-7 breast cancer cells. Proc. Natl. Acad. Sci. USA 1998, 95, 15665–15670. [Google Scholar] [CrossRef] [Green Version]
- Miyake, K.; Mickley, L.; Litman, T.; Zhan, Z.; Robey, R.; Cristensen, B.; Brangi, M.; Greenberger, L.; Dean, M.; Fojo, T.; et al. Molecular cloning of cDNAs which are highly overexpressed in mitoxantrone-resistant cells: Demonstration of homology to ABC transport genes. Cancer Res. 1999, 59, 8–13. [Google Scholar]
- Aronica, E.; Gorter, J.A.; Redeker, S.; van Vliet, E.; Ramkema, M.; Scheffer, G.L.; Scheper, R.J.; Van Der Valk, P.; Leenstra, S.; Baayen, J.C.; et al. Localization of breast cancer resistance protein (BCRP) in microvessel endothelium of human control and epileptic brain. Epilepsia 2005, 46, 849–857. [Google Scholar] [CrossRef]
- Fetsch, P.A.; Abati, A.; Litman, T.; Morisaki, K.; Honjo, Y.; Mittal, K.; Bates, S.E. Localization of the ABCG2 mitoxantrone resistance-associated protein in normal tissues. Cancer Lett. 2006, 235, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Maliepaard, M.; Scheffer, G.L.; Faneyte, I.F.; van Gastelen, M.A.; Pijnenborg, A.C.; Schinkel, A.H.; van De Vijver, M.J.; Scheper, R.J.; Schellens, J.H. Subcellular localization and distribution of the breast cancer resistance protein transporter in normal human tissues. Cancer Res. 2001, 61, 3458–3464. [Google Scholar]
- Noguchi, K.; Katayama, K.; Sugimoto, Y. Human ABC transporter ABCG2/BCRP expression in chemoresistance: Basic and clinical perspectives for molecular cancer therapeutics. Pharm. Pers. Med. 2014, 7, 53–64. [Google Scholar] [CrossRef] [Green Version]
- Robey, R.; Medina-Pérez, W.Y.; Nishiyama, K.; Lahusen, T.; Miyake, K.; Litman, T.; Senderowicz, A.M.; Ross, D.D.; Bates, S.E. Overexpression of the ATP-binding cassette half-transporter, ABCG2 (Mxr/BCrp/ABCP1), in flavopiridol-resistant human breast cancer cells. Clin. Cancer Res. 2001, 7, 145–152. [Google Scholar]
- Maliepaard, M.; van Gastelen, M.; Tohgo, A.; Hausheer, F.H.; van Waardenburg, R.C.; de Jong, L.A.; Pluim, D.; Beijnen, J.H.; Schellens, J.H. Circumvention of breast cancer resistance protein (BCRP)-mediated resistance to camptothecins in vitro using non-substrate drugs or the BCRP inhibitor GF120918. Clin. Cancer Res. 2001, 7, 935–941. [Google Scholar] [PubMed]
- Burger, H.; van Tol, H.; Boersma, A.W.; Brok, M.; Wiemer, E.A.; Stoter, G.; Nooter, K. Imatinib mesylate (STI571) is a substrate for the breast cancer resistance protein (BCRP)/ABCG2 drug pump. Blood 2004, 104, 2940–2942. [Google Scholar] [CrossRef]
- Elkind, N.B.; Szentpétery, Z.; Apáti, A.; Ozvegy-Laczka, C.; Várady, G.; Ujhelly, O.; Szabó, K.; Homolya, L.; Váradi, A.; Buday, L.; et al. Multidrug transporter ABCG2 prevents tumor cell death induced by the epidermal growth factor receptor inhibitor Iressa (ZD1839, gefitinib). Cancer Res. 2005, 65, 1770–1777. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rabindran, S.K.; Ross, D.D.; Doyle, L.A.; Yang, W.; Greenberger, L.M. Fumitremorgin C reverses multidrug resistance in cells transfected with the breast cancer resistance protein. Cancer Res. 2000, 60, 47–50. [Google Scholar] [PubMed]
- Allen, J.D.; van Loevezijn, A.; Lakhai, J.M.; van der Valk, M.; van Tellingen, O.; Reid, G.; Schellens, J.H.; Koomen, G.J.; Schinkel, A.H. Potent and specific inhibition of the breast cancer resistance protein multidrug transporter in vitro and in mouse intestine by a novel analogue of fumitremorgin C. Mol. Cancer Ther. 2002, 1, 417–425. [Google Scholar]
- de Bruin, M.; Miyake, K.; Litman, T.; Robey, R.; Bates, S.E. Reversal of resistance by GF120918 in cell lines expressing the ABC half-transporter, MXR. Cancer Lett. 1999, 146, 117–126. [Google Scholar] [CrossRef]
- Robey, R.W.; Steadman, K.; Polgar, O.; Morisaki, K.; Blayney, M.; Mistry, P.; Bates, S.E. Pheophorbide a is a specific probe for ABCG2 function and inhibition. Cancer Res. 2004, 64, 1242–1246. [Google Scholar] [CrossRef] [Green Version]
- Gupta, A.; Dai, Y.; Vethanayagam, R.R.; Hebert, M.F.; Thummel, K.E.; Unadkat, J.D.; Ross, D.D.; Mao, Q. Cyclosporin A, tacrolimus and sirolimus are potent inhibitors of the human breast cancer resistance protein (ABCG2) and reverse resistance to mitoxantrone and topotecan. Cancer Chemother. Pharmacol. 2006, 58, 374–383. [Google Scholar] [CrossRef]
- Chearwae, W.; Shukla, S.; Limtrakul, P.; Ambudkar, S.V. Modulation of the function of the multidrug resistance-linked ATP-binding cassette transporter ABCG2 by the cancer chemopreventive agent curcumin. Mol. Cancer Ther. 2006, 5, 1995–2006. [Google Scholar] [CrossRef] [Green Version]
- Anreddy, N.; Patel, A.; Zhang, Y.; Wang, Y.J.; Shukla, S.; Kathawala, R.J.; Kumar, P.; Gupta, P.; Ambudkar, S.V.; Wurpel, J.N.D.; et al. A-803467, a tetrodotoxin-resistant sodium channel blocker, modulates ABCG2-mediated MDR in vitro and in vivo. Oncotarget 2015, 6, 39276–39291. [Google Scholar] [CrossRef]
- Ji, N.; Yang, Y.; Lei, Z.N.; Cai, C.Y.; Wang, J.Q.; Gupta, P.; Xian, X.; Yang, D.H.; Kong, D.; Chen, Z.S. Ulixertinib (BVD-523) antagonizes ABCB1- and ABCG2-mediated chemotherapeutic drug resistance. Biochem. Pharmacol. 2018, 158, 274–285. [Google Scholar] [CrossRef]
- Miyata, H.; Takada, T.; Toyoda, Y.; Matsuo, H.; Ichida, K.; Suzuki, H. Identification of febuxostat as a new strong ABCG2 inhibitor: Potential applications and risks in clinical situations. Front. Pharmacol. 2016, 7, 518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murakami, M.; Ohnuma, S.; Fukuda, M.; Chufan, E.E.; Kudoh, K.; Kanehara, K.; Sugisawa, N.; Ishida, M.; Naitoh, T.; Shibata, H.; et al. Synthetic analogs of curcumin modulate the function of multidrug resistance-linked ATP-binding cassette transporter ABCG2. Drug Metab. Dispos. 2017, 45, 1166–1177. [Google Scholar] [CrossRef] [Green Version]
- Westover, D.; Ling, X.; Lam, H.; Welch, J.; Jin, C.; Gongora, C.; Del Rio, M.; Wani, M.; Li, F. FL118, a novel camptothecin derivative, is insensitive to ABCG2 expression and shows improved efficacy in comparison with irinotecan in colon and lung cancer models with ABCG2-induced resistance. Mol. Cancer 2015, 14, 92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, W.; Fan, Y.F.; Cai, C.Y.; Wang, J.Q.; Teng, Q.X.; Lei, Z.N.; Zeng, L.; Gupta, P.; Chen, Z.S. Olmutinib (BI1482694/HM61713), a novel epidermal growth factor receptor tyrosine kinase inhibitor, reverses ABCG2-mediated multidrug resistance in cancer cells. Front. Pharmacol. 2018, 9, 1097. [Google Scholar] [CrossRef] [Green Version]
- Peña-Solórzano, D.; Stark, S.A.; König, B.; Sierra, C.A.; Ochoa-Puentes, C. ABCG2/BCRP: Specific and nonspecific modulators. Med. Res. Rev. 2017, 37, 987–1050. [Google Scholar] [CrossRef] [PubMed]
- Henrich, C.J.; Bokesch, H.R.; Dean, M.; Bates, S.E.; Robey, R.W.; Goncharova, E.I.; Wilson, J.A.; McMahon, J.B. A high-throughput cell-based assay for inhibitors of ABCG2 activity. J. Biomol. Screen. 2006, 11, 176–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henrich, C.J.; Robey, R.W.; Bokesch, H.R.; Bates, S.E.; Shukla, S.; Ambudkar, S.V.; Dean, M.; McMahon, J.B. New inhibitors of ABCG2 identified by high-throughput screening. Mol. Cancer Ther. 2007, 6, 3271–3278. [Google Scholar] [CrossRef] [Green Version]
- Strouse, J.J.; Ivnitski-Steele, I.; Khawaja, H.M.; Perez, D.; Ricci, J.; Yao, T.; Weiner, W.S.; Schroeder, C.E.; Simpson, D.S.; Maki, B.E.; et al. A selective ATP-binding cassette subfamily G member 2 efflux inhibitor revealed via high-throughput flow cytometry. J. Biomol. Screen. 2013, 18, 26–38. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Byun, Y.; Ren, Y.R.; Liu, J.O.; Laterra, J.; Pomper, M.G. Identification of inhibitors of ABCG2 by a bioluminescence imaging-based high-throughput assay. Cancer Res. 2009, 69, 5867–5875. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sugisawa, N.; Ohnuma, S.; Ueda, H.; Murakami, M.; Sugiyama, K.; Ohsawa, K.; Kano, K.; Tokuyama, H.; Doi, T.; Aoki, J.; et al. Novel potent ABCB1 modulator, Phenethylisoquinoline alkaloid, reverses multidrug resistance in cancer cell. Mol. Pharm. 2018, 15, 4021–4030. [Google Scholar] [CrossRef]
- Nakanishi, T.; Ross, D.D. Breast cancer resistance protein (BCRP/ABCG2): Its role in multidrug resistance and regulation of its gene expression. Chin. J. Cancer 2012, 31, 73–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishiyama, M.; Kuga, T. Central effects of the neurotropic mycotoxin fumitremorgin A in the rabbit (I). Effects on the spinal cord. Jpn. J. Pharmacol. 1989, 50, 167–173. [Google Scholar] [CrossRef]
- Rabindran, S.K.; He, H.; Singh, M.; Brown, E.; Collins, K.I.; Annable, T.; Greenberger, L.M. Reversal of a novel multidrug resistance mechanism in human colon carcinoma cells by fumitremorgin C. Cancer Res. 1998, 58, 5850–5858. [Google Scholar] [PubMed]
- Weidner, L.D.; Zoghbi, S.S.; Lu, S.; Shukla, S.; Ambudkar, S.V.; Pike, V.W.; Mulder, J.; Gottesman, M.M.; Innis, R.B.; Hall, M.D. The inhibitor Ko143 is not specific for ABCG2. J. Pharmacol. Exp. Ther. 2015, 354, 384–393. [Google Scholar] [CrossRef] [Green Version]
- Aslanis, V.; Zhang, J.; Lomeli, B.; Grosch, K.; Ouatas, T. Effect of cyclosporine coadministration on the pharmacokinetics of eltrombopag in healthy volunteers. Cancer Chemother. Pharmacol. 2018, 82, 847–855. [Google Scholar] [CrossRef]
- Huguet, J.; Lu, J.; Gaudette, F.; Chiasson, J.L.; Hamet, P.; Michaud, V.; Turgeon, J. No effects of pantoprazole on the pharmacokinetics of rosuvastatin in healthy subjects. Eur. J. Clin. Pharmacol. 2016, 72, 925–931. [Google Scholar] [CrossRef]
- Zhang, X.N.; Ma, Z.J.; Wang, Y.; Sun, B.; Guo, X.; Pan, C.Q.; Chen, L.M. Angelica dahurica ethanolic extract improves impaired wound healing by activating angiogenesis in diabetes. PLoS ONE 2017, 12, e0177862. [Google Scholar]
- Kim, Y.K.; Kim, Y.S.; Ryu, S.Y. Antiproliferative effect of furanocoumarins from the root of Angelica dahurica on cultured human tumor cell lines. Phytotherapy Res. 2007, 21, 288–290. [Google Scholar] [CrossRef]
- Lechner, D.; Stavri, M.; Oluwatuyi, M.; Pereda-Miranda, R.; Gibbons, S. The anti-staphylococcal activity of Angelica dahurica (Bai Zhi). Phytochemistry 2004, 65, 331–335. [Google Scholar] [CrossRef] [PubMed]
- Ban, H.S.; Lim, S.S.; Suzuki, K.; Jung, S.H.; Lee, S.; Lee, Y.S.; Shin, K.H.; Ohuchi, K. Inhibitory effects of furanocoumarins isolated from the roots of Angelica dahurica on prostaglandin E2 production. Planta Med. 2003, 69, 408–412. [Google Scholar]
- Shukla, S.; Wu, C.-P.; Nandigama, K.; Ambudkar, S.V. The naphthoquinones, vitamin K3 and its structural analogue plumbagin, are substrates of the multidrug resistance linked ATP binding cassette drug transporter ABCG2. Mol. Cancer Ther. 2007, 6, 3279–3286. [Google Scholar] [CrossRef] [Green Version]
- Ambudkar, S.V. Drug-stimulatable ATPase activity in crude membranes of human MDR1-transfected mammalian cells. Methods Enzymol 1998, 292, 504–514. [Google Scholar]
- Kage, K.; Tsukahara, S.; Sugiyama, T.; Asada, S.; Ishikawa, E.; Tsuruo, T.; Sugimoto, Y. Dominant-negative inhibition of breast cancer resistance protein as drug efflux pump through the inhibition of S-S dependent homodimerization. Int. J. Cancer 2002, 97, 626–630. [Google Scholar] [CrossRef] [PubMed]
- Katayama, K.; Shibata, K.; Mitsuhashi, J.; Noguchi, K.; Sugimoto, Y. Pharmacological interplay between breast cancer resistance protein and gefitinib in epidermal growth factor receptor signaling. Anticancer Res. 2009, 29, 1059–1065. [Google Scholar] [PubMed]
- Oshima, Y.; Kikuchi, H. Developments toward the production of diverse natural-product-like compounds: Diversity-oriented synthesis and diversity-enhanced extracts. Heterocycles 2018, 96, 1509. [Google Scholar] [CrossRef]
- Kikuchi, H.; Sakurai, K.; Oshima, Y. Development of Diversity-Enhanced Extracts of Curcuma zedoaria and Their New Sesquiterpene-like compounds. Org. Lett. 2014, 16, 1916–1919. [Google Scholar] [CrossRef]
- Kobayashi, M.; Funayama, R.; Ohnuma, S.; Unno, M.; Nakayama, K. Wnt-beta-catenin signaling regulates ABCC3 (MRP3) transporter expression in colorectal cancer. Cancer Sci. 2016, 107, 1776–1784. [Google Scholar] [CrossRef] [Green Version]
- Yamazaki, R.; Nishiyama, Y.; Furuta, T.; Hatano, H.; Igarashi, Y.; Asakawa, N.; Kodaira, H.; Takahashi, H.; Aiyama, R.; Matsuzaki, T.; et al. Novel acrylonitrile derivatives, YHO-13177 and YHO-13351, reverse BCRP/ABCG2-mediated drug resistance in vitro and in vivo. Mol. Cancer Ther. 2011, 10, 1252–1263. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| IC50 ± SE (µM) a | Fold Reversal b | IC50 ± SE (µM) a | Fold Reversal b | |
|---|---|---|---|---|
| HCT-116 | HCT-116/BCRP | |||
| Medium | 0.11 ± 0.0037 | 1.0 | 2.59 ± 0.24 | 1.0 |
| Ko143 1 µM | 0.11 ± 0.0036 | 1.0 | 0.26 ± 0.027 | 10.1 |
| PFC 5 µM | 0.077 ± 0.022 | 1.4 | 0.36 ± 0.11 | 7.3 |
| PFC 10 µM | 0.057 ± 0.013 | 1.9 | 0.19 ± 0.018 | 13.8 |
| IC50 ± SE (µM) a | ||
|---|---|---|
| HCT-116 | HCT-116/BCRP | |
| Ko143 1 µM | 77.97 ± 2.33 | 115.79 ± 4.85 |
| PFC 10 µM | 42.70 ± 2.42 | 43.07 ± 3.18 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kokubo, S.; Ohnuma, S.; Murakami, M.; Kikuchi, H.; Funayama, S.; Suzuki, H.; Kajiwara, T.; Yamamura, A.; Karasawa, H.; Sugisawa, N.; et al. A Phenylfurocoumarin Derivative Reverses ABCG2-Mediated Multidrug Resistance In Vitro and In Vivo. Int. J. Mol. Sci. 2021, 22, 12502. https://doi.org/10.3390/ijms222212502
Kokubo S, Ohnuma S, Murakami M, Kikuchi H, Funayama S, Suzuki H, Kajiwara T, Yamamura A, Karasawa H, Sugisawa N, et al. A Phenylfurocoumarin Derivative Reverses ABCG2-Mediated Multidrug Resistance In Vitro and In Vivo. International Journal of Molecular Sciences. 2021; 22(22):12502. https://doi.org/10.3390/ijms222212502
Chicago/Turabian StyleKokubo, Shoji, Shinobu Ohnuma, Megumi Murakami, Haruhisa Kikuchi, Shota Funayama, Hideyuki Suzuki, Taiki Kajiwara, Akihiro Yamamura, Hideaki Karasawa, Norihiko Sugisawa, and et al. 2021. "A Phenylfurocoumarin Derivative Reverses ABCG2-Mediated Multidrug Resistance In Vitro and In Vivo" International Journal of Molecular Sciences 22, no. 22: 12502. https://doi.org/10.3390/ijms222212502






