Equine Chorionic Gonadotropin as an Effective FSH Replacement for In Vitro Ovine Follicle and Oocyte Development
Abstract
1. Introduction
2. Results
2.1. eCG Dose Effects on Follicular and Oocyte In Vitro Growth Cultured in FBS-Supplemented Medium
2.1.1. Follicle Growth Parameters in FBS-Supplemented Medium
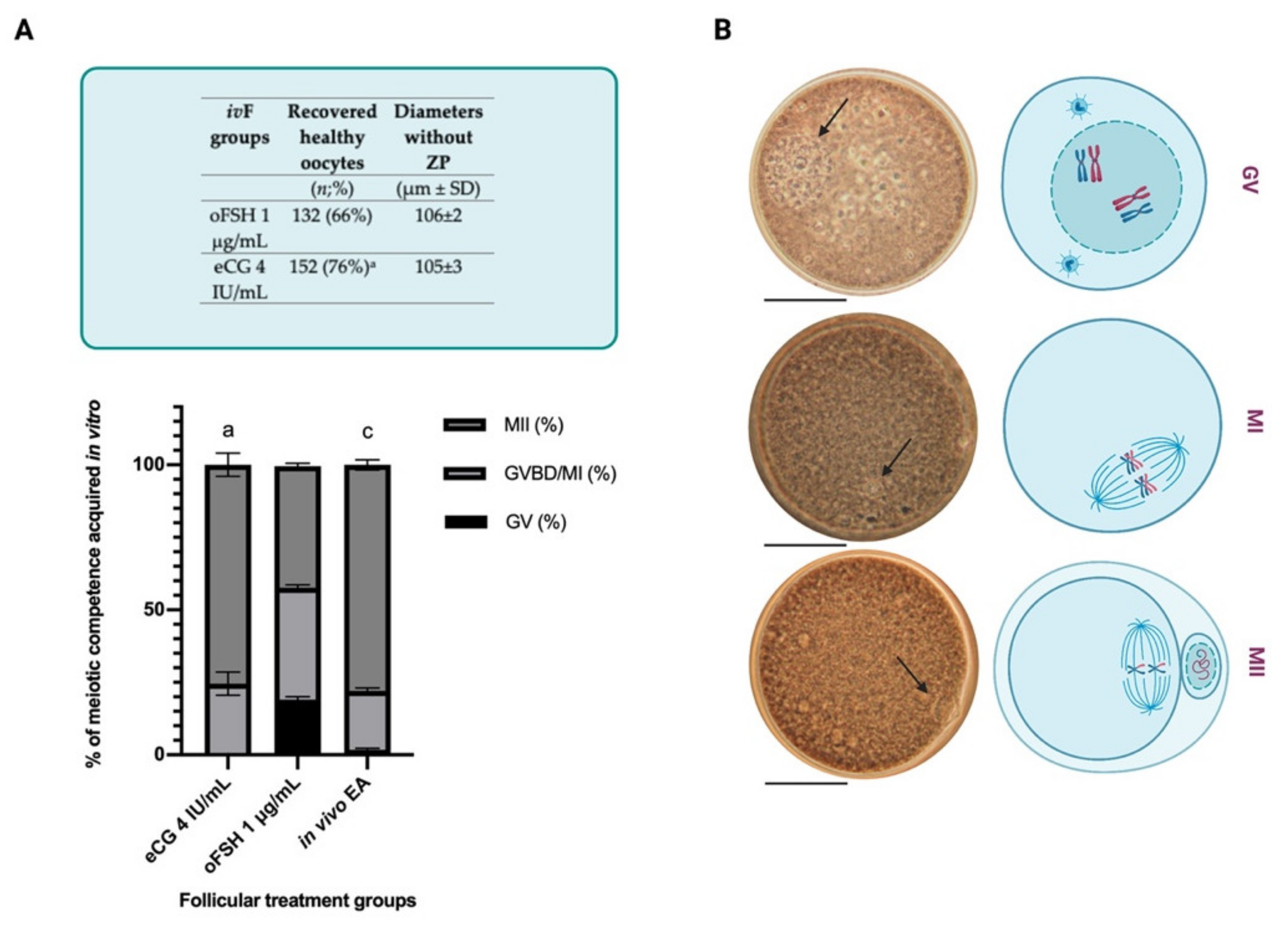
2.1.2. Oocyte Meiotic Competence in FBS-Supplemented Medium
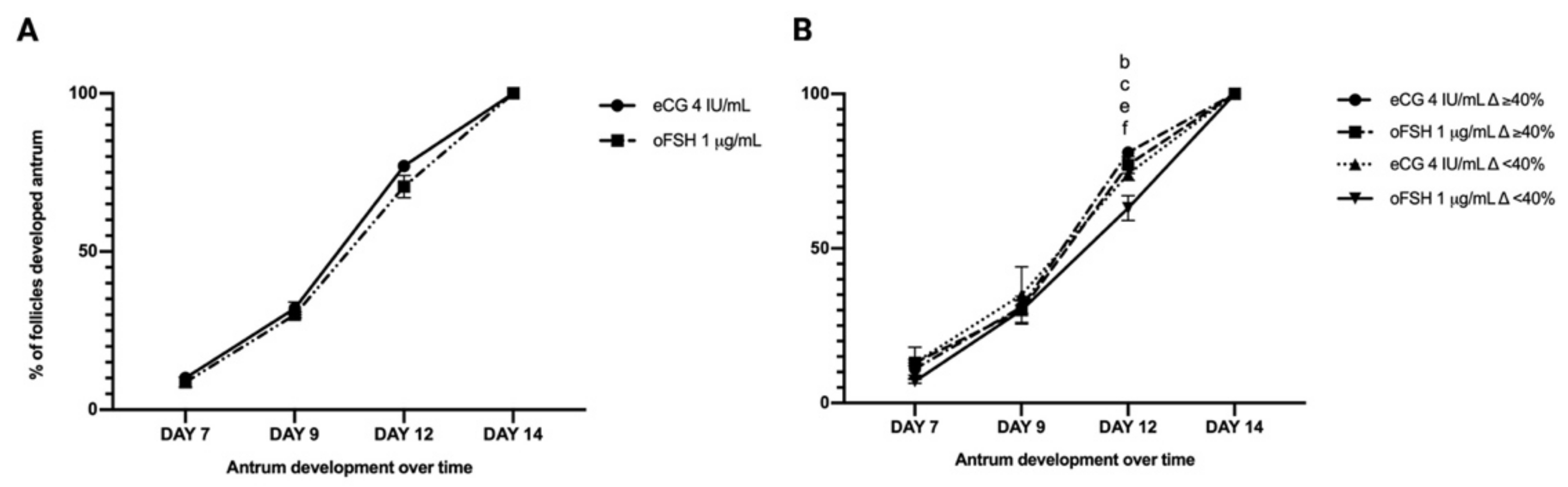
2.2. eCG and oFSH-Induced ivF Performance in PA Follicle Cultured in FBS-Supplemented Medium
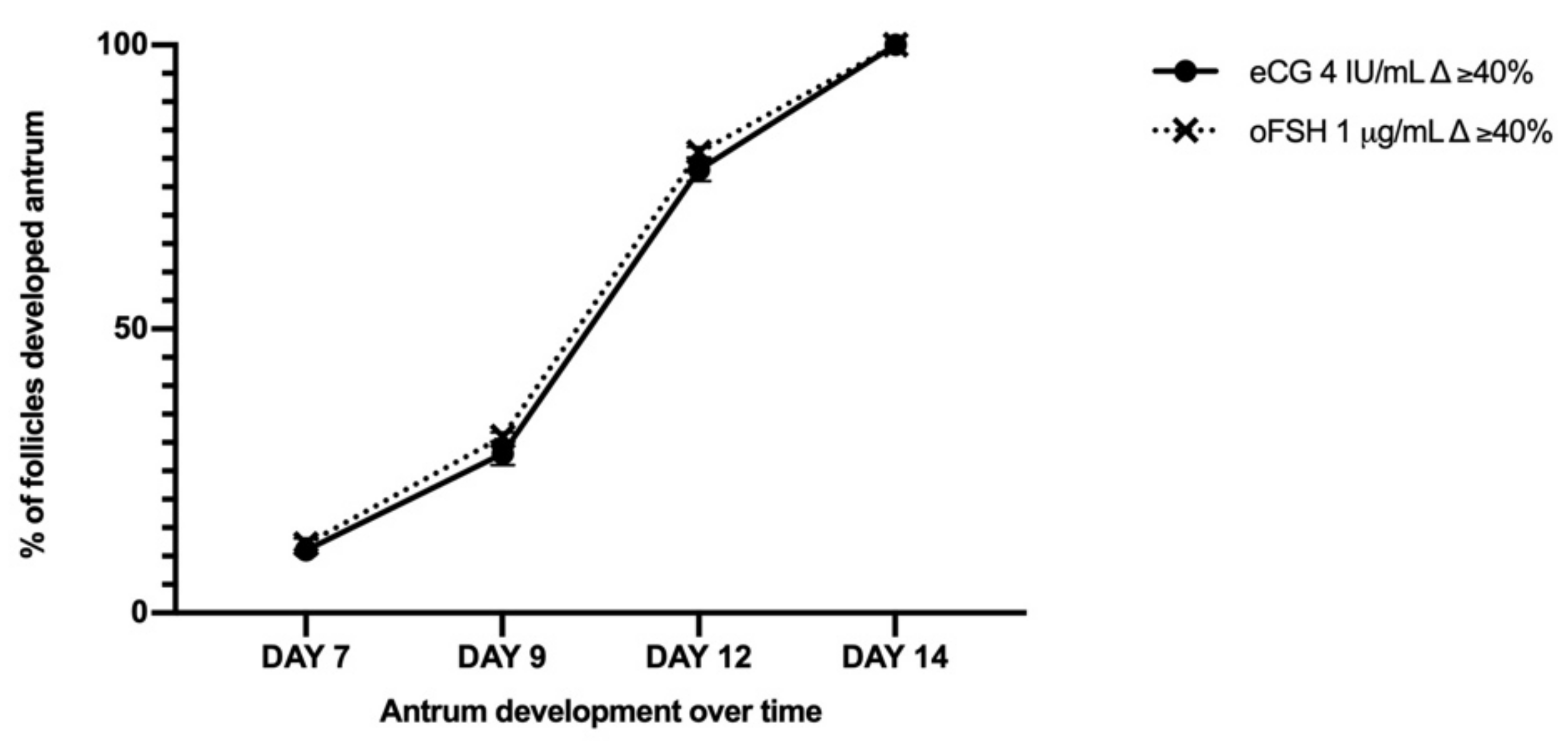
2.2.1. Follicle Growth Parameters Promoted in eCG and oFSH in FBS-Supplemented Medium
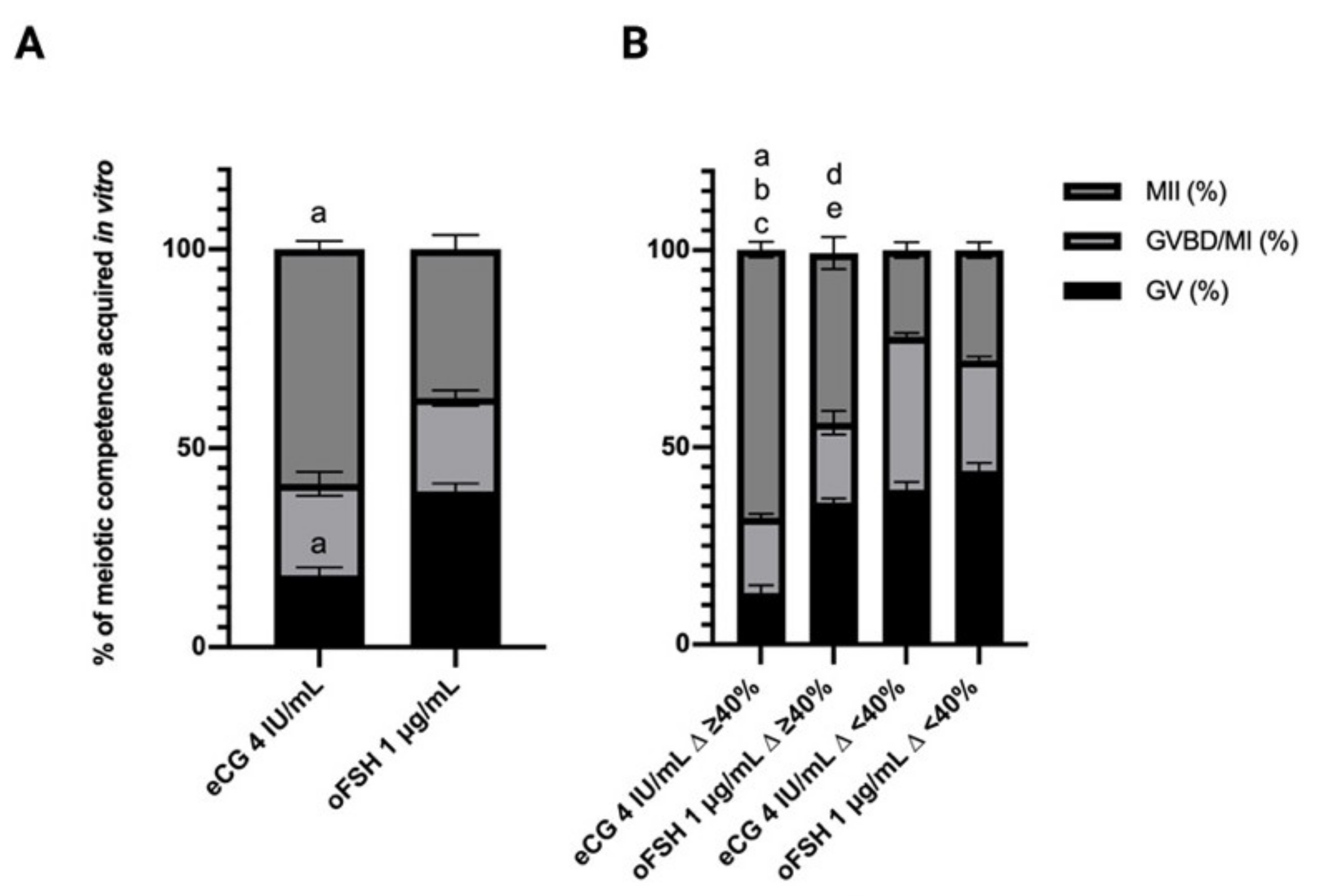
2.2.2. Oocyte Meiotic Competence Promoted in eCG and oFSH ivF Grown Oocytes
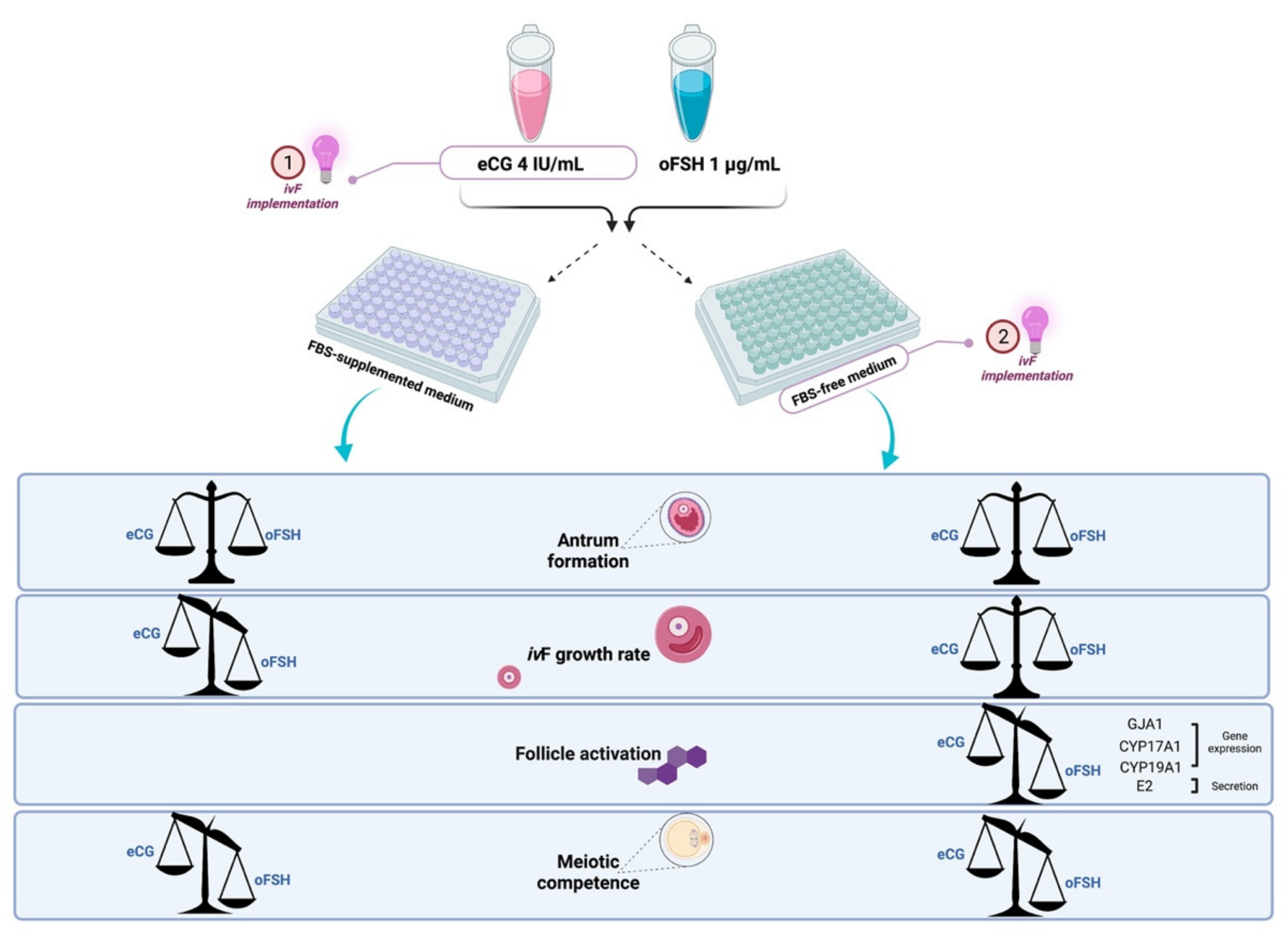
2.3. Comparison between eCG and oFSH in Follicles Cultured in FBS-Free Medium
2.3.1. PA Performances in eCG and oFSH In Vitro Grown PA Follicles Cultured in FBS-Free Medium
2.3.2. Influence of eCG and oFSH on Oocytes Collected from ivF Carried Out in FBS Free Medium
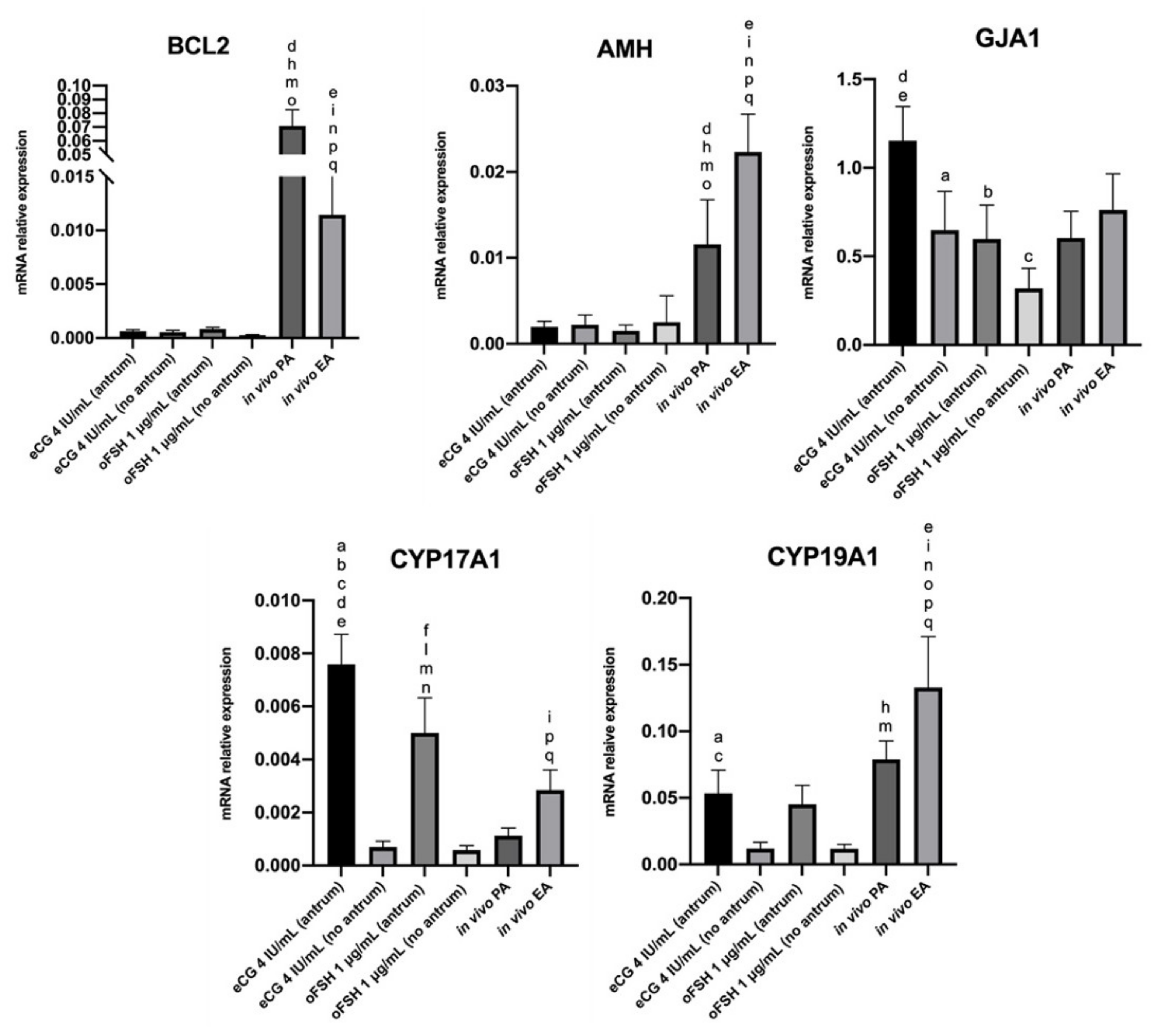
2.4. Steroidogenic Transcript Program Activation on ivF Culture
2.4.1. Comparison In Vitro (eCG vs. oFSH, Antrum vs. No Antrum)
2.4.2. Comparison In Vivo (PA vs. EA)
2.4.3. Comparison In Vitro vs. In Vivo
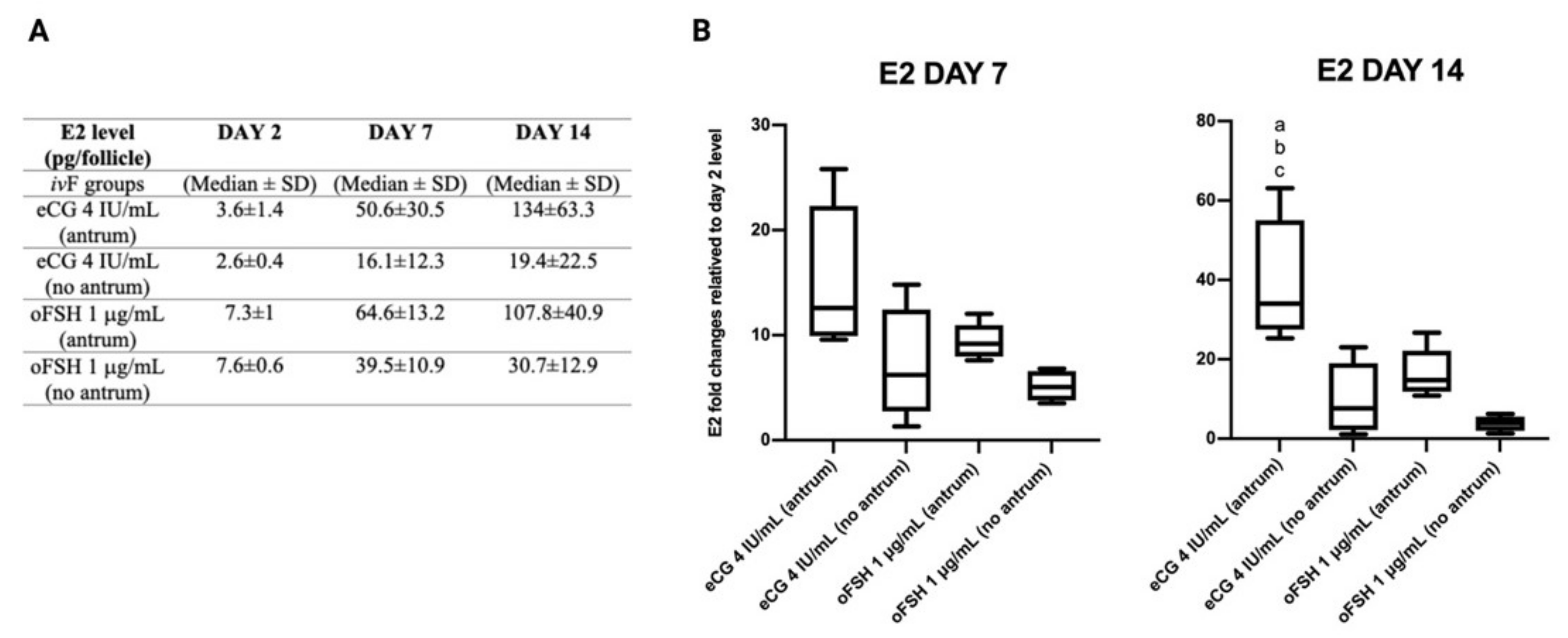
2.5. Follicular Secretion of Estradiol (E2) during ivF Culture
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Isolation, Morphological Evaluation, and In Vitro Culture of PA Follicles
4.3. In Vitro Culture of PA Follicles
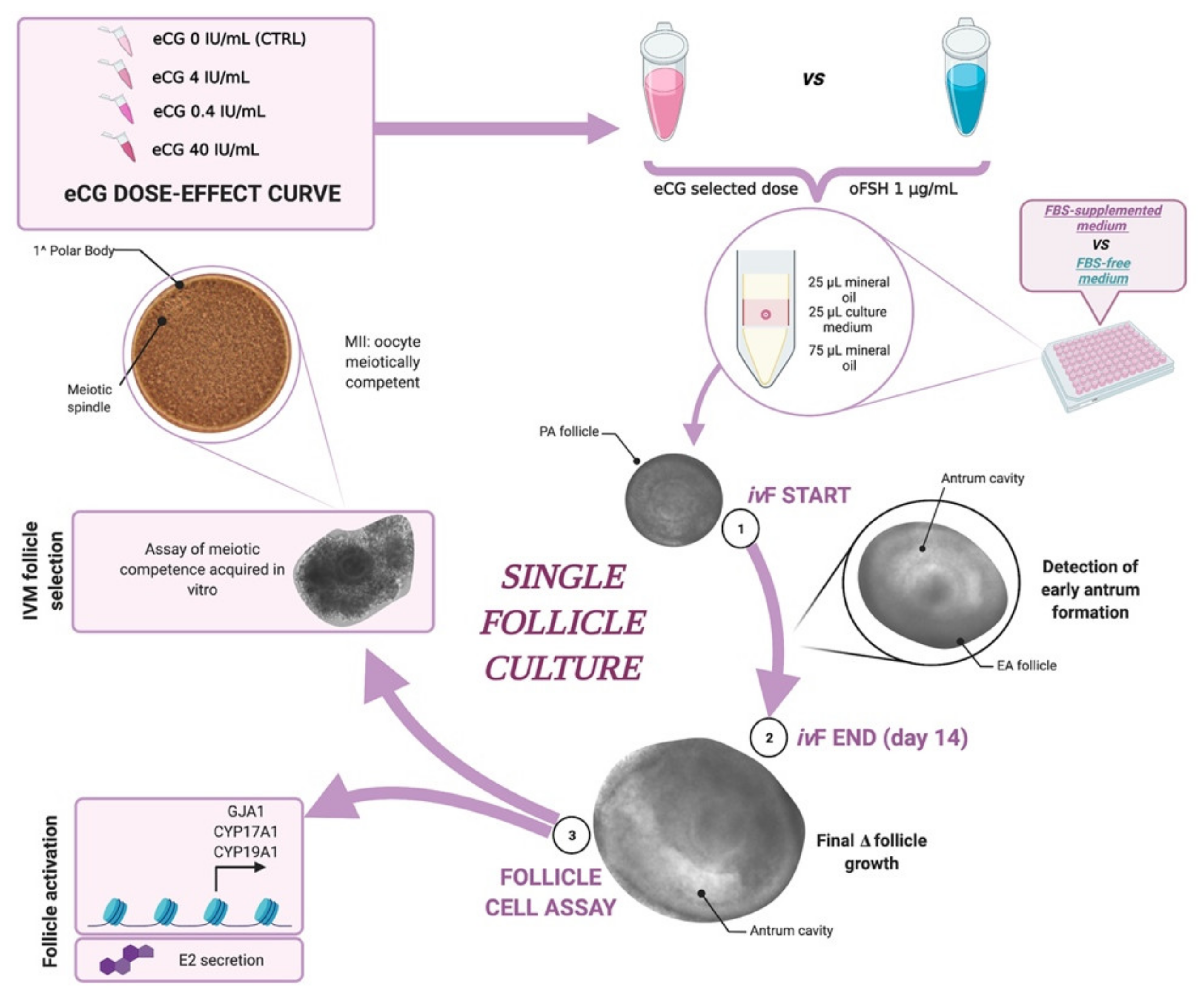
4.4. Experimental Plan
4.4.1. Comparison between eCG vs. oFSH
4.4.2. IvF Outcomes
Morphological Analysis of In Vitro Follicle Development
4.4.3. Oocytes IVM of In Vitro Grown (IVG) Follicles
4.4.4. Oocyte Meiotic Competency Assessment
4.4.5. Detection of Estradiol in the Culture Medium (Estradiol Production Assay)
4.4.6. Real-Time qPCR
4.5. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Gougeon, A. Regulation of ovarian follicular development in primates: Facts and hypotheses. Endocr. Rev. 1996, 17, 121–155. [Google Scholar] [CrossRef] [PubMed]
- Scaramuzzi, R.J.; Baird, D.T.; Campbell, B.K.; Driancourt, M.A.; Dupont, J.; Fortune, J.E.; Gilchrist, R.B.; Martin, G.B.; McNatty, K.P.; McNeilly, A.S.; et al. Regulation of folliculogenesis and the determination of ovulation rate in ruminants. Reprod. Fertil. Dev. 2011, 23, 444–467. [Google Scholar] [CrossRef] [PubMed]
- Cecconi, S.; Ciccarelli, C.; Barberi, M.; Macchiarelli, G.; Canipari, R. Granulosa cell-oocyte interactions. Eur. J. Obstet. Gynecol. Reprod. Biol. 2004, 115, 19–22. [Google Scholar] [CrossRef] [PubMed]
- Saraiva, M.V.A.; Celestino, J.J.H.; Araújo, V.R.; Chaves, R.N.; Almeida, A.P.; Lima-Verde, I.B.; Duarte, A.B.G.; Silva, G.M.; Martins, F.S.; Bruno, J.B.; et al. Expression of follicle-stimulating hormone receptor (FSHR) in goat ovarian follicles and the impact of sequential culture medium on in vitro development of caprine preantral follicles. Zygote 2011, 19, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Vanacker, J.; Luyckx, V.; Amorim, C.; Dolmans, M.M.; van Langendonckt, A.; Donnez, J.; Camboni, A. Should we isolate human preantral follicles before or after cryopreservation of ovarian tissue? Fertil. Steril. 2013, 99, 13–18. [Google Scholar] [CrossRef]
- Cortvrindt, R.; Smitz, J.; van Steirteghem, A.C. A morphological and functional study of the effect of slow freezing followed by complete in-vitro maturation of primary mouse ovarian follicles. Hum. Reprod. 1996, 11, 2648–2655. [Google Scholar] [CrossRef]
- Eppig, J.J. Development in vitro of mouse oocytes from primordial follicles. Biol. Reprod. 1996, 54, 197–207. [Google Scholar] [CrossRef]
- Gupta, P.S.P.; Ramesh, H.S.; Manjunatha, B.M.; Nandi, S.; Ravindra, J.P. Production of buffalo embryos using oocytes from in vitro grown preantral follicles. Zygote 2008, 16, 57–63. [Google Scholar] [CrossRef]
- Gupta, P.S.P.; Nandi, S. Isolation and culture of preantral follicles for retrieving oocytes for the embryo production: Present status in domestic animals. Reprod. Domest. Anim. 2012, 47, 513–519. [Google Scholar] [CrossRef]
- Antonino, D.D.C.; Soares, M.M.; Júnior, J.D.M.; de Alvarenga, P.B.; Mohallem, R.D.F.F.; Rocha, C.D.; Vieira, L.A.; de Souza, A.G.; Beletti, M.E.; Alves, B.G.; et al. Three-dimensional levitation culture improves in-vitro growth of secondary follicles in bovine model. Reprod. Biomed. Online 2019, 38, 300–311. [Google Scholar] [CrossRef]
- Wu, J.; Emery, B.R.; Carrell, D.T. In Vitro Growth, Maturation, Fertilization, and Embryonic Development of Oocytes from Porcine Preantral Follicles. Biol. Reprod. 2001, 64, 375–381. [Google Scholar] [CrossRef]
- Magalhães, D.M.; Duarte, A.B.G.; Araújo, V.R.; Brito, I.R.; Soares, T.G.; Lima, I.M.T.; Lopes, C.A.P.; Campello, C.C.; Rodrigues, A.P.R.; Figueiredo, J.R. In vitro production of a caprine embryo from a preantral follicle cultured in media supplemented with growth hormone. Theriogenology 2011, 75, 182–188. [Google Scholar] [CrossRef]
- Luz, V.B.; Araújo, V.R.; Duarte, A.B.G.; Celestino, J.J.H.; Silva, T.F.P.; Magalhães-Padilha, D.M.; Chaves, R.N.; Brito, I.R.; Almeida, A.P.; Campello, C.C.; et al. Eight-cell parthenotes originated from in vitro grown sheep preantral follicles. Reprod. Sci. 2012, 19, 1219–1225. [Google Scholar] [CrossRef]
- Arunakumari, G.; Shanmugasundaram, N.; Rao, V.H. Development of morulae from the oocytes of cultured sheep preantral follicles. Theriogenology 2010, 74, 884–894. [Google Scholar] [CrossRef]
- Xu, M.; West-Farrell, E.R.; Stouffer, R.L.; Shea, L.D.; Woodruff, T.K.; Zelinski, M.B. Encapsulated Three-Dimensional Culture Supports Development of Nonhuman Primate Secondary Follicles1. Biol. Reprod. 2009, 81, 587–594. [Google Scholar] [CrossRef]
- McLaughlin, M.; Albertini, D.F.; Wallace, W.H.B.; Anderson, R.A.; Telfer, E.E. Metaphase II oocytes from human unilaminar follicles grown in a multistep culture system. Mol. Hum. Reprod. 2018, 24, 135–142. [Google Scholar] [CrossRef]
- Barboni, B.; Russo, V.; Cecconi, S.; Curini, V.; Colosimo, A.; Garofalo, M.L.A.; Capacchietti, G.; Giacinto, O.; Mattioli, M. In Vitro grown sheep preantral follicles yield oocytes with normal nuclear-epigenetic maturation. PLoS ONE 2011, 6, e27550. [Google Scholar] [CrossRef]
- Xu, J.; Lawson, M.S.; Yeoman, R.R.; Pau, K.Y.; Barrett, S.L.; Zelinski, M.B.; Stouffer, R.L. Secondary follicle growth and oocyte maturation during encapsulated three-dimensional culture in rhesus monkeys: Effects of gonadotrophins, oxygen and fetuin. Hum. Reprod. 2011, 26, 1061–1072. [Google Scholar] [CrossRef]
- Silva, J.R.V.; van den Hurk, R.; Figueiredo, J.R. Ovarian follicle development in vitro and oocyte competence: Advances and challenges for farm animals. Domest. Anim. Endocrinol. 2016, 55, 123–135. [Google Scholar] [CrossRef]
- Newton, H.; Picton, H.; Gosden, R.G. From Cryopreserved Ovine Tissue. Science 1997, 115, 141–150. [Google Scholar] [CrossRef]
- Sun, J.; Li, X. Growth and antrum formation of bovine primary follicles in long-term culture in vitro. Reprod. Biol. 2013, 13, 221–228. [Google Scholar] [CrossRef]
- Da Silva, G.M.; Rossetto, R.; Chaves, R.N.; Duarte, A.B.G.; Araújo, V.R.; Feltrin, C.; Bernuci, M.P.; Anselmo-Franci, J.A.; Xu, M.; Woodruff, T.K.; et al. In vitro development of secondary follicles from pre-pubertal and adult goats cultured in two-dimensional or three-dimensional systems. Zygote 2015, 23, 475–484. [Google Scholar] [CrossRef]
- Cecconi, S.; Barboni, B.; Coccia, M.; Mattioli, M. In vitro development of sheep preantral follicles. Biol. Reprod. 1999. [Google Scholar] [CrossRef][Green Version]
- Costa, S.L.; Costa, E.P.; Pereira, E.C.M.; Benjamin, L.A.; Rodrigues, M.T.; Mendes, V.R.A.; Silva, T.F. Influence of insulin-like growth factor I (IGF-I) on the survival and the in vitro development of caprine preantral follicles. Pesqui. Vet. Bras. 2014. [Google Scholar] [CrossRef][Green Version]
- Telfer, E.E.; McLaughlin, M.; Ding, C.; Thong, K.J. A two-step serum-free culture system supports development of human oocytes from primordial follicles in the presence of activin. Hum. Reprod. 2008, 23, 1151–1158. [Google Scholar] [CrossRef]
- Chaves, R.N.; Duarte, A.B.G.; Rodrigues, G.Q.; Celestino, J.J.H.; Silva, G.M.; Lopes, C.A.P.; Almeida, A.P.; Donato, M.A.M.; Peixoto, C.A.; Moura, A.A.A.; et al. The Effects of Insulin and Follicle-Simulating Hormone (FSH) During In Vitro Development of Ovarian Goat Preantral Follicles and the Relative mRNA Expression for Insulin and FSH Receptors and Cytochrome P450 Aromatase in Cultured Follicles1. Biol. Reprod. 2012, 87, 1–11. [Google Scholar] [CrossRef]
- Adams, G.P.; Singh, J.; Baerwald, A.R. Large animal models for the study of ovarian follicular dynamics in women. Theriogenology 2012, 78, 1733–1748. [Google Scholar] [CrossRef]
- De Figueiredo, J.R.; Cadenas, J.; de Lima, L.F.; Santos, R.R. Advances in in vitro folliculogenesis in domestic ruminants. Anim. Reprod. 2018, 16, 52–65. [Google Scholar] [CrossRef]
- Barros, V.R.P.; Monte, A.P.O.; Lins, T.L.B.G.; Santos, J.M.; Menezes, V.G.; Cavalcante, A.Y.P.; Araújo, V.R.; Gouveia, B.B.; Matos, M.H.T. In vitro survival, growth, and maturation of sheep oocytes from secondary follicles cultured in serum-free conditions: Impact of a constant or a sequential medium containing recombinant human FSH. Domest. Anim. Endocrinol. 2019, 67, 71–79. [Google Scholar] [CrossRef]
- Cadoret, V.; Frapsauce, C.; Jarrier, P.; Maillard, V.; Bonnet, A.; Locatelli, Y.; Royère, D.; Monniaux, D.; Guérif, F.; Monget, P. Molecular evidence that follicle development is accelerated in vitro compared to in vivo. Reproduction 2017, 153, 493–508. [Google Scholar] [CrossRef]
- Courbiere, B.; Massardier, J.; Salle, B.; Mazoyer, C.; Guerin, J.F.; Lornage, J. Follicular viability and histological assessment after cryopreservation of whole sheep ovaries with vascular pedicle by vitrification. Fertil. Steril. 2005, 84, 1065–1071. [Google Scholar] [CrossRef] [PubMed]
- Sauvat, F.; Bouilly, J.; Capito, C.; Lefèvre, A.; Blachère, T.; Borenstein, N.; Sarnacki, S.; Dandolo, L.; Binart, N. Ovarian function is restored after grafting of cryopreserved immature ovary in ewes. FASEB J. 2013, 27, 1511–1518. [Google Scholar] [CrossRef] [PubMed]
- Baird, D.T.; Webb, R.; Campbell, B.K.; Harkness, L.M.; Gosden, R.G. Long-term ovarian function in sheep after ovariectomy and transplantation of autografts stored at −196 C. Endocrinology 1999, 140, 462–471. [Google Scholar] [CrossRef] [PubMed]
- Campbell, B.K.; Telfer, E.E.; Webb, R.; Baird, D.T. Ovarian autografts in sheep as a model for studying folliculogenesis. Mol. Cell. Endocrinol. 2000, 163, 131–139. [Google Scholar] [CrossRef]
- Cognie, Y.; Benoit, F.; Poulin, N.; Khatir, H.; Driancourt, M.A. Effect of follicle size and of the Fec(B) Booroola gene on oocyte function in sheep. J. Reprod. Fertil. 1998, 112, 379–386. [Google Scholar] [CrossRef]
- McCaffery, F.H.; Leask, R.; Riley, S.C.; Telfer, E.E. Culture of bovine preantral follicles in a serum-free system: Markers for assessment of growth and development. Biol. Reprod. 2000, 63, 267–273. [Google Scholar] [CrossRef]
- Dalbies-Tran, R.; Cadoret, V.; Desmarchais, A.; Elis, S.; Maillard, V.; Monget, P.; Monniaux, D.; Reynaud, K.; Saint-Dizier, M.; Uzbekova, S. A Comparative Analysis of Oocyte Development in Mammals. Cells 2020, 9, 1002. [Google Scholar] [CrossRef]
- Yang, Q.; Zhu, L.; Jin, L. Human Follicle in vitro Culture Including Activation, Growth, and Maturation: A Review of Research Progress. Front. Endocrinol. 2020, 11, 1–9. [Google Scholar] [CrossRef]
- Filatov, M.; Khramova, Y.; Parshina, E.; Bagaeva, T.; Semenova, M. Influence of gonadotropins on ovarian follicle growth and development in vivo and in vitro. Zygote 2017, 25, 235–243. [Google Scholar] [CrossRef]
- Ferreira, A.C.A.; Maside, C.; Sá, N.A.R.; Guerreiro, D.D.; Correia, H.H.V.; Leiva-Revilla, J.; Lobo, C.H.; Araújo, V.R.; Apgar, G.A.; Brandão, F.Z.; et al. Balance of insulin and FSH concentrations improves the in vitro development of isolated goat preantral follicles in medium containing GH. Anim. Reprod. Sci. 2016, 165, 1–10. [Google Scholar] [CrossRef]
- Monte, A.P.O.; Barros, V.R.P.; Santos, J.M.; Menezes, V.G.; Cavalcante, A.Y.P.; Gouveia, B.B.; Bezerra, M.E.S.; Macedo, T.J.S.; Matos, M.H.T. Immunohistochemical localization of insulin-like growth factor-1 (IGF-1) in the sheep ovary and the synergistic effect of IGF-1 and FSH on follicular development in vitro and LH receptor immunostaining. Theriogenology 2019, 129, 61–69. [Google Scholar] [CrossRef]
- Wallace, J.M.; Martin, G.B.; McNeilly, A.S. Changes in the secretion of LH pulses, FSH and prolactin during the preovulatory phase of the oestrous cycle of the ewe and the influence of treatment with bovine follicular fluid during the luteal phase. J. Endocrinol. 1988, 116, 123–135. [Google Scholar] [CrossRef]
- Orisaka, M.; Miyazaki, Y.; Shirafuji, A.; Tamamura, C.; Tsuyoshi, H.; Tsang, B.K.; Yoshida, Y. The role of pituitary gonadotropins and intraovarian regulators in follicle development: A mini-review. Reprod. Med. Biol. 2021, 20, 169–175. [Google Scholar] [CrossRef]
- Orisaka, M.; Tajima, K.; Tsang, B.K.; Kotsuji, F. Oocyte-granulosa-theca cell interactions during preantral follicular development. J. Ovarian Res. 2009, 2, 1–7. [Google Scholar] [CrossRef]
- McGee, E.A.; Hsueh, A.J.W. Initial and cyclic recruitment of ovarian follicles. Endocr. Rev. 2000, 21, 200–214. [Google Scholar] [CrossRef]
- McGee, E.A.; Raj, R.S. Regulators of ovarian preantral follicle development. Semin. Reprod. Med. 2015, 33, 179–184. [Google Scholar] [CrossRef]
- Saraiva, M.V.A.; Brito, I.R.; Rodrigues, G.Q.; Almeida, A.P.; Lima, I.M.T.; Rossetto, R.; Frota, I.M.A.; Leitão, C.C.F.; Costa, J.J.N.; Silva, J.R.V.; et al. FSH and LH enhance the development of goat pre antral follicles cultured in vitro.pdf. Anim. Reprod. 2012, 9, 71–79. [Google Scholar]
- Sharma, G.T.; Dubey, P.K.; Meur, S.K. Survival and developmental competence of buffalo preantral follicles using three-dimensional collagen gel culture system. Anim. Reprod. Sci. 2009, 114, 115–124. [Google Scholar] [CrossRef]
- Brito, I.R.; Saraiva, M.V.A.; Araújo, V.R.; Celestino, J.J.H.; Magalhães-Padilha, D.M.; Lima, I.M.T.; van den Hurk, R.; Figueiredo, J.R.; Silva, J.R.V. The effect of IGF-1 and FSH on the in vitro development of caprine secondary follicles and on the IGF-1, IGFR-I and FSHR mRNA levels. Res. Vet. Sci. 2012, 93, 729–732. [Google Scholar] [CrossRef]
- Magalhães-Padilha, D.M.; Andrade, P.M.; Sales, E.T.; Araujo, V.R.; Lima, I.M.T.; Castro, S.V.; Faustino, L.R.; Lopes, C.A.P.; Campello, C.C.; Báo, S.N.; et al. Effect of sequential medium on in vitro culture of goat ovarian cortical tissue. Anim. Reprod. Sci. 2012, 132, 159–168. [Google Scholar] [CrossRef][Green Version]
- Thomas, F.H.; Vanderhyden, B.C. Oocyte-granulosa cell interactions during mouse follicular development: Regulation of kit ligand expression and its role in oocyte growth. Reprod. Biol. Endocrinol. 2006, 4, 1–8. [Google Scholar] [CrossRef]
- Ferreira, A.C.A.; Sá, N.A.R.; Cadenas, J.; Correia, H.H.V.; Guerreiro, D.D.; Alves, B.G.; Lima, L.F.; Celestino, J.J.H.; Rodrigues, A.P.P.R.; Gastal, E.L.; et al. Pituitary porcine FSH, and recombinant bovine and human FSH differentially affect growth and relative abundances of mRNA transcripts of preantral and early developing antral follicles in goats. Anim. Reprod. Sci. 2020, 219. [Google Scholar] [CrossRef]
- Committee, T.P.; Society, A.; Medicine, R. Gonadotropin preparations: Past, present, and future perspectives. Fertil. Steril. 2008, 90, 13–20. [Google Scholar] [CrossRef]
- Park, S.J.; Kim, T.S.; Kim, J.M.; Chang, K.T.; Lee, H.S.; Lee, D.S. Repeated superovulation via PMSG/hCG administration induces 2-Cys peroxiredoxins expression and overoxidation in the reproductive tracts of female mice. Mol. Cells 2015, 38, 1071–1078. [Google Scholar] [CrossRef]
- Gomes, D.S.; Aragaõ, L.B.; Lima Neto, M.F.; Barroso, P.A.A.; Paulino, L.R.F.M.; Silva, B.R.; Souza, A.L.P.; Vasconcelos, G.L.; Silva, A.W.B.; Silva, J.R.V. Supplementation of culture medium with knockout serum replacement improves the survival of bovine secondary follicles when compared with other protein sources during in vitro culture. Zygote 2019, 28, 32–36. [Google Scholar] [CrossRef]
- Jin, J.X.; Lee, S.; Setyawan, E.M.N.; Taweechaipaisankul, A.; Kim, G.A.; Han, H.J.; Ahn, C.; Lee, B.C. A potential role of knockout serum replacement as a porcine follicular fluid substitute for in vitro maturation: Lipid metabolism approach. J. Cell. Physiol. 2018, 233, 6984–6995. [Google Scholar] [CrossRef]
- Park, Y.H.; Gong, S.P.; Kim, H.Y.; Kim, G.A.; Choi, J.H.; Ahn, J.Y.; Lim, J.M. Development of a serum-free defined system employing growth factors for preantral follicle culture. Mol. Reprod. Dev. 2013, 80, 725–733. [Google Scholar] [CrossRef]
- Da Silva, A.F.B.; De Lima, L.F.; De Figueiredo, J.R. Estratégias para a melhoria da eficiência do cultivo folicular in vitro: Importância da suplementação do meio e estudo das alterações epigenéticas. Res. Soc. Dev. 2021, 10, e22910918022. [Google Scholar] [CrossRef]
- Fisch, B.; Abir, R. Female fertility preservation: Past, present and future. Reproduction 2018, 156, F11–F27. [Google Scholar] [CrossRef]
- Herta, A.C.; Lolicato, F.; Smitz, J.E.J. In vitro follicle culture in the context of IVF. Reproduction 2018, 156, F59–F73. [Google Scholar] [CrossRef]
- De Vos, M.; Smitz, J.; Woodruff, T.K. Fertility preservation in women with cancer. Lancet 2014, 384, 1302–1310. [Google Scholar] [CrossRef]
- Martinez, F.; International Society for Fertility Preservation–ESHRE–ASRM Expert Working Group. Update on fertility preservation from the Barcelona International Society for Fertility Preservation–ESHRE–ASRM 2015 expert meeting: Indications, results and future perspectives. Fertil. Steril. 2017, 108, 407–415.e11. [Google Scholar] [CrossRef] [PubMed]
- Cecconi, S.; Capacchietti, G.; Russo, V.; Berardinelli, P.; Mattioli, M.; Barboni, B. In Vitro Growth of Preantral Follicles Isolated from Cryopreserved Ovine Ovarian Tissue. Biol. Reprod. 2004, 70, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Fazleabas, A.T.; Shikanov, A.; Jackson, E.; Barrett, S.L.; Hirshfeld-Cytron, J.; Kiesewetter, S.E.; Shea, L.D.; Woodruff, T.K. In Vitro Oocyte Maturation and Preantral Follicle Culture from the Luteal-Phase Baboon Ovary Produce Mature Oocytes. Biol. Reprod. 2011, 84, 689–697. [Google Scholar] [CrossRef]
- Vilanova, X.M.; de Briyne, N.; Beaver, B.; Turner, P.V. Horse welfare during equine chorionic gonadotropin (eCG) production. Animals 2019, 9, 1053. [Google Scholar] [CrossRef]
- Goodman, N.; Cobin, R.H. Reproductive disorders. In Evidence-Based Endocrinology, 3rd ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2012; pp. 190–228. [Google Scholar]
- Quintero-Elisea, J.A.; Macías-Cruz, U.; Álvarez-Valenzuela, F.D.; Correa-Calderón, A.; González-Reyna, A.; Lucero-Magaña, F.A.; Soto-Navarro, S.A.; Avendaño-Reyes, L. The effects of time and dose of pregnant mare serum gonadotropin (PMSG) on reproductive efficiency in hair sheep ewes. Trop. Anim. Health Prod. 2011, 43, 1567–1573. [Google Scholar] [CrossRef]
- Behringer, R.; Gertsenstein, M.; Nagy, K.V.; Nagy, A. Administration of gonadotropins for superovulation in mice. Cold Spring Harb. Protoc. 2018, 2018, 24–27. [Google Scholar] [CrossRef]
- Popova, E.; Krivokharchenko, A.; Ganten, D.; Bader, M. Comparison between PMSG- and FSH-induced superovulation for the generation of transgenic rats. Mol. Reprod. Dev. 2002, 63, 177–182. [Google Scholar] [CrossRef]
- Somanjaya, R.; Fuah, A.M.; Rahayu, S.; Setiadi, M.A.; Abdullah, L. PMSG in ewes: A Practical and Efficient Step for Superovulation. IOP Conf. Ser. Earth Environ. Sci. 2021, 748, 012010. [Google Scholar] [CrossRef]
- Ryan, J.P.; Hunton, J.R.; Maxwell, W.M.C. Time of ovulation in merino ewes superovulated with pmsg and fsh-p. Reprod. Fertil. Dev. 1992, 4, 91–97. [Google Scholar] [CrossRef]
- Driancourt, M.A.; Fry, R.C. Effect of superovulation with pFSH or PMSG on growth and maturation of the ovulatory follicles in sheep. Anim. Reprod. Sci. 1992, 27, 279–292. [Google Scholar] [CrossRef]
- Pendleton, R.J.; Youngs, C.R.; Rorie, R.W.; Pool, S.H.; Memon, M.A.; Godke, R.A. Follicle stimulating hormone versus pregnant mare serum gonadotropin for superovulation of dairy goats. Small Rumin. Res. 1992, 8, 217–224. [Google Scholar] [CrossRef]
- Gonzalez, A. Superovulation pregnant gonadotrophin: Antipregnant gonadotrophin. Can. Vet. J. Rev. Vet. Can. 1994, 35, 158–162. [Google Scholar]
- González-Menció, F.; Manns, J.; Murphy, B.D. FSH and LH activity of PMSG from mares at different stages of gestation. Anim. Reprod. Sci. 1978, 1, 137–144. [Google Scholar] [CrossRef]
- Guthrie, H.D.; Henricks, D.M.; Handlin, D.L. Plasma hormone levels and fertility in pigs induced to superovulate with PMSG. J. Reprod. Fertil. 1974, 41, 361–370. [Google Scholar] [CrossRef]
- Nikiforov, D.; Russo, V.; Nardinocchi, D.; Bernabò, N.; Mattioli, M.; Barboni, B. Innovative multi-protectoral approach increases survival rate after vitrification of ovarian tissue and isolated follicles with improved results in comparison with conventional method. J. Ovarian Res. 2018, 11, 1–15. [Google Scholar] [CrossRef]
- Endo, M.; Kawahara-Miki, R.; Cao, F.; Kimura, K.; Kuwayama, T.; Monji, Y.; Iwata, H. Estradiol supports in vitro development of bovine early antral follicles. Reproduction 2013, 145, 85–96. [Google Scholar] [CrossRef]
- West-Farrell, E.R.; Xu, M.; Gomberg, M.A.; Chow, Y.H.; Woodruff, T.K.; Shea, L.D. The mouse follicle microenvironment regulates antrum formation and steroid production: Alterations in gene expression profiles. Biol. Reprod. 2009, 80, 432–439. [Google Scholar] [CrossRef][Green Version]
- Wei, S.C.; Gong, Z.D.; Zhao, H.W.; Liang, H.Q.; Lai, L.J.; Deng, Y.Y. Equine chorionic gonadotropin influence on sheep oocyte in vitro maturation, apoptosis, and follicle-stimulating hormone receptor and luteinizing hormone receptor expression. Genet. Mol. Res. 2016, 15, 1–13. [Google Scholar] [CrossRef]
- Sheela Rani, C.S.; Moudgal, N.R. Differences in the behavior of luteinizing hormones of various species at the rat gonadal cell receptor site. Endocrinology 1985, 116, 597–603. [Google Scholar] [CrossRef]
- Steelman, S.L.; Pohley, F.M. Assay of the follicle stimulating hormone based on the augmentation with human chorionic gonadotropin. Endocrinology 1953, 53, 604–616. [Google Scholar] [CrossRef] [PubMed]


| ivF Groups | N° of ivF PA | Unhealthy Follicles | Recovered Healthy Follicles | Degree of PA Growth | PA Diameter | EA Diameter |
|---|---|---|---|---|---|---|
| (n) | (%) | (n;%) | (Δ%) | (μm ± SD) | (μm ± SD) | |
| eCG 40 IU/mL | 90 | 60 (67%) d | 30 (33%) d | 38% | 243.8 ± 11 | 337 ± 29 a |
| eCG 4 IU/mL | 90 | 36 (40%) b,d,f | 54 (60%) b,d,f | 41% | 257.6 ± 23 | 362 ± 50 |
| eCG 0.4 IU/mL | 90 | 59 (66%) f | 31 (34%) f | 46% | 252.3 ± 19 | 364.2 ± 44 |
| eCG 0 IU/mL (CTRL) | 90 | 61 (68%) a,c,f | 29 (32%) a,c,f | 10% | 233.2 ± 3 | 256.2 ± 2 a |
| ivF Groups | N° of ivF PA | Recovered Healthy Oocytes | Oocyte Diameters without ZP | GV | GVBD/MI | MII |
|---|---|---|---|---|---|---|
| (n) | (n;%) | (μm ± SD) | (%) | (%) | (%) | |
| eCG 40 IU | 90 | 30 (33%) | 103 ± 5 a | 69.3% d,e | 10% | 20.7% |
| eCG 4 IU | 90 | 54 (60%) i | 106 ± 4 b | 5.6% | 27.7% b,d,f | 66.7% b,e,h,i |
| eCG 0.4 IU | 90 | 31 (34%) | 105 ± 6 c | 42% | 10% | 48% c,f |
| eCG 0 IU (CTRL–) | 90 | 29 (32%) | 93 ± 2 | 71% a,b,c | 7% | 22% |
| in vivo EA (CTRL+) | 90 | 80 (89%) i | 106 ± 3 d | 1.7% | 20.6% d,g,l | 77.7% d,g,l |
| ivF Groups | N° of ivF PA | Recovered Healthy Follicles | ∆ ≥ 40% | PA Diameter | EA Diameter |
|---|---|---|---|---|---|
| (n) | (n;%) | (n;%) | (μm ± SD) | (μm ± SD) | |
| oFSH 1 μg/mL | 200 | 116 (58%) | 60% | 254 ± 26 | 380 ± 70 |
| eCG 4 IU/mL | 200 | 122 (61%) | 81% a | 256 ± 25 | 410 ± 68 |
| ivF Groups | Recovered Healthy Oocytes | Diameters without ZP |
|---|---|---|
| (n;%) | (μm ± SD) | |
| eCG 4 IU/mL (αω) | 122 (61%) | 107 ± 5 |
| ∆ > 40% | 99 (81%) a | |
| ∆ < 40% | 23 (19%) f | |
| oFSH 1 μg/mL (αω) | 116 (58%) | 105 ± 7 |
| ∆ > 40% | 70 (60%) | |
| ∆ < 40% | 46 (40%) |
| ivF Groups | N° of ivF PA | Recovered Healthy Follicles | Degree of PA Growth | PA Starting Diameter | Final Diameter |
|---|---|---|---|---|---|
| (n) | (n;%) | (∆ > 40%) | (μm ± SD) | (μm ± SD) | |
| oFSH 1 μg/mL | 200 | 132 (66%) | 68.7% | 254 ± 17 | 413 ± 25 |
| eCG 4 IU/mL | 200 | 152 (76%) a | 70% | 256 ± 15 | 434 ± 31 |
| Gene | Forward Sequence | Reverse Sequence |
|---|---|---|
| BCL2 | 5′-CCTTCTTTGAGTTCGGAG-3′ | 5′-CTGGCTGTCTCTGAAGG-3′ |
| AMH | 5′-GTGGTGCTGCTGCTAAAGATG-3′ | 5′-CTCCTCATCAGCCTGTCCGA-3′ |
| GJA1 | 5′-CACTTGAAGCAGATTGAA-3′ | 5′-AGGATACTGATGATGTAGG-3′ |
| CYP17A1 | 5′-CTTACCATTGACAAAGGCACAGAC-3′ | 5′-CAACTCATCTCGCCATCATTAAGC-3′ |
| CYP19A1 | 5′-TCGTCCTGGTCAACCCTTCTG-3′ | 5′-CCAGACGAGACCAGAGACCG-3′ |
| GAPDH | 5′-TCGGAGTGAACGGATTTGGC-3′ | 5′-CCGTTCTCTGCCTTGACTGT-3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Berardino, C.; Peserico, A.; Capacchietti, G.; Crociati, M.; Monaci, M.; Tosi, U.; Mauro, A.; Russo, V.; Bernabò, N.; Barboni, B. Equine Chorionic Gonadotropin as an Effective FSH Replacement for In Vitro Ovine Follicle and Oocyte Development. Int. J. Mol. Sci. 2021, 22, 12422. https://doi.org/10.3390/ijms222212422
Di Berardino C, Peserico A, Capacchietti G, Crociati M, Monaci M, Tosi U, Mauro A, Russo V, Bernabò N, Barboni B. Equine Chorionic Gonadotropin as an Effective FSH Replacement for In Vitro Ovine Follicle and Oocyte Development. International Journal of Molecular Sciences. 2021; 22(22):12422. https://doi.org/10.3390/ijms222212422
Chicago/Turabian StyleDi Berardino, Chiara, Alessia Peserico, Giulia Capacchietti, Martina Crociati, Maurizio Monaci, Umberto Tosi, Annunziata Mauro, Valentina Russo, Nicola Bernabò, and Barbara Barboni. 2021. "Equine Chorionic Gonadotropin as an Effective FSH Replacement for In Vitro Ovine Follicle and Oocyte Development" International Journal of Molecular Sciences 22, no. 22: 12422. https://doi.org/10.3390/ijms222212422
APA StyleDi Berardino, C., Peserico, A., Capacchietti, G., Crociati, M., Monaci, M., Tosi, U., Mauro, A., Russo, V., Bernabò, N., & Barboni, B. (2021). Equine Chorionic Gonadotropin as an Effective FSH Replacement for In Vitro Ovine Follicle and Oocyte Development. International Journal of Molecular Sciences, 22(22), 12422. https://doi.org/10.3390/ijms222212422












