Osr1 Is Required for Mesenchymal Derivatives That Produce Collagen in the Bladder
Abstract
:1. Introduction
2. Results
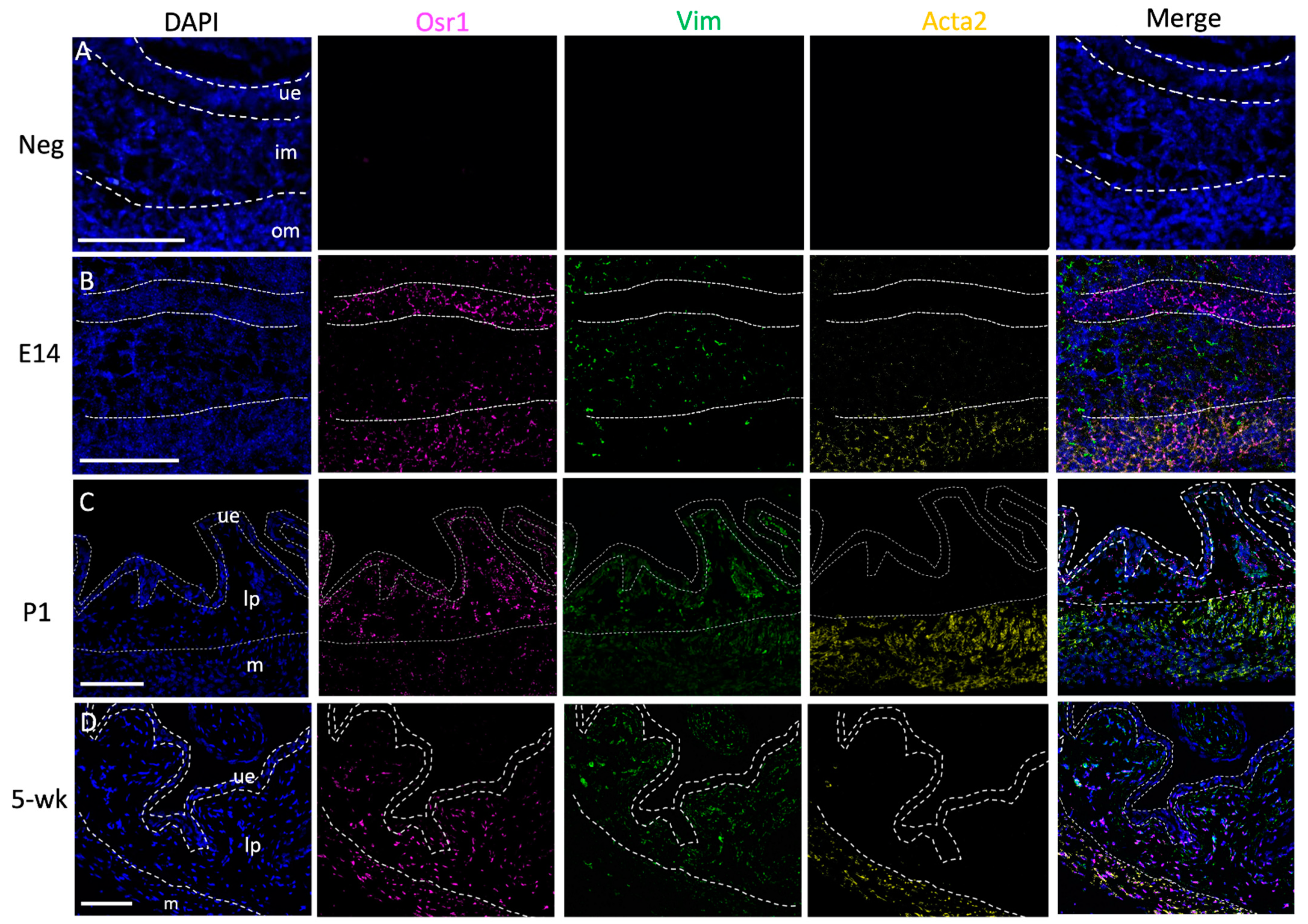
2.1. Osr1 Expression Changes during Bladder Development
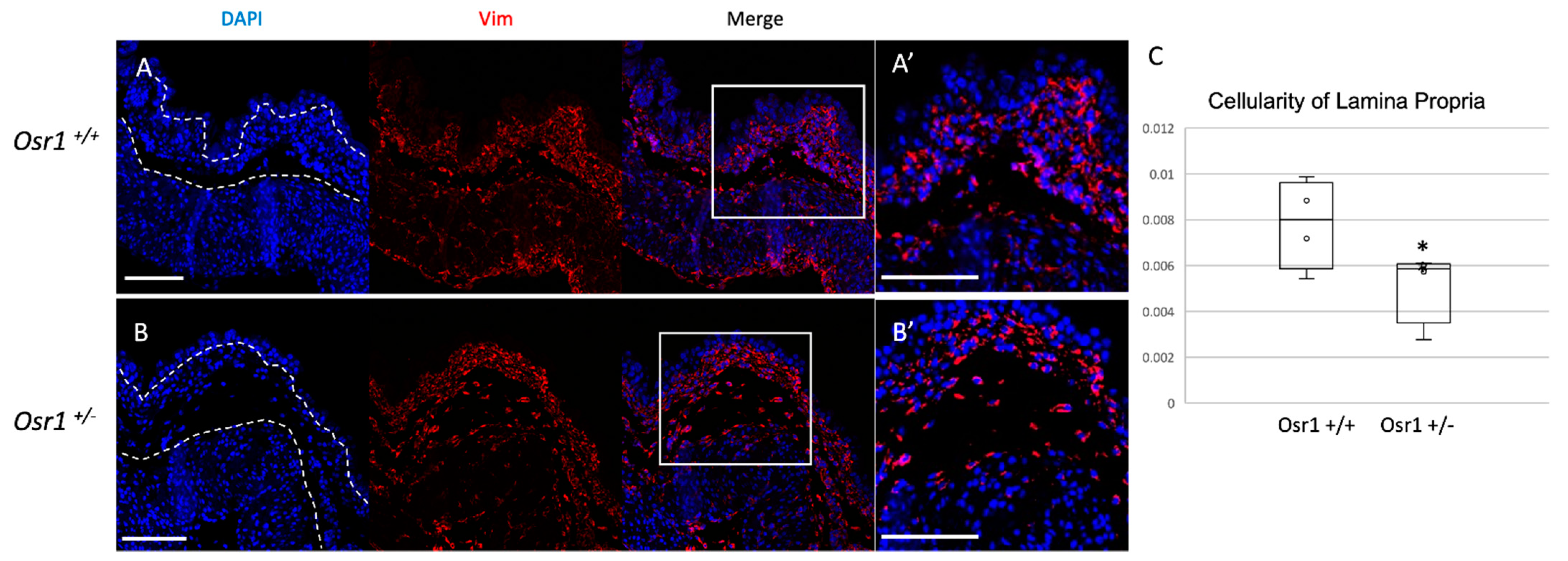
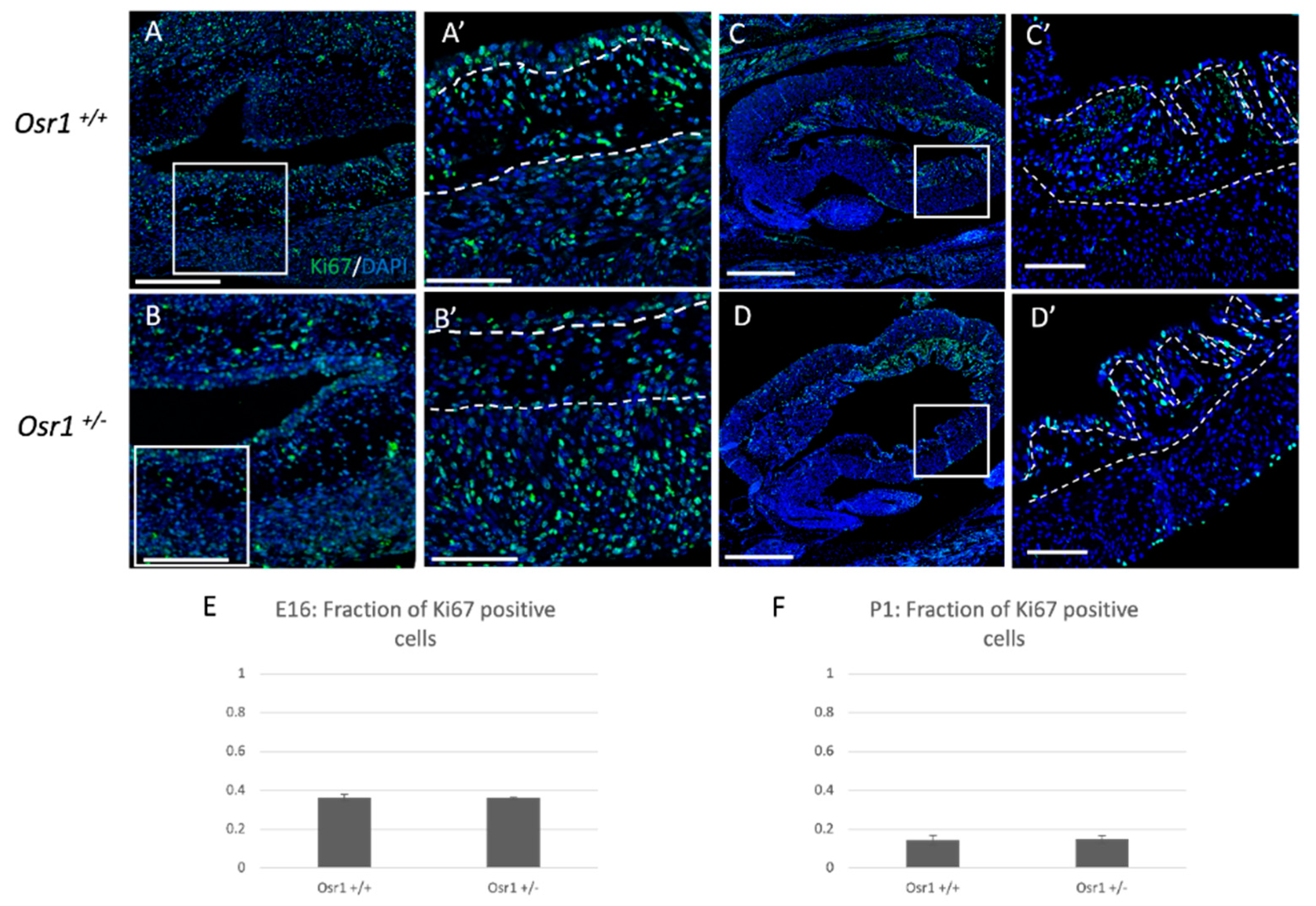
2.2. Osr1+/− Newborn Mice Exhibit Decreased Cellularity in the Lamina Propria Layer
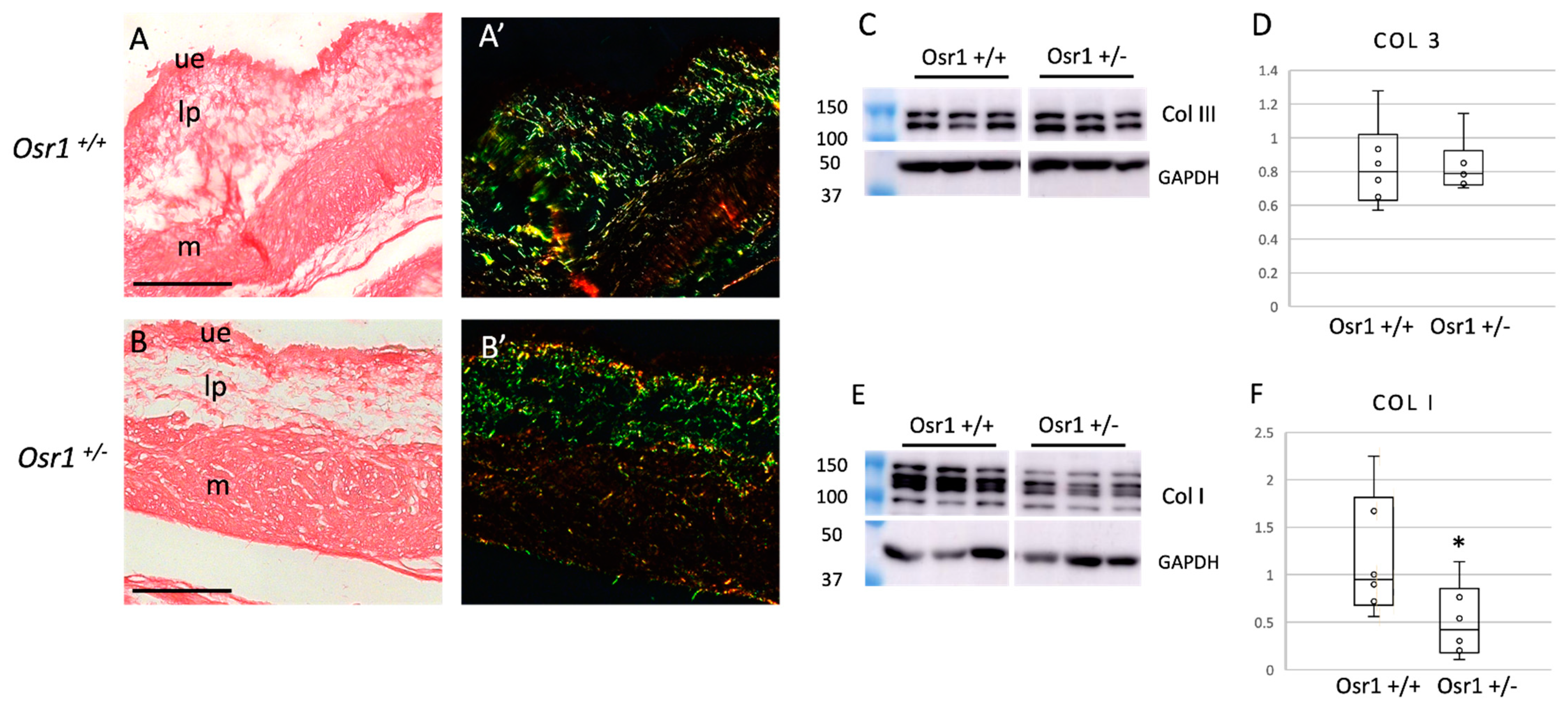
2.3. Osr1+/− Newborn Mice Exhibit Decreased Collagen in the Bladder
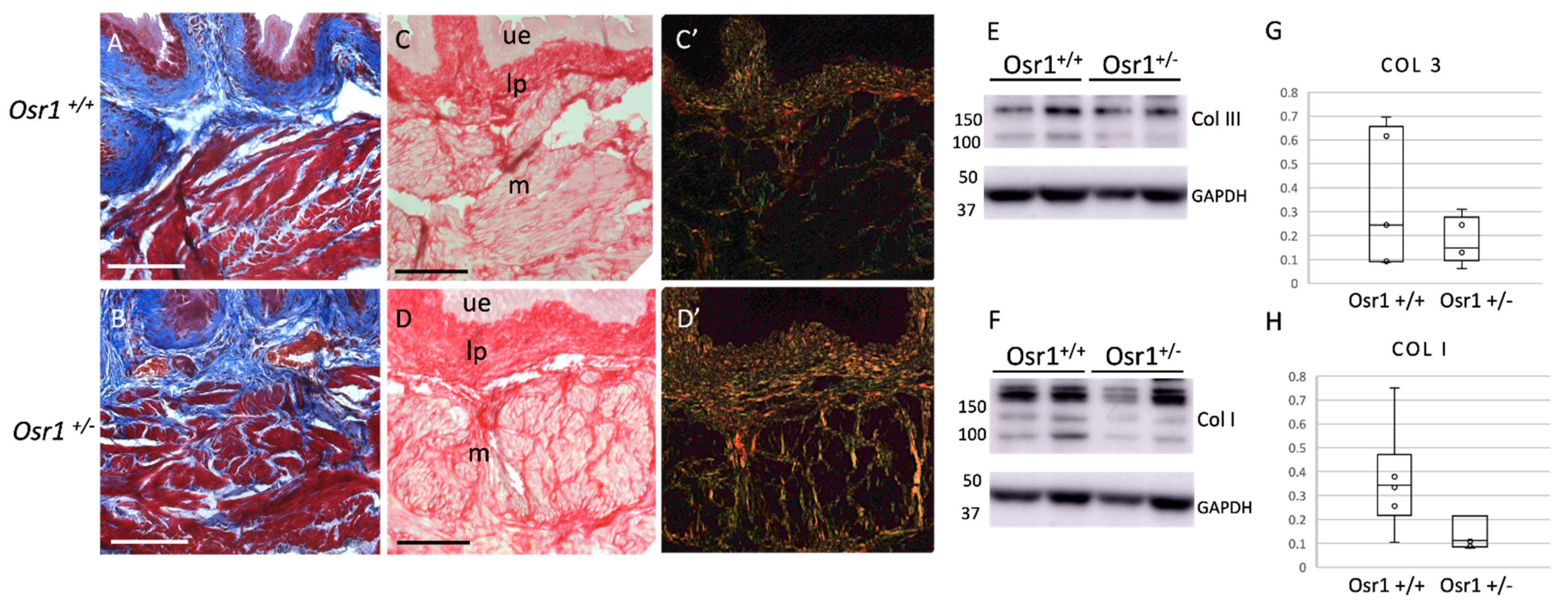
2.4. Osr1+/− Adult Mice Exhibit Increased Collagen in the Bladder
2.5. Osr1+/− Adult Mice Have Poor Bladder Function
3. Discussion
4. Methods
4.1. Mouse Lines
4.2. Tissue Collection and Processing
4.3. RNAScope for mRNA Expression
4.4. Sirius Red Staining
4.5. Immunofluorescent Staining
4.6. TUNEL
4.7. Western Blot Analysis
4.8. Cystometry
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Andersson, K.-E.; McCloskey, K.D. Lamina propria: The functional center of the bladder? Neurourol. Urodyn. 2014, 33, 9–16. [Google Scholar] [CrossRef]
- Cheng, F.; Birder, L.; Kullmann, F.A.; Hornsby, J.; Watton, P.; Watkins, S.C.; Thompson, M.; Robertson, A.M. Layer dependent role of collagen recruitment during loading of the rat bladder wall. Biomech. Model. Mechanobiol. 2018, 17, 403–417. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.L.; Chung, J.S.; Yeung, M.K.; Howard, P.S.; Macarak, E.J. Roles of the lamina propria and the detrusor in tension transfer during bladder filling. Scand. J. Urol. Nephrol. Suppl. 1999, 201, 38–45. [Google Scholar] [PubMed]
- Chang, S.L.; Howard, P.S.; Koo, H.P.; Macarak, E.J. Role of type III collagen in bladder filling. Neurourol. Urodyn. 1998, 17, 135–145. [Google Scholar] [CrossRef]
- Macarak, E.J.; Howard, P.S. The role of collagen in bladder filling. Adv. Exp. Med. Biol. 1999, 462, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Foditsch, E.E.; Roider, K.; Patras, I.; Hutu, I.; Bauer, S.; Janetschek, G.; Zimmermann, R. Structural Changes of the Urinary Bladder After Chronic Complete Spinal Cord Injury in Minipigs. Int. Neurourol. J. 2017, 21, 12–19. [Google Scholar] [CrossRef] [Green Version]
- Anumanthan, G.; Tanaka, S.; Adams, C.; Thomas, J.; Wills, M.; Adams, M.; Hayward, S.; Matusik, R.; Bhowmick, N.; Brock, J.; et al. Bladder Stromal Loss of Transforming Growth Factor Receptor II Decreases Fibrosis After Bladder Obstruction. J. Urol. 2009, 182, 1775–1780. [Google Scholar] [CrossRef] [Green Version]
- Jiang, X.; Chen, Y.; Zhu, H.; Wang, B.; Qu, P.; Chen, R.; Sun, X. Sodium Tanshinone IIA Sulfonate Ameliorates Bladder Fibrosis in a Rat Model of Partial Bladder Outlet Obstruction by Inhibiting the TGF-β/Smad Pathway Activation. PLoS ONE 2015, 10, e0129655. [Google Scholar] [CrossRef] [Green Version]
- Zwaans, B.M.M.; Wegner, K.A.; Bartolone, S.N.; Vezina, C.M.; Chancellor, M.B.; Lamb, L.E. Radiation cystitis modeling: A comparative study of bladder fibrosis radio-sensitivity in C57BL/6, C3H, and BALB/c mice. Physiol. Rep. 2020, 8, e14377. [Google Scholar] [CrossRef] [Green Version]
- Brenner-Anantharam, A.; Cebrian, C.; Guillaume, R.; Hurtado, R.; Sun, T.-T.; Herzlinger, D. Tailbud-derived mesenchyme promotes urinary tract segmentation via BMP4 signaling. Development 2007, 134, 1967–1975. [Google Scholar] [CrossRef] [Green Version]
- Haraguchi, R.; Mo, R.; Hui, C.; Motoyama, J.; Makino, S.; Shiroishi, T.; Gaffield, W.; Yamada, G. Unique functions of Sonic hedgehog signaling during external genitalia development. Development 2001, 128, 4241–4250. [Google Scholar] [CrossRef] [PubMed]
- Liaw, A.; Cunha, G.R.; Shen, J.; Cao, M.; Liu, G.; Sinclair, A.; Baskin, L. Development of the human bladder and ureterovesical junction. Differentiation 2018, 103, 66–73. [Google Scholar] [CrossRef]
- Ikeda, Y.; Zabbarova, I.; Schaefer, C.M.; Bushnell, D.; De Groat, W.C.; Kanai, A.; Bates, C.M. Fgfr2 is integral for bladder mesenchyme patterning and function. Am. J. Physiol. Renal Physiol. 2017, 312, F607–F618. [Google Scholar] [CrossRef]
- Islam, S.S.; Mokhtari, R.B.; Kumar, S.; Maalouf, J.; Arab, S.; Yeger, H.; Farhat, W.A. Spatio-Temporal Distribution of Smads and Role of Smads/TGF-β/BMP-4 in the Regulation of Mouse Bladder Organogenesis. PLoS ONE 2013, 8, e61340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, W.; Yeung, C.K.; Ng, Y.K.; Zhang, J.R.; Hui, C.C.; Kim, P.C. Sonic Hedgehog Mediator Gli2 Regulates Bladder Mesenchymal Patterning. J. Urol. 2008, 180, 1543–1550. [Google Scholar] [CrossRef]
- Zhou, L.; Liu, J.; Olson, P.; Zhang, K.; Wynne, J.; Xie, L. Tbx5 and Osr1 interact to regulate posterior second heart field cell cycle progression for cardiac septation. J. Mol. Cell. Cardiol. 2015, 85, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- James, R.G.; Kamei, C.N.; Wang, Q.; Jiang, R.; Schultheiss, T.M. Odd-skipped related 1 is required for development of the metanephric kidney and regulates formation and differentiation of kidney precursor cells. Development 2006, 133, 2995–3004. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Lan, Y.; Xu, J.; Chang, C.-F.; Brugmann, S.A.; Jiang, R. Odd-skipped related-1 controls neural crest chondrogenesis during tongue development. Proc. Natl. Acad. Sci. USA 2013, 110, 18555–18560. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Lan, Y.; Cho, E.-S.; Maltby, K.M.; Jiang, R. Odd-skipped related 1 (Odd 1) is an essential regulator of heart and urogenital development. Dev. Biol. 2005, 288, 582–594. [Google Scholar] [CrossRef] [Green Version]
- Vallecillo-García, P.; Orgeur, M.; vom Hofe-Schneider, S.; Stumm, J.; Kappert, V.; Ibrahim, D.M.; Börno, S.T.; Hayashi, S.; Relaix, F.; Hildebrandt, K.; et al. Odd skipped-related 1 identifies a population of embryonic fibro-adipogenic progenitors regulating myogenesis during limb development. Nat. Commun. 2017, 8, 1218. [Google Scholar] [CrossRef]
- Rankin, S.A.; Gallas, A.L.; Neto, A.; Gómez-Skarmeta, J.L.; Zorn, A.M. Suppression of Bmp4 signaling by the zinc-finger repressors Osr1 and Osr2 is required for Wnt/β-catenin-mediated lung specification in Xenopus. Dev. Camb. Engl. 2012, 139, 3010–3020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, L.; Xu, J.; Grigg, E.; Slack, M.; Chaturvedi, P.; Jiang, R.; Zorn, A.M. Osr1 functions downstream of Hedgehog pathway to regulate foregut development. Dev. Biol. 2017, 427, 72–83. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.; Gupta, I.; Park, J.; Bram, Y.; Schwartz, R.E. Hedgehog Signaling Demarcates a Niche of Fibrogenic Peribiliary Mesenchymal Cells. Gastroenterology 2020, 159, 624–638. [Google Scholar] [CrossRef] [PubMed]
- Stumm, J.; Vallecillo-García, P.; Vom Hofe-Schneider, S.; Ollitrault, D.; Schrewe, H.; Economides, A.N.; Marazzi, G.; Sassoon, D.A.; Stricker, S. Odd skipped-related 1 (Osr1) identifies muscle-interstitial fibro-adipogenic progenitors (FAPs) activated by acute injury. Stem Cell Res. 2018, 32, 8–16. [Google Scholar] [CrossRef]
- Fillion, M.-L.; El Andalousi, J.; Tokhmafshan, F.; Murugapoopathy, V.; Watt, C.L.; Murawski, I.J.; Capolicchio, J.-P.; El-Sherbiny, M.; Jednak, R.; Gupta, I.R. Heterozygous loss-of-function mutation in Odd-skipped related 1 (Osr1) is associated with vesicoureteric reflux, duplex systems, and hydronephrosis. Am. J. Physiol.-Ren. Physiol. 2017, 313, F1106–F1115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smeulders, N.; Woolf, A.S.; Wilcox, D.T. Extracellular matrix protein expression during mouse detrusor development. J. Pediatr. Surg. 2003, 38, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Liao, J.; Chen, Y.; Zou, C.; Zhang, H.; Cheng, J.; Liu, D.; Li, T.; Zhang, Q.; Li, J.; et al. Single-Cell Transcriptomic Map of the Human and Mouse Bladders. J. Am. Soc. Nephrol. JASN 2019, 30, 2159–2176. [Google Scholar] [CrossRef]
- Fry, C.H.; Sui, G.-P.; Kanai, A.J.; Wu, C. The function of suburothelial myofibroblasts in the bladder. Neurourol. Urodyn. 2007, 26, 914–919. [Google Scholar] [CrossRef]
- Lattouf, R.; Younes, R.; Lutomski, D.; Naaman, N.; Godeau, G.; Senni, K.; Changotade, S. Picrosirius Red Staining: A Useful Tool to Appraise Collagen Networks in Normal and Pathological Tissues. J. Histochem. Cytochem. 2014, 62, 751–758. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.J.; Kim, J.; Na, Y.G.; Kim, K.H. Irreversible Bladder Remodeling Induced by Fibrosis. Int. Neurourol. J. 2021, 25, S3–S7. [Google Scholar] [CrossRef]
- Nagy, A.; Gertsenstein, M.; Vintersten, K.; Behringer, R. Embedding Mouse Embryos and Tissues in Wax. Cold Spring Harb. Protoc. 2007, 2007, pdb.prot4815. [Google Scholar] [CrossRef] [PubMed]
- Velasquez Flores, M.; Mossa, A.H.; Cammisotto, P.; Campeau, L. Succinate decreases bladder function in a rat model associated with metabolic syndrome. Neurourol. Urodyn. 2018, 37, 1549–1558. [Google Scholar] [CrossRef] [PubMed]

| Osr1+/+ | Osr1+/− | p Value | |
|---|---|---|---|
| Basal pressure (cm H2O) | 11.42 (6.50) | 13.58 (9.09) | 0.758 |
| Intermicturition pressure (cm H2O) | 26.06 (8.66) | 23.78 (13.97) | 0.46 |
| Threshold pressure (cm H2O) | 44.94 (12.20) | 44.17 (20.44) | 0.951 |
| Maximum pressure (cm H2O) | 110.66 (32.84) | 97.08 (51.85) | 0.389 |
| Micturition volume (mL) | 0.065 (0.033) | 0.032 (0.007) | 0.001 * |
| Intercontraction interval (seconds) | 165.06 (54.57) | 82.34 (26.37) | <0.001 * |
| Spontaneous activity (cm H2O) | 14.64 (5.03) | 10.20 (9.39) | 0.065 |
| Bladder capacity (mL) | 0.075 (0.025) | 0.036 (0.011) | <0.001 * |
| Residual volume (mL) | 0.013 (0.007) | 0.007 (0.008) | 0.121 |
| Bladder compliance (mL/cm H2O) | 0.003 (0.001) | 0.002 (0.001) | 0.018 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murugapoopathy, V.; Cammisotto, P.G.; Mossa, A.H.; Campeau, L.; Gupta, I.R. Osr1 Is Required for Mesenchymal Derivatives That Produce Collagen in the Bladder. Int. J. Mol. Sci. 2021, 22, 12387. https://doi.org/10.3390/ijms222212387
Murugapoopathy V, Cammisotto PG, Mossa AH, Campeau L, Gupta IR. Osr1 Is Required for Mesenchymal Derivatives That Produce Collagen in the Bladder. International Journal of Molecular Sciences. 2021; 22(22):12387. https://doi.org/10.3390/ijms222212387
Chicago/Turabian StyleMurugapoopathy, Vasikar, Philippe G. Cammisotto, Abubakr H. Mossa, Lysanne Campeau, and Indra R. Gupta. 2021. "Osr1 Is Required for Mesenchymal Derivatives That Produce Collagen in the Bladder" International Journal of Molecular Sciences 22, no. 22: 12387. https://doi.org/10.3390/ijms222212387
APA StyleMurugapoopathy, V., Cammisotto, P. G., Mossa, A. H., Campeau, L., & Gupta, I. R. (2021). Osr1 Is Required for Mesenchymal Derivatives That Produce Collagen in the Bladder. International Journal of Molecular Sciences, 22(22), 12387. https://doi.org/10.3390/ijms222212387









