An Enhanced Water Solubility and Stability of Anthocyanins in Mulberry Processed with Hot Melt Extrusion
Abstract
:1. Introduction
2. Results and Discussions
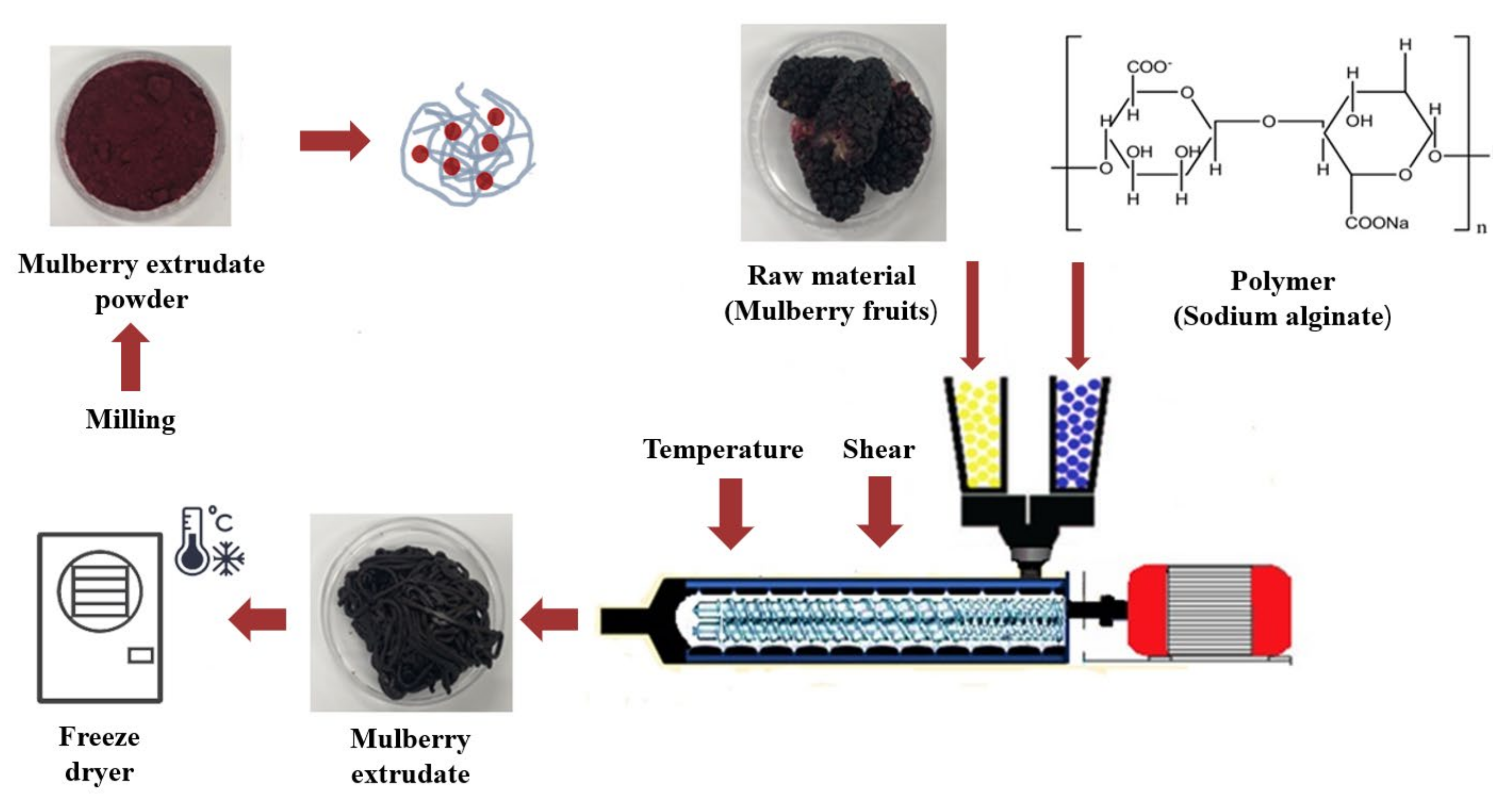
2.1. Colloidal Solid Dispersion Systems by HME
2.2. An Analysis of Total Flavonoids, Phenolic Content from Different MUL Formulations
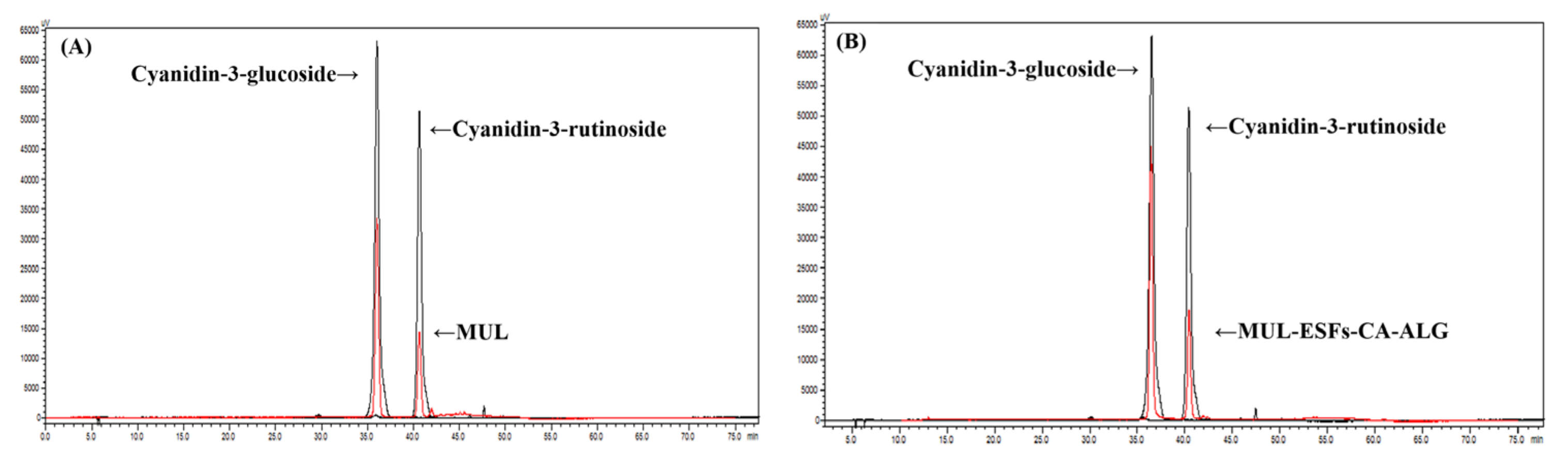
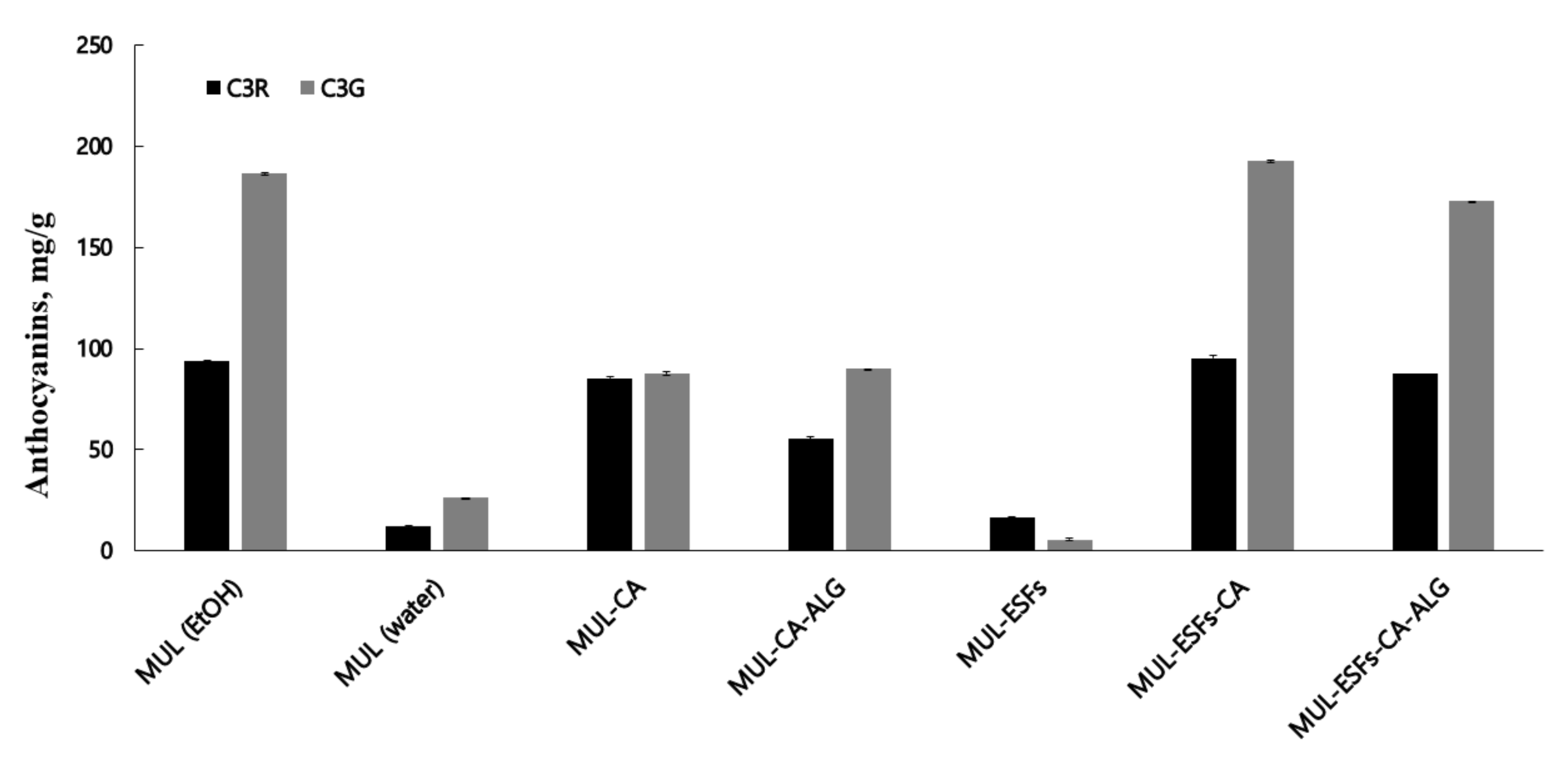
2.3. An Analysis of ATC Content Obtained from MUL-ESFs
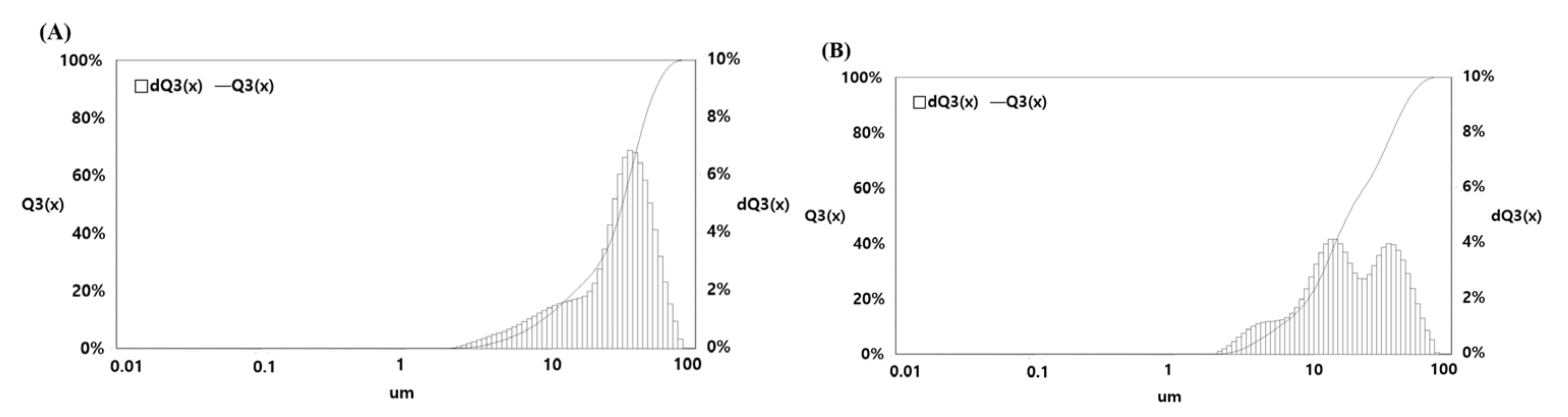
2.4. Particle Size and Solubility Analysis
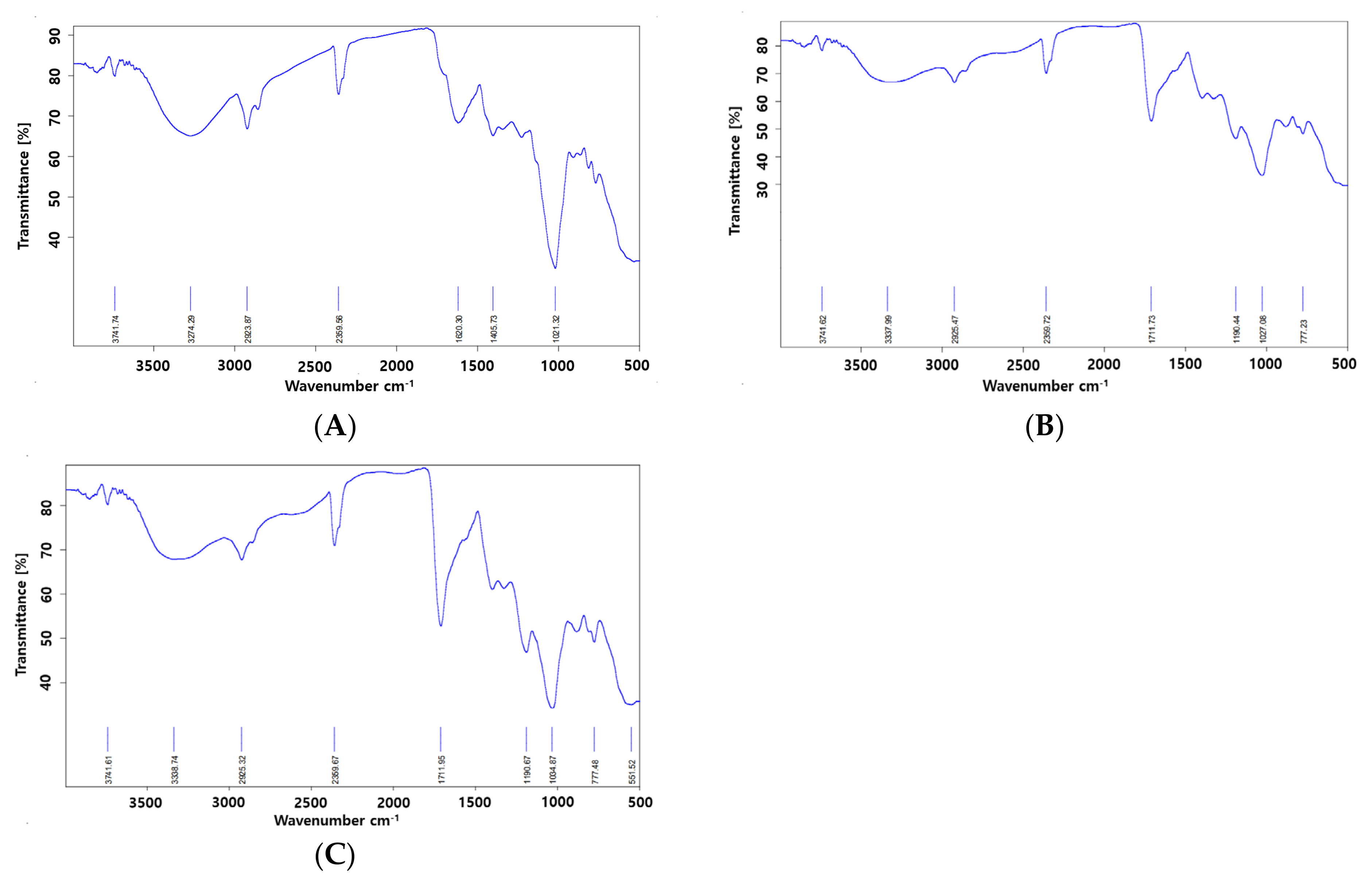
2.5. FT-IR of Different Formulations
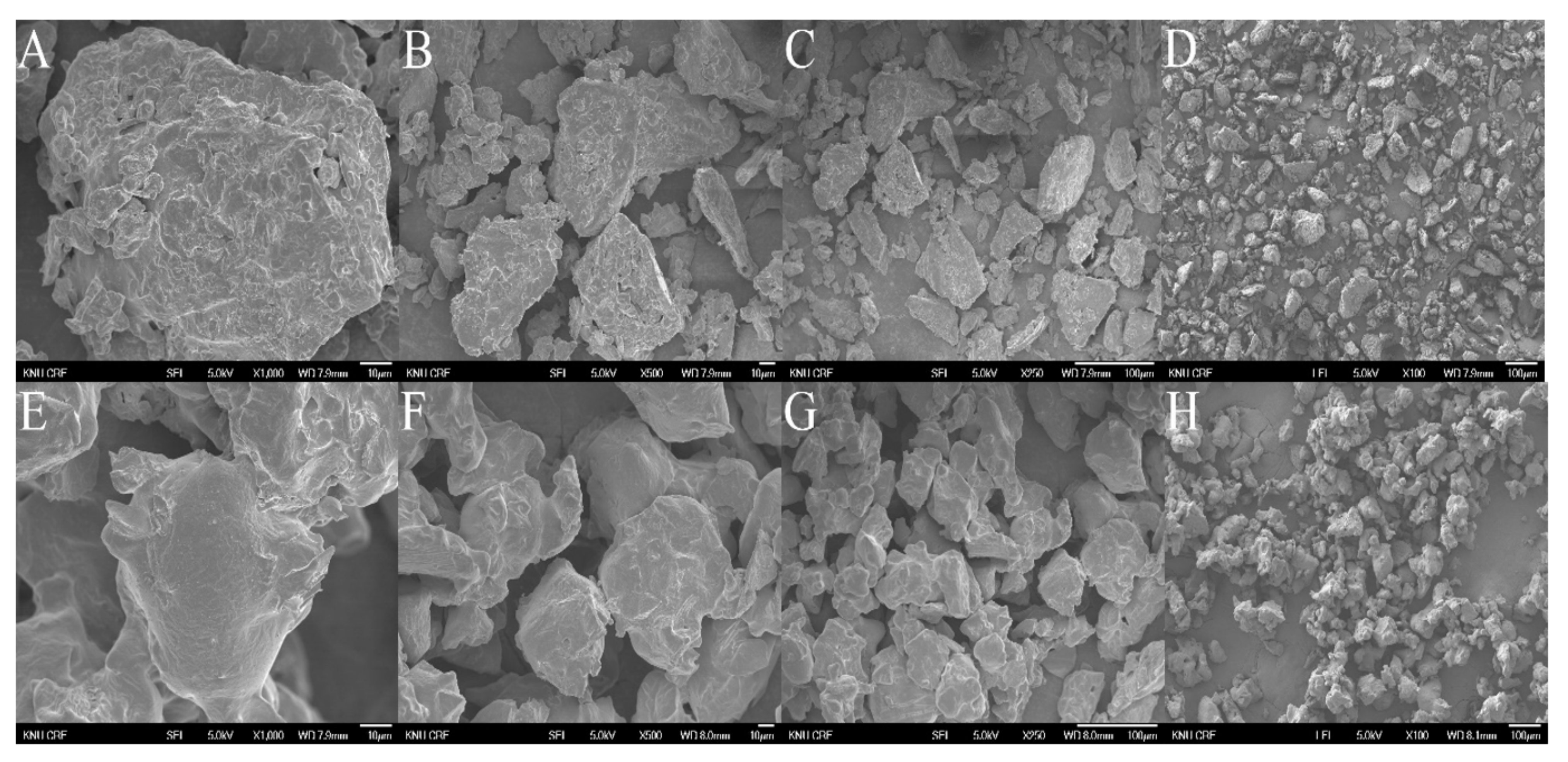
2.6. The Morphology of MUL Formulations
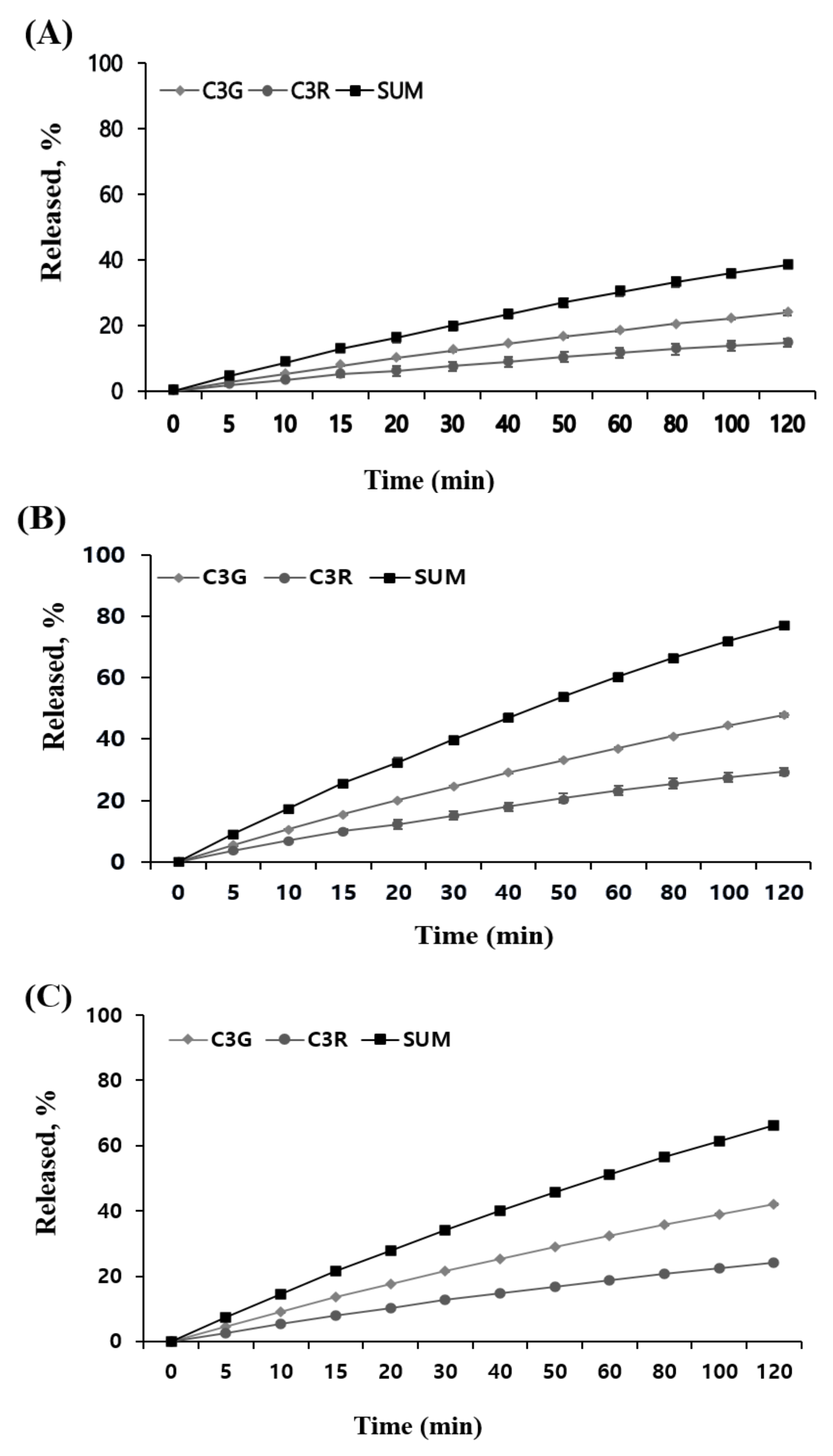
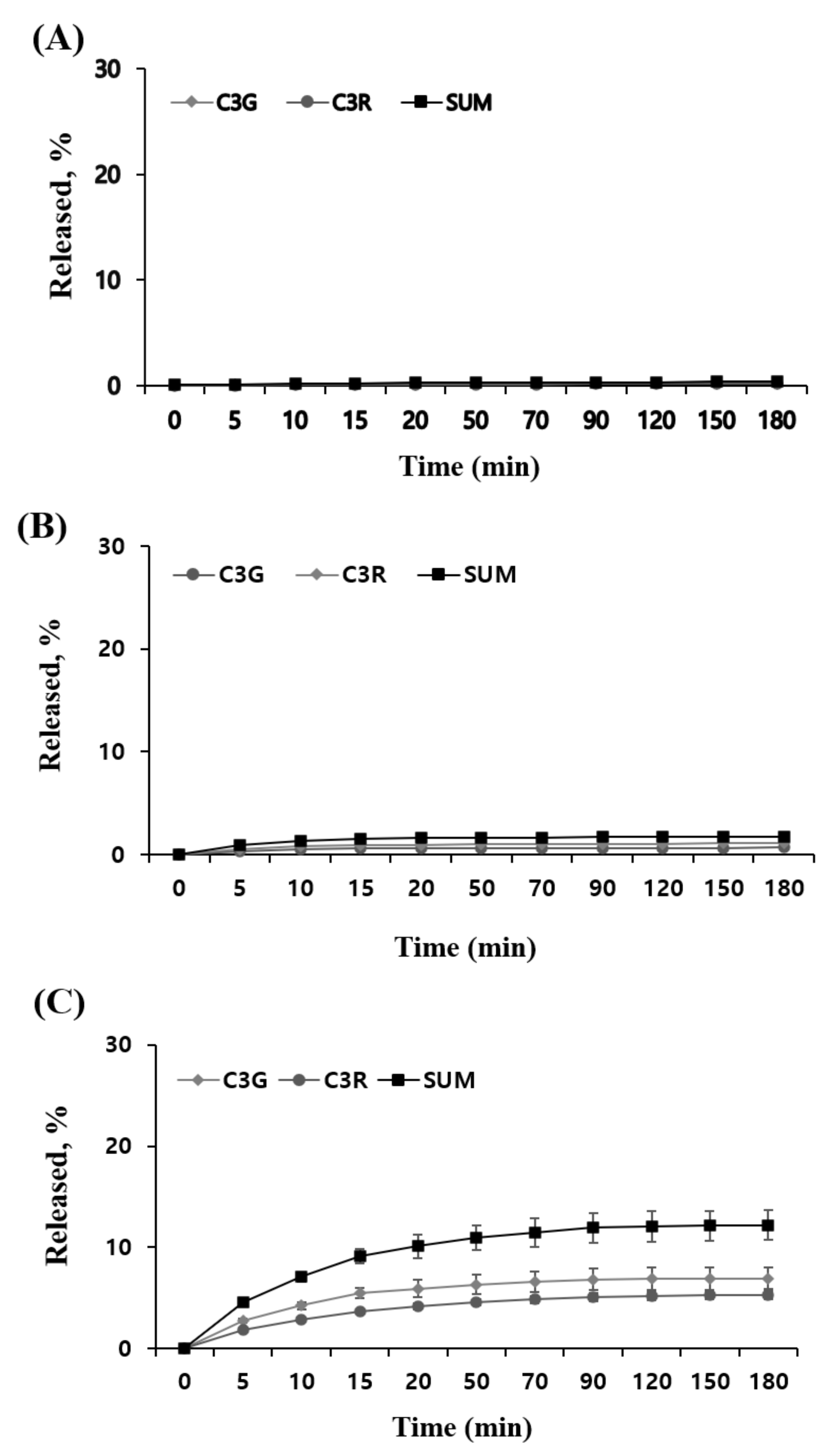
2.7. An In Vitro Release in Different MUL Formulations
3. Materials and Methods
3.1. Materials
3.2. The Preparation of Colloidal Solid Dispersion Systems by HME
3.3. The Total Flavonoid Content Analysis of HME-ESFs
3.4. The Total Phenol Content Analysis of Different MUL Formulations
3.5. High Pressure Liquid Chromatography (HPLC) Analysis of Different MUL Formulations
3.6. The Total ATC Content Analysis of Different MUL Formulations
3.7. The Chromatic Difference Analysis in Different MUL Formulations
3.8. Particle Characterization
3.8.1. Particle Size Analysis
3.8.2. Water Solubility
3.8.3. Fourier-Transform Infrared Spectroscopy (FT-IR) Analysis
3.8.4. Scanning Electron Microscopy (SEM) Analysis
3.9. In Vitro Release Study
3.9.1. Simulated Gastric Fluid (SGF)
3.9.2. Simulated Intestinal Fluid (SIF)
3.10. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lu, C.; Li, C.; Chen, B.; Shen, Y. Composition and antioxidant, antibacterial, and anti-HepG2 cell activities of polyphenols from seed coat of amygdalus pedunculata Pall. Food Chem. 2018, 265, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Francis, F.J.; Markakis, P.C. Food colorants: Anthocyanins. Crit. Rev. Food Sci. Nutr. 1989, 27, 273–314. [Google Scholar] [CrossRef] [PubMed]
- Clifford, M.N. Anthocyanins-nature, occurrence and dietary burden. J. Sci. Food Agric. 2000, 80, 1063–1072. [Google Scholar] [CrossRef]
- Giusti, M.M.; Wrolstad, R.E. Characterization of red radish anthocyanins. J. Food Sci. 1996, 61, 322–326. [Google Scholar] [CrossRef]
- Pool-Zobel, B.L.; Bub, A.; Schröder, N.; Rechkemmer, G. Anthocyanins are potent antioxidants in model systems but do not reduce endogenous oxidative DNA damage in human colon cells. Eur. J. Nutr. 1999, 38, 227–234. [Google Scholar] [CrossRef]
- Kay, C.D.; Holub, B.J. The effect of wild blueberry (Vaccinium angustifolium) consumption on postprandial serum antioxidant status in human subjects. Br. J. Nutr. 2002, 88, 389–397. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Nair, M.G.; Strasburg, G.M.; Chang, Y.C.; Booren, A.M.; Gray, J.I.; deWitt, D.L. Antioxidant and antiinflammatory activities of anthocyanins and their aglycon, cyanidin, from tart cherries. J. Nat. Prod. 1999, 62, 294–296. [Google Scholar] [CrossRef]
- Tsuda, T.; Horio, F.; Uchida, K.; Aoki, H.; Osawa, T. Dietary cyanidin-3-o-β-D-glucoside-rich purple corn color prevents obesity and ameliorates hyperglycemia in mice. J. Nutr. 2003, 133, 2125–2130. [Google Scholar] [CrossRef]
- Canter, P.H.; Ernst, E. Anthocyanosides of Vaccinium myrtillus (bilberry) for nightvision-a systematic review of placebo-controlled trials. Surv. Ophthalmol. 2004, 49, 38–50. [Google Scholar] [CrossRef]
- Janssens, S.; Van den Mooter, G. Review: Physical chemistry of solid dispersions. J. Pharm. Pharmacol. 2009, 61, 1571–1586. [Google Scholar] [CrossRef]
- Kong, J.M.; Chia, L.S.; Goh, N.K.; Chia, T.F.; Brouillard, R. Analysis and biological activities of anthocyanins. Phytochemistry 2003, 64, 923–933. [Google Scholar] [CrossRef]
- Prior, R.L. Absorption and metabolism of anthocyanins: Potential health effects. In Phytochemicals: Mechanism of Action; CRC Press: Boca Raton, FL, USA, 2004; pp. 1–19. [Google Scholar]
- Nielsen, I.L.F.; Dragested, L.O.; Ravn-Haren, G.; Freese, R.; Rasmussen, S.E. Absorption and excretion of black currant anthocyanins in humans and watanabe heritable hyperlipidemic rabbits. J. Agric. Food Chem. 2003, 51, 2813–2820. [Google Scholar] [CrossRef]
- Felgines, C.; Talavéra, S.; Gonthier, M.P.; Texier, O.; Scalbert, A.; Lamaison, J.L.; Rémésy, C. Strawberry anthocyanins are recovered in urine as glucuro- and sulfoconjugates in humans. J. Nutr. 2003, 133, 1296–1301. [Google Scholar] [CrossRef] [Green Version]
- Huh, H.W.; Na, Y.G.; Kang, H.C.; Kim, M.; Han, M.; Pham, T.M.A.; Lee, H.; Baek, J.S.; Lee, H.K.; Cho, C.W. Novel self-floating tablet for enhanced oral bioavailability of metformin based on cellulose. Int. J. Pharm. 2021, 592, 120113. [Google Scholar] [CrossRef]
- Ryu, S.; Park, S.; Lee, H.Y.; Lee, H.; Cho, W.C.; Baek, J.S. Biodegradable nanoparticles-loaded PLGA microcapsule for the enhances encapsulation efficiency and controlled release of hydrophilic drug. Int. J. Mol. Sci. 2021, 22, 2792. [Google Scholar] [CrossRef]
- Na, Y.G.; Byeon, J.J.; Kim, M.K.; Han, M.G.; Cho, C.W.; Baek, J.S.; Lee, H.K.; Shin, Y.G. Pharmacokinetic/pharmacodynamic modeling to predict the antiplatelet effect of the ticagrelor-loaded self-microemulsifying drug delivery system in rats. Mol. Pharm. 2020, 17, 1079–1089. [Google Scholar] [CrossRef]
- Kim, M.K.; Ki, D.H.; Na, Y.G.; Lee, H.S.; Baek, J.S.; Lee, J.Y.; Lee, H.K.; Che, C.W. Optimization of mesoporous silica nanoparticles through statistical design of experiment and the application for the anticancer drug. Pharmaceutics 2021, 13, 184. [Google Scholar] [CrossRef]
- Desai, K.G.H.; Park, H.J. Recent developments in microencapsulation of food ingredients. Dry. Technol. 2005, 23, 1361–1394. [Google Scholar] [CrossRef]
- Breitenbach, J. Melt extrusion: From process to drug delivery technology. Eur. J. Pharm. Biopharm. 2002, 54, 107–117. [Google Scholar] [CrossRef]
- Azad, M.O.K.; Adnan, M.; Sung, I.J.; Lim, J.D.; Baek, J.S.; Lim, Y.S.; Park, C.H. Development of value-added functional food by fusion of colored potato and buckwheat flour through hot-melt extrusion. J. Food Process. Preserv. 2021, e15312. [Google Scholar] [CrossRef]
- Maniruzzaman, M.; Boateng, J.S.; Snowden, M.J.; Douroumis, D. A review of hot-melt extrusion: Process thechnology to pharmaceutical products. ISRN Pharm. 2012, 2012, 436763. [Google Scholar] [CrossRef] [Green Version]
- Ottoboni, M.; Spranghers, T.; Pinotti, L.; Baldi, A.; Jaeghere, W.D.; Eeckhout, M. Inclusion of Hermetia illucens larvae or prepupae in an experimental extruded feed: Process optimization and impact on in vitro digestibility. Ital. J. Anim. Sci. 2018, 17, 418–427. [Google Scholar] [CrossRef] [Green Version]
- Chokshi, R.; Zia, H. Hot-melt extrusion technique: A review. Iran. J. Pharm. Res. 2004, 3, 3–16. [Google Scholar]
- Ghebremeskel, A.N.; Vemavarapu, C.; Lodaya, M. Use of surfactants as plasticizers in preparing solid dispersions of poorly soluble API: Selection of polymer-surfactant combinations using solubility parameters and testing the processability. Int. J. Pharm. 2007, 328, 119–129. [Google Scholar] [CrossRef]
- Thiry, J.; Lebrun, P.; Vinassa, C.; Adam, M.; Netchacovitch, L.; Ziemons, E.; Hubert, P.; Krier, F.; Evrard, B. Continuous production of itraconazole-based solid dispersions by hot melt extrusion: Preformulation, optimization and design space determination. Int. J. Pharm. 2016, 515, 114–124. [Google Scholar] [CrossRef]
- Amidon, G.E.; He, X.; Hageman, M.J. Physicochemical characterization and principles of oral doses form selection. Burg. Med Chem. Drug Discov. 2003, 5, 15–19. [Google Scholar] [CrossRef]
- Grunhagen, H.H.; Muller, O.N. Melt extrusion technology. Pharm. Manuf. Pack. Sourcer 1995, 1, 167–170. [Google Scholar]
- Knels, L.; Valtink, M.; Piazena, H.; de la Vega Marin, J.; Gommel, K.; Lupp, A.; Roehlecke, C.; Mehner, M.; Funk, R.H.W. Effects of narrow-band IR-A and of water-filtered infrared A on fibroblasts. Photochem. Photobiol. 2016, 92, 475–487. [Google Scholar] [CrossRef]
- Azad, M.O.K.; Kang, W.S.; Lim, J.D.; Park, C.H. Bio-fortification of Angelica gigas Nakai nano-powder using bio-polymer by hot melt extrusion to enhance the bioaccessibility and functionality of nutraceutical compounds. Pharmaceuticals 2019, 13, 3. [Google Scholar] [CrossRef] [Green Version]
- Miguel, M.G. Anthocyanin: Antioxidant and/or anti-inflammatory activities. J. Appl. Pharm. Sci. 2011, 1, 7–15. [Google Scholar]
- Piao, J.; Lee, J.Y.; Weon, J.B.; Ma, C.J.; Ko, H.J.; Kim, D.D.; Kang, W.S.; Cho, H.J. Angelica gigas Nakai and soluplus based solid formulations prepared by hot-melting extrusion: Oral absorption enhancing and memory ameliorating effects. PLoS ONE 2015, 10, e0124447. [Google Scholar] [CrossRef]
- Haghi, G.; Hatami, A. Simultaneous quantification of flavonoids and phenolic acids in plant materials by a newly developed isocratic high-perfomance liquid chromatography approach. J. Agric. Food Chem. 2010, 58, 10812–10816. [Google Scholar] [CrossRef] [PubMed]
- Davidov-Pardo, G.; Arozarena, I.; Marin-Arroyo, M.R. Stability of polyphenolic extracts from grape seeds after thermal treatments. Eur. Food Res. Technol. 2011, 232, 211–220. [Google Scholar] [CrossRef]
- Andersen, O.M.; Markham, K.R. Flavonoids: Chemistry, Biochemistry and Applications; CRC Press: Boca Raton, FL, USA, 2005; pp. 58–63. [Google Scholar]
- Ruvinov, E.; Cohen, S. Alginate biomaterial for the treatment of myocardial infarction: Progress, translational strategies, and clinical outlook from ocean algae to patient bedside. Adv. Drug Deliv. Rev. 2016, 96, 54–76. [Google Scholar] [CrossRef]
- Lee, S.Y.; Nam, S.Y.; Choi, Y.H.; Kim, M.J.; Koo, J.S.; Chae, B.J.; Kang, W.S.; Cho, H.J. Fabrication and characterizations of hot-melt extruded nanocomposites based on zinc sulfate monohydrate and soluplus. J. Appl. Sci. 2017, 7, 902. [Google Scholar] [CrossRef] [Green Version]
- Tallury, P.; Payton, K.; Santra, S. Silica-based multimodal/multifunctional nanoparticles for bioimaging and biosensing applications. Nanomedicine 2008, 3, 579–592. [Google Scholar] [CrossRef]
- Noyes, A.A.; Whitney, W.R. The rate of solution of solid substances in their own solutions. J. Am. Chem. Soc. 1897, 19, 930–934. [Google Scholar] [CrossRef] [Green Version]
- Kawabata, Y.; Wada, K.; Nakatani, M.; Yamada, S.; Onoue, S. Formulation design for poorly water-soluble drugs based on biopharmaceutics classification system: Basic approaches and practical applications. Int. J. Pharm. 2011, 420, 1–10. [Google Scholar] [CrossRef]
- Fox, S.C. Remington Education Pharmaceutics; Pharmaceutical Press: London, UK, 2014; pp. 28–53. [Google Scholar]
- Silverstein, R.M.; Webster, F.X.; Kiemle, D.J. Identificacao Espectrométrica de Compostos Orgânicos/Spectrometric Identification of Organic Compounds; LTC: Rio de Janeiro, Brazil, 2006; pp. 425–456. [Google Scholar]
- Correia, L.P.; Procopio, J.V.V.; de Santana, C.P.; Santos, A.F.O.; Cavalcante, H.M.M.; Macedo, R.O. Characterization of herbal medicine with different particle sizes using pyrolysis GC/MS, SEM, and thermal techniques. J. Therm. Anal. Calorim. 2013, 111, 1691–1698. [Google Scholar] [CrossRef]
- Karaman, R. Prodrugs design based on inter- and intramolecular chemical processes. Chem. Biol. Drug Des. 2013, 82, 643–668. [Google Scholar] [CrossRef]
- Patel, N.; Lalwani, D.; Gollmer, S.; Injeti, E.; Sari, Y.; Nesamony, J. Development and evaluation of a calcium alginate based oral ceftriaxone sodium formulation. Prog. Biomater. 2016, 5, 117–133. [Google Scholar] [CrossRef] [Green Version]
- Guan, J.; Zhang, Y.; Liu, Q.; Zhang, X.; Chokshi, R.; Mao, S. Exploration of alginates as potential stabilizers of nanosuspension. AAPS PharmSciTech 2017, 18, 3172–3181. [Google Scholar] [CrossRef]
- Zhuang, Y.; Zhou, W. Microencapsulation of anthocyanins through two-step emulsification and release characteristics during in vitro digestion. Food Chem. 2019, 278, 357–363. [Google Scholar] [CrossRef]
- Merisko-Liversidge, E.M.; Liversidge, G.G. Drug nanoparticles: Formulating poorly water-soluble compounds. Toxicol. Pathol. 2008, 36, 43–48. [Google Scholar] [CrossRef]
- Dong, L.M.; Luan, N.T.; Thuy, D.T.K. Enhancing the viability rate of probiotic by co-encapsulating with prebiotic in alginate microcapsules supplemented to cupcake production. Microbiol. Biotechnol. Lett. 2020, 48, 113–120. [Google Scholar] [CrossRef]
- Singhal, S.; Lohar, V.K.; Arora, V. Hot-melt extrusion technique. Pharm. Sci. 2011, 2, WMC001459. [Google Scholar] [CrossRef]
| Sample | Total Flavonoid Contents (mg/100 g) | Total Phenol Contents (mg/100 g) | Total Anthocyanin Contents (mg/100 g) | |
|---|---|---|---|---|
| EtOH (7) | MUL (1) | 547.48 ± 14.71 a* | 1109.77 ± 79.43 b | 247.87 ± 8.34 b |
| Water (8) | MUL | 46.47 ± 10.83 f | 813.91 ± 10.55 f | 95.60 ± 3.33 f |
| MUL-CA (2) | 52.81 ± 9.96 c | 2225.28 ± 50.51 e | 264.63 ± 6.28 d | |
| MUL-CA-ALG (3) | 61.95 ± 39.00 d | 1489.78 ± 101.98 d | 149.60 ± 5.00 e | |
| MUL-ESFs (4) | 114.10 ± 7.60 e | 3380.27 ± 53.47 g | 259.73 ± 6.71 g | |
| MUL-ESFs-CA (5) | 79.20 ± 4.45 a | 2668.72 ± 25.35 a | 331.08 ± 10.15 a | |
| MUL-ESFs-CA-ALG (6) | 68.59 ± 1.20 b | 3076.80 ± 37.10 c | 308.59 ± 8.08 c | |
| Sample | Color | L*, a*, b* | |
|---|---|---|---|
| EtOH (7) | MUL |  | L*; 31.12, a*; 37.50, b*; 17.50 |
| Water (8) | MUL (1) |  | L*; 30.64, a*; 34.67, b*; 22.53 |
| MUL-CA (2) |  | L*; 32.73, a*; 56.64, b*; 48.48 | |
| MUL-CA-ALG (3) |  | L*; 35.67, a*; 56.03, b*; 41.24 | |
| MUL-ESFs (4) |  | L*; 18.46, a*; 35.20, b*; 14.64 | |
| MUL-ESFs-CA (5) |  | L*; 32.87, a*; 57.17, b*; 48.08 | |
| MUL-ESFs-CA-ALG (6) |  | L*; 29.90, a*; 57.25, b*; 48.64 | |
| Formulation | Solubility (%) |
|---|---|
| MUL (1) | 13.26 ± 0.41% d* |
| MUL-CA (2) | 13.63 ± 0.18% cd |
| MUL-CA-ALG (3) | 13.41 ± 0.25% d |
| MUL-ESFs (4) | 13.96 ± 0.33% c |
| MUL-ESFs-CA (5) | 16.08 ± 0.21% b |
| MUL-ESFs-CA-ALG (6) | 17.03 ± 0.32% a |
| HPLC System | ||
|---|---|---|
| Column | YMC-Triart C18 | |
| Flow rate | 1.0 mL/min | |
| Injection volume | 10 μL | |
| Oven temperature | 30 °C | |
| Detector wavelength | 535 nm | |
| Mobile phase | Gradient | |
| Solvent A | Water:formic acid (90:10) | |
| Solvent B | Acetonitrile:methanol:water:formic acid (22.5:22.5:40:10) | |
| Elution time (min) | A | B |
| 0 | 93 | 7 |
| 35 | 75 | 25 |
| 45 | 35 | 65 |
| 46 | 0 | 100 |
| 50 | 35 | 65 |
| 60 | 75 | 25 |
| 70 | 93 | 7 |
| 75 | 93 | 7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Go, E.-J.; Ryu, B.-R.; Ryu, S.-J.; Kim, H.-B.; Lee, H.-T.; Kwon, J.-W.; Baek, J.-S.; Lim, J.-D. An Enhanced Water Solubility and Stability of Anthocyanins in Mulberry Processed with Hot Melt Extrusion. Int. J. Mol. Sci. 2021, 22, 12377. https://doi.org/10.3390/ijms222212377
Go E-J, Ryu B-R, Ryu S-J, Kim H-B, Lee H-T, Kwon J-W, Baek J-S, Lim J-D. An Enhanced Water Solubility and Stability of Anthocyanins in Mulberry Processed with Hot Melt Extrusion. International Journal of Molecular Sciences. 2021; 22(22):12377. https://doi.org/10.3390/ijms222212377
Chicago/Turabian StyleGo, Eun-Ji, Byeong-Ryeol Ryu, Su-Ji Ryu, Hyun-Bok Kim, Hyun-Tai Lee, Jin-Woo Kwon, Jong-Suep Baek, and Jung-Dae Lim. 2021. "An Enhanced Water Solubility and Stability of Anthocyanins in Mulberry Processed with Hot Melt Extrusion" International Journal of Molecular Sciences 22, no. 22: 12377. https://doi.org/10.3390/ijms222212377
APA StyleGo, E.-J., Ryu, B.-R., Ryu, S.-J., Kim, H.-B., Lee, H.-T., Kwon, J.-W., Baek, J.-S., & Lim, J.-D. (2021). An Enhanced Water Solubility and Stability of Anthocyanins in Mulberry Processed with Hot Melt Extrusion. International Journal of Molecular Sciences, 22(22), 12377. https://doi.org/10.3390/ijms222212377







