Aquatic Toxicity Effects and Risk Assessment of ‘Form Specific’ Product-Released Engineered Nanomaterials
Abstract
:1. Introduction
2. Results and Discussion
2.1. Toxicity Effects of PR-ENMs and Ions (% v/v)
2.1.1. Dose–Response
P. subcapitata
S. polyrhiza
D. magna
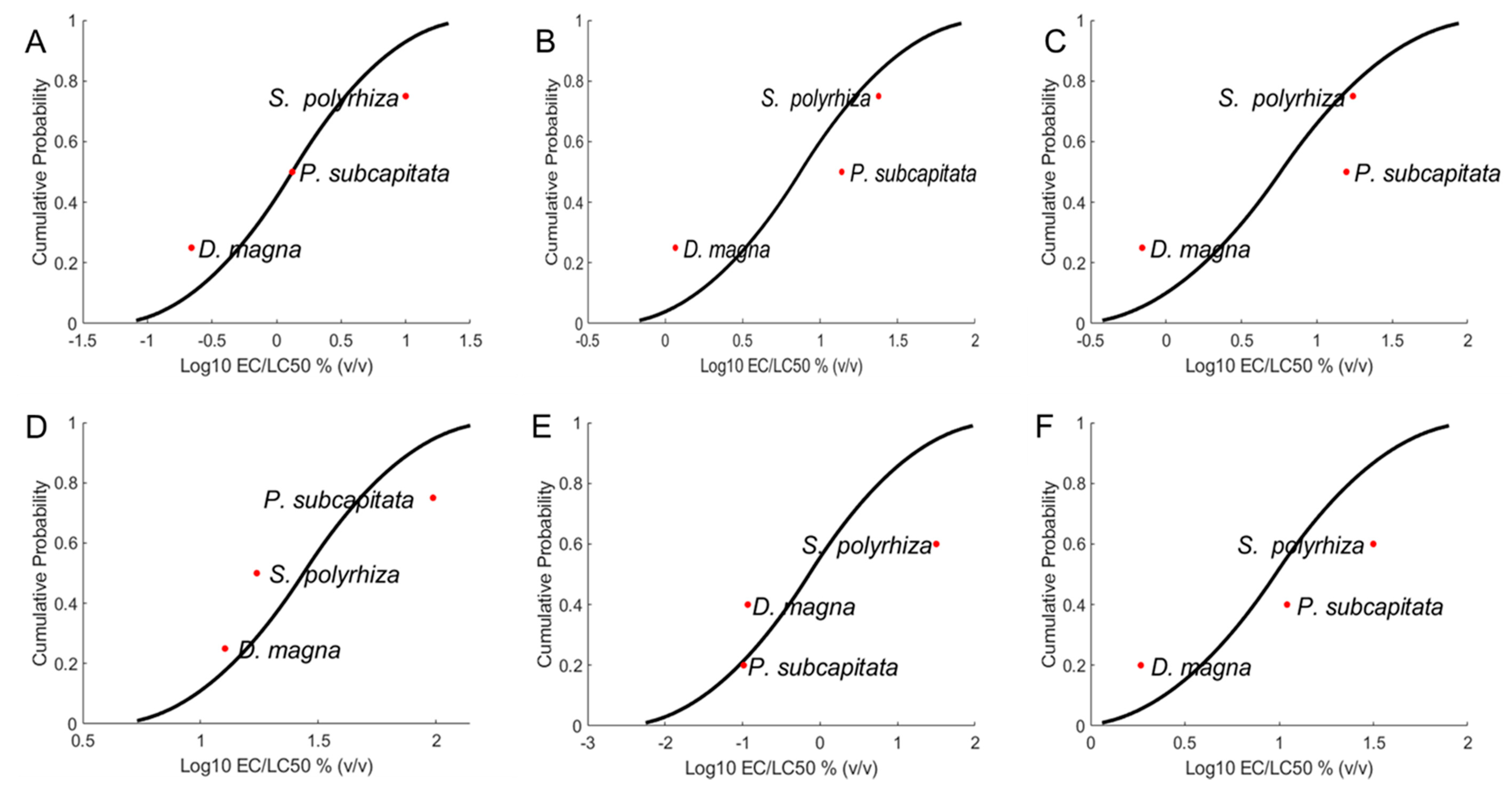
2.1.2. Species Sensitivity
2.2. Toxicity Assessment of PR-ENMs (Relative to Quantified Amounts)
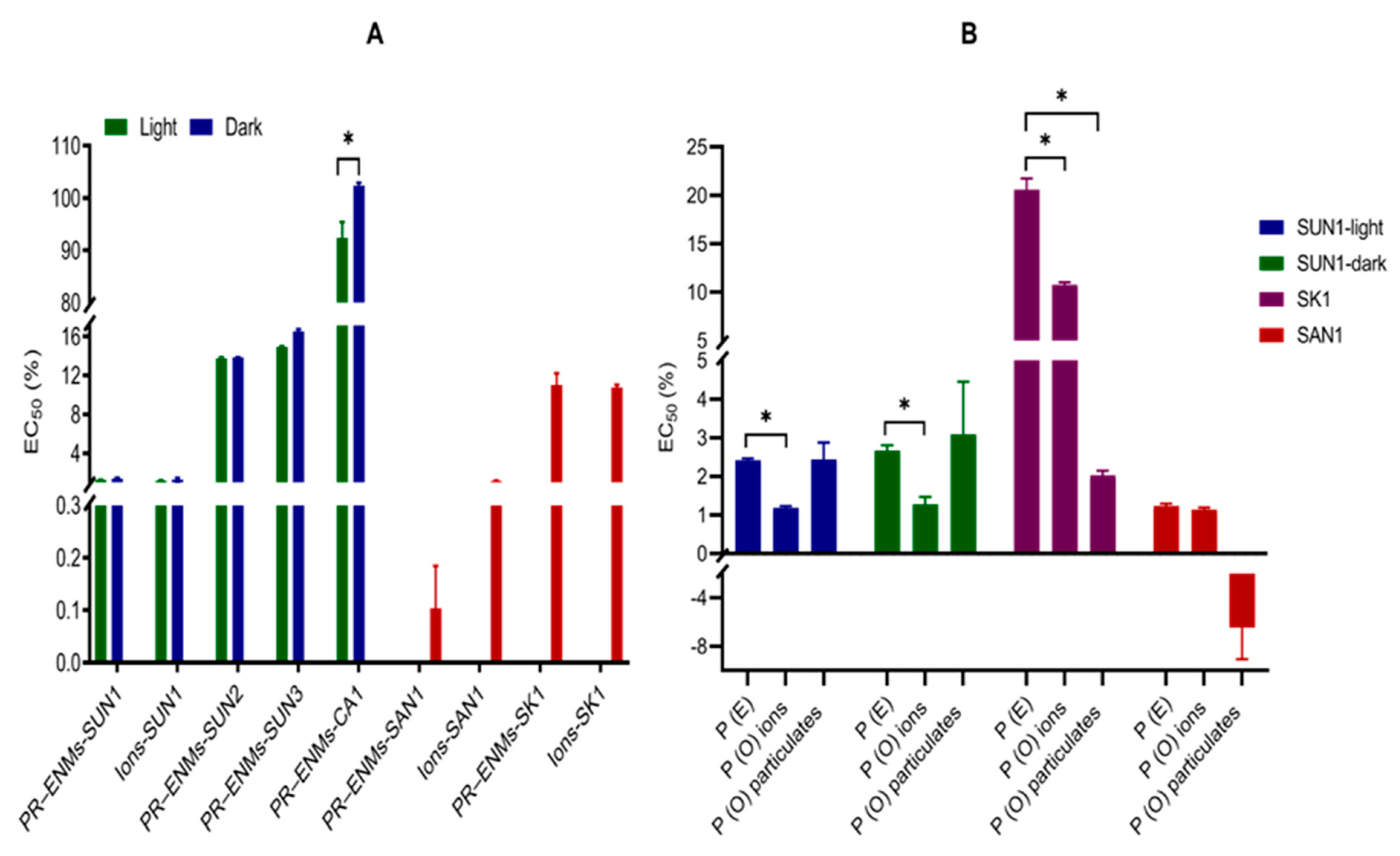
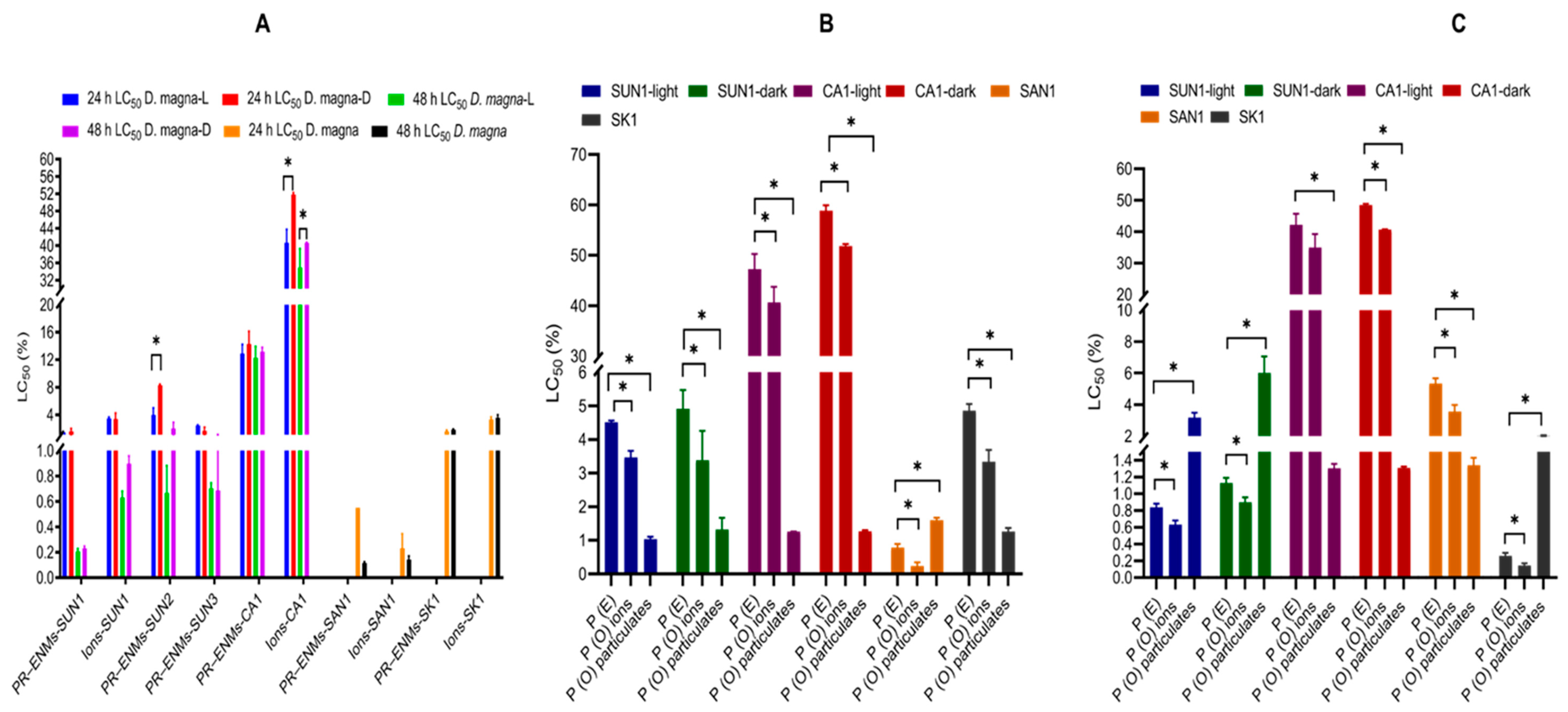
2.2.1. Toxicity Evaluation of Relative Contribution of Binary PR-ENMs
2.2.2. Half-Maximal Effective or -Lethal Concentration
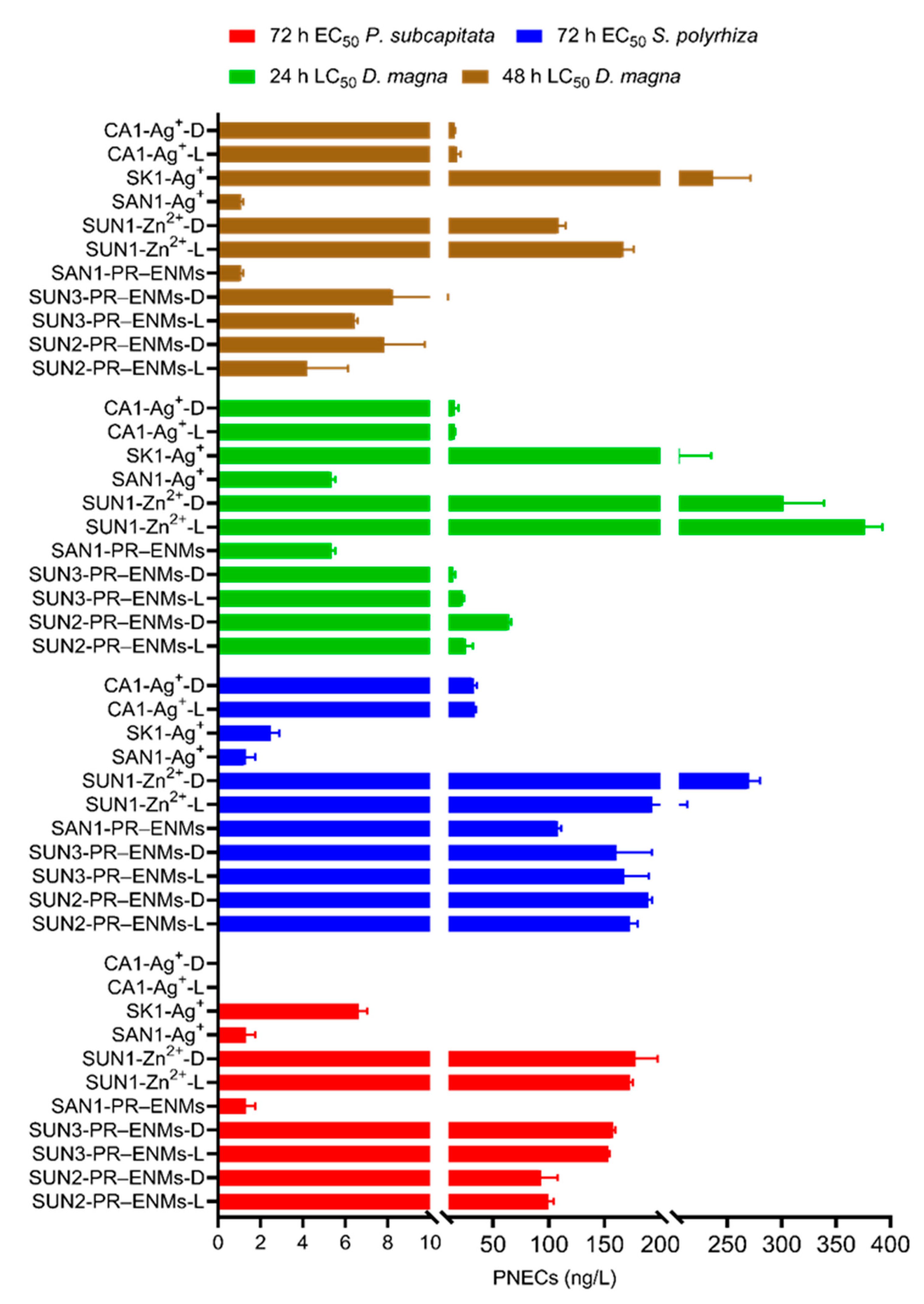
2.2.3. Predicted No Effects Concentrations of PR-ENMs
2.2.4. Risk Characterisation of PR-ENMs
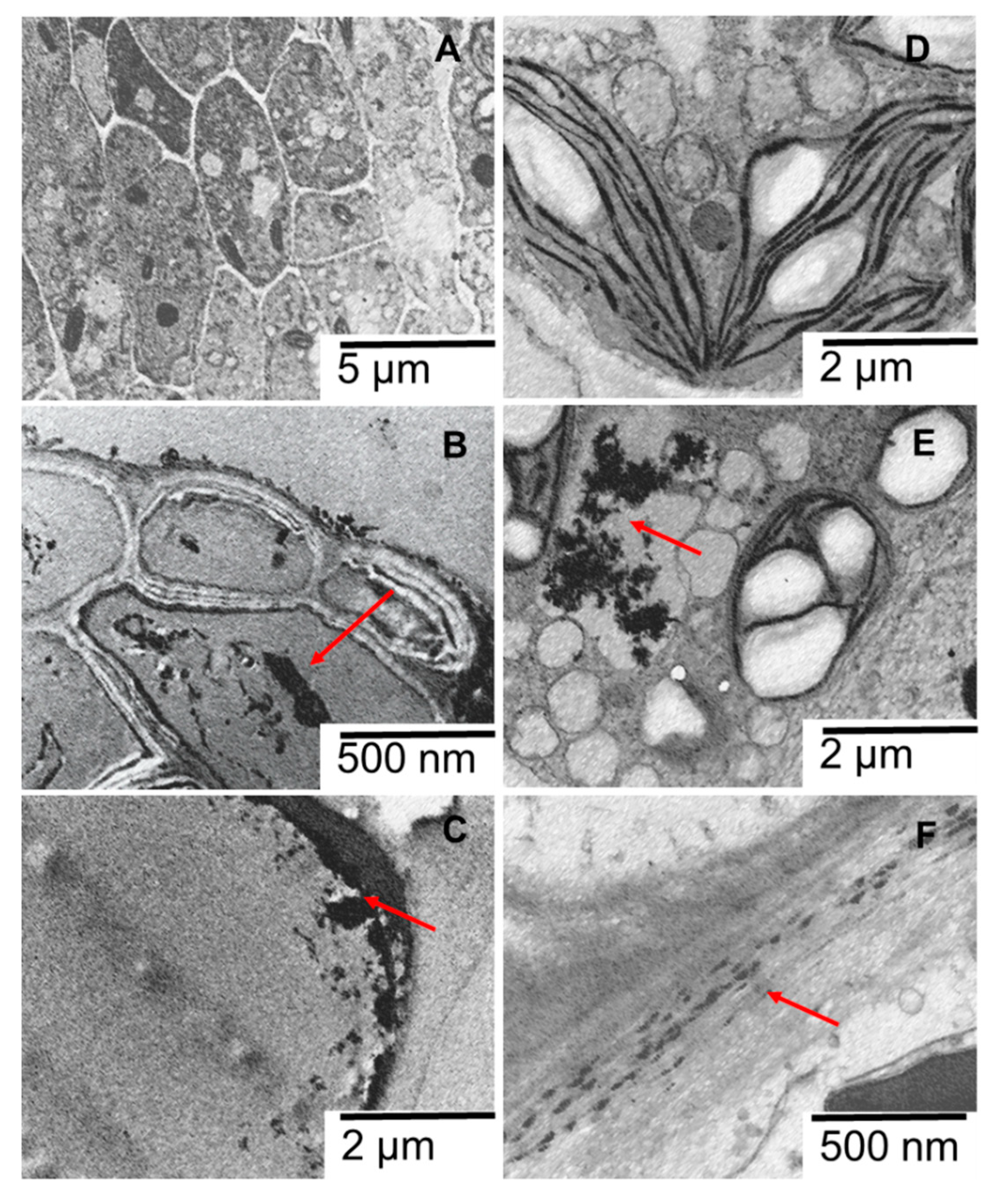
2.3. Sunscreen PR-ENMs Interaction with Biota
2.3.1. Interaction with P. subcapitata
2.3.2. Interaction with S. polyrhiza
2.3.3. Interaction with D. magna
3. Materials and Methods
3.1. Preparation of PR-ENMs’ Samples
3.2. Toxicity Effects Assessment
3.2.1. Dose–Response
P. subcapitata
D. magna
S. polyrhiza
3.2.2. Evaluation of Relative Toxicity Contributions between Mixtures
3.3. Species Sensitivity Distribution
3.4. Risk Characterisation
3.5. PR-ENMs Interaction with Test Organisms
Electron Microscopy Analysis Sample Preparation
3.6. Data Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Westerhoff, P.; Song, G.; Hristovski, K.; Kiser, M.A.; Moulin, P.; Yang, F.L.; Dhillon, A.P.; Thompson, R.P.H.; Bottero, J.Y.; Labille, J.; et al. Occurrence and removal of titanium at full scale wastewater treatment plants: Implications for TiO2 nanomaterials. J. Environ. Monit. 2011, 13, 1195. [Google Scholar] [CrossRef]
- Mueller, N.C.; Nowack, B. Exposure Modeling of Engineered Nanoparticles in the Environment. EMPA Act. 2008, 42, 63. [Google Scholar] [CrossRef]
- Keller, A.A.; McFerran, S.; Lazareva, A.; Suh, S. Global life cycle releases of engineered nanomaterials. J. Nanopart. Res. 2013, 15, 1692. [Google Scholar] [CrossRef]
- Gagnon, V.; Button, M.; Boparai, H.K.; Nearing, M.; O’Carroll, D.M.; Weber, K.P.; Nearing, M.; Weber, K.P.; Boparai, H.K.; Button, M.; et al. Influence of realistic wearing on the morphology and release of silver nanomaterials from textiles. Environ. Sci. Nano 2018, 6, 411–424. [Google Scholar] [CrossRef]
- Geranio, L.; Heuberger, M.; Nowack, B. The Behavior of Silver Nanotextiles during Washing. Environ. Sci. Technol. 2009, 43, 8113–8118. [Google Scholar] [CrossRef] [Green Version]
- Lombi, E.; Donner, E.; Scheckel, K.G.; Sekine, R.; Lorenz, C.; Von Goetz, N.; Nowack, B. Silver speciation and release in commercial antimicrobial textiles as influenced by washing. Chemosphere 2014, 111, 352–358. [Google Scholar] [CrossRef] [Green Version]
- Lorenz, C.; Windler, L.; von Goetz, N.; Lehmann, R.P.P.; Schuppler, M.; Hungerbühler, K.; Heuberger, M.; Nowack, B. Characterization of silver release from commercially available functional (nano)textiles. Chemosphere 2012, 89, 817–824. [Google Scholar] [CrossRef]
- Limpiteeprakan, P.; Babel, S.; Lohwacharin, J.; Takizawa, S. Release of silver nanoparticles from fabrics during the course of sequential washing. Environ. Sci. Pollut. Res. 2016, 23, 22810–22818. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.J.B.; Yoon, T.H. Determination of silver nanoparticle species released from textiles into artificial sweat and laundry wash for a risk assessment. Hum. Ecol. Risk Assess. Int. J. 2017, 23, 741–750. [Google Scholar] [CrossRef]
- Windler, L.; Lorenz, C.; Von Goetz, N.; Hungerbühler, K.; Amberg, M.; Heuberger, M.; Nowack, B. Release of titanium dioxide from textiles during washing. Environ. Sci. Technol. 2012, 46, 8181–8188. [Google Scholar] [CrossRef]
- Mackevica, A.; Olsson, M.E.; Hansen, S.F. Quantitative characterization of TiO2 nanoparticle release from textiles by conventional and single particle ICP-MS. J. Nanopart. Res. 2018, 20, 6. [Google Scholar] [CrossRef]
- Kaegi, R.; Sinnet, B.; Zuleeg, S.; Hagendorfer, H.; Mueller, E.; Vonbank, R.; Boller, M.; Burkhardt, M. Release of silver nanoparticles from outdoor facades. Environ. Pollut. 2010, 158, 2900–2905. [Google Scholar] [CrossRef]
- Adeleye, A.S.; Oranu, E.A.; Tao, M.; Keller, A.A. Release and detection of nanosized copper from a commercial antifouling paint. Water Res. 2016, 102, 374–382. [Google Scholar] [CrossRef] [Green Version]
- Azimzada, A.; Farner, J.M.; Jreije, I.; Hadioui, M.; Liu-Kang, C.; Tufenkji, N.; Shaw, P.; Wilkinson, K.J. Single- and Multi-Element Quantification and Characterization of TiO2 Nanoparticles Released From Outdoor Stains and Paints. Front. Environ. Sci. 2020, 8, 91. [Google Scholar] [CrossRef]
- Lehutso, R.F.; Thwala, M. Assessment of Nanopollution in Water Environments from Commercial Products. Nanomaterials 2021, 11, 2537. [Google Scholar] [CrossRef]
- Botta, C.; Labille, J.; Auffan, M.; Borschneck, D.; Miche, H.H.; Cabié, M.; Masion, A.; Rose, J.; Bottero, J.-Y.Y. TiO2-based nanoparticles released in water from commercialized sunscreens in a life-cycle perspective: Structures and quantities. Environ. Pollut. 2011, 159, 1543–1550. [Google Scholar] [CrossRef]
- Nthwane, Y.B.; Tancu, Y.; Maity, A.; Thwala, M. Characterisation of titanium oxide nanomaterials in sunscreens obtained by extraction and release exposure scenarios. SN Appl. Sci. 2019, 1, 312. [Google Scholar] [CrossRef] [Green Version]
- Jeon, S.K.; Kim, E.J.; Lee, J.; Lee, S. Potential risks of TiO2 and ZnO nanoparticles released from sunscreens into outdoor swimming pools. J. Hazard. Mater. 2016, 317, 312–318. [Google Scholar] [CrossRef]
- Wong, S.W.Y.; Zhou, G.J.; Leung, P.T.Y.; Han, J.; Lee, J.S.; Kwok, K.W.H.; Leung, K.M.Y. Sunscreens containing zinc oxide nanoparticles can trigger oxidative stress and toxicity to the marine copepod Tigriopus japonicus. Mar. Pollut. Bull. 2020, 154, 111078. [Google Scholar] [CrossRef]
- Mitrano, D.M.; Motellier, S.; Clavaguera, S.; Nowack, B. Review of nanomaterial aging and transformations through the life cycle of nano-enhanced products. Environ. Int. 2015, 77, 132–147. [Google Scholar] [CrossRef] [Green Version]
- Adam, V.; Caballero-Guzman, A.; Nowack, B. Considering the forms of released engineered nanomaterials in probabilistic material flow analysis. Environ. Pollut. 2018, 243, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Lehutso, R.F.; Tancu, Y.; Maity, A.; Thwala, M. Aquatic toxicity of transformed and product-released engineered nanomaterials: An overview of the current state of knowledge. Process Saf. Environ. Prot. 2020, 138, 39–56. [Google Scholar] [CrossRef]
- Koivisto, A.J.; Jensen, A.C.Ø.; Kling, K.I.; Nørgaard, A.; Brinch, A.; Christensen, F.; Jensen, K.A. Quantitative material releases from products and articles containing manufactured nanomaterials: Towards a release library. NanoImpact 2017, 5, 119–132. [Google Scholar] [CrossRef]
- Gondikas, A.P.; Von Der Kammer, F.; Reed, R.B.; Wagner, S.; Ranville, J.F.; Hofmann, T.; von der Kammer, F.; Reed, R.B.; Wagner, S.; Ranville, J.F.; et al. Release of TiO2 nanoparticles from sunscreens into surface waters: A one-year survey at the old danube recreational lake. Environ. Sci. Technol. 2014, 48, 5415–5422. [Google Scholar] [CrossRef] [PubMed]
- Long, C.-L.; Yang, Z.-G.; Yuan, Y.; Li, H.-P.; Wang, Q. Determination of gold nanoparticles in natural water using single particle-ICP-MS. J. Cent. South Univ. 2016, 23, 1611–1617. [Google Scholar] [CrossRef]
- Yang, Y.; Long, C.-L.; Li, H.-P.; Wang, Q.; Yang, Z.-G.; Wang, Q. Analysis of silver and gold nanoparticles in environmental water using single particle-inductively coupled plasma-mass spectrometry. Sci. Total Environ. Yang 2016, 563564, 996–1007. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.J.; Shih, Y.H.; Su, C.H.; Ho, H.C. Comparison of three analytical methods to measure the size of silver nanoparticles in real environmental water and wastewater samples. J. Hazard. Mater. 2017, 322, 95–104. [Google Scholar] [CrossRef]
- Bäuerlein, P.S.; Emke, E.; Tromp, P.; Hofman, J.A.M.H.; Carboni, A.; Schooneman, F.; de Voogt, P.; van Wezel, A.P. Is there evidence for man-made nanoparticles in the Dutch environment? Sci. Total Environ. 2017, 576, 273–283. [Google Scholar] [CrossRef]
- Gondikas, A.; Von Der Kammer, F.; Kaegi, R.; Borovinskaya, O.; Neubauer, E.; Navratilova, J.; Praetorius, A.; Cornelis, G.; Hofmann, T. Where is the nano? Analytical approaches for the detection and quantification of TiO2 engineered nanoparticles in surface waters. Environ. Sci. Nano 2018, 5, 313–326. [Google Scholar] [CrossRef]
- Peters, R.J.B.B.; van Bemmel, G.; Milani, N.B.L.L.; den Hertog, G.C.T.T.; Undas, A.K.; van der Lee, M.; Bouwmeester, H. Detection of nanoparticles in Dutch surface waters. Sci. Total Environ. 2018, 621, 210–218. [Google Scholar] [CrossRef]
- Venkatesan, A.K.; Reed, R.B.; Lee, S.; Bi, X.; Hanigan, D.; Yang, Y.; Ranville, J.F.; Herckes, P.; Westerhoff, P. Detection and Sizing of Ti-Containing Particles in Recreational Waters Using Single Particle ICP-MS. Bull. Environ. Contam. Toxicol. 2018, 100, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Phalyvong, K.; Sivry, Y.; Pauwels, H.; Gélabert, A.; Tharaud, M.; Wille, G.; Bourrat, X.; Benedetti, M.F. Occurrence and Origins of Cerium Dioxide and Titanium Dioxide Nanoparticles in the Loire River (France) by Single Particle ICP-MS and FEG-SEM Imaging. Front. Environ. Sci. 2020, 8, 141. [Google Scholar] [CrossRef]
- Rand, L.N.; Bi, Y.; Poustie, A.; Bednar, A.J.; Hanigan, D.J.; Westerhoff, P.; Ranville, J.F. Quantifying temporal and geographic variation in sunscreen and mineralogic titanium-containing nanoparticles in three recreational rivers. Sci. Total Environ. 2020, 743, 140845. [Google Scholar] [CrossRef] [PubMed]
- Slomberg, D.L.; Auffan, M.; Guéniche, N.; Angeletti, B.; Campos, A.; Borschneck, D.; Aguerre-Chariol, O.; Rose, J. Anthropogenic Release and Distribution of Titanium Dioxide Particles in a River Downstream of a Nanomaterial Manufacturer Industrial Site. Front. Environ. Sci. 2020, 8, 76. [Google Scholar] [CrossRef]
- Wang, J.; Nabi, M.M.; Mohanty, S.K.; Afrooz, A.N.; Cantando, E.; Aich, N.; Baalousha, M. Detection and quantification of engineered particles in urban runoff. Chemosphere 2020, 248, 126070. [Google Scholar] [CrossRef]
- Wu, S.; Zhang, S.; Gong, Y.; Shi, L.; Zhou, B. Identification and quantification of titanium nanoparticles in surface water: A case study in Lake Taihu, China. J. Hazard. Mater. 2020, 382, 121045. [Google Scholar] [CrossRef]
- Suhendra, E.; Chang, C.H.; Hou, W.C.; Hsieh, Y.C. A review on the environmental fate models for predicting the distribution of engineered nanomaterials in surface waters. Int. J. Mol. Sci. 2020, 21, 4554. [Google Scholar] [CrossRef]
- Ellis, L.A.; Kissane, S.; Hoffman, E.; Brown, J.B.; Valsami-Jones, E.; Colbourne, J.; Lynch, I. Multigenerational Exposures of Daphnia Magna to Pristine and Aged Silver Nanoparticles: Epigenetic Changes and Phenotypical Ageing Related Effects. Small 2020, 16, 2000301. [Google Scholar] [CrossRef]
- Temizel-Sekeryan, S.; Hicks, A.L. Developing physicochemical property-based ecotoxicity characterization factors for silver nanoparticles under mesocosm conditions for use in life cycle assessment. Environ. Sci. Nano 2021, 8, 1786–1800. [Google Scholar] [CrossRef]
- Reed, R.B.; Zaikova, T.; Barber, A.; Simonich, M.; Lankone, R.; Marco, M.; Hristovski, K.; Herckes, P.; Passantino, L.; Fairbrother, D.H.; et al. Potential Environmental Impacts and Antimicrobial Efficacy of Silver- and Nanosilver-Containing Textiles. Environ. Sci. Technol. 2016, 50, 4018–4026. [Google Scholar] [CrossRef]
- Gao, J.; Sepúlveda, M.S.; Klinkhamer, C.; Wei, A.; Gao, Y.; Mahapatra, C.T. Nanosilver-coated socks and their toxicity to zebrafish (Danio rerio) embryos. Chemosphere 2015, 119, 948–952. [Google Scholar] [CrossRef]
- Corinaldesi, C.; Damiani, E.; Marcellini, F.; Falugi, C.; Tiano, L.; Brugè, F.; Danovaro, R. Sunscreen products impair the early developmental stages of the sea urchin Paracentrotus lividus. Sci. Rep. 2017, 7, 7815. [Google Scholar] [CrossRef] [Green Version]
- Dedman, C.J.; King, A.; Christie-Oleza, J.; Davies, G.-L. Environmentally relevant concentrations of titanium dioxide nanoparticles pose negligible risk to marine microbes. Environ. Sci. Nano 2021, 8, 1236–1255. [Google Scholar] [CrossRef]
- Galletti, A.; Seo, S.; Joo, S.H.S.; Su, C.; Blackwelder, P. Effects of titanium dioxide nanoparticles derived from consumer products on the marine diatom thalassiosira pseudonana. Environ. Sci. 2016, 23, 21113–21122. [Google Scholar] [CrossRef]
- Hanigan, D.; Truong, L.; Schoepf, J.; Nosaka, T.; Mulchandani, A.; Tanguay, R.L.; Westerhoff, P. Trade-offs in ecosystem impacts from nanomaterial versus organic chemical ultraviolet filters in sunscreens. Water Res. 2018, 139, 281–290. [Google Scholar] [CrossRef]
- Baek, S.; Joo, S.H.; Kumar, N.; Toborek, M. Antibacterial effect and toxicity pathways of industrial and sunscreen ZnO nanoparticles on Escherichia coli. J. Environ. Chem. Eng. 2017, 5, 3024–3032. [Google Scholar] [CrossRef]
- Schiavo, S.; Oliviero, M.; Philippe, A.; Manzo, S. Nanoparticles based sunscreens provoke adverse effects on marine microalgae Dunaliella tertiolecta. Environ. Sci. Nano 2018, 5, 3011–3022. [Google Scholar] [CrossRef]
- Spisni, E.; Seo, S.; Joo, S.; Su, C. Release and toxicity comparison between industrial-and sunscreen-derived nano-ZnO particles. Int. J. Environ. 2016, 13, 2485–2494. [Google Scholar] [CrossRef] [Green Version]
- Farkas, J.; Peter, H.; Christian, P.; Gallego Urrea, J.A.; Hassellöv, M.; Tuoriniemi, J.; Gustafsson, S.; Olsson, E.; Hylland, K.; Thomas, K.V. Characterization of the effluent from a nanosilver producing washing machine. Environ. Int. 2011, 37, 1057–1062. [Google Scholar] [CrossRef] [PubMed]
- Echavarri-Bravo, V.; Paterson, L.; Aspray, T.J.; Porter, J.S.; Winson, M.K.; Hartl, M.G.J. Natural marine bacteria as model organisms for the hazard-assessment of consumer products containing silver nanoparticles. Mar. Environ. Res. 2017, 130, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Auffan, M.; Liu, W.; Brousset, L.; Scifo, L.; Pariat, A.; Sanles, M.; Chaurand, P.; Angeletti, B.; Thiéry, A.; Masion, A.; et al. Environmental exposure of a simulated pond ecosystem to a CuO nanoparticle-based wood stain throughout its life cycle. Environ. Sci. Nano 2018, 5, 2579–2589. [Google Scholar] [CrossRef] [Green Version]
- Künniger, T.; Gerecke, A.C.; Ulrich, A.; Huch, A.; Vonbank, R.; Heeb, M.; Wichser, A.; Haag, R.; Kunz, P.; Faller, M. Release and environmental impact of silver nanoparticles and conventional organic biocides from coated wooden façades. Environ. Pollut. 2014, 184, 464–471. [Google Scholar] [CrossRef]
- Zheng, Y.; Mutzner, L.; Ort, C.; Kaegi, R.; Gottschalk, F. Modelling engineered nanomaterials in wet-weather discharges. NanoImpact 2019, 16, 100188. [Google Scholar] [CrossRef]
- Coll, C.; Notter, D.; Gottschalk, F.; Sun, T.; Som, C.; Nowack, B. Probabilistic environmental risk assessment of five nanomaterials (nano-TiO2, nano-Ag, nano-ZnO, CNT, and fullerenes). Nanotoxicology 2016, 10, 436–444. [Google Scholar] [CrossRef]
- Blaser, S.A.; Scheringer, M.; MacLeod, M.; Hungerbühler, K. Estimation of cumulative aquatic exposure and risk due to silver: Contribution of nano-functionalized plastics and textiles. Sci. Total Environ. 2008, 390, 396–409. [Google Scholar] [CrossRef]
- Gottschalk, F.; Sonderer, T.; Scholz, R.W.; Nowack, B. Modeled Environmental Concentrations of Engineered Nanomaterials (TiO2, ZnO, Ag, CNT, Fullerenes) for Different Regions. Environ. Sci. Technol. 2009, 43, 9216–9222. [Google Scholar] [CrossRef]
- Kjølholt, J.; Gottschalk, F.; Brinch, A.; Lüftzhøft, H.H.; Hartmann, N.B.; Nowack, B.; Baun, A. Environmental Assessment of Nanomaterial Use in Denmark; The Danish Environmental Protection Agency: Copenhagen, Denmark, 2015; ISBN 9788793352711. [Google Scholar]
- Wang, Y.; Kalinina, A.; Sun, T.; Nowack, B. Probabilistic modeling of the flows and environmental risks of nano-silica. Sci. Total Environ. 2016, 545, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Musee, N. Comment on “Risk Assessments Show Engineered Nanomaterials To Be of Low Environmental Concern”. Environ. Sci. Technol. 2018, 52, 6723–6724. [Google Scholar] [CrossRef]
- Sun, T.Y.; Bornhöft, N.A.; Hungerbühler, K.; Nowack, B. Dynamic Probabilistic Modeling of Environmental Emissions of Engineered Nanomaterials. Environ. Sci. Technol. 2016, 50, 4701–4711. [Google Scholar] [CrossRef]
- Musee, N. Simulated environmental risk estimation of engineered nanomaterials: A case of cosmetics in Johannesburg City. Hum. Exp. Toxicol. 2011, 30, 1181–1195. [Google Scholar] [CrossRef]
- Garner, K.L.; Suh, S.; Keller, A.A. Assessing the Risk of Engineered Nanomaterials in the Environment: Development and Application of the nanoFate Model. Environ. Sci. Technol. 2017, 51, 5541–5551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ellis, L.J.A.; Baalousha, M.; Valsami-Jones, E.; Lead, J.R. Seasonal variability of natural water chemistry affects the fate and behaviour of silver nanoparticles. Chemosphere 2018, 191, 616–625. [Google Scholar] [CrossRef] [PubMed]
- Hong, H.; Adam, V.; Nowack, B. Form-Specific and Probabilistic Environmental Risk Assessment of Three Engineered Nanomaterials (nano-Ag, nano-TiO2 and nano-ZnO) in European Freshwaters. Environ. Toxicol. Chem. 2021, 40, 2629–2639. [Google Scholar] [CrossRef] [PubMed]
- Nowack, B.; Mitrano, D.M. Procedures for the production and use of synthetically aged and product released nanomaterials for further environmental and ecotoxicity testing. NanoImpact 2018, 10, 70–80. [Google Scholar] [CrossRef]
- Slomberg, D.L.; Catalano, R.; Ziarelli, F.; Viel, S.; Bartolomei, V.; Labille, J.; Masion, A. Aqueous aging of a silica coated TiO2 UV filter used in sunscreens: Investigations at the molecular scale with dynamic nuclear polarization NMR. RSC Adv. 2020, 10, 8266–8274. [Google Scholar] [CrossRef]
- Auffan, M.; Pedeutour, M.; Rose, J.; Masion, A.; Ziarelli, F.; Borschneck, D.; Chaneac, C.; Botta, C.; Chaurand, P.; Labille, J.; et al. Structural Degradation at the Surface of a TiO2-Based Nanomaterial Used in Cosmetics. Environ. Sci. Technol. 2010, 44, 2689–2694. [Google Scholar] [CrossRef]
- Labille, J.; Feng, J.; Botta, C.; Borschneck, D.; Sammut, M.; Cabie, M.; Auffan, M.; Rose, J.; Bottero, J.-Y. Aging of TiO2 nanocomposites used in sunscreen. Dispersion and fate of the degradation products in aqueous environment. Environ. Pollut. 2010, 158, 3482–3489. [Google Scholar] [CrossRef]
- Impellitteri, C.A.; Tolaymat, T.M.; Scheckel, K.G. The Speciation of Silver Nanoparticles in Antimicrobial Fabric Before and After Exposure to a Hypochlorite/Detergent Solution. J. Environ. Qual. 2009, 38, 1528–1530. [Google Scholar] [CrossRef]
- Zhang, W.; Xiao, B.; Fang, T. Chemical transformation of silver nanoparticles in aquatic environments: Mechanism, morphology and toxicity. Chemosphere 2018, 191, 324–334. [Google Scholar] [CrossRef]
- Abbas, Q.; Yousaf, B.; Ali, A.M.U.; Munir, M.A.M.; El-Naggar, A.; Rinklebe, J.; Naushad, M. Transformation pathways and fate of engineered nanoparticles (ENPs) in distinct interactive environmental compartments: A review. Environ. Int. 2020, 138, 105646. [Google Scholar] [CrossRef]
- Moeta, P.J.; Wesley-Smith, J.; Maity, A.; Thwala, M. Nano-enabled products in South Africa and the assessment of environmental exposure potential for engineered nanomaterials. SN Appl. Sci. 2019, 1, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Nasser, F.; Lynch, I. Updating traditional regulatory tests for use with novel materials: Nanomaterial toxicity testing with Daphnia magna. Saf. Sci. 2019, 118, 497–504. [Google Scholar] [CrossRef]
- Ceschin, S.; Bellini, A.; Scalici, M. Aquatic plants and ecotoxicological assessment in freshwater ecosystems: A review. Environ. Sci. Pollut. Res. 2020, 28, 4975–4988. [Google Scholar] [CrossRef]
- Ma, H.; Williams, P.L.; Diamond, S.A. Ecotoxicity of manufactured ZnO nanoparticles—A review. Environ. Pollut. 2013, 172, 76–85. [Google Scholar] [CrossRef]
- Turan, N.B.; Erkan, H.S.; Engin, G.O.; Bilgili, M.S. Nanoparticles in the aquatic environment: Usage, properties, transformation and toxicity—A review. Process Saf. Environ. Prot. 2019, 130, 238–249. [Google Scholar] [CrossRef]
- Caloni, S.; Durazzano, T.; Franci, G.; Marsili, L. Sunscreens’ UV Filters Risk for Coastal Marine Environment Biodiversity: A Review. Divers 2021, 13, 374. [Google Scholar] [CrossRef]
- Sureda, A.; Capó, X.; Busquets-Cortés, C.; Tejada, S. Acute exposure to sunscreen containing titanium induces an adaptive response and oxidative stress in Mytillus galloprovincialis. Ecotoxicol. Environ. Saf. 2018, 149, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Araújo, C.V.M.; Rodríguez-Romero, A.; Fernández, M.; Sparaventi, E.; Medina, M.M.; Tovar-Sánchez, A. Repellency and mortality effects of sunscreens on the shrimp Palaemon varians: Toxicity dependent on exposure method. Chemosphere 2020, 257, 127190. [Google Scholar] [CrossRef]
- Vimercati, L.; Cavone, D.; Caputi, A.; De Maria, L.; Tria, M.; Prato, E.; Ferri, G.M. Nanoparticles: An Experimental Study of Zinc Nanoparticles Toxicity on Marine Crustaceans. General Overview on the Health Implications in Humans. Front. Public Health 2020, 8, 192. [Google Scholar] [CrossRef]
- ISO 20227:2017—Water Quality—Determination of the Growth Inhibition Effects of Waste Waters, Natural Waters and Chemicals on the Duckweed Spirodela polyrhiza—Method Using a Stock Culture Independent Microbiotest. Available online: https://www.iso.org/standard/67326.html (accessed on 16 July 2019).
- OECD. OECD TEST 202 Test No. 202: Daphnia sp. Acute Immobilisation Test|READ Online. Available online: http://www.oecd-ilibrary.org/environment/test-no-202-daphnia-sp-acute-immobilisation-test_9789264069947-en (accessed on 25 October 2017).
- OECD. OECD 201 Test No. 201: Freshwater Alga and Cyanobacteria, Growth Inhibition Test | en | OECD. Available online: https://www.oecd-ilibrary.org/docserver/9789264069923-en.pdf?expires=1563277198&id=id&accname=guest&checksum=7A8EEE762DBC807803461DE10E3B270C (accessed on 26 October 2017).
- Ye, N.; Wang, Z.; Wang, S.; Peijnenburg, W.J.G.M. Toxicity of mixtures of zinc oxide and graphene oxide nanoparticles to aquatic organisms of different trophic level: Particles outperform dissolved ions. Nanotoxicology 2018, 12, 423–438. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, J.; Li, X.; Shao, J.; Peijnenburg, W.J.G.M. Aquatic toxicity of nanosilver colloids to different trophic organisms: Contributions of particles and free silver ion. Environ. Toxicol. Chem. 2012, 31, 2408–2413. [Google Scholar] [CrossRef]
- Wang, D.; Lin, Z.; Wang, T.; Yao, Z.; Qin, M.; Zheng, S.; Lu, W. Where does the toxicity of metal oxide nanoparticles come from: The nanoparticles, the ions, or a combination of both? J. Hazard. Mater. 2016, 308, 328–334. [Google Scholar] [CrossRef]
- Du, J.; Tang, J.; Xu, S.; Ge, J.; Dong, Y.; Li, H.; Jin, M. A review on silver nanoparticles-induced ecotoxicity and the underlying toxicity mechanisms. Regul. Toxicol. Pharmacol. 2018, 98, 231–239. [Google Scholar] [CrossRef]
- Ribeiro, F.; Gallego-Urrea, J.A.; Jurkschat, K.; Crossley, A.; Hassellöv, M.; Taylor, C.; Soares, A.M.V.M.; Loureiro, S. Silver nanoparticles and silver nitrate induce high toxicity to Pseudokirchneriella subcapitata, Daphnia magna and Danio rerio. Sci. Total Environ. 2014, 466–467, 232–241. [Google Scholar] [CrossRef] [PubMed]
- Bicherel, P.; Thomas, P.C. Aquatic Toxicity Calculation of Mixtures: A Chemical Activity Approach Incorporating a Bioavailability Reduction Concept. Environ. Sci. Technol. 2021, 55, 11183–11191. [Google Scholar] [CrossRef] [PubMed]
- Fastelli, P.; Renzi, M. Exposure of key marine species to sunscreens: Changing ecotoxicity as a possible indirect effect of global warming. Mar. Pollut. Bull. 2019, 149, 110517. [Google Scholar] [CrossRef] [PubMed]
- Blasco, J.; Trombini, C.; Sendra, M.; Araujo, C.V.M. Environmental Risk Assessment of Sunscreens; Springer: Cham, Switzerland, 2020; pp. 163–184. [Google Scholar]
- Arvidsson, R. Risk Assessments Show Engineered Nanomaterials to Be of Low Environmental Concern. Environ. Sci. Technol. 2018, 52, 2436–2437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gottschalk, F.; Sun, T.; Nowack, B. Environmental concentrations of engineered nanomaterials: Review of modeling and analytical studies. Environ. Pollut. 2013, 181, 287–300. [Google Scholar] [CrossRef]
- Tarrahi, R.; Movafeghi, A.; Khataee, A.; Rezanejad, F.; Gohari, G. Evaluating the Toxic Impacts of Cadmium Selenide Nanoparticles on the Aquatic Plant Lemna minor. Molecules 2019, 24, 410. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.; Hua, T.; Xiao, F.; Chen, C.; Gersberg, R.M.; Liu, Y.; Ng, W.J.; Tan, S.K. Uptake and accumulation of CuO nanoparticles and CdS/ZnS quantum dot nanoparticles by Schoenoplectus tabernaemontani in hydroponic mesocosms. Ecol. Eng. 2014, 70, 114–123. [Google Scholar] [CrossRef]
- Jiang, H.S.; Qiu, X.N.; Li, G.B.; Li, W.; Yin, L.Y. Silver nanoparticles induced accumulation of reactive oxygen species and alteration of antioxidant systems in the aquatic plant Spirodela polyrhiza. Environ. Toxicol. Chem. 2014, 33, 1398–1405. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Li, J.; Zhang, X.; Wei, H.; Cui, L. Effects of heat acclimation pretreatment on changes of membrane lipid peroxidation, antioxidant metabolites, and ultrastructure of chloroplasts in two cool-season turfgrass species under heat stress. Environ. Exp. Bot. 2006, 56, 274–285. [Google Scholar] [CrossRef]
- Skyba, M.; Petijová, L.; Košuth, J.; Koleva, D.P.; Ganeva, T.G.; Kapchina-Toteva, V.M.; Čellárová, E. Oxidative stress and antioxidant response in Hypericum perforatum L. plants subjected to low temperature treatment. J. Plant Physiol. 2012, 169, 955–964. [Google Scholar] [CrossRef]
- Manke, A.; Wang, L.; Rojanasakul, Y. Mechanisms of nanoparticle-induced oxidative stress and toxicity. Biomed. Res. Int. 2013, 2013, 942916. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhuvaneshwari, M.; Sagar, B.; Doshi, S.; Chandrasekaran, N.; Mukherjee, A. Comparative study on toxicity of ZnO and TiO2 nanoparticles on Artemia salina: Effect of pre-UV-A and visible light irradiation. Environ. Sci. Pollut. Res. 2017, 24, 5633–5646. [Google Scholar] [CrossRef]
- Wang, F.; Guan, W.; Xu, L.; Ding, Z.; Ma, H.; Ma, A.; Terry, N. Effects of Nanoparticles on Algae: Adsorption, Distribution, Ecotoxicity and Fate. Appl. Sci. 2019, 9, 1534. [Google Scholar] [CrossRef] [Green Version]
- Van Hoecke, K.; De Schamphelaere, K.A.C.; Van der Meeren, P.; Lucas, S.; Janssen, C.R. Ecotoxicity of Silica Nanoparticles to the Green Alga Pseudokirchneriella Subcapitata: Importance of Surface Area. Environ. Toxicol. Chem. 2008, 27, 1948. [Google Scholar] [CrossRef] [PubMed]
- Van Hoecke, K.; Quik, J.T.K.; Mankiewicz-Boczek, J.; De Schamphelaere, K.A.C.; Elsaesser, A.; Van Der Meeren, P.; Barnes, C.; Mckerr, G.; Howard, C.V.; Van De Meent, D.; et al. Fate and effects of CeO2 nanoparticles in aquatic ecotoxicity tests. Environ. Sci. Technol. 2009, 43, 4537–4546. [Google Scholar] [CrossRef]
- Rastogi, A.; Zivcak, M.; Sytar, O.; Kalaji, H.M.; He, X.; Mbarki, S.; Brestic, M. Impact of metal and metal oxide nanoparticles on plant: A critical review. Front. Chem. 2017, 5, 78. [Google Scholar] [CrossRef] [Green Version]
- Tkalec, M.; Peharec Štefani, C.B.P.; Balen, B. Phytotoxicity of silver nanoparticles and defence mechanisms. Compr. Anal. Chem. 2019, 84, 145–198. [Google Scholar] [CrossRef]
- Yan, A.; Chen, Z. Impacts of silver nanoparticles on plants: A focus on the phytotoxicity and underlying mechanism. Int. J. Mol. Sci. 2019, 20, 1003. [Google Scholar] [CrossRef]
- Glenn, J.B.; White, S.A.; Klaine, S.J. Interactions of gold nanoparticles with freshwater aquatic macrophytes are size and species dependent. Environ. Toxicol. Chem. 2012, 31, 194–201. [Google Scholar] [CrossRef]
- Li, L.; Sillanpää, M.; Tuominen, M.; Lounatmaa, K.; Schultz, E. Behavior of titanium dioxide nanoparticles in Lemna minor growth test conditions. Ecotoxicol. Environ. Saf. 2013, 88, 89–94. [Google Scholar] [CrossRef]
- Ma, X.; Geiser-Lee, J.; Deng, Y.; Kolmakov, A. Interactions between engineered nanoparticles (ENPs) and plants: Phytotoxicity, uptake and accumulation. Sci. Total Environ. 2010, 408, 3053–3061. [Google Scholar] [CrossRef] [PubMed]
- Harris, A.T.; Bali, R. On the formation and extent of uptake of silver nanoparticles by live plants. J. Nanopart. Res. 2008, 10, 691–695. [Google Scholar] [CrossRef]
- Thwala, M.; Klaine, S.J.; Musee, N. Interactions of metal-based engineered nanoparticles with aquatic higher plants: A review of the state of current knowledge. Environ. Toxicol. Chem. 2016, 35, 1677–1694. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, H.-S.S.; Li, M.; Chang, F.-Y.Y.; Li, W.; Yin, L.-Y.Y. Physiological analysis of silver nanoparticles and AgNO3 toxicity to Spirodela polyrhiza. Environ. Toxicol. Chem. 2012, 31, 1880–1886. [Google Scholar] [CrossRef]
- Kumari, M.; Khan, S.S.; Pakrashi, S.; Mukherjee, A.; Chandrasekaran, N. Cytogenetic and genotoxic effects of zinc oxide nanoparticles on root cells of Allium cepa. J. Hazard. Mater. 2011, 190, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Pereira, S.P.P.; Jesus, F.; Aguiar, S.; de Oliveira, R.; Fernandes, M.; Ranville, J.; Nogueira, A.J.A. Phytotoxicity of silver nanoparticles to Lemna minor: Surface coating and exposure period-related effects. Sci. Total Environ. 2018, 618, 1389–1399. [Google Scholar] [CrossRef]
- Perreault, F.; Popovic, R.; Dewez, D. Different toxicity mechanisms between bare and polymer-coated copper oxide nanoparticles in Lemna gibba. Environ. Pollut. 2014, 185, 219–227. [Google Scholar] [CrossRef]
- Rajput, V.D.; Minkina, T.; Sushkova, S.; Mandzhieva, S.; Fedorenko, A.; Lysenko, V.; Bederska-Błaszczyk, M.; Olchowik, J.; Tsitsuashvili, V.; Chaplygin, V. Structural and Ultrastructural Changes in Nanoparticle Exposed Plants. In Nanoscience for Sustainable Agriculture; Springer International Publishing: New York, NY, USA, 2019; pp. 281–295. ISBN 9783319978529. [Google Scholar]
- Wan, J.; Wang, R.; Wang, R.; Ju, Q.; Wang, Y.; Xu, J. Comparative Physiological and Transcriptomic Analyses Reveal the Toxic Effects of ZnO Nanoparticles on Plant Growth. Environ. Sci. Technol. 2019, 53, 4235–4244. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Cao, X.; Liu, X.; Wang, Z.; Zhang, C.; White, J.C.; Xing, B. Interactions of CuO nanoparticles with the algae Chlorella pyrenoidosa: Adhesion, uptake, and toxicity. Nanotoxicology 2016, 10, 1297–1305. [Google Scholar] [CrossRef] [PubMed]
- Thwala, M.; Klaine, S.; Musee, N. Exposure Media and Nanoparticle Size Influence on the Fate, Bioaccumulation, and Toxicity of Silver Nanoparticles to Higher Plant Salvinia minima. Molecules 2021, 26, 2305. [Google Scholar] [CrossRef]
- Lehutso, R.F.; Tancu, Y.; Maity, A.; Thwala, M. Characterisation of engineered nanomaterials in nano-enabled products exhibiting priority environmental exposure. Molecules 2021, 26, 1370. [Google Scholar] [CrossRef] [PubMed]
- Slabbert, J.L. Methods for Direct Estimation of Ecological Effect potential (DEEEP); Water Research Commission: Pretoria, South Africa, 2004; ISBN 1770052410. [Google Scholar]
- Rodrigues, R.; Arenzon, A.; Raya-Rodriguez, M.T.; Fontoura, N.F. Algal density assessed by spectrophotometry: A calibration curve for the unicellular algae Pseudokirchneriella subcapitata. J. Environ. Chem. Ecotoxicol. 2011, 3, 225–228. [Google Scholar]
- Li, M.; Liu, W.; Slaveykova, V.I. Effects of mixtures of engineered nanoparticles and metallic pollutants on aquatic organisms. Environments 2020, 7, 27. [Google Scholar] [CrossRef] [Green Version]
- Naasz, S.; Altenburger, R.; Kühnel, D. Environmental mixtures of nanomaterials and chemicals: The Trojan-horse phenomenon and its relevance for ecotoxicity. Sci. Total Environ. 2018, 635, 1170–1181. [Google Scholar] [CrossRef]
- EPA Center for Computational Toxicology and Exposure. EPA’s (2020): Species Sensitivity Distribution (SSD) Toolbox. The United States Environmental Protection Agency’s Center for Computational Toxicology and Exposure Software. Available online: https://epa.figshare.com/articles/code/Species_Sensitivity_Distribution_SSD_Toolbox/11971392 (accessed on 23 October 2021).
- De Bruijn, J.; Hansen, B.; Johansson, S.; Luotamo, M.; Munn, S.; Musset, C.; Olsen, S.; Olsson, H.; Paya-Perez, A.; Pedersen, F.; et al. Technical Guidance Document on risk Assessment. Part 1. Part 2. EUR 20418 EN, JRC23785. 2002. Available online: https://publications.jrc.ec.europa.eu/repository/handle/JRC23785 (accessed on 23 October 2021).
- Hristozov, D.; Malsch, I. Hazards and Risks of engineered nanoparticles for the environment and human health. Sustainability 2009, 1, 1161–1194. [Google Scholar] [CrossRef] [Green Version]
- Sekine, R.; Moore, K.L.; Matzke, M.; Vallotton, P.; Jiang, H.; Hughes, G.M.; Kirby, J.K.; Donner, E.; Grovenor, C.R.M.; Svendsen, C.; et al. Complementary Imaging of Silver Nanoparticle Interactions with Green Algae: Dark-Field Microscopy, Electron Microscopy, and Nanoscale Secondary Ion Mass Spectrometry. ACS Nano 2017, 11, 37. [Google Scholar] [CrossRef] [Green Version]



| Sample Name | Toxicant Type | 72 h EC50 | 72 h EC50 | 24 h LC50 | 48 h LC50 | ||||
|---|---|---|---|---|---|---|---|---|---|
| P. subcapitata | S. polyrhiza | D. magna | D. magna | ||||||
| Light | Dark | Light | Dark | Light | Dark | Light | Dark | ||
| SUN1 | Zn2+ | 173.1 ± 2.5 | 177.8 ± 20 | 239.1 ± 68 | 292.1 ± 40 | 376.4 ± 16 | 301.9 ± 37 | 167.0 ± 9.1 | 109.1 ± 6.0 |
| SUN2 | PR–nTiO2 | 99.50 ± 5.1 | 93.20 ± 15 | 173.0 ± 6.6 | 189.0 ± 3.4 | 26.03 ± 5.8 | 64.59 ± 2.1 | 4.205 ± 1.9 | 7.856 ± 1.9 |
| SUN3 | PR–nTiO2 | 153.5 ± 1.4 | 157.7 ± 2.2 | 167.5 ± 22 | 161.1 ± 31 | 23.18 ± 1.0 | 14.01 ± 2.4 | 6.453 ± 0.12 | 7.243 ± 0.57 |
| CA1 | Ag+ | nd | nd | 33.94 ± 1.2 | 32.25 ± 4.0 | 15.50 ± 0.7 | 15.80 ± 3.5 | 17.9 ± 3.0 | 15.89 ± 0.36 |
| SK1 | Ag+ | 663.5 ± 39 | 2494 ± 397 | 206.2 ± 29 | 236.9 ± 34 | ||||
| SAN1 | PR–nAg | 1.315 ± 0.45 | 108.2 ± 3.1 | 5.367 ± 0.17 | 1.110 ± 0.08 | ||||
| Ag+ | 9.387 ± 0.26 | 71.74 ± 5.0 | 2.089 ± 0.65 | 1.065 ± 0.31 | |||||
| Sample Name | ENMs Type | Target Analyte | Concentration (mg/L) | ENMs Shape | ENMs Size (nm) | |
|---|---|---|---|---|---|---|
| Light Condition | Dark Condition | |||||
| SUN1 | nTiO2 + nZnO | Ti | 6.99 ± 0.06 | 8.51 ± 0.209 | elongated | 13 × 69 |
| Zn | 26.8 ± 0.39 | 27.00 ± 0.84 | angular | 34 × 30 | ||
| Zn2+ | 13.7 ± 0.42 | 13.84 ± 0.55 | ||||
| SUN2 | nTiO2 | Ti | 0.68 ± 0.03 | 0.78 ± 0.02 | angular | 40 × 21 |
| SUN3 | nTiO2 | Ti | 0.96 ± 0.02 | 0.93 ± 0.009 | angular | 30 × 21 |
| CA1 * | nTiO2 + nAg | Ti | 39.52 ± 0.82 | 30.71 ± 0.71 | angular + elongated | 8 × 34, 17 × 93 |
| Ag | 56.19 ± 3.0 | 52.38 ± 0.8 | angular | 22 × 20 | ||
| Ag+ | 39.05 ± 1.6 | 33.33 ± 6.4 | ||||
| SAN1 | nAg | Ag | 0.95 ± 0.03 | spherical | 23 × 20, 9 × 10 | |
| Ag+ | 0.82 ± 0.01 | |||||
| SK1 | nTiO2 + nAg | Ti | 5.73 ± 0.23 | angular | 62 × 51 | |
| Ag | 6.00 ± 0.4 | spherical + angular | 8 × 8 nm, 89 × 95 | |||
| Ag+ | 6.40 ± 0.4 | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lehutso, R.F.; Wesley-Smith, J.; Thwala, M. Aquatic Toxicity Effects and Risk Assessment of ‘Form Specific’ Product-Released Engineered Nanomaterials. Int. J. Mol. Sci. 2021, 22, 12468. https://doi.org/10.3390/ijms222212468
Lehutso RF, Wesley-Smith J, Thwala M. Aquatic Toxicity Effects and Risk Assessment of ‘Form Specific’ Product-Released Engineered Nanomaterials. International Journal of Molecular Sciences. 2021; 22(22):12468. https://doi.org/10.3390/ijms222212468
Chicago/Turabian StyleLehutso, Raisibe Florence, James Wesley-Smith, and Melusi Thwala. 2021. "Aquatic Toxicity Effects and Risk Assessment of ‘Form Specific’ Product-Released Engineered Nanomaterials" International Journal of Molecular Sciences 22, no. 22: 12468. https://doi.org/10.3390/ijms222212468
APA StyleLehutso, R. F., Wesley-Smith, J., & Thwala, M. (2021). Aquatic Toxicity Effects and Risk Assessment of ‘Form Specific’ Product-Released Engineered Nanomaterials. International Journal of Molecular Sciences, 22(22), 12468. https://doi.org/10.3390/ijms222212468






