A Hypothetical Model Suggesting Some Possible Ways That the Progesterone Receptor May Be Involved in Cancer Proliferation
Abstract
:1. Knowledge of Transplant Immunology Has Led to Some Effective Anticancer Therapies
Autologous Immunotherapy
2. The Developing of Malignant Tumors with Neoantigens May Utilize Systems That Provide the Body a Mechanism to Control Self-Tolerance Check-Point Inhibitors
2.1. Cytotoxic T-Lymphocyte Associated Molecule-4 (CTLA-4)
2.2. Programmed Cell Death (PD-1) and Programmed Cell Death Ligand 1 and Ligand 2
3. Survival of the Fetal-Placental Unit and Malignant Tumors
3.1. Similarities between Trophoblast and Cancer Cells
3.2. The Role of Distinct Lymphocyte Cell Population in Regulating Feto-Maternal Tolerance and Tolerance to Malignant Tumors
3.3. Innate Lymphoid Cells (ILCs) in the Decidua of the Placenta and Cancer Cells
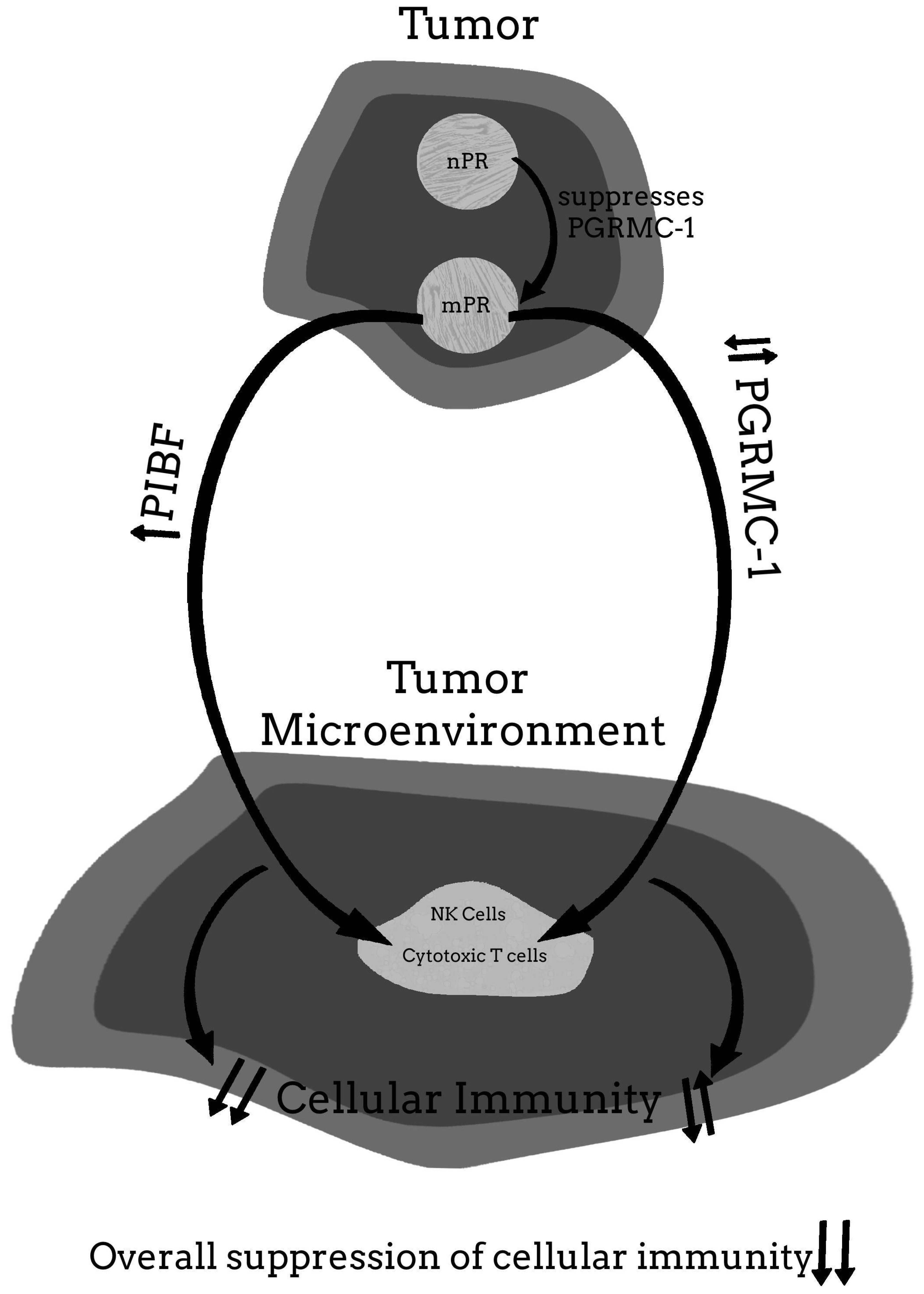
4. Progesterone Associated Molecules That the Cytoplasmic 34 kDa Splice Variant of PIBF Plays a Role in Fetal-Placental and Malignant Tumor Proliferation and Metastases
4.1. The Importance of Progesterone (P) to Prevent Miscarriage
4.2. The Progesterone Induced Blocking Factor (Alternate Name Progesterone Immunomodulatory Binding Factor 1)
4.3. Evidence That the Cytoplasmic 34 kDa Splice Variant of PIBF Plays a Role in Preventing Immune Rejection of the Fetal-Placental Unit
4.4. Cancer Cell Line Studies to Evaluate Whether PIBF Is Secreted by Cancer Cells and the Effect of P and Anti-PR Drugs on PIBF up and Downregulation
5. Animal and Clinical Trials Using Mifepristone to Inhibit Cancer Progression
5.1. Early Human Clinical Trials with Cancers Positive for the Classical Nuclear P Receptor
5.2. Controlled Studies Evaluating Efficacy of Mifepristone in Improving Quality and Length of Life in Spontaneous Murine Cancers Not Known to Be Associated with the Classical nPR
5.3. Human Experience in Treating Various Advanced Cancers Not Associated with the Classical nPR with Mifepristone
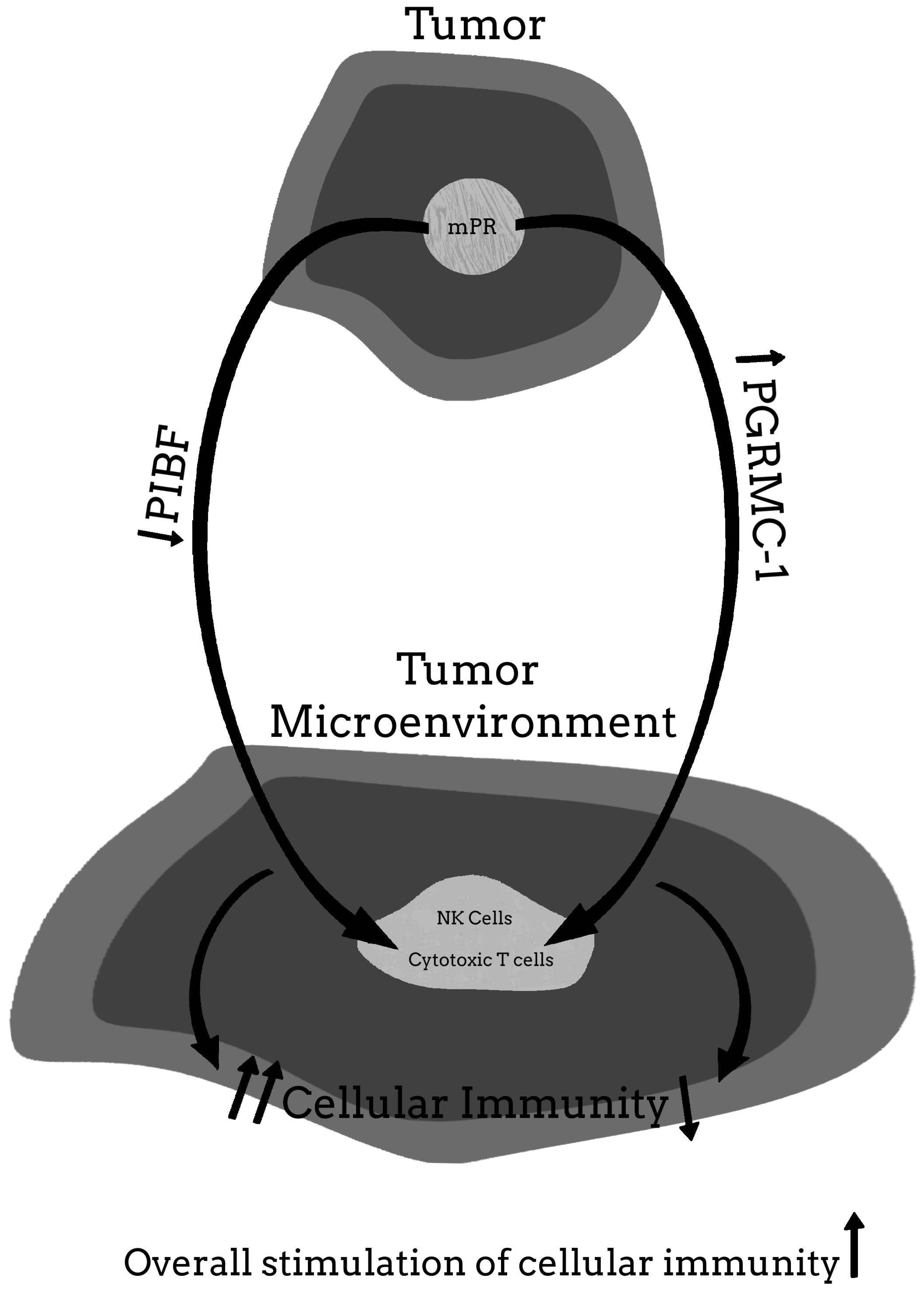
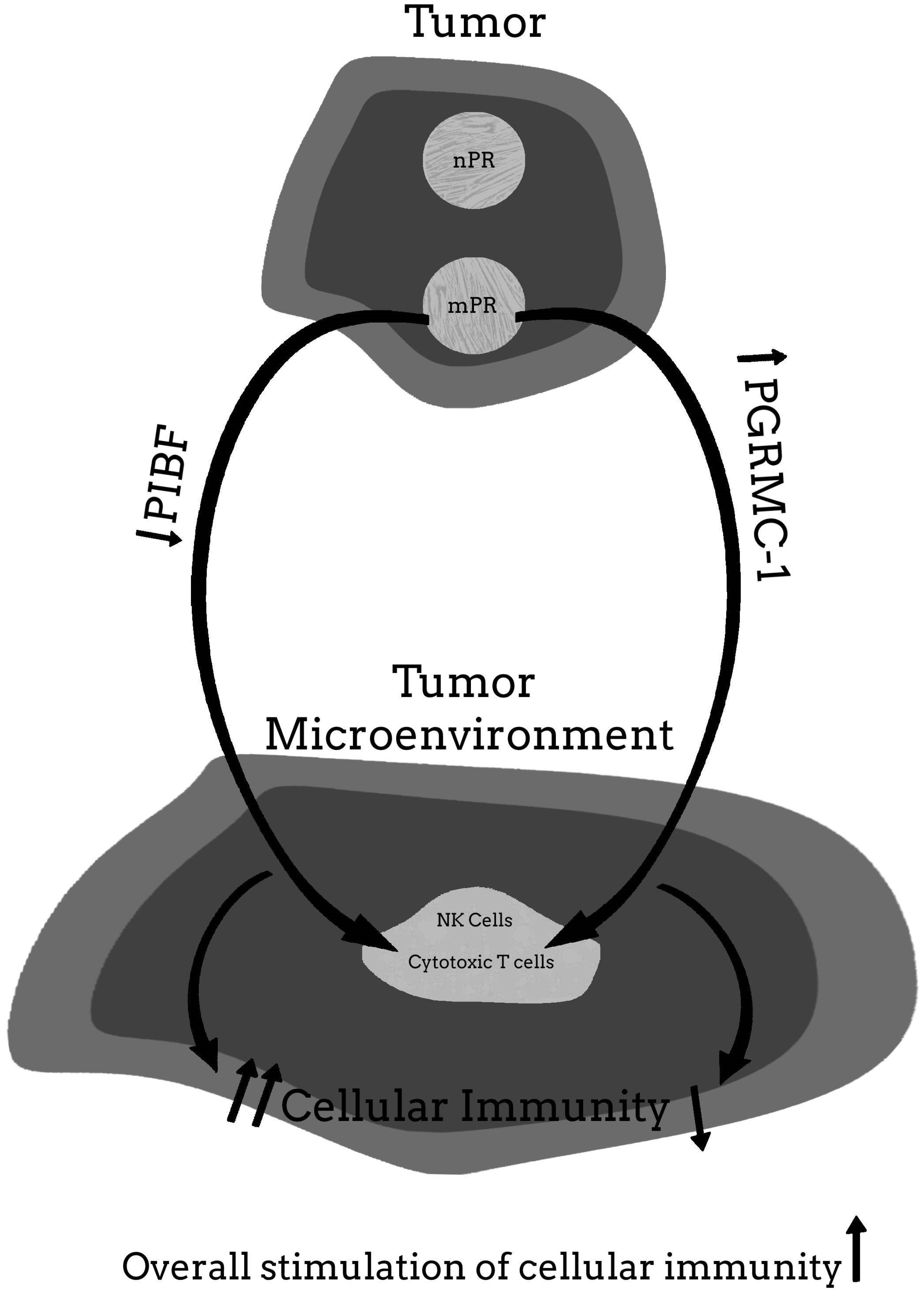
5.4. The Possibility That the Benefits of Mifepristone Treatment for Cancer May Involve Some Protein or Pathway Other Than PIBF
6. Conclusions and Final Thoughts
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Sahin, U.; Özlem, T. Personalized vaccines for cancer immunotherapy. Science 2018, 359, 1355–1360. [Google Scholar] [CrossRef] [Green Version]
- Coulie, P.G.; Van den Eynde, B.J.; Van Der Bruggen, P.; Boon-Falleur, T. Tumour antigens recognized by T lymphocytes: At the core of cancer immunotherapy. Nat. Rev. Cancer 2014, 14, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Bossi, G.; Trambas, C.; Booth, S.; Clark, R.; Stinchcombe, J.; Griffiths, G.M. The secretory synapse: The secrets of a serial killer. Immunol. Rev. 2002, 189, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Durgeau, A.; Virk, Y.; Corgnac, S.; Mami-Chouaib, F. Recent Advances in Targeting CD8 T-Cell Immunity for More Effective Cancer Immunotherapy. Front. Immunol. 2018, 9, 14. [Google Scholar] [CrossRef] [PubMed]
- Boon, T.; Coulie, P.G.; Eynde, B.V.D. Tumor antigens recognized by T cells. Immunol. Today 1997, 18, 267–268. [Google Scholar] [CrossRef]
- Weynants, P.; Thonnard, J.; Marchand, M.; Delos, M.; Boon, T.; Coulie, P.G. Derivation of Tumor-specific Cytolytic T-Cell Clones from Two Lung Cancer Patients with Long Survival. Am. J. Respir. Crit. Care Med. 1999, 159, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Karanikas, V.; Colau, D.; Baurain, J.F.; Chiari, R.; Thonnard, J.; Gutierrez-Roelens, I.; Goffinet, C.; Van Schaftingen, E.V.; Weynants, P.; Boon, T.; et al. High frequency of cytolytic T lymphocytes directed against a tumor-specific mutated antigen detectable with HLA tetramers in the blood of a lung carcinoma patient with long survival. Cancer Res. 2001, 61, 3718–3724. [Google Scholar]
- Davis, S.J.; Ikemizu, S.; Evans, E.J.; Fugger, L.; Bakker, T.R.; Van Der Merwe, P.A. The nature of molecular recognition by T cells. Nat. Immunol. 2003, 4, 217–224. [Google Scholar] [CrossRef]
- Gross, L. Intradermal immunization of C3H mice against a sarcoma that originated in an animal of the same line. Cancer Res. 1943, 3, 323–326. [Google Scholar]
- Baldwin, R.W. Immunity to Methylcholanthrene-Induced Tumours in Inbred Rats Following Atrophy and Regression of the Implanted Tumours. Br. J. Cancer 1955, 9, 652–657. [Google Scholar] [CrossRef] [Green Version]
- Prehn, R.T.; Main, J.M. Immunity to Methylcholanthrene-Induced Sarcomas. J. Natl. Cancer Inst. 1957, 18, 769–778. [Google Scholar] [CrossRef] [PubMed]
- Klein, G.; Sjogren, H.O.; Klein, E.; Hellstrom, K.E. Demonstration of resistance against methylcholanthrene-induced sarcomas in the primary autochthonous host. Cancer Res. 1960, 20, 1561–1572. [Google Scholar] [PubMed]
- Check, J.H.; Childs, T.C.; Brady, L.W.; Derasse, A.R.; Fuscaldo, K.E. Protection against spontaneous mouse mammary adenocarcinoma by inoculation of heat-treated syngeneic mammary tumor cells. Int. J. Cancer 1971, 7, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Check, J.H.; Brady, L.W.; Leipold, P.L.; O’Neill, E.A. Protection against transplanted and spontaneous lymphoma by inoculation of heat-altered syngeneic tumor cells in splenectomized mice. Cancer 1974, 34, 197–203. [Google Scholar] [CrossRef]
- Check, J.H.; Brady, L.W. Differences in the Protective Effect of Complete Freund’s Adjuvant in Spontaneous versus Transplanted Lymphomas in AKR Mice1. Prog. Tumor Res. 1974, 19, 217–221. [Google Scholar] [CrossRef]
- Check, J.H.; Silverman, G.A.; Dryden, R.L.; Brady, L.W. Inhibition of spontaneous akr leukemia by multiple inoculations ofCorynebacterium parvum. Cancer 1979, 44, 488–491. [Google Scholar] [CrossRef]
- Tamura, Y.; Peng, P.; Liu, K.; Daou, M.; Srivastava, P.K. Immunotherapy of Tumors with Autologous Tumor-Derived Heat Shock Protein Preparations. Science 1997, 278, 117–120. [Google Scholar] [CrossRef]
- Sato, K.; Torimoto, Y.; Tamura, Y.; Shindo, M.; Shinzaki, H.; Hirai, K.; Kohgo, Y. Immunotherapy using heat-shock protein preparations of leukemia cells after syngeneic bone marrow transplantation in mice. Blood 2001, 98, 1852–1857. [Google Scholar] [CrossRef] [Green Version]
- Dudley, M.E.; Wunderlich, J.R.; Robbins, P.F.; Yang, J.C.; Hwu, P.; Schwartzentruber, D.J.; Topalian, S.L.; Sherry, R.; Restifo, N.P.; Hubicki, A.M.; et al. Cancer Regression and Autoimmunity in Patients After Clonal Repopulation with Antitumor Lymphocytes. Science 2002, 298, 850–854. [Google Scholar] [CrossRef] [Green Version]
- Rosenberg, S.A.; Yang, J.C.; Sherry, R.M.; Kammula, U.S.; Hughes, M.S.; Phan, G.Q.; Citrin, D.; Restifo, N.P.; Robbins, P.F.; Wunderlich, J.R.; et al. Durable Complete Responses in Heavily Pretreated Patients with Metastatic Melanoma Using T-Cell Transfer Immunotherapy. Clin. Cancer Res. 2011, 17, 4550–4557. [Google Scholar] [CrossRef] [Green Version]
- Chandran, S.; Somerville, R.P.T.; Yang, J.C.; Sherry, R.M.; Klebanoff, C.; Goff, S.L.; Wunderlich, J.R.; Danforth, D.N.; Zlott, D.; Paria, B.C.; et al. Treatment of metastatic uveal melanoma with adoptive transfer of tumour-infiltrating lymphocytes: A single-centre, two-stage, single-arm, phase 2 study. Lancet Oncol. 2017, 18, 792–802. [Google Scholar] [CrossRef]
- Lu, H.; Zhao, X.; Li, Z.; Hu, Y.; Wang, H. From CAR-T cells to CAR-NK cells: A developing immunotherapy method for mematological malignancies. Front. Oncol. 2021, 11, 1–13. [Google Scholar] [CrossRef]
- Kochenderfer, J.N.; Rosenberg, S.A. Treating B-cell cancer with T cells expressing anti-CD19 chimeric antigen receptors. Nat. Rev. Clin. Oncol. 2013, 10, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Kalos, M.; Levine, B.L.; Porter, D.L.; Katz, S.; Grupp, S.A.; Bagg, A.; June, C.H. T Cells with Chimeric Antigen Receptors Have Potent Antitumor Effects and Can Establish Memory in Patients with Advanced Leukemia. Sci. Transl. Med. 2011, 3, 95ra73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brentjens, R.J.; Davila, M.L.; Riviere, I.; Park, J.; Wang, X.; Cowell, L.G.; Bartido, S.; Stefanski, J.; Taylor, C.; Olszewska, M.; et al. CD19-Targeted T Cells Rapidly Induce Molecular Remissions in Adults with Chemotherapy-Refractory Acute Lymphoblastic Leukemia. Sci. Transl. Med. 2013, 5, 177ra38. [Google Scholar] [CrossRef] [Green Version]
- Maude, S.L.; Frey, N.; Shaw, P.A.; Aplenc, R.; Barrett, D.M.; Bunin, N.J.; Chew, A.; Gonzalez, V.E.; Zheng, Z.; Lacey, S.F.; et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N. Engl. J. Med. 2014, 371, 1507–1517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Snyder, A.; Makarov, V.; Merghoub, T.; Yuan, J.; Zaretsky, J.M.; Desrichard, A.; Walsh, L.A.; Postow, M.A.; Wong, P.; Ho, T.S.; et al. Genetic Basis for Clinical Response to CTLA-4 Blockade in Melanoma. N. Engl. J. Med. 2014, 371, 2189–2199. [Google Scholar] [CrossRef] [Green Version]
- Van Allen, E.M.; Miao, D.; Schilling, B.; Shukla, S.A.; Blank, C.; Zimmer, L.; Sucker, A.; Hillen, U.; Foppen, M.H.G.; Goldinger, S.M.; et al. Genomic correlates of response to CTLA-4 blockade in metastatic melanoma. Science 2015, 350, 207–211. [Google Scholar] [CrossRef] [Green Version]
- Munn, D.H.; Bronte, V. Immune suppressive mechanisms in the tumor microenvironment. Curr. Opin. Immunol. 2016, 39, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Zhao, H.; Özen, M.; Wong, R.J.; Stevenson, D.K. Heme oxygenase-1 in pregnancy and cancer: Similarities in cellular invasion, cytoprotection, angiogenesis, and immunomodulation. Front. Pharmacol. 2015, 5, 295. [Google Scholar] [CrossRef] [Green Version]
- Holtan, S.G.; Creedon, D.J.; Haluska, P.; Markovic, S.N. Cancer and Pregnancy: Parallels in Growth, Invasion, and Immune Modulation and Implications for Cancer Therapeutic Agents. In Proceedings of the Mayo Clinic Proceedings; Elsevier BV: Amsterdam, The Netherlands, 2009; Volume 84, pp. 985–1000. [Google Scholar]
- Mullen, C.A. Review: Analogies between Trophoblastic and Malignant Cells. Am. J. Reprod. Immunol. 1998, 39, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Billiard, F.; Litvinova, E.; Saadoun, D.; Djelti, F.; Klatzmann, D.; Cohen, J.L.; Marodon, G.; Salomon, B.L. Regulatory and Effector T Cell Activation Levels Are Prime Determinants of In Vivo Immune Regulation. J. Immunol. 2006, 177, 2167–2174. [Google Scholar] [CrossRef] [Green Version]
- Aluvihare, V.R.; Betz, A.G. The role of regulatory T cells in alloantigen tolerance. Immunol. Rev. 2006, 212, 330–343. [Google Scholar] [CrossRef] [PubMed]
- Li, X.C.; Rothstein, D.M.; Sayegh, M.H. Costimulatory pathways in transplantation: Challenges and new developments. Immunol. Rev. 2009, 229, 271–293. [Google Scholar] [CrossRef]
- D’Addio, F.; Riella, L.V.; Mfarrej, B.; Chabtini, L.; Adams, L.T.; Yeung, M.; Yagita, H.; Azuma, M.; Sayegh, M.H.; Guleria, I. The Link between the PDL1 Costimulatory Pathway and Th17 in Fetomaternal Tolerance. J. Immunol. 2011, 187, 4530–4541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moffett-King, A. Natural killer cells and pregnancy. Nat. Rev. Immunol. 2002, 2, 656–663. [Google Scholar] [CrossRef]
- Crome, S.; Nguyen, L.T.; Lopez-Verges, S.; Yang, S.Y.C.; Martin, B.; Yam, J.Y.; Johnson, D.J.; Nie, J.; Pniak, M.; Yen, P.H.; et al. A distinct innate lymphoid cell population regulates tumor-associated T cells. Nat. Med. 2017, 23, 368–375. [Google Scholar] [CrossRef]
- Crome, S.; Lang, P.A.; Lang, K.; Ohashi, P.S. Natural killer cells regulate diverse T cell responses. Trends Immunol. 2013, 34, 342–349. [Google Scholar] [CrossRef]
- Gasteiger, G.; Rudensky, A.Y. Interactions between innate and adaptive lymphocytes. Nat. Rev. Immunol. 2014, 14, 631–639. [Google Scholar] [CrossRef]
- Artis, D.; Spits, H. The biology of innate lymphoid cells. Nat. Cell Biol. 2015, 517, 293–301. [Google Scholar] [CrossRef]
- Corner, G.W.; Allen, W.M. Physiology of the corpus luteum. Am. J. Obstet. Gynecol. 2005, 193, 1574. [Google Scholar] [CrossRef]
- Check, J.; Liss, J.; Check, D. The Beneficial Effect of Luteal Phase Support on Pregnancy Rates in Women with Unexplained Infertility. Fertil. Steril. 2013, 99, S23. [Google Scholar] [CrossRef]
- Baulieu, E.-E. Contragestion and Other Clinical Applications of RU 486, an Antiprogesterone at the Receptor. Science 1989, 245, 1351–1357. [Google Scholar] [CrossRef]
- Szekeres-Bartho, J. The Role of Progesterone in Feto-Maternal Immunological Cross Talk. Med. Princ. Pract. 2018, 27, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Check, J.H.; Cohen, R. The role of progesterone and the progesterone receptor in human reproduction and cancer. Expert Rev. Endocrinol. Metab. 2013, 8, 469–484. [Google Scholar] [CrossRef]
- Check, J.; Nazari, P.; Goldberg, J.; Yuen, W.; Angotti, D. A model for potential tumor immunotherapy based on knowledge of immune mechanisms responsible for spontaneous abortion. Med. Hypotheses 2001, 57, 337–343. [Google Scholar] [CrossRef]
- Polgar, B.; Kispal, G.; Lachmann, M.; Paar, G.; Nagy, E.; Csere, P.; Miko, E.; Szereday, L.; Varga, P.; Szekeres-Bartho, J. Molecular Cloning and Immunologic Characterization of a Novel cDNA Coding for Progesterone-Induced Blocking Factor. J. Immunol. 2003, 171, 5956–5963. [Google Scholar] [CrossRef] [Green Version]
- Lachmann, M.; Gelbmann, D.; Kálmán, E.; Polgár, B.; Buschle, M.; von Gabain, A.; Szekeres-Barthó, J.; Nagy, E. PIBF(progesterone induced blocking factor) is overexpressed in highly proliferating cells and associated with the centrosome. Int. J. Cancer 2004, 112, 51–60. [Google Scholar] [CrossRef]
- Kim, K.; Lee, K.; Rhee, K. CEP90 is Required for the Assembly and Centrosomal Accumulation of Centriolar Satellites, Which Is Essential for Primary Cilia Formation. PLoS ONE 2012, 7, e48196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, M.; Xu, H.; Zhang, D.; Si, C.; Zhou, X.; Zhao, H.; Liu, Q.; Xu, B.; Zhang, A. Decreased PIBF1/IL6/p-STAT3 during the mid-secretory phase inhibits human endometrial stromal cell proliferation and decidualization. J. Adv. Res. 2021, 30, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Rhee, K. The pericentriolar satellite protein CEP90 is crucial for integrity of the mitotic spindle pole. J. Cell Sci. 2011, 124, 338–347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miko, E.; Halasz, M.; Jericevic-Mulac, B.; Wicherek, L.; Arck, P.; Arató, G.; Magierlo, J.S.; Rukavina, D.; Szekeres-Bartho, J. Progesterone-induced blocking factor (PIBF) and trophoblast invasiveness. J. Reprod. Immunol. 2011, 90, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Halasz, M.; Polgar, B.; Berta, G.; Czimbalek, L.; Szekeres-Bartho, J. Progesterone-induced blocking factor differentially regulates trophoblast and tumor invasion by altering matrix metalloproteinase activity. Cell. Mol. Life Sci. 2013, 70, 4617–4630. [Google Scholar] [CrossRef] [Green Version]
- Balassa, T.; Berta, G.; Jakab, L.; Bohonyi, N.; Szekeres-Barthó, J. The effect of the Progesterone-Induced Blocking Factor (PIBF) on E-cadherin expression, cell motility and invasion of primary tumour cell lines. J. Reprod. Immunol. 2018, 125, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Szekeres-Bartho, J.; Wegmann, T. A progesterone-dependent immunomodulatory protein alters the Th1Th2 balance. J. Reprod. Immunol. 1996, 31, 81–95. [Google Scholar] [CrossRef]
- Raghupathy, R.; Al Mutawa, E.; Makhseed, M.; Azizieh, F.; Szekeres-Bartho, J. Modulation of cytokine production by dydrogesterone in lymphocytes from women with recurrent miscarriage. BJOG Int. J. Obstet. Gynaecol. 2005, 112, 1096–1101. [Google Scholar] [CrossRef]
- Raghupathy, R.; Al-Mutawa, E.; Al-Azemi, M.; Makhseed, M.; Azizieh, F.; Szekeres-Bartho, J. Progesterone-induced blocking factor (PIBF) modulates cytokine production by lymphocytes from women with recurrent miscarriage or preterm delivery. J. Reprod. Immunol. 2009, 80, 91–99. [Google Scholar] [CrossRef]
- Kozma, N.; Halasz, M.; Polgar, B.; Poehlmann, T.G.; Markert, U.R.; Palkovics, T.; Keszei, M.; Par, G.; Kiss, K.; Szeberenyi, J.; et al. Progesterone-Induced Blocking Factor Activates STAT6 via Binding to a Novel IL-4 Receptor. J. Immunol. 2006, 176, 819–826. [Google Scholar] [CrossRef] [Green Version]
- Koopman, L.A.; Kopcow, H.D.; Rybalov, B.; Boyson, J.E.; Orange, J.; Schatz, F.; Masch, R.; Lockwood, C.J.; Schachter, A.D.; Park, P.J.; et al. Human Decidual Natural Killer Cells Are a Unique NK Cell Subset with Immunomodulatory Potential. J. Exp. Med. 2003, 198, 1201–1212. [Google Scholar] [CrossRef] [Green Version]
- Bogdan, A.; Berta, G.; Szekeres-Bartho, J. PIBF positive uterine NK cells in the mouse decidua. J. Reprod. Immunol. 2017, 119, 38–43. [Google Scholar] [CrossRef]
- Faust, Z.; Laskarin, G.; Rukavina, D.; Szekeres-Bartho, J. Progesterone-induced blocking factor inhibits degranulation of natural killer cells. Am. J. Reprod. Immunol. 1999, 42, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, K.R. Steroid receptor regulated transcription of specific genes and gene networks. Annu. Rev. Genet. 1985, 19, 209–252. [Google Scholar] [CrossRef] [PubMed]
- Daniel, A.R.; Hagan, C.R.; Lange, C.A. Progesterone receptor action: Defining a role in breast cancer. Expert Rev. Endocrinol. Metab. 2011, 6, 359–369. [Google Scholar] [CrossRef] [Green Version]
- Szekeres-Bartho, J.; Kilar, F.; Falkay, G.; Csernus, V.; Török, A.; Pacsa, A. The Mechanism of the Inhibitory Effect of Progesterone on Lymphocyte Cytotoxicity: I. Progesterone-Treated Lymphocytes Release a Substance Inhibiting Cytotoxicity and Prostaglandin Synthesis. Am. J. Reprod. Immunol. 1985, 9, 15–18. [Google Scholar] [CrossRef] [PubMed]
- Szekeres-Bartho, J.; Autran, B.; Debre, P.; Andreu, G.; Denver, L.; Chaouat, G. Immunoregulatory effects of a suppressor factor from healthy pregnant women’s lymphocytes after progesterone induction. Cell. Immunol. 1989, 122, 281–294. [Google Scholar] [CrossRef]
- Szekeres-Bartho, J.; Faust, Z.; Varga, P. The Expression of a Progesterone-Induced Immunomodulatory Protein in Pregnancy Lymphocytes. Am. J. Reprod. Immunol. 1995, 34, 342–348. [Google Scholar] [CrossRef]
- Szekeres-Bartho, J.; Reznikoff-Etievant, M.; Varga, P.; Pichon, M.-F.; Varga, Z.; Chaouat, G. Lymphocytic progesterone receptors in normal and pathological human pregnancy. J. Reprod. Immunol. 1989, 16, 239–247. [Google Scholar] [CrossRef]
- Szekeres-Bartho, J.; Szekeres, G.; Debre, P.; Autran, B.; Chaouat, G. Reactivity of lymphocytes to a progesterone receptor-specific monoclonal antibody. Cell. Immunol. 1990, 125, 273–283. [Google Scholar] [CrossRef]
- Polgar, B.; Barakonyi, A.; Xynos, I.; Szekeres-Bartho, J. The Role of γ/δ T Cell Receptor Positive Cells in Pregnancy. Am. J. Reprod. Immunol. 1999, 41, 239–244. [Google Scholar] [CrossRef]
- Szekeres-Bartho, J.; Faust, Z.; Varga, P.; Szereday, L.; Kelemen, K. The Immunological Pregnancy Protective Effect of Progesterone Is Manifested via Controlling Cytokine Production. Am. J. Reprod. Immunol. 1996, 35, 348–351. [Google Scholar] [CrossRef]
- Szekeres-Bartho, J.; Par, G.; Dombay, G.; Smart, Y.; Volgyi, Z. The Antiabortive Effect of Progesterone-Induced Blocking Factor in Mice Is Manifested by Modulating NK Activity. Cell. Immunol. 1997, 177, 194–199. [Google Scholar] [CrossRef]
- Szekeres-Bartho, J.; Kinsky, R.; Chaouat, G. The effect of a progesterone induced immunologic blocking factor on NK-mediated resorption. Am. J. Reprod. Immunol. 1990, 24, 105–107. [Google Scholar] [CrossRef] [PubMed]
- Check, J.H.; Szekeres-Bartho, J.; O’Shaughnessy, A. Progesterone Induced Blocking Factor Seen in Pregnancy Lymphocytes Soon After Implantation. Am. J. Reprod. Immunol. 1996, 35, 277–280. [Google Scholar] [CrossRef] [PubMed]
- Check, J.H.; Arwitz, M.; Gross, J.; Szekeres-Bartho, J.; Wu, C.H. Evidence that the Expression of Progesterone-Induced Blocking Factor by Maternal T-Lymphocytes Is Positively Correlated with Conception. Am. J. Reprod. Immunol. 1997, 38, 6–8. [Google Scholar] [CrossRef] [PubMed]
- Check, J.H.; Szekeres-Barthó, J.; Nazari, P.; Katz, Y.; Check, M.L. A Corpus Luteum Is Not a Prerequisite for the Expression of Progesterone Induced Blocking Factor by T-Lymphocytes a Week After Implantation. J. Assist. Reprod. Genet. 2001, 18, 603–607. [Google Scholar] [CrossRef]
- Check, J.H.; Levin, E.; Bollendorf, A.; Locuniak, J. Miscarriage in the first trimester according to the presence or absence of the progesterone-induced blocking factor at three to five weeks from conception in progesterone supplemented women. Clin. Exp. Obstet. Gynecol. 2005, 32, 13–14. [Google Scholar]
- Srivastava, M.D.; Thomas, A.; Srivastava, B.I.S.; Check, J.H. Expression and modulation of progesterone induced blocking factor (PIBF) and innate immune factors in human leukemia cell lines by progesterone and mifepristone. Leuk. Lymphoma 2007, 48, 1610–1617. [Google Scholar] [CrossRef]
- Kyurkchiev, B.; Naydenov, E.; Tumangelova-Yuzeir, K.; Ivanova-Todorova, E.; Belemezova, K.; Bochev, I.; Minkin, K.; Mourdjeva, M.; Velikova, T.; Nachev, S.; et al. Cells Isolated from Human Glioblastoma Multiforme Express Progesterone-Induced Blocking Factor (PIBF). Cell. Mol. Neurobiol. 2014, 34, 479–489. [Google Scholar] [CrossRef]
- González-Arenas, A.; Valadez-Cosmes, P.; Jiménez-Arellano, C.; López-Sánchez, M.; Camacho-Arroyo, I. Progesterone-induced blocking factor is hormonally regulated in human astrocytoma cells, and increases their growth through the IL-4R/JAK1/STAT6 pathway. J. Steroid Biochem. Mol. Biol. 2014, 144, 463–470. [Google Scholar] [CrossRef]
- Acevedo, H.F.; Tong, J.Y.; Hartsock, R.J. Human chorionic gonadotropin-beta subunit gene expression in cultured human fetal and cancer cells of different types and origins. Cancer 1995, 76, 1467–1475. [Google Scholar] [CrossRef]
- Check, J.H.; Sarumi, M.; DiAntonio, A.; Hunter, K.; Simpkins, G.; Duroseau, M. Serum levels of the progesterone induced blocking factor do not precipitously rise in women with gynecologic cancer in contrast to women exposed to progesterone. Clin. Exp. Obstet. Gynecol. 2015, 42, 563–567. [Google Scholar] [PubMed]
- Check, J.H.; Rosenberg, A.; Check, D.L.; DiAntonio, A.; Rui, H.; Cohen, R.; DiAntonio, G. Serum levels of the immunomodulatory protein, the progesterone induced blocking factor (PIBF) which is found in high levels during pregnancy is not higher in women with progesterone (P) receptor (R) positive vs. negative breast cancer. Clin. Exp. Obstet. Gynecol. 2017, 44, 187–189. [Google Scholar]
- Klijn, J.G.; De Jong, F.H.; Bakker, G.H.; Lamberts, S.W.; Rodenburg, C.J.; Alexieva-Figusch, J. Antiprogestins, a new form of endocrine therapy for human breast cancer. Cancer Res. 1989, 49, 2851–2856. [Google Scholar]
- Philibert, D. RU 38486: An Original Multifaceted Antihormone In Vivo. In Adrenal Steroid Antagonism; Agarwal, M.K., Ed.; Walter de Gruyter & Co.: Berlin, Germany, 1984; pp. 77–101. [Google Scholar]
- Perrault, D.; Eisenhauer, E.A.; Pritchard, K.I.; Panasci, L.; Norris, B.; Vandenberg, T.; Fisher, B. Phase II study of the progesterone antagonist mifepristone in patients with untreated metastatic breast carcinoma: A National Cancer Institute of Canada Clinical Trials Group study. J. Clin. Oncol. 1996, 14, 2709–2712. [Google Scholar] [CrossRef] [PubMed]
- Robertson, J.; Willsher, P.; Winterbottom, L.; Blamey, R.; Thorpe, S. Onapristone, a progesterone receptor antagonist, as first-line therapy in primary breast cancer. Eur. J. Cancer 1999, 35, 214–218. [Google Scholar] [CrossRef]
- Jonat, W.; Bachelot, T.; Ruhstaller, T.; Kuss, I.; Reimann, U.; Robertson, J.F.R. Randomized phase II study of lonaprisan as second-line therapy for progesterone receptor-positive breast cancer. Ann. Oncol. 2013, 24, 2543–2548. [Google Scholar] [CrossRef]
- Rocereto, T.F.; Saul, H.M.; Aikins, J.A.; Paulson, J. Phase II Study of Mifepristone (RU486) in Refractory Ovarian Cancer. Gynecol. Oncol. 2000, 77, 429–432. [Google Scholar] [CrossRef] [PubMed]
- Rocereto, T.F.; Brady, W.E.; Shahin, M.S.; Hoffman, J.S.; Small, L.; Rotmensch, J.; Mannel, R.S. A phase II evaluation of mifepristone in the treatment of recurrent or persistent epithelial ovarian, fallopian or primary peritoneal cancer: A gynecologic oncology group study. Gynecol. Oncol. 2010, 116, 332–334. [Google Scholar] [CrossRef]
- Ramondetta, L.M.; Johnson, A.J.; Sun, C.C.; Atkinson, N.; Smith, J.A.; Jung, M.S.; Broaddus, R.; Iyer, R.B.; Burke, T. Phase 2 trial of mifepristone (RU-486) in advanced or recurrent endometrioid adenocarcinoma or low-grade endometrial stromal sarcoma. Cancer 2009, 115, 1867–1874. [Google Scholar] [CrossRef]
- Check, J.H.; Sansoucie, L.; Chern, J.; Amadi, N.; Katz, Y. Mifepristone treatment improves length and quality of survival of mice with spontaneous leukemia. Anticancer Res. 2009, 29, 2977–2980. [Google Scholar]
- Check, J.H.; Sansoucie, L.; Chern, J.; Dix, E. Mifepristone treatment improves length and quality of survival of mice with spontaneous lung cancer. Anticancer Res. 2010, 30, 119–122. [Google Scholar]
- Check, J.H.; Dix, E.; Wilson, C.; Check, D. Progesterone receptor antagonist therapy has therapeutic potential even in cancer restricted to males as evidenced from murine testicular and prostate cancer studies. Anticancer Res. 2010, 30, 4921–4924. [Google Scholar]
- Check, D.L.; Check, J.H.; Poretta, T. Conservative laparoscopic surgery plus mifepristone for treating multifocal renal cell carcinoma. Cancer Sci. Res. 2020, 3, 1–4. [Google Scholar]
- Check, J.H.; Check, D. Therapy Aimed to Suppress the Production of the Immunosuppressive Protein Progesterone Induced Blocking Factor (PIBF) May Provide Palliation and/or Increased Longevity for Patients With a Variety of Different Advanced cancers—A review. Anticancer Res. 2019, 39, 3365–3372. [Google Scholar] [CrossRef] [PubMed]
- Check, J.H.; Wilson, C.; Lofberg, P. Long-term High-quality Survival with Single-agent Mifepristone Treatment despite Advanced Cancer. Anticancer Res. 2016, 36, 6511–6514. [Google Scholar] [CrossRef] [PubMed]
- Check, J.H.; Check, D.L.; Dougherty, M.P. Progesterone receptor antagonists—A novel treatment for severe hyponatremia from the endocrine paraneoplastic syndrome. J. Endocrinol. Res. 2021, 3, 40–43. [Google Scholar]
- Check, D.L.; Check, J.H. Significant palliative benefits of single agent mifepristone for advanced lung cancer that previously failed standard therapy. Med. Clin. Sci. 2019, 1, 1–5. [Google Scholar] [CrossRef]
- Check, J.H.; Check, D.; Poretta, T. Mifepristone Extends Both Length and Quality of Life in a Patient with Advanced Non-small Cell Lung Cancer that Has Progressed despite Chemotherapy and a Check-point Inhibitor. Anticancer Res. 2019, 39, 1923–1926. [Google Scholar] [CrossRef] [Green Version]
- Check, D.L.; Check, J.H.; Poretta, T.; Aikins, J.; Wilson, C. Prolonged high-quality life in patients with non-small cell lung cancer treated with mifepristone who advanced despite osimertinib. Cancer Sci. Res. 2020, 3, 1–5. [Google Scholar]
- Check, J.H.; Wilson, C.; Cohen, R.; Sarumi, M. Evidence that Mifepristone, a progesterone receptor antagonist, can cross the blood brain barrier and provide palliative benefits for glioblastoma multiforme grade IV. Anticancer Res. 2014, 34, 2385–2388. [Google Scholar]
- Check, J.H.; Check, D.; Srivastava, M.D.; Poretta, T.; Aikins, J.K. Treatment with Mifepristone Allows a Patient with End-stage Pancreatic Cancer in Hospice on a Morphine Drip to Restore a Decent Quality of Life. Anticancer Res. 2020, 40, 6997–7001. [Google Scholar] [CrossRef]
- Check, J.H.; Dix, E.; Sansoucie, L.; Check, D. Mifepristone may halt progression of extensively metastatic human adenocarcinoma of the colon—Case report. Anticancer Res. 2009, 29, 1611–1613. [Google Scholar] [PubMed]
- Check, J.H.; Dix, E.; Cohen, R.; Check, D.; Wilson, C. Efficacy of the progesterone receptor antagonist mifepristone for palliative therapy of patients with a variety of advanced cancer types. Anticancer Res. 2010, 30, 623–628. [Google Scholar] [PubMed]
- Check, J.H.; Check, D.; Poretta, T.; Wilson, C. Palliative Benefits of Oral Mifepristone for the Treatment of Metastatic Fibroblastic Osteosarcoma. Anticancer Res. 2021, 41, 2111–2115. [Google Scholar] [CrossRef] [PubMed]
- Check, J.H. The role of progesterone and the progesterone receptor in cancer. Expert Rev. Endocrinol. Metab. 2017, 12, 187–197. [Google Scholar] [CrossRef]
- Cahill, M.A.; Jazayeri, J.A.; Catalano, S.M.; Toyokuni, S.; Kovacevic, Z.; Richardson, D.R. The emerging role of progesterone receptor membrane component 1 (PGRMC1) in cancer biology. Biochim. Biophys. Acta (BBA) Bioenerg. 2016, 1866, 339–349. [Google Scholar] [CrossRef]
- Mifsud, W.; Bateman, A. Membrane-bound progesterone receptors contain a cytochrome b5-like ligand-binding domain. Genome Biol. 2002, 3, 1–5. [Google Scholar] [CrossRef]
- Hampton, K.K.; Stewart, R.; Napier, D.; Claudio, P.P.; Craven, R.J. PGRMC1 Elevation in Multiple Cancers and Essential Role in Stem Cell Survival. Adv. Lung Cancer 2015, 4, 37–51. [Google Scholar] [CrossRef] [Green Version]
- Ruan, X.; Zhang, Y.; Mueck, A.O.; Willibald, M.; Seeger, H.; Fehm, T.; Brucker, S.; Neubauer, H. Increased expression of progesterone receptor membrane component 1 is associated with aggressive phenotype and poor prognosis in ER-positive and negative breast cancer. Menopause 2017, 24, 203–209. [Google Scholar] [CrossRef]
- Peluso, J.J.; Liu, X.; Saunders, M.M.; Claffey, K.P.; Phoenix, K. Regulation of Ovarian Cancer Cell Viability and Sensitivity to Cisplatin by Progesterone Receptor Membrane Component-1. J. Clin. Endocrinol. Metab. 2008, 93, 1592–1599. [Google Scholar] [CrossRef] [Green Version]
- Ponikwicka-Tyszko, D.; Chrusciel, M.; Stelmaszewska, J.; Bernaczyk, P.; Chrusciel, P.; Sztachelska, M.; Scheinin, M.; Bidzinski, M.; Szamatowicz, J.; Huhtaniemi, I.T.; et al. Molecular mechanisms underlying mifepristone’s agonistic action on ovarian cancer progression. EBioMedicine 2019, 47, 170–183. [Google Scholar] [CrossRef] [Green Version]
- Craven, R.J. Ag-205 for the Treatment of Breast Cancer. U.S. Patent 9,724,337, 8 August 2017. [Google Scholar]
- Szekeres-Bartho, J.; Polgar, B. PIBF: The Double Edged Sword. Pregnancy and Tumor. Am. J. Reprod. Immunol. 2010, 64, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Check, J.H. Pros and cons of the use of progesterone to reduce miscarriage rates. Clin. Exp. Obstet. Gynecol. 2018, 45, 652–655. [Google Scholar]
- Check, J.H.; Aly, J. Improving the chance of successful implantation—Part 2—CIRCUMVENTING immune rejection and the fetal semi-allograft. Clin. Exp. Obst. Gyn. 2018, 45, 9–13. [Google Scholar]
- Cohen, R.A.; Check, J.H.; Dougherty, M.P. Evidence that exposure to progesterone alone is a sufficient stimulus to cause a precipitous rise in the immunomodulatory protein the progesterone induced blocking factor (PIBF). J. Assist. Reprod. Genet. 2016, 33, 221–229. [Google Scholar] [CrossRef] [Green Version]
- Check, D.L.; Check, J.H. Novel methods of improving fecundity and various pathological disorders based on a hypothetical model of embryo implantation. Gynecol. Reprod. Health 2020, 4, 1–15. [Google Scholar] [CrossRef]
- Check, J.H.; Check, D. Mifepristone may be the best single pharmaceutical agent for treatment of a variety of advanced cancers. Cancer Sci. Res. 2021, 4, 1–6. [Google Scholar]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Check, J.H.; Check, D.L. A Hypothetical Model Suggesting Some Possible Ways That the Progesterone Receptor May Be Involved in Cancer Proliferation. Int. J. Mol. Sci. 2021, 22, 12351. https://doi.org/10.3390/ijms222212351
Check JH, Check DL. A Hypothetical Model Suggesting Some Possible Ways That the Progesterone Receptor May Be Involved in Cancer Proliferation. International Journal of Molecular Sciences. 2021; 22(22):12351. https://doi.org/10.3390/ijms222212351
Chicago/Turabian StyleCheck, Jerome H., and Diane L. Check. 2021. "A Hypothetical Model Suggesting Some Possible Ways That the Progesterone Receptor May Be Involved in Cancer Proliferation" International Journal of Molecular Sciences 22, no. 22: 12351. https://doi.org/10.3390/ijms222212351
APA StyleCheck, J. H., & Check, D. L. (2021). A Hypothetical Model Suggesting Some Possible Ways That the Progesterone Receptor May Be Involved in Cancer Proliferation. International Journal of Molecular Sciences, 22(22), 12351. https://doi.org/10.3390/ijms222212351




