Silver Nanoparticles Modulate the Epithelial-to-Mesenchymal Transition in Estrogen-Dependent Breast Cancer Cells In Vitro
Abstract
1. Introduction
2. Results
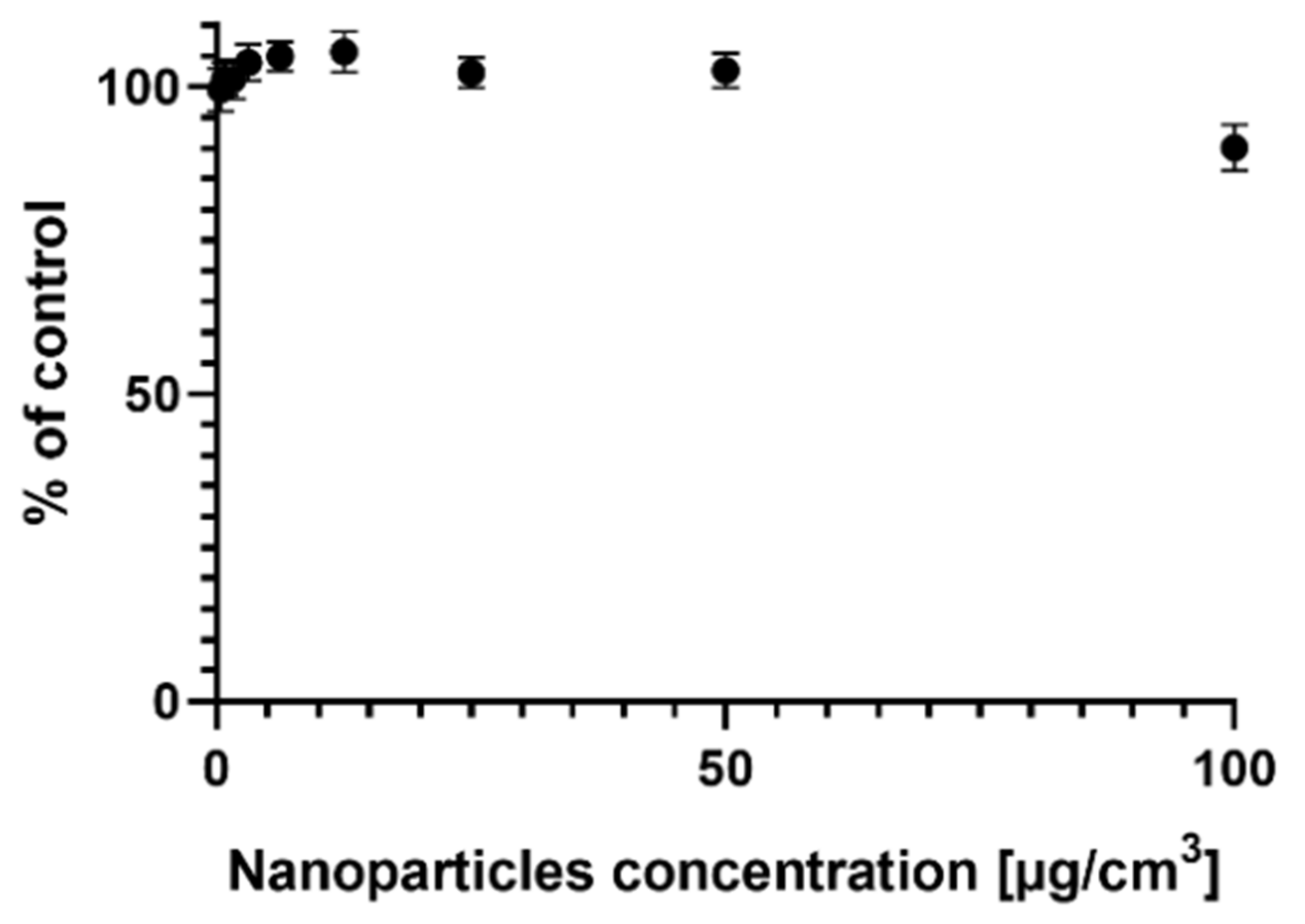
2.1. Survival of the MCF-7 Cells Treated with AgNPs
2.2. Assessment of the Migration Capacity of MCF-7 Cells via Wound Healing Test
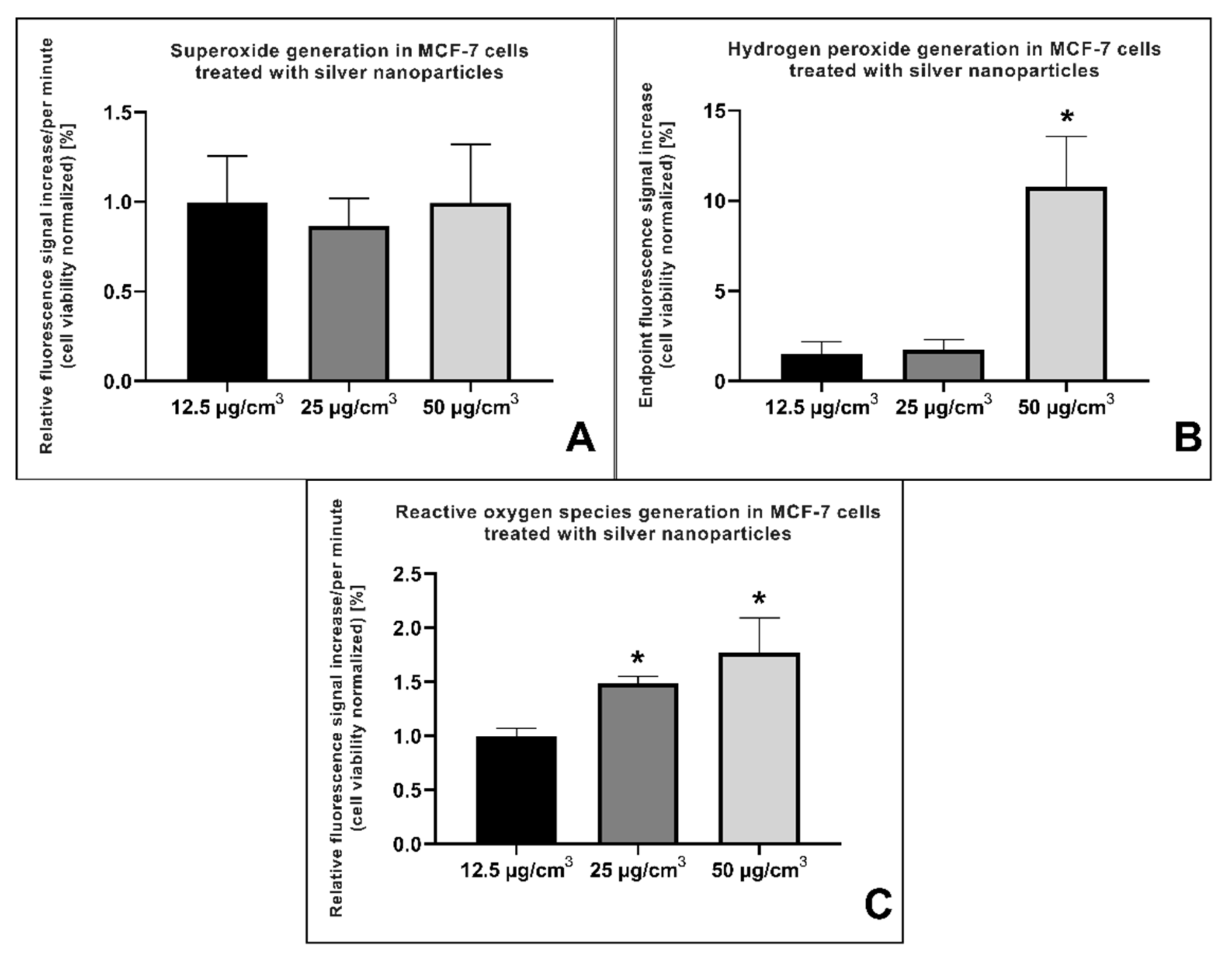
2.3. Reactive Oxygen Species (ROS) Generation
2.4. Cell Cycle Measurement
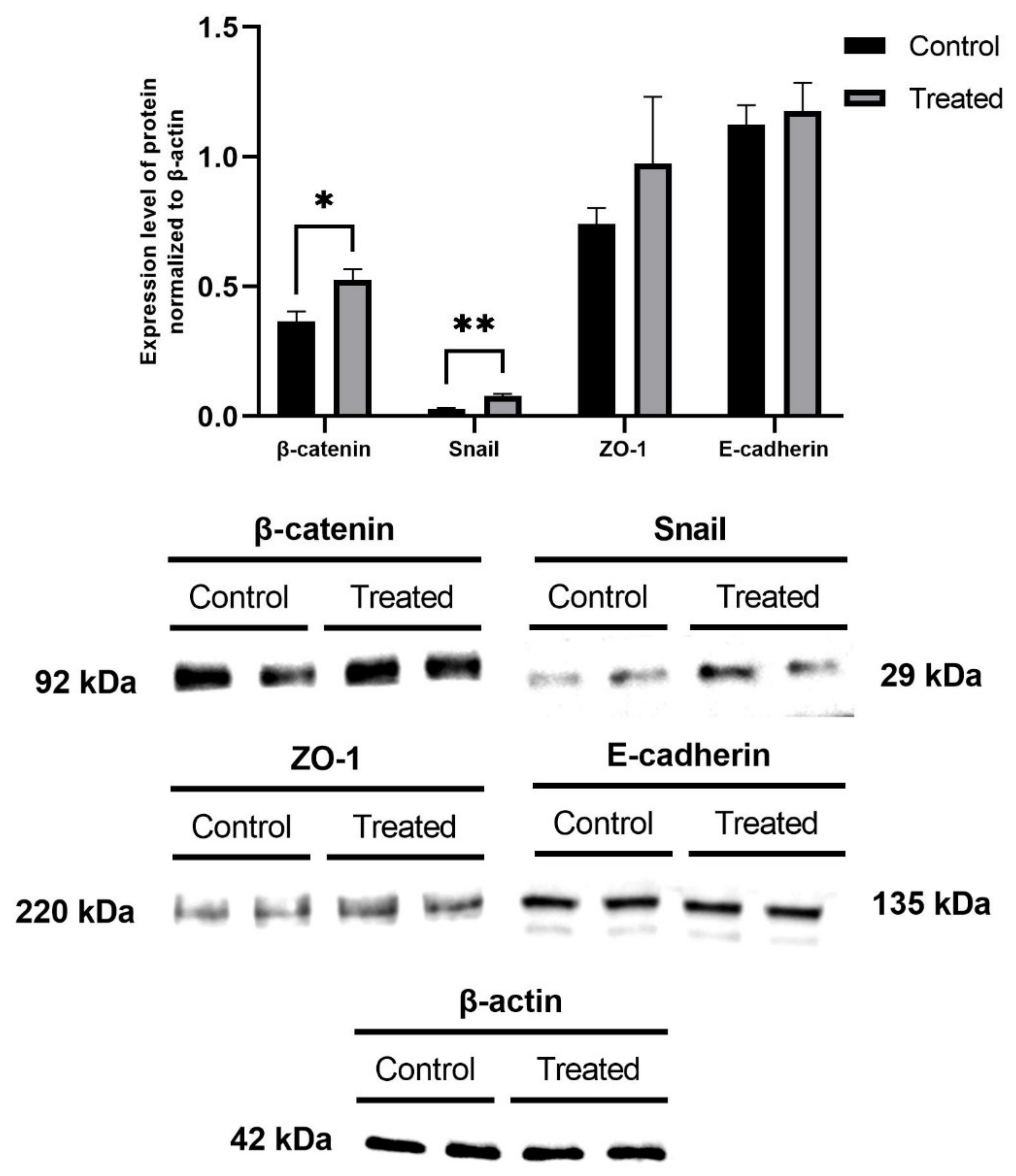
2.5. Measurement of EMT Marker Proteins and MTA Protein Levels
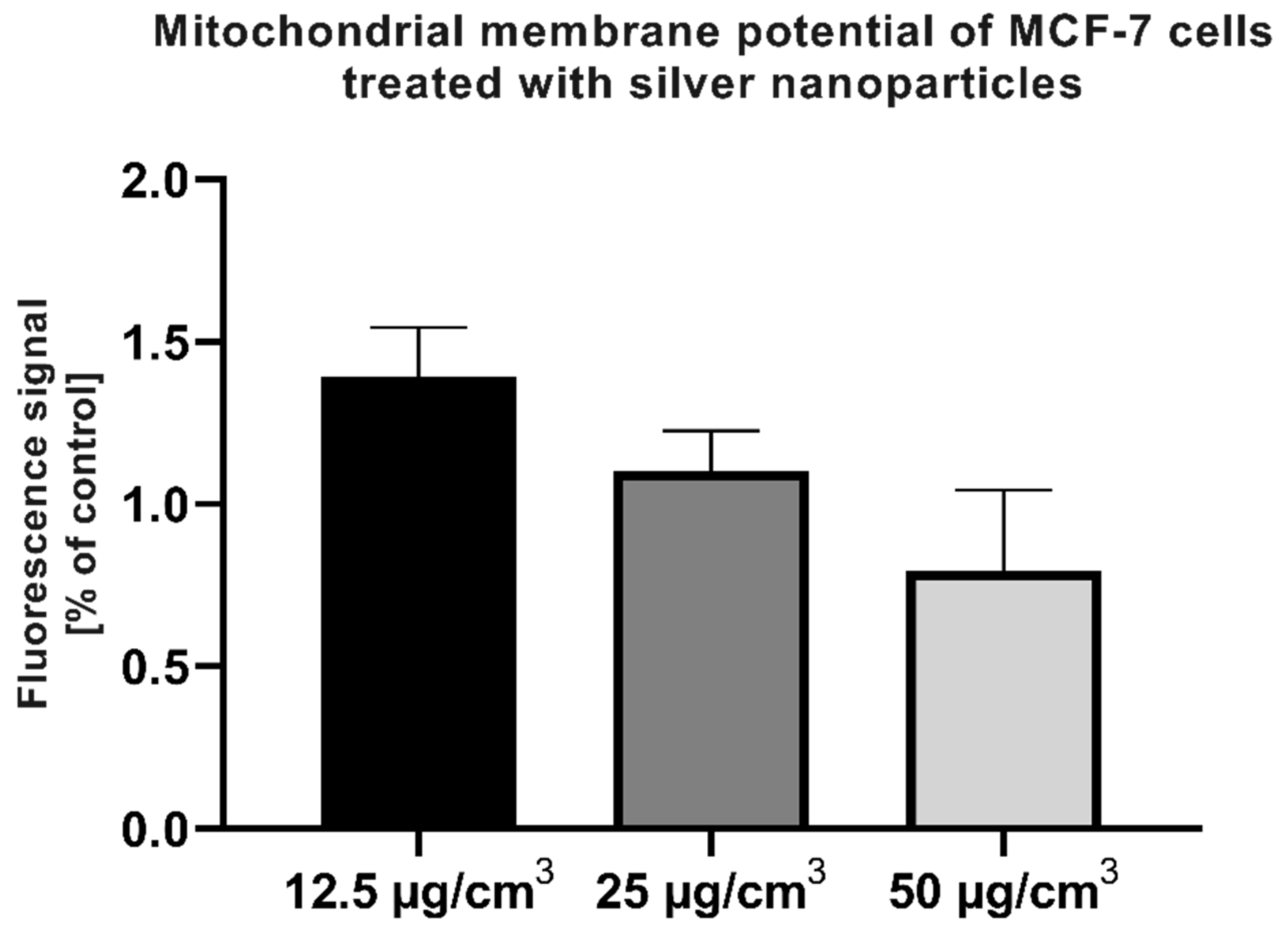
2.6. Assessment of Mitochondrial Membrane Potential
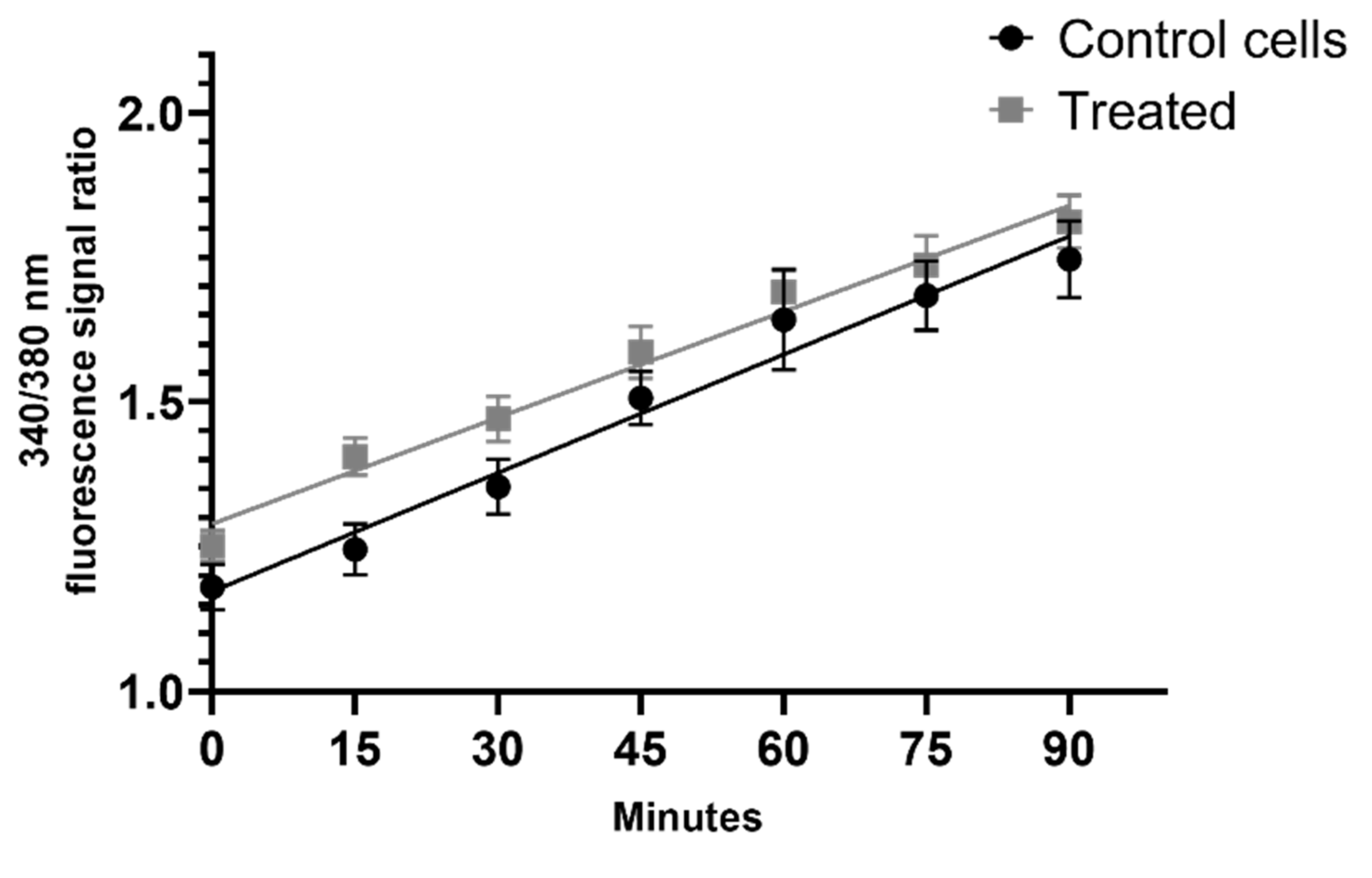
2.7. Measurement of the Intracellular Calcium Level after the Addition of AgNPs
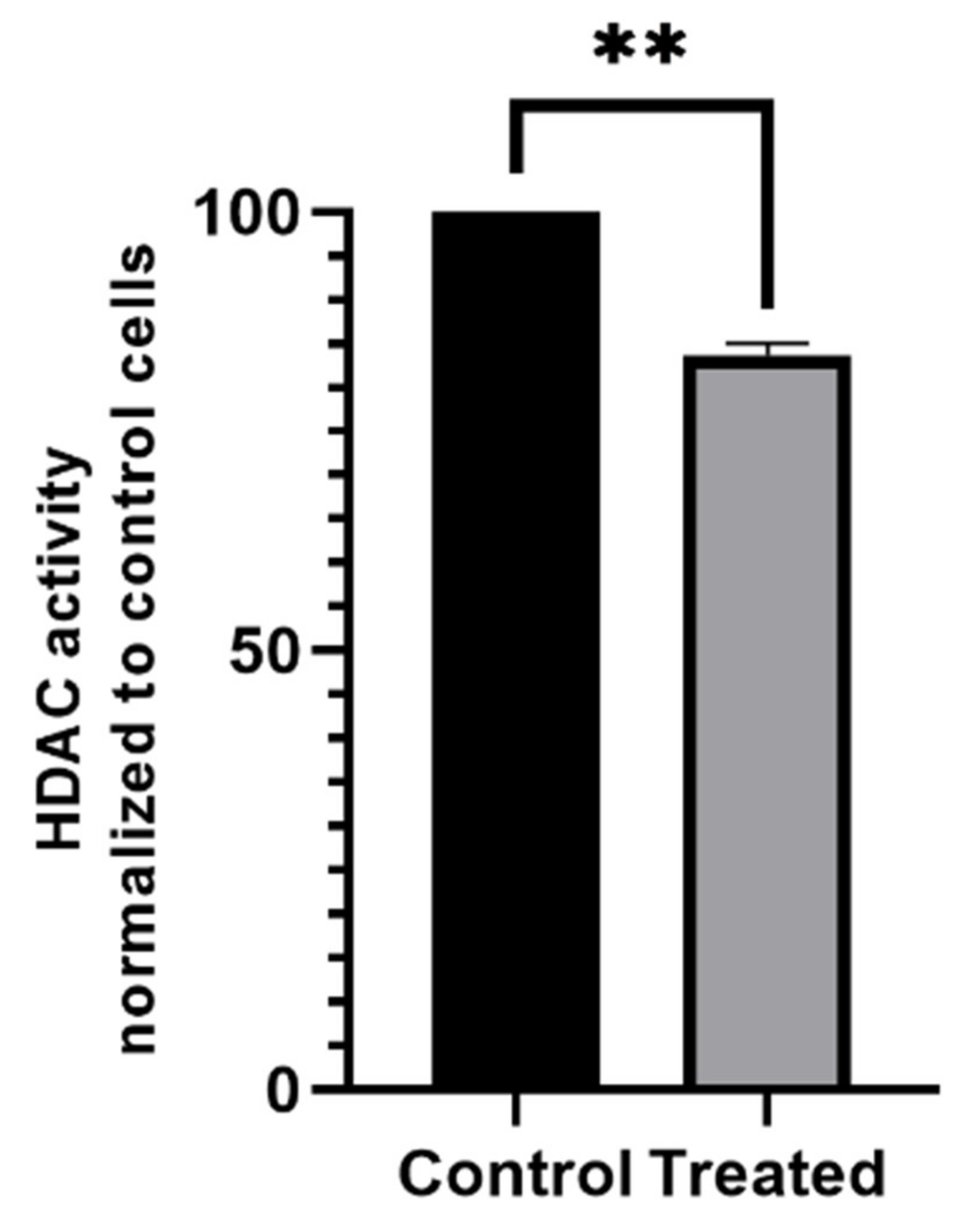
2.8. Histone Deacetylase (HDAC) Activity
3. Discussion
4. Materials and Methods
4.1. Preparation of the AgNP Solution
4.2. Cell Culture
4.3. Determination of Cell Viability Using the SRB Method
4.4. Wound Healing Test
4.5. Measurement of Free Radical and Reactive Oxygen Species Generation
4.6. Cell Cycle Analysis Using Flow Cytometry
4.7. Determination of the Expression Level of Selected EMT Marker Proteins and the MTA3 Protein Using the Western Blot Method
4.8. Mitochondrial Membrane Potential Measurement
4.9. Measurement of the Intracellular Calcium Flux in MCF-7 Cells Treated with AgNPs
4.10. Determination of the Histone Deacetylase (HDAC) Activity
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sousa, B.; Moser, E.; Cardoso, F. An Update on Male Breast Cancer and Future Directions for Research and Treatment. Eur. J. Pharmacol. 2013, 717, 71–83. [Google Scholar] [CrossRef]
- Youlden, D.R.; Cramb, S.M.; Dunn, N.A.M.; Muller, J.M.; Pyke, C.M.; Baade, P.D. The Descriptive Epidemiology of Female Breast Cancer: An International Comparison of Screening, Incidence, Survival and Mortality. Cancer Epidemiol. 2012, 36, 237–248. [Google Scholar] [CrossRef]
- Stuckey, A. Breast Cancer. Clin. Obstet. Gynecol. 2011, 54, 96–102. [Google Scholar] [CrossRef]
- Collaborative Group on Hormonal Factors in Breast Cancer. Breast Cancer and Hormonal Contraceptives: Collaborative Reanalysis of Individual Data on 53,297 Women with Breast Cancer and 100,239 Women without Breast Cancer from 54 Epidemiological Studies. Int. J. Gynecol. Obstet. 1996, 55, 320–321. [Google Scholar] [CrossRef]
- Al Zaman, A.; Mughal, S.; Al Zaman, Y.; Al Zaman, E. Correlation between Hormone Receptor Status and Age, and Its Prognostic Implications in Breast Cancer Patients in Bahrain. Saudi Med. J. 2016, 37, 37–42. [Google Scholar] [CrossRef]
- Yang, X.R.; Chang-Claude, J.; Goode, E.L.; Couch, F.J.; Nevanlinna, H.; Milne, R.L.; Gaudet, M.; Schmidt, M.K.; Broeks, A.; Cox, A.; et al. Associations of Breast Cancer Risk Factors With Tumor Subtypes: A Pooled Analysis From the Breast Cancer Association Consortium Studies. J. Natl. Cancer Inst. 2011, 103, 250–263. [Google Scholar] [CrossRef] [PubMed]
- Thiery, J.P.; Acloque, H.; Huang, R.Y.J.; Nieto, M.A. Epithelial-Mesenchymal Transitions in Development and Disease. Cell 2009, 139, 871–890. [Google Scholar] [CrossRef]
- Kalluri, R.; Neilson, E.G. Epithelial-Mesenchymal Transition and Its Implications for Fibrosis. J. Clin. Investig. 2003, 112, 1776–1784. [Google Scholar] [CrossRef] [PubMed]
- Peinado, H.; Olmeda, D.; Cano, A. Snail, ZEB and BHLH Factors in Tumour Progression: An Alliance against the Epithelial Phenotype? Nat. Rev. Cancer 2007, 7, 415–428. [Google Scholar] [CrossRef] [PubMed]
- Manke, A.; Wang, L.; Rojanasakul, Y. Mechanisms of Nanoparticle-Induced Oxidative Stress and Toxicity. BioMed Res. Int. 2013, 2013, 942916. [Google Scholar] [CrossRef]
- AshaRani, P.V.; Low Kah Mun, G.; Hande, M.P.; Valiyaveettil, S. Cytotoxicity and Genotoxicity of Silver Nanoparticles in Human Cells. ACS Nano 2009, 3, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Kuiper, G.G.J.M.; Carlsson, B.; Grandien, K.; Enmark, E.; Haäggblad, J.; Nilsson, S.; Gustafsson, J.-A. Comparison of the Ligand Binding Specificity and Transcript Tissue Distribution of Estrogen Receptors α and β. Endocrinology 1997, 138, 863–870. [Google Scholar] [CrossRef] [PubMed]
- Julin, B.; Wolk, A.; Bergkvist, L.; Bottai, M.; Åkesson, A. Dietary Cadmium Exposure and Risk of Postmenopausal Breast Cancer: A Population-Based Prospective Cohort Study. Cancer Res. 2012, 72, 1459–1466. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Q.; Xu, L.; Ma, J.; Wei, B.; An, Z.; Wu, W.; Liu, S. Silver Nanoparticles Compromise the Development of Mouse Pubertal Mammary Glands through Disrupting Internal Estrogen Signaling. Nanotoxicology 2020, 14, 740–756. [Google Scholar] [CrossRef]
- Ernst, M.; Schmid, C.; Froesch, E.R. Phenol Red Mimics Biological Actions of Estradiol: Enhancement of Osteoblast Proliferation In Vitro and of Type I Collagen Gene Expression in Bone and Uterus of Rats In Vivo. J. Steroid Biochem. 1989, 33, 907–914. [Google Scholar] [CrossRef]
- Kruszewski, M.; Grądzka, I.; Bartłomiejczyk, T.; Chwastowska, J.; Sommer, S.; Grzelak, A.; Zuberek, M.; Lankoff, A.; Dusinska, M.; Wojewódzka, M. Oxidative DNA Damage Corresponds to the Long Term Survival of Human Cells Treated with Silver Nanoparticles. Toxicol. Lett. 2013, 219, 151–159. [Google Scholar] [CrossRef]
- Khan, M.S.; Alomari, A.; Tabrez, S.; Hassan, I.; Wahab, R.; Bhat, S.A.; Alafaleq, N.O.; Altwaijry, N.; Shaik, G.M.; Zaidi, S.K.; et al. Anticancer Potential of Biogenic Silver Nanoparticles: A Mechanistic Study. Pharmaceutics 2021, 13, 707. [Google Scholar] [CrossRef]
- Roszak, J.; Smok-Pieniążek, A.; Spryszyńska, S.; Kowalczyk, K.; Domeradzka-Gajda, K.; Świercz, R.; Grobelny, J.; Tomaszewska, E.; Ranoszek-Soliwoda, K.; Celichowski, G.; et al. Cytotoxic Effects in Transformed and Non-Transformed Human Breast Cell Lines after Exposure to Silver Nanoparticles in Combination with Selected Aluminium Compounds, Parabens or Phthalates. J. Hazard. Mater. 2020, 392, 122442. [Google Scholar] [CrossRef]
- Cronholm, P.; Karlsson, H.L.; Hedberg, J.; Lowe, T.A.; Winnberg, L.; Elihn, K.; Wallinder, I.O.; Möller, L. Intracellular Uptake and Toxicity of Ag and CuO Nanoparticles: A Comparison Between Nanoparticles and Their Corresponding Metal Ions. Small 2013, 9, 970–982. [Google Scholar] [CrossRef] [PubMed]
- Sangour, M.H.; Ali, I.M.; Atwan, Z.W.; Al, A.A. Effect of Ag Nanoparticles on Viability of MCF-7 and Vero Cell Lines and Gene Expression of Apoptotic Genes. Egypt. J. Med. Hum. Genet. 2021, 22, 9. [Google Scholar] [CrossRef]
- Jaje, J.; Wolcott, H.N.; Fadugba, O.; Cripps, D.; Yang, A.J.; Mather, I.H.; Thorpe, C. A Flavin-Dependent Sulfhydryl Oxidase in Bovine Milk. Biochemistry 2007, 46, 13031–13040. [Google Scholar] [CrossRef][Green Version]
- Chen, J.L.; Steele, T.W.J.; Stuckey, D.C. Metabolic Reduction of Resazurin; Location within the Cell for Cytotoxicity Assays. Biotechnol. Bioeng. 2018, 115, 351–358. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, J.; Wilson, I.; Orton, T.; Pognan, F. Investigation of the Alamar Blue (Resazurin) Fluorescent Dye for the Assessment of Mammalian Cell Cytotoxicity. Eur. J. Biochem. 2000, 267, 5421–5426. [Google Scholar] [CrossRef] [PubMed]
- Bernas, T.; Dobrucki, J.W. The Role of Plasma Membrane in Bioreduction of Two Tetrazolium Salts, MTT, and CTC. Arch. Biochem. Biophys. 2000, 380, 108–116. [Google Scholar] [CrossRef]
- Rodríguez-Razón, C.; Yañez-Sánchez, I.; Ramos-Santillan, V.O.; Velásquez-Ordóñez, C.; Gutiérrez-Rubio, S.A.; García-García, M.; López-Roa, R.; Sánchez-Hernández, P.E.; Daneri-Navarro, A.; García-Iglesias, T. Adhesion, Proliferation, and Apoptosis in Different Molecular Portraits of Breast Cancer Treated with Silver Nanoparticles and Its Pathway-Network Analysis. Int. J. Nanomed. 2018, 13, 1081–1095. [Google Scholar] [CrossRef]
- Sathishkumar, P.; Vennila, K.; Jayakumar, R.; Yusoff, A.R.M.; Hadibarata, T.; Palvannan, T. Phyto-Synthesis of Silver Nanoparticles Using Alternanthera Tenella Leaf Extract: An Effective Inhibitor for the Migration of Human Breast Adenocarcinoma (MCF-7) Cells. Bioprocess Biosyst. Eng. 2016, 39, 651–659. [Google Scholar] [CrossRef] [PubMed]
- Fehaid, A.; Taniguchi, A. Size-Dependent Effect of Silver Nanoparticles on the Tumor Necrosis Factor α-Induced Dna Damage Response. Int. J. Mol. Sci. 2019, 20, 1038. [Google Scholar] [CrossRef]
- Gliga, A.R.; Skoglund, S.; Odnevall Wallinder, I.; Fadeel, B.; Karlsson, H.L. Size-Dependent Cytotoxicity of Silver Nanoparticles in Human Lung Cells: The Role of Cellular Uptake, Agglomeration and Ag Release. Part. Fibre Toxicol. 2014, 11, 11. [Google Scholar] [CrossRef]
- Perde-Schrepler, M.; Florea, A.; Brie, I.; Virag, P.; Fischer-Fodor, E.; Vâlcan, A.; Gurzǎu, E.; Lisencu, C.; Maniu, A. Size-Dependent Cytotoxicity and Genotoxicity of Silver Nanoparticles in Cochlear Cells In Vitro. J. Nanomater. 2019, 2019, 6090259. [Google Scholar] [CrossRef]
- AshaRani, P.; Hande, M.P.; Valiyaveettil, S. Anti-Proliferative Activity of Silver Nanoparticles. BMC Cell Biol. 2009, 10, 65. [Google Scholar] [CrossRef]
- Li, Z.-W.; Li, C.-W.; Wang, Q.; Shi, S.-J.; Hu, M.; Zhang, Q.; Cui, H.-H.; Sun, J.-B.; Zhou, M.; Wu, G.-L.; et al. The Cellular and Molecular Mechanisms Underlying Silver Nanoparticle/Chitosan Oligosaccharide/Poly(Vinyl Alcohol) Nanofiber-Mediated Wound Healing. J. Biomed. Nanotechnol. 2017, 13, 17–34. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.B.; Dananjaya, S.H.S.; Nikapitiya, C.; Park, B.K.; Gooneratne, R.; Kim, T.-Y.; Lee, J.; Kim, C.-H.; de Zoysa, M. Silver Nanoparticles Enhance Wound Healing in Zebrafish (Danio Rerio). Fish Shellfish. Immunol. 2017, 68, 536–545. [Google Scholar] [CrossRef] [PubMed]
- Ullah, I.; Khalil, A.T.; Ali, M.; Iqbal, J.; Ali, W.; Alarifi, S.; Shinwari, Z.K. Green-Synthesized Silver Nanoparticles Induced Apoptotic Cell Death in MCF-7 Breast Cancer Cells by Generating Reactive Oxygen Species and Activating Caspase 3 and 9 Enzyme Activities. Oxidative Med. Cell. Longev. 2020, 2020, 1215395. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-F.; Liu, Z.-G.; Shen, W.; Gurunathan, S. Silver Nanoparticles: Synthesis, Characterization, Properties, Applications, and Therapeutic Approaches. Int. J. Mol. Sci. 2016, 17, 1534. [Google Scholar] [CrossRef]
- Oparka, M.; Walczak, J.; Malinska, D.; van Oppen, L.M.P.E.; Szczepanowska, J.; Koopman, W.J.H.; Wieckowski, M.R. Quantifying ROS levels using CM-H2DCFDA and HyPer. Methods 2016, 109, 3–11. [Google Scholar] [CrossRef]
- Gorowiec, M.R.; Borthwick, L.A.; Parker, S.M.; Kirby, J.A.; Saretzki, G.C.; Fisher, A.J. Free Radical Generation Induces Epithelial-to-Mesenchymal Transition in Lung Epithelium via a TGF-Β1-Dependent Mechanism. Free. Radic. Biol. Med. 2012, 52, 1024–1032. [Google Scholar] [CrossRef]
- Li, S.; Luo, W. Matrix Metalloproteinase 2 Contributes to Aggressive Phenotype, Epithelial-Mesenchymal Transition and Poor Outcome in Nasopharyngeal Carcinoma. OncoTargets Ther. 2019, 12, 5701. [Google Scholar] [CrossRef]
- Das, V.; Bhattacharya, S.; Chikkaputtaiah, C.; Hazra, S.; Pal, M. The Basics of Epithelial–Mesenchymal Transition (EMT): A Study from a Structure, Dynamics, and Functional Perspective. J. Cell. Physiol. 2019, 234, 14535–14555. [Google Scholar] [CrossRef]
- Scheau, C.; Badarau, I.A.; Costache, R.; Caruntu, C.; Mihai, G.L.; Didilescu, A.C.; Constantin, C.; Neagu, M. The Role of Matrix Metalloproteinases in the Epithelial-Mesenchymal Transition of Hepatocellular Carcinoma. Anal. Cell. Pathol. 2019, 2019, 9423907. [Google Scholar] [CrossRef]
- Wan, T.; Zhang, T.; Si, X.; Zhou, Y. Overexpression of EMT-Inducing Transcription Factors as a Potential Poor Prognostic Factor for Hepatocellular Carcinoma in Asian Populations: A Meta-Analysis. Oncotarget 2017, 8, 59500. [Google Scholar] [CrossRef]
- Miyoshi, A.; Kitajima, Y.; Sumi, K.; Sato, K.; Hagiwara, A.; Koga, Y.; Miyazaki, K. Snail and SIP1 Increase Cancer Invasion by Upregulating MMP Family in Hepatocellular Carcinoma Cells. Br. J. Cancer 2004, 90, 1265–1273. [Google Scholar] [CrossRef] [PubMed]
- Azhar, N.A.; Ghozali, S.Z.; Abu Bakar, S.A.; Lim, V.; Ahmad, N.H. Suppressing Growth, Migration, and Invasion of Human Hepatocellular Carcinoma HepG2 Cells by Catharanthus Roseus-silver Nanoparticles. Toxicol. In Vitro 2020, 67, 104910. [Google Scholar] [CrossRef] [PubMed]
- Agraval, H.; Yadav, U.C.S. MMP-2 and MMP-9 Mediate Cigarette Smoke Extract-Induced Epithelial-Mesenchymal Transition in Airway Epithelial Cells via EGFR/Akt/GSK3β/β-Catenin Pathway: Amelioration by Fisetin. Chem.-Biol. Interact. 2019, 314, 108846. [Google Scholar] [CrossRef]
- Jiang, J.; Wang, K.; Chen, Y.; Chen, H.; Nice, E.C.; Huang, C. Redox Regulation in Tumor Cell Epithelial–Mesenchymal Transition: Molecular Basis and Therapeutic Strategy. Signal Transduct. Target. Ther. 2017, 2, 17036. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Weinberg, R.A. Epithelial–Mesenchymal Plasticity: A Central Regulator of Cancer Progression. Trends Cell Biol. 2015, 25, 675–686. [Google Scholar] [CrossRef]
- Zhu, X.; Huang, S.; Zeng, L.; Ma, J.; Sun, S.; Zeng, F.; Kong, F.; Cheng, X. HMOX-1 Inhibits TGF-β-Induced Epithelial-Mesenchymal Transition in the MCF-7 Breast Cancer Cell Line. Int. J. Mol. Med. 2017, 40, 411–417. [Google Scholar] [CrossRef]
- Sun, Y.; Zhou, Q.-M.; Lu, Y.-Y.; Zhang, H.; Chen, Q.-L.; Zhao, M.; Su, S.-B. Resveratrol Inhibits the Migration and Metastasis of MDA-MB-231 Human Breast Cancer by Reversing TGF-Β1-Induced Epithelial-Mesenchymal Transition. Molecules 2019, 24, 1131. [Google Scholar] [CrossRef]
- Tam, W.L.; Weinberg, R.A. The Epigenetics of Epithelial-Mesenchymal Plasticity in Cancer. Nat. Med. 2013, 19, 1438–1449. [Google Scholar] [CrossRef]
- Kessenbrock, K.; Plaks, V.; Werb, Z. Matrix Metalloproteinases: Regulators of the Tumor Microenvironment. Cell 2010, 141, 52–67. [Google Scholar] [CrossRef]
- Ghahhari, N.M.; Babashah, S. Interplay between MicroRNAs and WNT/β-Catenin Signalling Pathway Regulates Epithelial–Mesenchymal Transition in Cancer. Eur. J. Cancer 2015, 51, 1638–1649. [Google Scholar] [CrossRef]
- Min, C.; Eddy, S.F.; Sherr, D.H.; Sonenshein, G.E. NF-ΚB and Epithelial to Mesenchymal Transition of Cancer. J. Cell. Biochem. 2008, 104, 733–744. [Google Scholar] [CrossRef]
- Cichon, M.A.; Radisky, D.C. ROS-Induced Epithelial-Mesenchymal Transition in Mammary Epithelial Cells Is Mediated by NF-ΚB-Dependent Activation of Snail. Oncotarget 2014, 5, 2827. [Google Scholar] [CrossRef]
- Naber, H.P.H.; Drabsch, Y.; Snaar-Jagalska, B.E.; ten Dijke, P.; van Laar, T. Snail and Slug, Key Regulators of TGF-β-Induced EMT, Are Sufficient for the Induction of Single-Cell Invasion. Biochem. Biophys. Res. Commun. 2013, 435, 58–63. [Google Scholar] [CrossRef]
- Overstreet, J.M.; Samarakoon, R.; Meldrum, K.K.; Higgins, P.J. Redox Control of P53 in the Transcriptional Regulation of TGF-Β1 Target Genes through SMAD Cooperativity. Cell. Signal. 2014, 26, 1427–1436. [Google Scholar] [CrossRef]
- Jaffer, O.A.; Carter, A.B.; Sanders, P.N.; Dibbern, M.E.; Winters, C.J.; Murthy, S.; Ryan, A.J.; Rokita, A.G.; Prasad, A.M.; Zabner, J.; et al. Mitochondrial-Targeted Antioxidant Therapy Decreases Transforming Growth Factor-β–Mediated Collagen Production in a Murine Asthma Model. Am. J. Respir. Cell Mol. Biol. 2015, 52, 106–115. [Google Scholar] [CrossRef]
- Liu, R.-M.; Desai, L.P. Reciprocal Regulation of TGF-β and Reactive Oxygen Species: A Perverse Cycle for Fibrosis. Redox Biol. 2015, 6, 565–577. [Google Scholar] [CrossRef]
- Pociask, D.A.; Sime, P.J.; Brody, A.R. Asbestos-Derived Reactive Oxygen Species Activate TGF-Β1. Lab. Investig. 2004, 84, 1013–1023. [Google Scholar] [CrossRef] [PubMed]
- Yoon, Y.-S.; Lee, J.-H.; Hwang, S.-C.; Choi, K.S.; Yoon, G. TGF Β1 Induces Prolonged Mitochondrial ROS Generation through Decreased Complex IV Activity with Senescent Arrest in Mv1Lu Cells. Oncogene 2005, 24, 1895–1903. [Google Scholar] [CrossRef] [PubMed]
- Martin, M.E.; Reaves, D.K.; Jeffcoat, B.; Enders, J.R.; Costantini, L.M.; Yeyeodu, S.T.; Botta, D.; Kavanagh, T.J.; Fleming, J.M. Silver Nanoparticles Alter Epithelial Basement Membrane Integrity, Cell Adhesion Molecule Expression, and TGF-Β1 Secretion. Nanomed. Nanotechnol. Biol. Med. 2019, 21, 102070. [Google Scholar] [CrossRef] [PubMed]
- Glozak, M.A.; Seto, E. Histone Deacetylases and Cancer. Oncogene 2007, 26, 5420–5432. [Google Scholar] [CrossRef] [PubMed]
- Wawruszak, A.; Kalafut, J.; Okon, E.; Czapinski, J.; Halasa, M.; Przybyszewska, A.; Miziak, P.; Okla, K.; Rivero-Muller, A.; Stepulak, A. Histone Deacetylase Inhibitors and Phenotypical Transformation of Cancer Cells. Cancers 2019, 11, 148. [Google Scholar] [CrossRef]
- Yao, D.; Dai, C.; Peng, S. Mechanism of the Mesenchymal–Epithelial Transition and Its Relationship with Metastatic Tumor Formation. Mol. Cancer Res. 2011, 9, 1608–1620. [Google Scholar] [CrossRef]
- Ji, M.; Lee, E.J.; Kim, K.B.; Kim, Y.; Sung, R.; Lee, S.-J.; Kim, D.S.; Park, S.M. HDAC Inhibitors Induce Epithelial-Mesenchymal Transition in Colon Carcinoma Cells. Oncol. Rep. 2015, 33, 2299–2308. [Google Scholar] [CrossRef]
- Sakamoto, T.; Kobayashi, S.; Yamada, D.; Nagano, H.; Tomokuni, A.; Tomimaru, Y.; Noda, T.; Gotoh, K.; Asaoka, T.; Wada, H.; et al. A Histone Deacetylase Inhibitor Suppresses Epithelial-Mesenchymal Transition and Attenuates Chemoresistance in Biliary Tract Cancer. PLoS ONE 2016, 11, e0145985. [Google Scholar] [CrossRef]
- Rhodes, L.V.; Tate, C.R.; Segar, H.C.; Burks, H.E.; Phamduy, T.B.; Hoang, V.; Elliott, S.; Gilliam, D.; Pounder, F.N.; Anbalagan, M.; et al. Suppression of Triple-Negative Breast Cancer Metastasis by Pan-DAC Inhibitor Panobinostat via Inhibition of ZEB Family of EMT Master Regulators. Breast Cancer Res. Treat. 2014, 145, 593–604. [Google Scholar] [CrossRef]
- Srivastava, R.K.; Kurzrock, R.; Shankar, S. MS-275 Sensitizes TRAIL-Resistant Breast Cancer Cells, Inhibits Angiogenesis and Metastasis, and Reverses Epithelial-Mesenchymal Transition In Vivo. Mol. Cancer Ther. 2010, 9, 3254–3266. [Google Scholar] [CrossRef]
- Shah, P.; Gau, Y.; Sabnis, G. Histone Deacetylase Inhibitor Entinostat Reverses Epithelial to Mesenchymal Transition of Breast Cancer Cells by Reversing the Repression of E-Cadherin. Breast Cancer Res. Treat. 2014, 143, 99–111. [Google Scholar] [CrossRef]
- Debeb, B.G.; Lacerda, L.; Xu, W.; Larson, R.; Solley, T.; Atkinson, R.; Sulman, E.P.; Ueno, N.T.; Krishnamurthy, S.; Reuben, J.M.; et al. Histone Deacetylase Inhibitors Stimulate Dedifferentiation of Human Breast Cancer Cells Through WNT/β-Catenin Signaling. Stem Cells 2012, 30, 2366–2377. [Google Scholar] [CrossRef] [PubMed]
- Cooper, R.J.; Menking-Colby, M.N.; Humphrey, K.A.; Victory, J.H.; Kipps, D.W.; Spitzer, N. Involvement of β-Catenin in Cytoskeleton Disruption Following Adult Neural Stem Cell Exposure to Low-Level Silver Nanoparticles. Neurotoxicology 2019, 71, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Mylavarapu, S.; Kumar, H.; Kumari, S.; Sravanthi, L.S.; Jain, M.; Basu, A.; Biswas, M.; Mylavarapu, S.V.S.; Das, A.; Roy, M. Activation of Epithelial-Mesenchymal Transition and Altered β-Catenin Signaling in a Novel Indian Colorectal Carcinoma Cell Line. Front. Oncol. 2019, 9, 54. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shi, J.; Chai, K.; Ying, X.; Zhou, B. The Role of Snail in EMT and Tumorigenesis. Curr. Cancer Drug Targets 2013, 13, 963–972. [Google Scholar] [CrossRef]
- Gavert, N.; Ben-Ze’ev, A. Epithelial–Mesenchymal Transition and the Invasive Potential of Tumors. Trends Mol. Med. 2008, 14, 199–209. [Google Scholar] [CrossRef]
- Vincent, T.; Neve, E.P.A.; Johnson, J.R.; Kukalev, A.; Rojo, F.; Albanell, J.; Pietras, K.; Virtanen, I.; Philipson, L.; Leopold, P.L.; et al. A SNAIL1–SMAD3/4 Transcriptional Repressor Complex Promotes TGF-β Mediated Epithelial–Mesenchymal Transition. Nat. Cell Biol. 2009, 11, 943–950. [Google Scholar] [CrossRef] [PubMed]
- Yook, J.I.; Li, X.-Y.; Ota, I.; Hu, C.; Kim, H.S.; Kim, N.H.; Cha, S.Y.; Ryu, J.K.; Choi, Y.J.; Kim, J.; et al. A Wnt–Axin2–GSK3β Cascade Regulates Snail1 Activity in Breast Cancer Cells. Nat. Cell Biol. 2006, 8, 1398–1406. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Jeon, H.M.; Ju, M.K.; Kim, C.H.; Yoon, G.; Han, S.I.; Park, H.G.; Kang, H.S. Wnt/Snail Signaling Regulates Cytochrome c Oxidase and Glucose Metabolism. Cancer Res. 2012, 72, 3607–3617. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Wang, L.; Chen, Q.; Chen, C. Cytotoxic Potential of Silver Nanoparticles. Yonsei Med. J. 2014, 55, 283–291. [Google Scholar] [CrossRef]
- Bouallegui, Y.; Ben Younes, R.; Oueslati, R.; Sheehan, D. Role of Endocytotic Uptake Routes in Impacting the ROS-Related Toxicity of Silver Nanoparticles to Mytilus Galloprovincialis: A Redox Proteomic Investigation. Aquat. Toxicol. 2018, 200, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Totta, P.; Pesiri, V.; Enari, M.; Marino, M.; Acconcia, F. Clathrin Heavy Chain Interacts With Estrogen Receptor α and Modulates 17β-Estradiol Signaling. Mol. Endocrinol. 2015, 29, 739–755. [Google Scholar] [CrossRef]
- Choo, W.H.; Park, C.H.; Jung, S.E.; Moon, B.; Ahn, H.; Ryu, J.S.; Kim, K.S.; Lee, Y.H.; Yu, I.J.; Oh, S.M. Long-Term Exposures to Low Doses of Silver Nanoparticles Enhanced In Vitro Malignant Cell Transformation in Non-Tumorigenic BEAS-2B Cells. Toxicol. In Vitro 2016, 37, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Liu, X.; Li, Z.; Huang, Q.; Li, F.; Li, C.-Y. Caspase-3 Regulates the Migration, Invasion and Metastasis of Colon Cancer Cells. Int. J. Cancer 2018, 143, 921–930. [Google Scholar] [CrossRef] [PubMed]
- Dhasarathy, A.; Kajita, M.; Wade, P.A. The Transcription Factor Snail Mediates Epithelial to Mesenchymal Transitions by Repression of Estrogen Receptor-α. Mol. Endocrinol. 2007, 21, 2907–2918. [Google Scholar] [CrossRef]
- Julien-Grille, S.; Bellacosa, A.; Larue, L. MTA3, a Mi-2/NuRD Complex Subunit, Regulates an Invasive Growth Pathway in Breast Cancer. Women’s Oncol. Rev. 2004, 4, 13–15. [Google Scholar] [CrossRef]
- Zuberek, M.; Wojciechowska, D.; Krzyzanowski, D.; Meczynska-Wielgosz, S.; Kruszewski, M.; Grzelak, A. Glucose Availability Determines Silver Nanoparticles Toxicity in HepG2. J. Nanobiotechnology 2015, 13, 72. [Google Scholar] [CrossRef] [PubMed]
- Vichai, V.; Kirtikara, K. Sulforhodamine B Colorimetric Assay for Cytotoxicity Screening. Nat. Protoc. 2006, 1, 1112–1116. [Google Scholar] [CrossRef] [PubMed]
- Diaz, L.; Gomes, A.; Herrera, G.; O’Connor, J.E.; Urios, A.; Felipo, V.; Montoliu, C. Real-time cytometric assay of nitric oxide and superoxide interaction in peripheral blood monocytes: A no-wash, no-lyse kinetic method. Cytom. Part B Clin. Cytom. 2017, 92, 211–217. [Google Scholar]
- Chen, J.; Rogers, S.C.; Kavdia, M. Analysis of kinetics of dihydroethidium fluorescence with superoxide using xanthine oxidase and hypoxanthine assay. Ann. Biomed. Eng. 2013, 41, 327–337. [Google Scholar] [CrossRef]
- Pendergrass, W.; Wolf, N.; Poot, M. Efficacy of MitoTracker Green™ and CMXrosamine to measure changes in mitochondrial membrane potentials in living cells and tissues. Cytom. Part A J. Int. Soc. Anal. Cytol. 2004, 61, 162–169. [Google Scholar] [CrossRef]



| Antigen | Antibody Clone 1° (Manufacturer) | Antibody Isotype | Antibody 2° |
|---|---|---|---|
| β-catenin | D10A8 (Cell Signaling) | Rabbit IgG1 | Goat anti-rIgG1 conjugate with HRP |
| E-cadherin | 24E10 (Cell Signaling) | Rabbit IgG1 | Goat anti-rIgG1 conjugate with HRP |
| Snail | C15D3 (Cell Signaling) | Rabbit IgG1 | Goat anti-rIgG1 conjugate with HRP |
| ZO-1 | D7D12 (Cell Signaling) | Rabbit IgG1 | Goat anti-rIgG1 conjugate with HRP |
| ZEB1 | D80D3 (Cell Signaling) | Rabbit IgG1 | Goat anti-rIgG1 conjugate with HRP |
| Claudin-1 | D5H1D (Cell Signaling) | Rabbit IgG1 | Goat anti-rIgG1 conjugate with HRP |
| β-actin | AC-74 (Sigma Aldrich) | Mouse IgG1 | Rabbit anti-mIgG1 conjugate with HRP |
| MTA3 | 428C2a (Santa Cruz Biotechnology) | Mouse IgG1 | Rabbit anti-mIgG1 conjugate with HRP |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rakowski, M.; Porębski, S.; Grzelak, A. Silver Nanoparticles Modulate the Epithelial-to-Mesenchymal Transition in Estrogen-Dependent Breast Cancer Cells In Vitro. Int. J. Mol. Sci. 2021, 22, 9203. https://doi.org/10.3390/ijms22179203
Rakowski M, Porębski S, Grzelak A. Silver Nanoparticles Modulate the Epithelial-to-Mesenchymal Transition in Estrogen-Dependent Breast Cancer Cells In Vitro. International Journal of Molecular Sciences. 2021; 22(17):9203. https://doi.org/10.3390/ijms22179203
Chicago/Turabian StyleRakowski, Michał, Szymon Porębski, and Agnieszka Grzelak. 2021. "Silver Nanoparticles Modulate the Epithelial-to-Mesenchymal Transition in Estrogen-Dependent Breast Cancer Cells In Vitro" International Journal of Molecular Sciences 22, no. 17: 9203. https://doi.org/10.3390/ijms22179203
APA StyleRakowski, M., Porębski, S., & Grzelak, A. (2021). Silver Nanoparticles Modulate the Epithelial-to-Mesenchymal Transition in Estrogen-Dependent Breast Cancer Cells In Vitro. International Journal of Molecular Sciences, 22(17), 9203. https://doi.org/10.3390/ijms22179203






