HLA-G Is Widely Expressed by Mast Cells in Regions of Organ Fibrosis in the Liver, Lung and Kidney
Abstract
:1. Introduction
2. Results
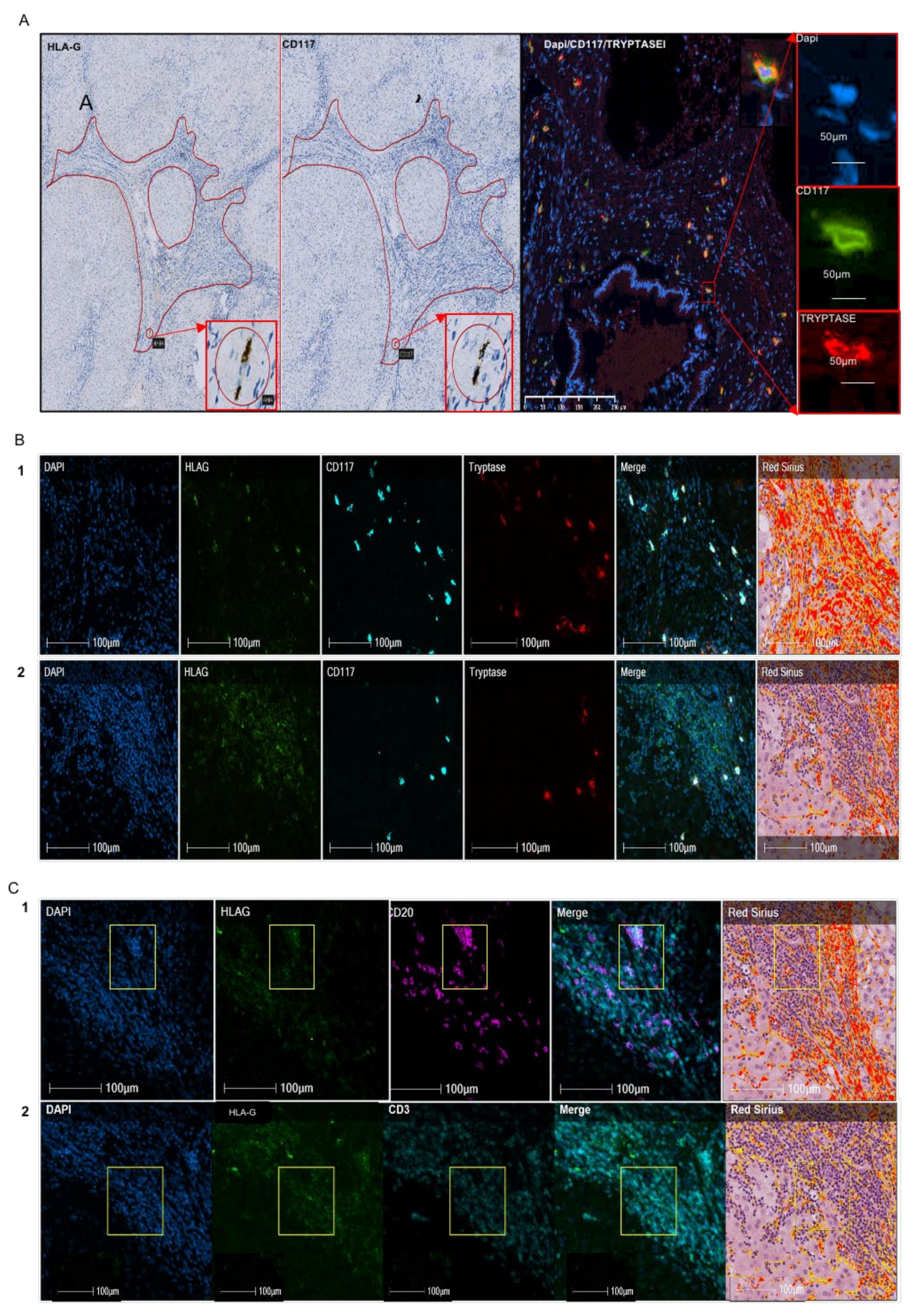
2.1. Quantitative Expression and the Nature of HLA-G+ Cells in Alcohol-Induced Fibrosis
2.2. Analysis of HLA-G+ Cells in IPF
2.3. Analysis of HLA-G+ Cells in Renal Fibrosis
3. Discussion
4. Patients and Methods
4.1. Patients
4.2. Methodology
Immunohistochemistry and Immunofluorescence
5. Conclusion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mutsaers, H.A.M.; Olinga, P. Editorial: Organ Fibrosis: Triggers, Pathways, and Cellular Plasticity. Front. Med. 2016, 3, 55. [Google Scholar] [CrossRef] [Green Version]
- Wynn, T.A.; Ramalingam, T.R. Mechanisms of fibrosis: Therapeutic translation for fibrotic disease. Nat. Med. 2012, 18, 1028–1040. [Google Scholar] [CrossRef] [Green Version]
- Rehm, J.; Taylor, B.; Mohapatra, S.; Irving, H.; Baliunas, D.; Patra, J.; Roerecke, M. Alcohol as a risk factor for liver cirrhosis: A systematic review and meta-analysis. Drug Alcohol Rev. 2010, 29, 437–445. [Google Scholar] [CrossRef]
- Le Jeune, I.; Gribbin, J.; West, J.; Smith, C.; Cullinan, P.; Hubbard, R. The incidence of cancer in patients with idiopathic pulmonary fibrosis and sarcoidosis in the UK. Respir. Med. 2007, 101, 2534–2540. [Google Scholar] [CrossRef] [Green Version]
- Djudjaj, S.; Boor, P. Cellular and molecular mechanisms of kidney fibrosis. Mol. Asp. Med. 2019, 65, 16–36. [Google Scholar] [CrossRef]
- Tsuzuki, T.; Iwata, H.; Murase, Y.; Takahara, T.; Ohashi, A. Renal tumors in end-stage renal disease: A comprehensive review. Int. J. Urol. 2018, 25, 780–786. [Google Scholar] [CrossRef] [Green Version]
- Friedman, S.L. Molecular Regulation of Hepatic Fibrosis, an Integrated Cellular Response to Tissue Injury. J. Biol. Chem. 2000, 275, 2247–2250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marvie, P.; Lisbonne, M.; L’Helgoualc’h, A.; Rauch, M.; Turlin, B.; Preisser, L.; Bourd-Boittin, K.; Théret, N.; Gascan, H.; Piquet-Pellorce, C.; et al. Interleukin-33 overexpression is associated with liver fibrosis in mice and humans. J. Cell. Mol. Med. 2009, 14, 1726–1739. [Google Scholar] [CrossRef] [Green Version]
- Amiot, L.; Vu, N.; Samson, M. Immunomodulatory Properties of HLA-G in Infectious Diseases. J. Immunol. Res. 2014, 2014, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Amiot, L.; Vu, N.; Rauch, M.; L’Helgoualc’h, A.; Chalmel, F.; Gascan, H.; Turlin, B.; Guyader, D.; Samson, M. Expression of HLA-G by mast cells is associated with hepatitis C virus-induced liver fibrosis. J. Hepatol. 2014, 60, 245–252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amiot, L.; Vu, N.; Samson, M. Biology of the immunomodulatory molecule HLA-G in human liver diseases. J. Hepatol. 2015, 62, 1430–1437. [Google Scholar] [CrossRef] [PubMed]
- Kovats, S.; Main, E.K.; Librach, C.; Stubblebine, M.; Fisher, S.J.; DeMars, R. A Class I Antigen, HLA-G, Expressed in Human Trophoblasts. Science 1990, 248, 220–223. [Google Scholar] [CrossRef]
- Le Discorde, M.; Moreau, P.; Sabatier, P.; Legeais, J.-M.; Carosella, E.D. Expression of HLA-G in human cornea, an immune-privileged tissue. Hum. Immunol. 2003, 64, 1039–1044. [Google Scholar] [CrossRef] [Green Version]
- Crisa, L.; McMaster, M.T.; Ishii, J.K.; Fisher, S.J.; Salomon, D.R. Identification of a Thymic Epithelial Cell Subset Sharing Expression of the Class Ib HLA-G Molecule with Fetal Trophoblasts. J. Exp. Med. 1997, 186, 289–298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cirulli, V.; Zalatan, J.; McMaster, M.; Prinsen, R.; Salomon, D.R.; Ricordi, C.; Torbett, B.E.; Meda, P.; Crisa, L. The Class I HLA Repertoire of Pancreatic Islets Comprises the Nonclassical Class Ib Antigen HLA-G. Diabetes 2006, 55, 1214–1222. [Google Scholar] [CrossRef] [Green Version]
- Brugière, O.; Thabut, G.; Pretolani, M.; Krawice-Radanne, I.; Dill, C.; Herbreteau, A.; Poras, I.; Moreau, P.; Colombat, M.; Danel, C.; et al. Immunohistochemical Study of HLA-G Expression in Lung Transplant Recipients. Arab. Archaeol. Epigr. 2009, 9, 1427–1438. [Google Scholar] [CrossRef]
- Ivanova-Todorova, E.; Mourdjeva, M.; Kyurkchiev, D.; Bochev, I.; Stoyanova, E.; Dimitrov, R.; Timeva, T.; Yunakova, M.; Bukarev, D.; Shterev, A.; et al. Original Article: HLA-G Expression Is Up-Regulated by Progesterone in Mesenchymal Stem Cells. Am. J. Reprod. Immunol. 2009, 62, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Onno, M.; Le Friec, G.; Pangault, C.; Amiot, L.; Guilloux, V.; Drénou, B.; Caulet-Maugendre, S.; André, P.; Fauchet, R. Modulation of HLA-G antigens expression in myelomonocytic cells. Hum. Immunol. 2000, 61, 1086–1094. [Google Scholar] [CrossRef]
- Yang, Y.; Chu, W.; Geraghty, D.E.; Hunt, J.S. Expression of HLA-G in human mononuclear phagocytes and selective induction by IFN-gamma. J. Immunol. 1996, 156, 4224–4231. [Google Scholar]
- Le Friec, G.; Gros, F.; Sebti, Y.; Guilloux, V.; Pangault, C.; Fauchet, R.; Amiot, L. Capacity of myeloid and plasmacytoid dendritic cells especially at mature stage to express and secrete HLA-G molecules. J. Leukoc. Biol. 2004, 76, 1125–1133. [Google Scholar] [CrossRef] [Green Version]
- Menier, C.; Rabreau, M.; Challier, J.-C.; Le Discorde, M.; Carosella, E.D.; Rouas-Freiss, N. Erythroblasts secrete the nonclassical HLA-G molecule from primitive to definitive hematopoiesis. Blood 2004, 104, 3153–3160. [Google Scholar] [CrossRef]
- Kisseleva, T.; Brenner, D.A. Fibrogenesis of Parenchymal Organs. Proc. Am. Thorac. Soc. 2008, 5, 338–342. [Google Scholar] [CrossRef] [Green Version]
- Bataller, R.; Brenner, D. Liver fibrosis. J. Clin. Investig. 2005, 115, 209–218. [Google Scholar] [CrossRef]
- Bringardner, B.D.; Baran, C.P.; Eubank, T.D.; Marsh, C.B. The Role of Inflammation in the Pathogenesis of Idiopathic Pulmonary Fibrosis. Antioxid. Redox Signal. 2008, 10, 287–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharif, R. Overview of idiopathic pulmonary fibrosis (IPF) and evidence-based guidelines. Am. J. Manag. Care 2017, 23, S176–S182. [Google Scholar] [PubMed]
- Komi, D.E.A.; Wöhrl, S.; Bielory, L. Mast Cell Biology at Molecular Level: A Comprehensive Review. Clin. Rev. Allergy Immunol. 2019, 58, 342–365. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Liu, C.; Zhou, D.; Zhang, L. TGF-β/SMAD Pathway and Its Regulation in Hepatic Fibrosis. J. Histochem. Cytochem. 2016, 64, 157–167. [Google Scholar] [CrossRef]
- Gruber, B.L.; Marchese, M.J.; Kew, R.R. Transforming growth factor-beta 1 mediates mast cell chemotaxis. J. Immunol. 1994, 152, 5860–5867. [Google Scholar]
- Fabre, T.; Molina, M.F.; Soucy, G.; Goulet, J.-P.; Willems, B.; Villeneuve, J.-P.; Bilodeau, M.; Shoukry, N.H. Type 3 cytokines IL-17A and IL-22 drive TGF-β–dependent liver fibrosis. Sci. Immunol. 2018, 3, eaar7754. [Google Scholar] [CrossRef] [Green Version]
- Gomez, G.; Ramirez, C.D.; Rivera, J.; Patel, M.; Norozian, F.; Wright, H.V.; Kashyap, M.V.; Barnstein, B.O.; Fischer-Stenger, K.; Schwartz, L.B.; et al. TGF-β1 Inhibits Mast Cell FcεRI Expression. J. Immunol. 2005, 174, 5987–5993. [Google Scholar] [CrossRef]
- Bissonnette, E.Y.; Enciso, J.A.; Befus, A.D. TGF-beta1 inhibits the release of histamine and tumor necrosis factor-alpha from mast cells through an autocrine pathway. Am. J. Respir. Cell Mol. Biol. 1997, 16, 275–282. [Google Scholar] [CrossRef]
- Theoharides, T.C.; Alysandratos, K.-D.; Angelidou, A.; Delivanis, D.-A.; Sismanopoulos, N.; Zhang, B.; Asadi, S.; Vasiadi, M.; Weng, Z.; Miniati, A.; et al. Mast cells and inflammation. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2012, 1822, 21–33. [Google Scholar] [CrossRef] [Green Version]
- Cha, S.-I.; Chang, C.S.; Kim, E.K.; Lee, J.W.; Matthay, M.A.; Golden, J.A.; Elicker, B.M.; Jones, K.; Collard, H.R.; Wolters, P.J. Lung mast cell density defines a subpopulation of patients with idiopathic pulmonary fibrosis. Histopathology 2012, 61, 98–106. [Google Scholar] [CrossRef] [Green Version]
- Ehara, T.; Shigematsu, H. Mast cells in the kidney. Review Article. Nephrology 2003, 8, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Pavone-Macaluso, M. Tissue Mast Cells in Renal Diseases. Acta Pathol. Microbiol. Scand. 1960, 50, 337–346. [Google Scholar] [CrossRef]
- Redegeld, F.A.; Yu, Y.; Kumari, S.; Charles, N.; Blank, U. Non-IgE mediated mast cell activation. Immunol. Rev. 2018, 282, 87–113. [Google Scholar] [CrossRef]
- Sugihara, A.; Tsujimura, T.; Fujita, Y.; Nakata, Y.; Terada, N. Evaluation of role of mast cells in the development of liver fibrosis using mast cell-deficient rats and mice. J. Hepatol. 1999, 30, 859–867. [Google Scholar] [CrossRef]
- Okazaki, T.; Hirota, S.; Xu, Z.D.; Maeyama, K.; Nakama, A.; Kawano, S.; Hori, M.; Kitamura, Y. Increase of mast cells in the liver and lung may be associated with but not a cause of fibrosis: Demonstration using mast cell-deficient Ws/Ws rats. Lab. Investig. 1998, 78, 1431–1438. [Google Scholar]
- Amiot, L.; Vu, N.; Drenou, B.; Scrofani, M.; Chalin, A.; Devisme, C.; Samson, M. The anti-fibrotic role of mast cells in the liver is mediated by HLA-G and interaction with hepatic stellate cells. Cytokine 2019, 117, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Presta, I.; Donato, A.; Zaffino, P.; Spadea, M.F.; Mancuso, T.; Malara, N.; Chiefari, E.; Donato, G. Does a polarization state exist for mast cells in cancer? Med. Hypotheses 2019, 131, 109281. [Google Scholar] [CrossRef] [PubMed]
- Sziksz, E.; Pap, D.; Lippai, R.; Béres, N.J.; Fekete, A.; Szabó, A.J.; Vannay, Á. Fibrosis Related Inflammatory Mediators: Role of the IL-10 Cytokine Family. Mediat. Inflamm. 2015, 2015, 1–15. [Google Scholar] [CrossRef]
- Miller, A.M.; Horiguchi, N.; Jeong, W.-I.; Radaeva, S.; Gao, B. Molecular Mechanisms of Alcoholic Liver Disease: Innate Immunity and Cytokines. Alcohol. Clin. Exp. Res. 2011, 35, 787–793. [Google Scholar] [CrossRef]
- Kucejko, W.; Chyczewska, E.; Naumnik, W.; Ossolinska, M. Concentration of surfactant protein D, Clara cell protein CC-16 and IL-10 in bronchoalveolar lavage (BAL) in patients with sarcoidosis, hypersensivity pneumonitis and idiopathic pulmonary fibrosis. Folia Histochem. Cytobiol. 2009, 47, 225–230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, Y.; Liu, R.; Xie, J.; Xiong, H.; He, J.C.; Chen, N. Interleukin-10 deficiency aggravates kidney inflammation and fibrosis in the unilateral ureteral obstruction mouse model. Lab. Investig. 2013, 93, 801–811. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodell, C.B.; Rai, R.; Faubel, S.; Burdick, J.A.; Soranno, D.E. Local immunotherapy via delivery of interleukin-10 and transforming growth factor β antagonist for treatment of chronic kidney disease. J. Control. Release 2015, 206, 131–139. [Google Scholar] [CrossRef]
- Jones, G.; Jones, S.A. Ectopic lymphoid follicles: Inducible centres for generating antigen-specific immune responses within tissues. Immunology 2015, 147, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Aloisi, F.; Borrell, R.P. Lymphoid neogenesis in chronic inflammatory diseases. Nat. Rev. Immunol. 2006, 6, 205–217. [Google Scholar] [CrossRef]
- François, A.; Chatelus, E.; Wachsmann, D.; Sibilia, J.; Bahram, S.; Alsaleh, G.; Gottenberg, J.-E. B lymphocytes and B-cell activating factor promote collagen and profibrotic markers expression by dermal fibroblasts in systemic sclerosis. Arthritis Res. Ther. 2013, 15, R168. [Google Scholar] [CrossRef] [Green Version]
- Naji, A.; Menier, C.; Morandi, F.; Agaugué, S.; Maki, G.; Ferretti, E.; Bruel, S.; Pistoia, V.; Carosella, E.D.; Rouas-Freiss, N. Binding of HLA-G to ITIM-Bearing Ig-like Transcript 2 Receptor Suppresses B Cell Responses. J. Immunol. 2014, 192, 1536–1546. [Google Scholar] [CrossRef] [Green Version]
- Gros, F.; Cabillic, F.; Toutirais, O.; Le Maux, A.; Sebti, Y.; Amiot, L. Soluble HLA-G molecules impair natural killer/dendritic cell crosstalkvia inhibition of dendritic cells. Eur. J. Immunol. 2008, 38, 742–749. [Google Scholar] [CrossRef]
- Monneret, G.; Voirin, N.; Krawice-Radanne, I.; Bohé, J.; Lepape, A.; Rouas-Freiss, N.; Carosella, E.D. Soluble human leukocyte antigen-G5 in septic shock: Marked and persisting elevation as a predictor of survival. Crit. Care Med. 2007, 35, 1942–1947. [Google Scholar] [CrossRef] [PubMed]


| HLA-G/mm2 | CD117/mm2 | ||
|---|---|---|---|
| Mean ± SD | Range | Mean ± SD | Range |
| 555 ± 699 | 41–2686 | 200 ± 271 | 12–1113 |
| % of HLA-G+ Cells | ||||
|---|---|---|---|---|
| CD117- Mast Cell Tryptase- | CD117+ | Mast Cell Tryptase+ | ||
| Mast cell tryptase+ CD117− | Mast cell tryptase+ CD117+ | Mast cell tryptase+ Total | ||
| 49 | 0 | 13 | 38 | 51 |
| % HLA-G Cells of Liver | ||
|---|---|---|
| Nodes | Fibrosis | |
| CD3- CD20- mast cell tryptase | 26 | 33 |
| CD3+ | 3 | 0 |
| CD20+ | 68 | 4 |
| Mast cell tryptase + | 3 | 63 |
| CD31 | 0 | 0 |
| CD1a | 0 | 0 |
| Nodes | Fibrosis | |
|---|---|---|
| HLA-G/mm2 | 849 | 99 |
| CD3/mm2 | 368 | 57 |
| CD20/mm2 | 3205 | 0 |
| Mast-cell tryptase/mm2 | 77 | 62 |
| Lung | |||
|---|---|---|---|
| HLA-G/mm2 | CD117/mm2 | ||
| Mean ± SD | range | Mean ± SD | range |
| 135 ± 62 | 55–215 | 227 ± 134 | 79–521 |
| Nodes | Fibrosis | |
|---|---|---|
| No CD3, no CD20, No Mast cell tryptase | 17 | 34 |
| CD3 | 0 | 3 |
| CD20 | 76 | 0 |
| Mast cell tryptase | 7 | 63 |
| CD31 | 0 | 0 |
| CD1a | 0 | 0 |
| HLA-G/mm2 | CD117/mm2 | ||
|---|---|---|---|
| Mean ± SD | range | Mean ± SD | range |
| 133 ± 102 | 21–289 | 426 ± 207 | 90–773 |
| Missing (N) | Mean (± std) or N (%) | |
|---|---|---|
| N = 42 | ||
| Age at transplant (yrs) | 0 | 60.1 ± 5.5 |
| Sex | 0 | |
| Male | 34 (80.9%) | |
| Female | 8 (19.1%) | |
| HCC | 0 | 17 (40.5%) |
| Metavir | 1 | |
| F0 | 1 (2.4%) | |
| F1 | 2 (4.8%) | |
| F2 | 0 (0%) | |
| F3 | 0 (0%) | |
| F4 | 38 (90.5%) | |
| Neuroinflammation | 19 | |
| A0 | 16 (38.1%) | |
| A1 | 7 (16.7%) | |
| A2 | 0 (0%) | |
| A3 | 0 (0%) | |
| A4 | 0 (0%) | |
| MELD | 0 | 20.9 ± 6.9 |
| CHILD | 2 | |
| A | 4 (9.5%) | |
| B | 8 (19%) | |
| C | 28 (66.7%) | |
| CMV + Recipient | 0 | 16 (38.1%) |
| CMV + Donor | 1 | 29 (69%) |
| Rejection | 15 | 8 (19%) |
| EAD | 0 | 12 (28.6%) |
| Missing (N) | Mean (± std) or N | |
|---|---|---|
| Age at diagnosis(years) | 0 | 65 ± 5 |
| Sex | ||
| Male (N) | 9 | |
| Female (N) | 1 | |
| Death (N) | 3 | |
| Evolution time before death (year) | In live at time (N = 7) | 4 ± 1 |
| Comorbidities | ||
| Body mass index | 2 | 27.31 ± 2.8 |
| Normal corpulence | 5 | |
| obesity | 5 | |
| Normal autoimmune tests | 6 | N = 3 |
| Smoking status | 3 | 7 |
| Forming smoker | 5 | |
| Current smoker | 1 | |
| Never smoker | 1 | |
| Vascular risk factors | 6 | 4 |
| Professional risk | 1 | |
| Histology | ||
| Common interstitial pneumonia equivalent to idiopathic pulmonary fibrosis N | 10 | |
| Pulmonary function tests | ||
| Tiffeneau’s ratio % | 0 | 76 ± 28 |
| FEV1 (forced expiratory volume in 1 s)% | 84 ± 18 | |
| Vital capacity% | 6 | 77 ± 5 |
| Forced vital capacity (FVC)% | 3 | 79 ± 24 |
| Maximum expiratory flow rate% | 4 | 97 ± 27 |
| Total lung capacity % | 0 | 67 ± 13 |
| Residual volume % | 4 | 61 ± 13 |
| DLCO diffusion capacity of lung for carbon monoxide(N) | 0 | 52 ± 17 |
| Restrictive syndrome (N) | N = 9 | |
| Isolated capillary alveolo diffusion disorder (N) | N = 1 | |
| Restrictive syndrome and Capillary alveolo diffusion disorder (N) | N = 3 |
| Missing (N) | Mean (± std) or N | |
|---|---|---|
| Age at diagnosis (years) | 63 ± 14 | |
| Sex | ||
| Male (N) | 4 | |
| Female (N) | 6 | |
| Death (N) | 0 | |
| Renal transplant(N) | 2 | |
| Comorbidities | ||
| Diabetes (N) | 3 | |
| HTA (N) | 2 | |
| Obesity (N) | 3 | |
| LMMC (N) | 1 | |
| Untreated rheumatoid arthritis (N) | ||
| comorbid association (N) | 3 | |
| Symptoms at diagnosis | ||
| Chronic renal failure | 8 | |
| progressive | 2 | |
| severe | 4 | |
| Acute renal failure | 2 | |
| Histology | ||
| Vascular nephropathy (N) | 4 | |
| tubulo-interstitial nephropathy (N) | 5 | |
| Immunoallergic nephropathy (N) | 1 | |
| Sclerous glomerules and tubulointertitiel nephropathy (N) | 1 | |
| Interstitial fibrosis and tubular atrophy (N) | ||
| 20% | 3 | |
| 30–40% | 4 | |
| 40–50% | 1 | |
| >50% | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mouchet, N.; Vu, N.; Turlin, B.; Rioux-Leclercq, N.; Jouneau, S.; Samson, M.; Amiot, L. HLA-G Is Widely Expressed by Mast Cells in Regions of Organ Fibrosis in the Liver, Lung and Kidney. Int. J. Mol. Sci. 2021, 22, 12490. https://doi.org/10.3390/ijms222212490
Mouchet N, Vu N, Turlin B, Rioux-Leclercq N, Jouneau S, Samson M, Amiot L. HLA-G Is Widely Expressed by Mast Cells in Regions of Organ Fibrosis in the Liver, Lung and Kidney. International Journal of Molecular Sciences. 2021; 22(22):12490. https://doi.org/10.3390/ijms222212490
Chicago/Turabian StyleMouchet, Nicolas, Nicolas Vu, Bruno Turlin, Nathalie Rioux-Leclercq, Stéphane Jouneau, Michel Samson, and Laurence Amiot. 2021. "HLA-G Is Widely Expressed by Mast Cells in Regions of Organ Fibrosis in the Liver, Lung and Kidney" International Journal of Molecular Sciences 22, no. 22: 12490. https://doi.org/10.3390/ijms222212490
APA StyleMouchet, N., Vu, N., Turlin, B., Rioux-Leclercq, N., Jouneau, S., Samson, M., & Amiot, L. (2021). HLA-G Is Widely Expressed by Mast Cells in Regions of Organ Fibrosis in the Liver, Lung and Kidney. International Journal of Molecular Sciences, 22(22), 12490. https://doi.org/10.3390/ijms222212490






