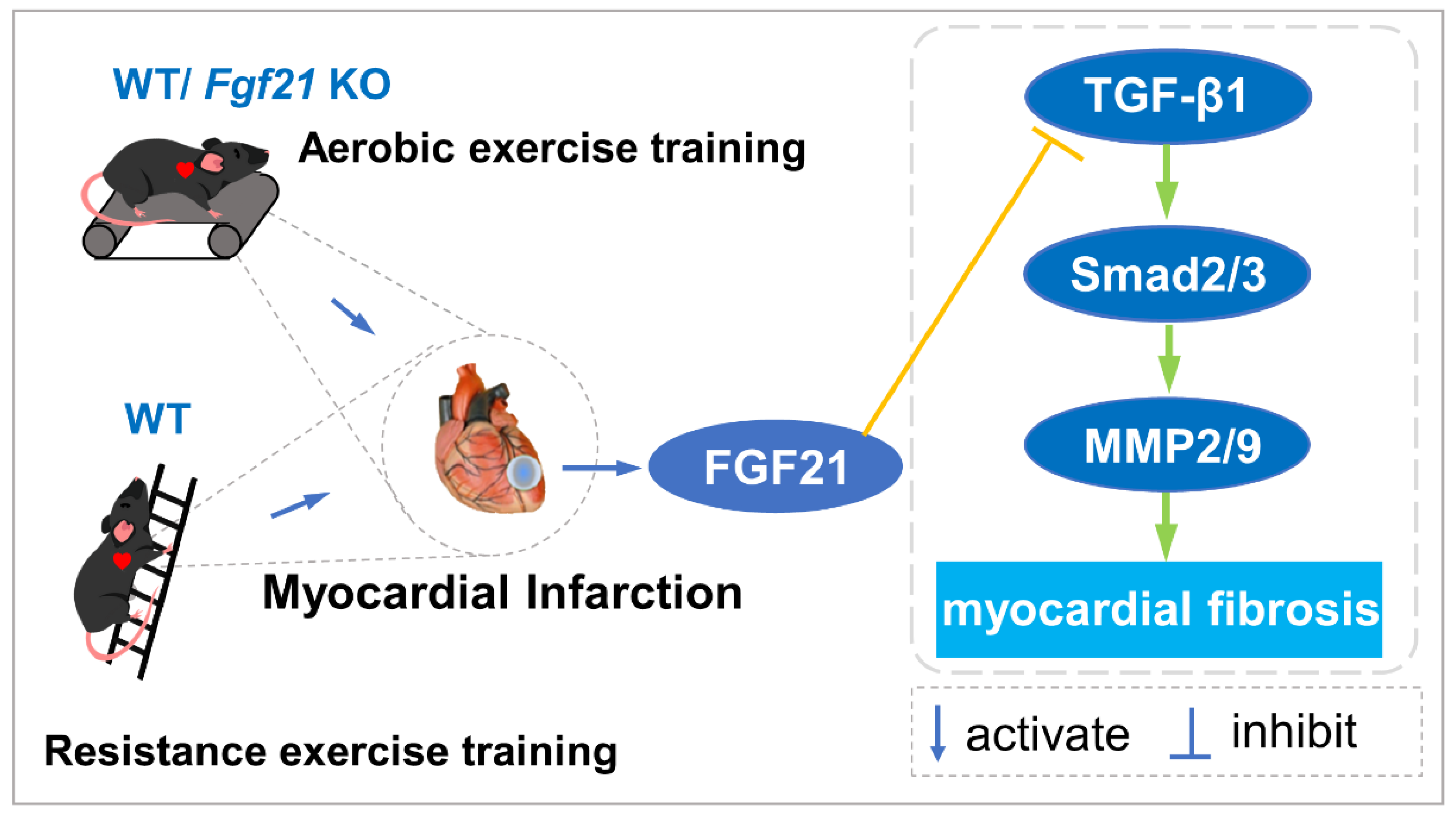
Exercise Training Alleviates Cardiac Fibrosis through Increasing Fibroblast Growth Factor 21 and Regulating TGF-β1-Smad2/3-MMP2/9 Signaling in Mice with Myocardial Infarction
Abstract
:1. Introduction
2. Results
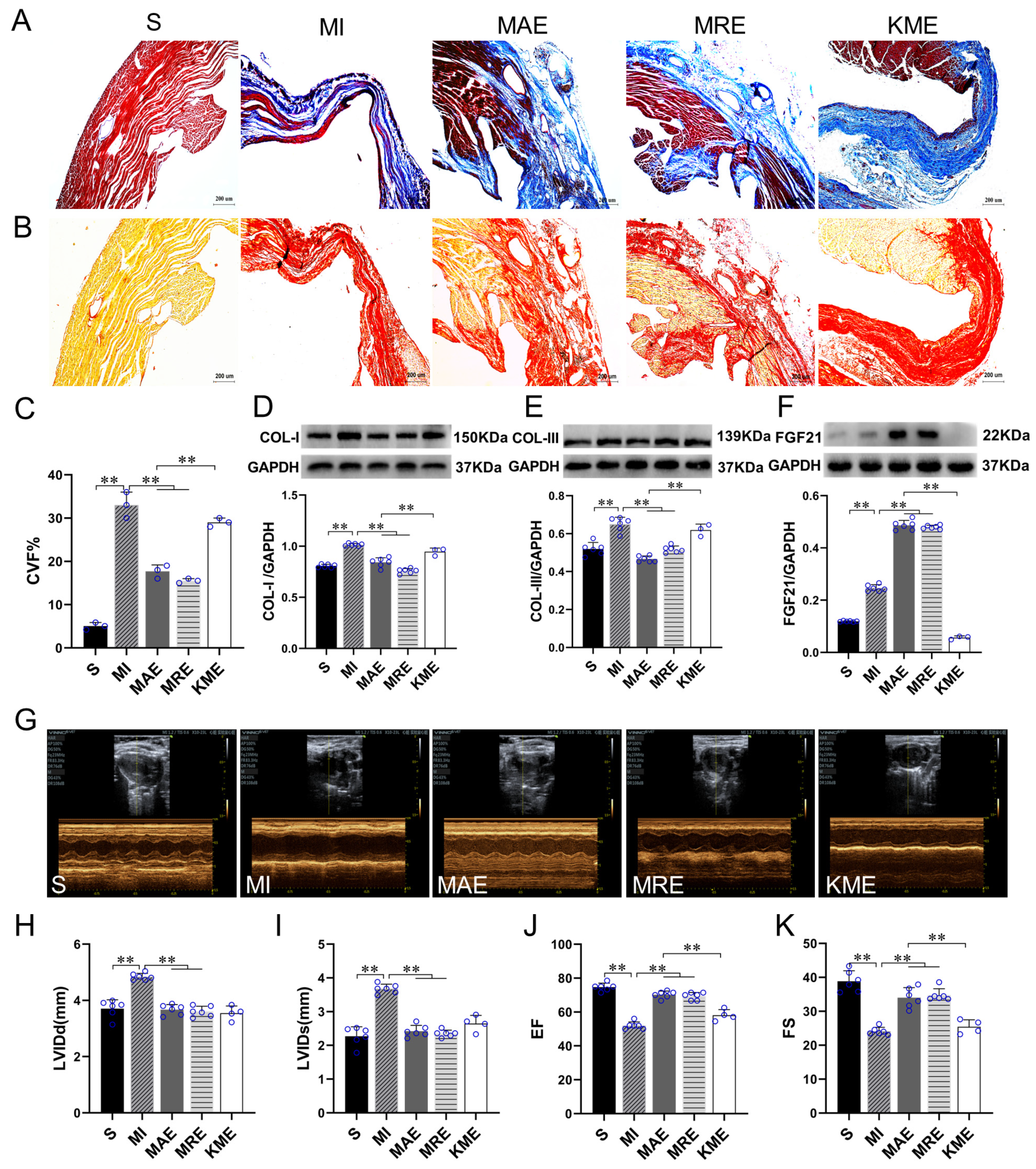
2.1. Exercise Training Up-Regulated FGF21 Expression and Alleviated Cardiac Dysfunction and Cardiac Fibrosis in the Infarcted Heart
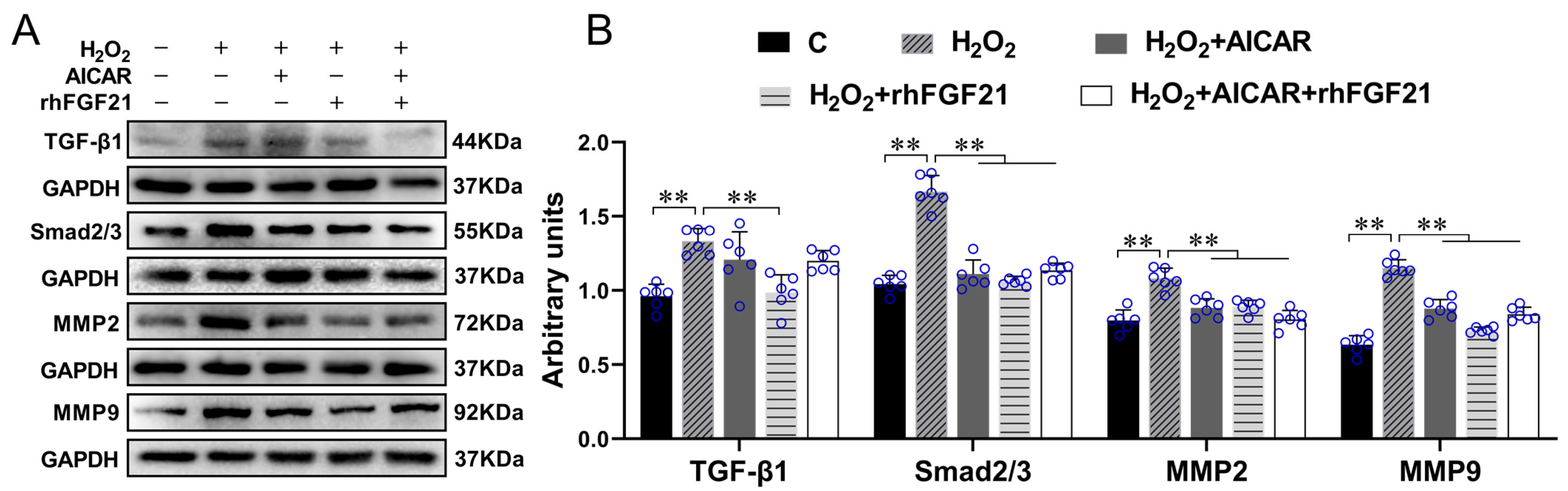
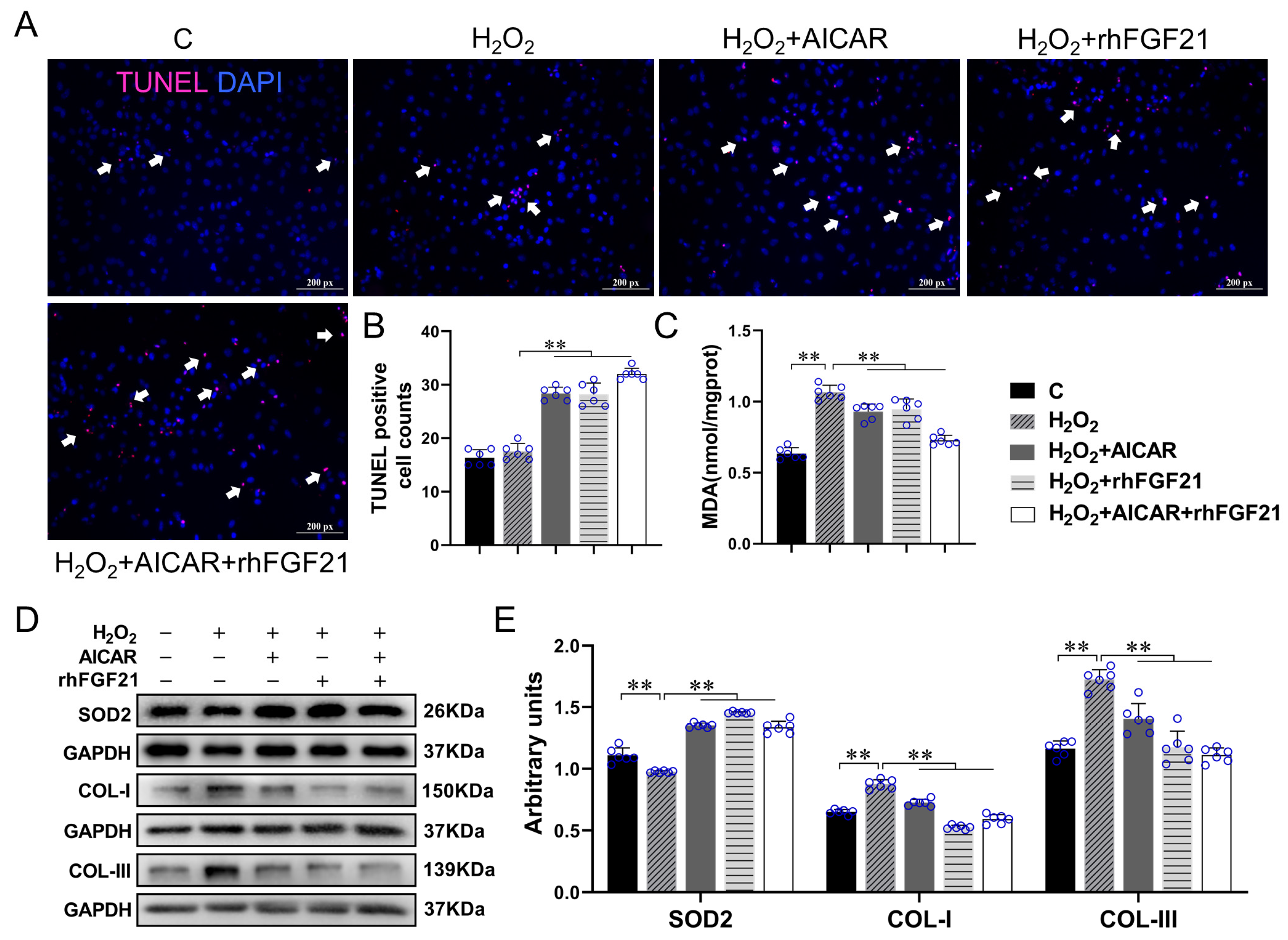
2.2. rhFGF21 and/or AICAR Inhibitedthe Activation of TGF-β1-Smad2/3-MMP2/9 Signaling Pathway, Promoted CFs Apoptosis and Antioxidant Capacity, Reduced Collagen Synthesis in CFs with H2O2-Treatment
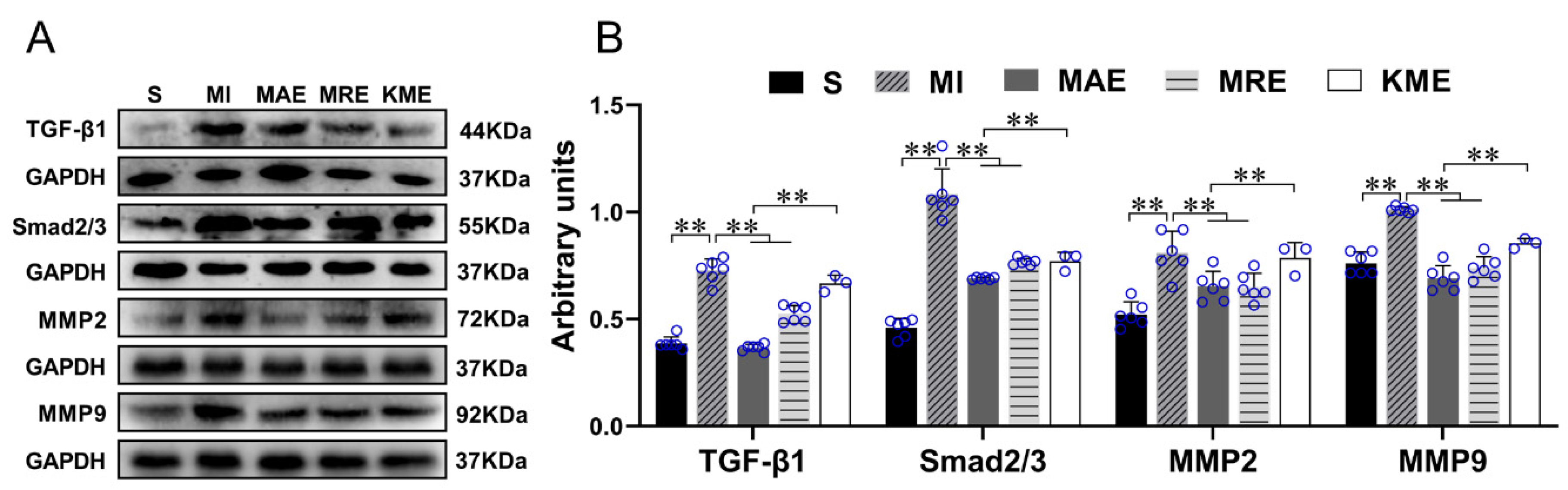
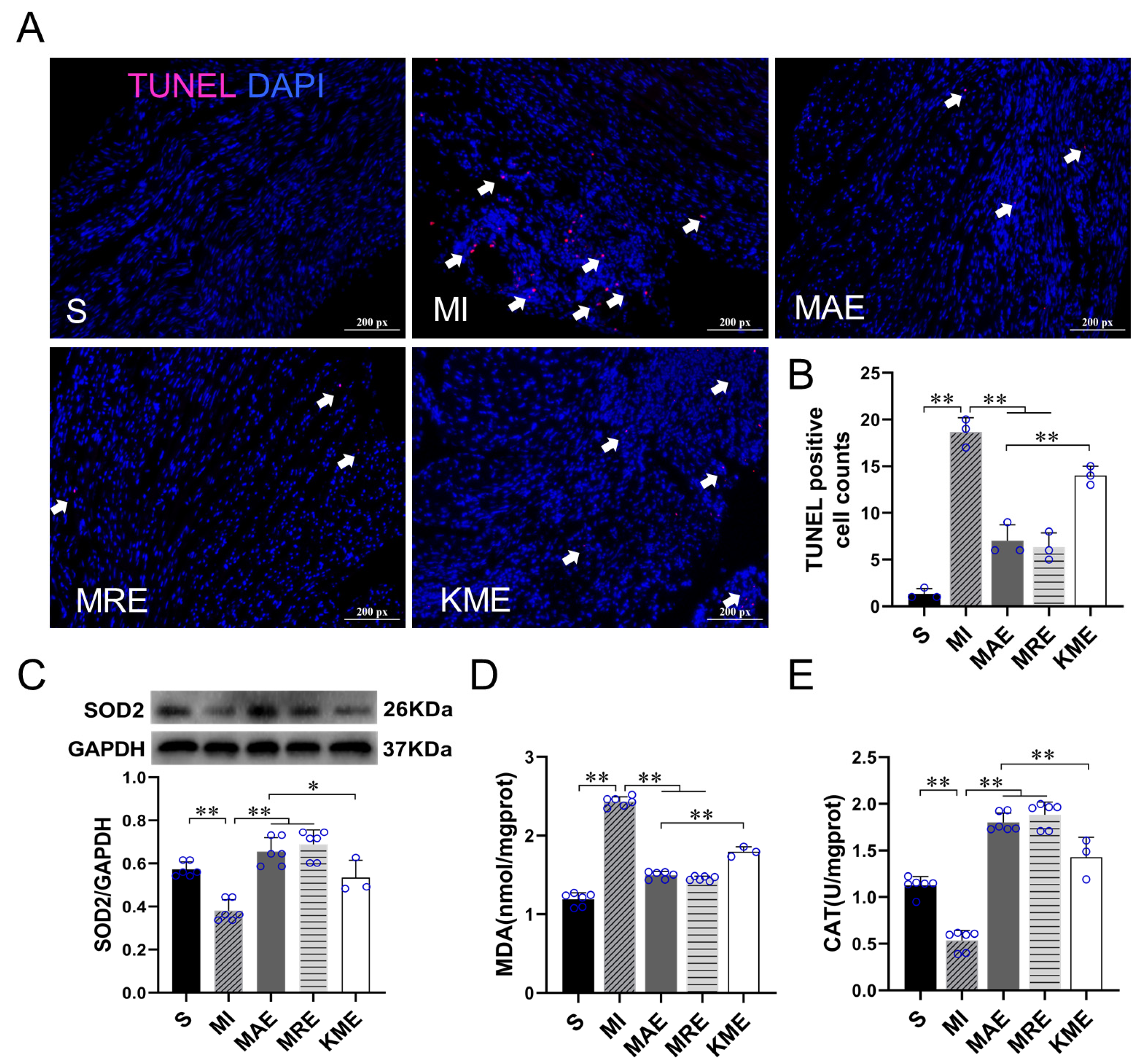
2.3. Exercise Training Inhibited the Activation of the TGF-β1-Smad2/3-MMP2/9 Signaling Pathway, Enhanced Antioxidant Capacity, and Reduced Cell Apoptosis via FGF21 in the Heart of Mice with MI
3. Discussion
4. Materials and Methods
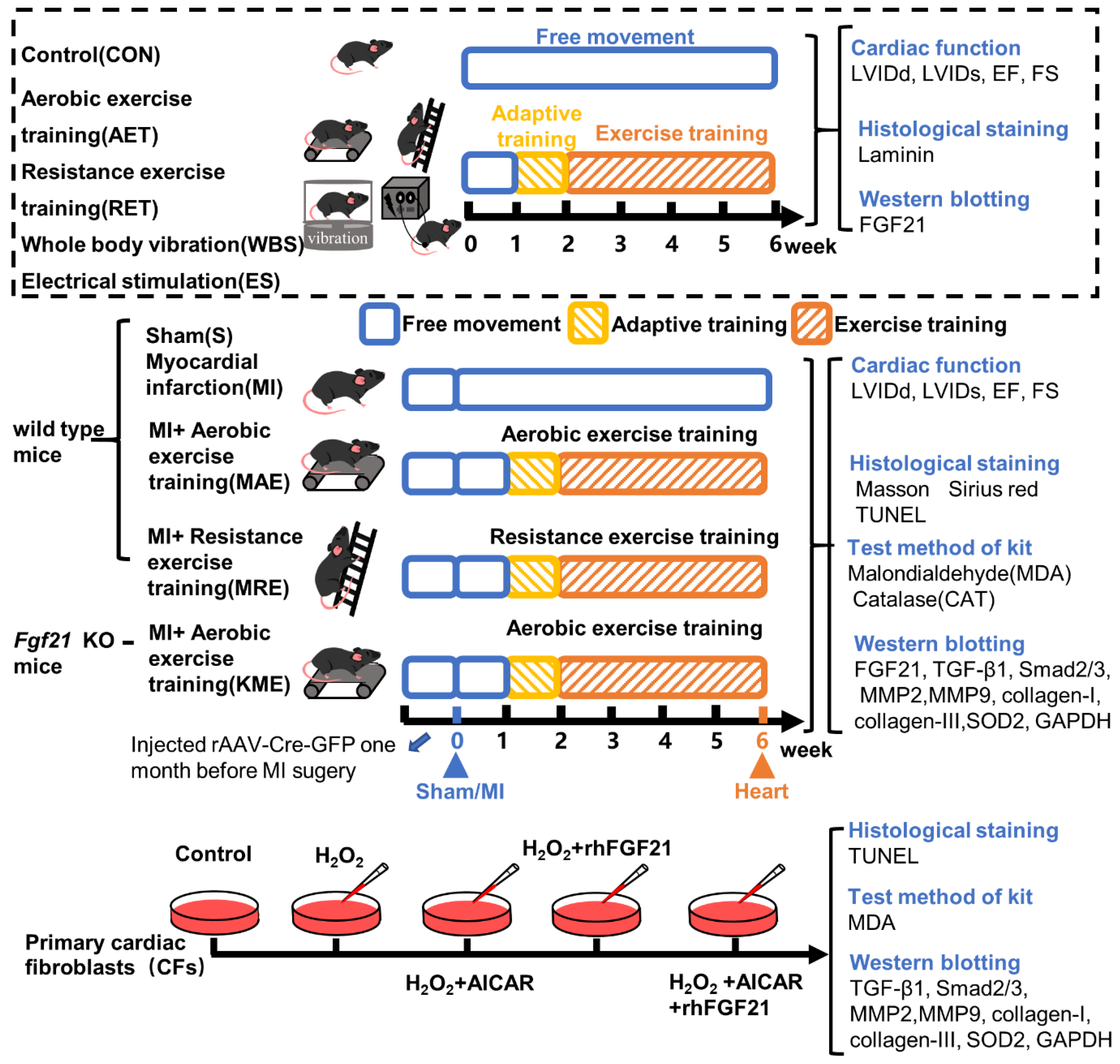
4.1. Animals
4.2. Exercise Protocols
4.3. Cardiac Fibroblast Isolation and Cell Culture
4.4. Echocardiographic Measurement
4.5. Histological Staining and Analysis
4.6. Immunofluorescence Staining
4.7. TUNEL Staining
4.8. Kit Assays
4.9. Western Blotting
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Virani, S.S.; Alonso, A.; Aparicio, H.J.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Cheng, S.; Delling, F.N.; et al. Heart Disease and Stroke Statistics-2021 Update: A Report from the American Heart Association. Circulation 2021, 143, e254–e743. [Google Scholar] [CrossRef] [PubMed]
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update from the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef] [PubMed]
- Li, H.L.; Zhuo, M.L.; Wang, D.; Wang, A.B.; Cai, H.; Sun, L.H.; Yang, Q.; Huang, Y.; Wei, Y.S.; Liu, P.P.; et al. Targeted cardiac overexpression of A20 improves left ventricular performance and reduces compensatory hypertrophy after myocardial infarction. Circulation 2007, 115, 1885–1894. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kong, P.; Christia, P.; Frangogiannis, N.G. The pathogenesis of cardiac fibrosis. Cell. Mol. Life Sci. 2014, 71, 549–574. [Google Scholar] [CrossRef] [Green Version]
- Frangogiannis, N.G. Regulation of the inflammatory response in cardiac repair. Circ. Res. 2012, 110, 159–173. [Google Scholar] [CrossRef]
- Xi, Y.; Hao, M.; Liang, Q.; Li, Y.; Gong, D.W.; Tian, Z. Dynamic resistance exercise increases skeletal muscle-derived FSTL1 inducing cardiac angiogenesis via DIP2A-Smad2/3 in rats following myocardial infarction. J. Sport Health Sci. 2021, 10, 594–603. [Google Scholar] [CrossRef]
- Liang, Q.; Cai, M.; Zhang, J.; Song, W.; Zhu, W.; Xi, L.; Tian, Z. Role of Muscle-Specific Histone Methyltransferase (Smyd1) in Exercise-Induced Cardioprotection against Pathological Remodeling after Myocardial Infarction. Int. J. Mol. Sci. 2020, 21, 7010. [Google Scholar] [CrossRef]
- Jia, D.; Hou, L.; Lv, Y.; Xi, L.; Tian, Z. Postinfarction exercise training alleviates cardiac dysfunction and adverse remodeling via mitochondrial biogenesis and SIRT1/PGC-1α/PI3K/Akt signaling. J. Cell. Physiol. 2019, 234, 23705–23718. [Google Scholar] [CrossRef]
- Shekarforoush, S.; Naghii, M.R. Whole-Body Vibration Training Increases Myocardial Salvage Against Acute Ischemia in Adult Male Rats. Arq. Bras. Cardiol. 2019, 112, 32–37. [Google Scholar] [CrossRef]
- Rucatti, A.L.; Jaenisch, R.B.; Rossato, D.D.; Bonetto, J.H.; Ferreira, J.; Xavier, L.L.; Sonza, A.; Dal Lago, P. Skeletal muscle electrical stimulation improves baroreflex sensitivity and heart rate variability in heart failure rats. Auton. Neurosci. 2015, 193, 92–96. [Google Scholar] [CrossRef]
- Acar, R.D.; Bulut, M.; Ergün, S.; Yesin, M.; Akçakoyun, M. Evaluation of the effect of cardiac rehabilitation on left atrial and left ventricular function and its relationship with changes in arterial stiffness in patients with acute myocardial infarction. Echocardiography 2015, 32, 443–447. [Google Scholar] [CrossRef]
- Grochulska, A.; Glowinski, S.; Bryndal, A. Cardiac Rehabilitation and Physical Performance in Patients after Myocardial Infarction: Preliminary Research. J. Clin. Med. 2021, 10, 2253. [Google Scholar] [CrossRef] [PubMed]
- Mehdipoor, M.; Damirchi, A.; Tousi, S.M.T.R.; Babaei, P. Concurrent vitamin D supplementation and exercise training improve cardiac fibrosis via TGF-beta/Smad signaling in myocardial infarction model of rats. J. Physiol. Biochem. 2021, 77, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Frangogiannis, N. Transforming growth factor-β in tissue fibrosis. J. Exp. Med. 2020, 217, e20190103. [Google Scholar] [CrossRef] [PubMed]
- Dobaczewski, M.; Chen, W.; Frangogiannis, N.G. Transforming growth factor (TGF)-β signaling in cardiac remodeling. J. Mol. Cell. Cardiol. 2011, 51, 600–606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dewald, O.; Ren, G.; Duerr, G.D.; Zoerlein, M.; Klemm, C.; Gersch, C.; Tincey, S.; Michael, L.H.; Entman, M.L.; Frangogiannis, N.G. Of mice and dogs: Species-specific differences in the inflammatory response following myocardial infarction. Am. J. Pathol. 2004, 164, 665–677. [Google Scholar] [CrossRef] [Green Version]
- Khalil, H.; Kanisicak, O.; Prasad, V.; Correll, R.N.; Fu, X.; Schips, T.; Vagnozzi, R.J.; Liu, R.; Huynh, T.; Lee, S.J. Fibroblast-specific TGF-β-Smad2/3 signaling underlies cardiac fibrosis. J. Clin. Investig. 2017, 127, 3770–3783. [Google Scholar] [CrossRef]
- Kharitonenkov, A.; DiMarchi, R. Fibroblast growth factor 21 night watch: Advances and uncertainties in the field. J Intern Med. 2017, 281, 233–246. [Google Scholar] [CrossRef]
- Phan, P.; Saikia, B.B.; Sonnaila, S.; Agrawal, S.; Alraawi, Z.; Kumar, T.K.S.; Iyer, S. The Saga of Endocrine FGFs. Cells 2021, 10, 2418. [Google Scholar] [CrossRef]
- Tezze, C.; Romanello, V.; Sandri, M. FGF21 as Modulator of Metabolism in Health and Disease. Front. Physiol. 2019, 10, 419. [Google Scholar] [CrossRef]
- Wang, S.; Wang, Y.; Zhang, Z.; Liu, Q.; Gu, J. Cardioprotective effects of fibroblast growth factor 21 against doxorubicin-induced toxicity via the SIRT1/LKB1/AMPK pathway. Cell Death Dis. 2017, 8, e3018. [Google Scholar] [CrossRef] [PubMed]
- Ferrer-Curriu, G.; Guitart-Mampel, M.; Rupérez, C.; Zamora, M.; Crispi, F.; Villarroya, F.; Fernández-Solà, J.; Garrabou, G.; Planavila, A. The protective effect of fibroblast growth factor-21 in alcoholic cardiomyopathy: A role in protecting cardiac mitochondrial function. J. Pathol. 2021, 253, 198–208. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhu, Z.; Xue, M.; Yi, X.; Liang, J.; Niu, C.; Chen, G.; Shen, Y.; Zhang, H.; Zheng, J.; et al. Fibroblast growth factor 21 protects the heart from angiotensin II-induced cardiac hypertrophy and dysfunction via SIRT1. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 1241–1252. [Google Scholar] [CrossRef]
- Planavila, A.; Redondo, I.; Hondares, E.; Vinciguerra, M.; Munts, C.; Iglesias, R.; Gabrielli, L.A.; Sitges, M.; Giralt, M.; van Bilsen, M.; et al. Fibroblast growth factor 21 protects against cardiac hypertrophy in mice. Nat. Commun. 2013, 4, 2019. [Google Scholar] [CrossRef]
- Yang, H.; Feng, A.; Lin, S.; Yu, L.; Lin, X.; Yan, X.; Lu, X.; Zhang, C. Fibroblast growth factor-21 prevents diabetic cardiomyopathy via AMPK-mediated antioxidation and lipid-lowering effects in the heart. Cell Death Dis. 2018, 9, 227. [Google Scholar] [CrossRef] [Green Version]
- Lin, S.; Yu, L.; Ni, Y.; He, L.; Weng, X.; Lu, X.; Zhang, C. Fibroblast Growth Factor 21 Attenuates Diabetes-Induced Renal Fibrosis by Negatively Regulating TGF-β-p53-Smad2/3-Mediated Epithelial-to-Mesenchymal Transition via Activation of AKT. Diabetes Metab. J. 2020, 44, 158–172. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Liu, Q.; Gui, Y.; Liao, C.; Xu, D. Exercise promotes cardiac-specific fibroblast growth factor 21 expression. Int. J. Cardiol. 2016, 203, 532–533. [Google Scholar] [CrossRef]
- Hansen, J.S.; Pedersen, B.K.; Xu, G.; Lehmann, R.; Weigert, C.; Plomgaard, P. Exercise-Induced Secretion of FGF21 and Follistatin Are Blocked by Pancreatic Clamp and Impaired in Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2016, 101, 2816–2825. [Google Scholar] [CrossRef] [Green Version]
- Morville, T.; Sahl, R.E.; Trammell, S.A.; Svenningsen, J.S.; Gillum, M.P.; Helge, J.W.; Clemmensen, C. Divergent effects of resistance and endurance exercise on plasma bile acids, FGF19, and FGF21 in humans. JCI Insight 2018, 3, e122737. [Google Scholar] [CrossRef]
- Bashey, R.I.; Martinez-Hernandez, A.; Jimenez, S.A. Isolation, characterization, and localization of cardiac collagen type VI. Associations with other extracellular matrix components. Circ. Res. 1992, 70, 1006–1017. [Google Scholar] [CrossRef] [Green Version]
- Sun, M.; Jin, L.; Bai, Y.; Wang, L.; Zhao, S.; Ma, C.; Ma, D. Fibroblast growth factor 21 protects against pathological cardiac remodeling by modulating galectin-3 expression. J. Cell. Biochem. 2019, 120, 19529–19540. [Google Scholar] [CrossRef] [PubMed]
- Ferrer-Curriu, G.; Redondo-Angulo, I.; Guitart-Mampel, M.; Ruperez, C.; Mas-Stachurska, A.; Sitges, M.; Garrabou, G.; Villarroya, F.; Fernández-Solà, J.; Planavila, A. Fibroblast growth factor-21 protects against fibrosis in hypertensive heart disease. J. Pathol. 2019, 248, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Zhao, J.; Rong, J. Pharmacological Modulation of Cardiac Remodeling after Myocardial Infarction. Oxid. Med. Cell. Longev. 2020, 2020, 8815349. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wang, Y.; Deng, Y.; Ni, H.; Shen, G.; Liu, X.; Li, J.; Wang, F. Epigenetic Control of circHNRNPH1 in Postischemic Myocardial Fibrosis through Targeting of TGF-β Receptor Type I. Mol. Ther. Nucleic Acids 2020, 25, 93–104. [Google Scholar] [CrossRef]
- Kang, G.J.; Kim, E.J.; Lee, C.H. Therapeutic Effects of Specialized Pro-Resolving Lipids Mediators on Cardiac Fibrosis via NRF2 Activation. Antioxidants 2020, 9, 1259. [Google Scholar] [CrossRef]
- Sunaga, H.; Koitabashi, N.; Iso, T.; Matsui, H.; Obokata, M.; Kawakami, R.; Murakami, M.; Yokoyama, T.; Kurabayashi, M. Activation of cardiac AMPK-FGF21 feed-forward loop in acute myocardial infarction: Role of adrenergic overdrive and lipolysis byproducts. Sci. Rep. 2019, 9, 11841. [Google Scholar] [CrossRef] [Green Version]
- Braith, R.W.; Stewart, K.J. Resistance exercise training: Its role in the prevention of cardiovascular disease. Circulation 2006, 113, 2642–2650. [Google Scholar] [CrossRef]
- Gomes-Neto, M.; Durães, A.R.; Conceição, L.S.R.; Roever, L.; Silva, C.M.; Alves, I.G.N.; Ellingsen, Ø.; Carvalho, V.O. Effect of combined aerobic and resistance training on peak oxygen consumption, muscle strength and health-related quality of life in patients with heart failure with reduced left ventricular ejection fraction: A systematic review and meta-analysis. Int. J. Cardiol. 2019, 293, 165–175. [Google Scholar] [CrossRef]
- Passino, C.; Severino, S.; Poletti, R.; Piepoli, M.F.; Mammini, C.; Clerico, A.; Gabutti, A.; Nassi, G.; Emdin, M. Aerobic training decreases B-type natriuretic peptide expression and adrenergic activation in patients with heart failure. J. Am. Coll. Cardiol. 2006, 47, 1835–1839. [Google Scholar] [CrossRef] [Green Version]
- Brand, C.S.; Lighthouse, J.K.; Trembley, M.A. Protective transcriptional mechanisms in cardiomyocytes and cardiac fibroblasts. J. Mol. Cell. Cardiol. 2019, 132, 1–12. [Google Scholar] [CrossRef]
- de Jong, S.; van Veen, T.A.; van Rijen, H.V.; de Bakker, J.M. Fibrosis and cardiac arrhythmias. J. Cardiovasc. Pharmacol. 2011, 57, 630–638. [Google Scholar] [CrossRef]
- Murtha, L.A.; Schuliga, M.J.; Mabotuwana, N.S.; Hardy, S.A.; Waters, D.W.; Burgess, J.K.; Knight, D.A.; Boyle, A.J. The Processes and Mechanisms of Cardiac and Pulmonary Fibrosis. Front. Physiol. 2017, 8, 777. [Google Scholar] [CrossRef] [Green Version]
- Disertori, M.; Masè, M.; Ravelli, F. Myocardial fibrosis predicts ventricular tachyarrhythmias. Trends Cardiovasc. Med. 2017, 27, 363–372. [Google Scholar] [CrossRef]
- Mukherjee, D.; Sen, S. Alteration of collagen phenotypes in ischemic cardiomyopathy. J. Clin. Investig. 1991, 88, 1141–1146. [Google Scholar] [CrossRef]
- Tanajak, P.; Chattipakorn, S.C.; Chattipakorn, N. Effects of fibroblast growth factor 21 on the heart. J. Endocrinol. 2015, 227, R13–R30. [Google Scholar] [CrossRef] [Green Version]
- Yan, X.; Chen, J.; Zhang, C.; Zhou, S.; Zhang, Z.; Chen, J.; Feng, W.; Li, X.; Tan, Y. FGF21 deletion exacerbates diabetic cardiomyopathy by aggravating cardiac lipid accumulation. J. Cell. Mol. Med. 2015, 19, 1557–1568. [Google Scholar] [CrossRef]
- Flanders, K.C. Smad3 as a mediator of the fibrotic response. Int. J. Exp. Pathol. 2004, 85, 47–64. [Google Scholar] [CrossRef] [PubMed]
- Ikeuchi, M.; Tsutsui, H.; Shiomi, T.; Matsusaka, H.; Matsushima, S.; Wen, J.; Kubota, T.; Takeshita, A. Inhibition of TGF-beta signaling exacerbates early cardiac dysfunction but prevents late remodeling after infarction. Cardiovasc. Res. 2004, 64, 526–535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rangarajan, S.; Bone, N.B.; Zmijewska, A.A.; Jiang, S.; Park, D.W.; Bernard, K.; Locy, M.L.; Ravi, S.; Deshane, J.; Mannon, R.B.; et al. Metformin reverses established lung fibrosis in a bleomycin model. Nat. Med. 2018, 24, 1121–1127. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zhao, W.; Meng, W.; Zhao, T.; Chen, Y.; Ahokas, R.A.; Liu, H.; Sun, Y. Platelet-derived growth factor blockade on cardiac remodeling following infarction. Mol. Cell. Biochem. 2014, 397, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Slavic, S.; Lauer, D.; Sommerfeld, M.; Kemnitz, U.R.; Grzesiak, A.; Trappiel, M.; Thöne-Reineke, C.; Baulmann, J.; Paulis, L.; Kappert, K.; et al. Cannabinoid receptor 1 inhibition improves cardiac function and remodelling after myocardial infarction and in experimental metabolic syndrome. J. Mol. Med. 2013, 91, 811–823. [Google Scholar] [CrossRef]
- Sonobe, T.; Tsuchimochi, H.; Schwenke, D.O.; Pearson, J.T.; Shirai, M. Treadmill running improves hindlimb arteriolar endothelial function in type 1 diabetic mice as visualized by X-ray microangiography. Cardiovasc. Diabetol. 2015, 14, 51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horii, N.; Uchida, M.; Hasegawa, N.; Fujie, S.; Oyanagi, E.; Yano, H.; Hashimoto, T.; Iemitsu, M. Resistance training prevents muscle fibrosis and atrophy via down-regulation of C1q-induced Wnt signaling in senescent mice. FASEB J. 2018, 32, 3547–3559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomes, M.J.; Pagan, L.U.; Lima, A.R.R.; Reyes, D.R.A.; Martinez, P.F.; Damatto, F.C.; Pontes, T.H.D.; Rodrigues, E.A.; Souza, L.M.; Tosta, I.F.; et al. Effects of aerobic and resistance exercise on cardiac remodelling and skeletal muscle oxidative stress of infarcted rats. J. Cell. Mol. Med. 2020, 24, 5352–5362. [Google Scholar] [CrossRef] [Green Version]
- Lin, C.I.; Huang, W.C.; Chen, W.C.; Kan, N.W.; Wei, L.; Chiu, Y.S.; Huang, C.C. Effect of whole-body vibration training on body composition, exercise performance and biochemical responses in middle-aged mice. Metabolism 2015, 64, 1146–1156. [Google Scholar] [CrossRef]
- Hu, L.; Klein, J.D.; Hassounah, F.; Cai, H.; Zhang, C.; Xu, P.; Wang, X.H. Low-frequency electrical stimulation attenuates muscle atrophy in CKD--a potential treatment strategy. J. Am. Soc. Nephrol. 2015, 26, 626–635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, F.; Li, Z.; Cai, M.; Xi, Y.; Xu, Z.; Zhang, Z.; Li, H.; Zhu, W.; Tian, Z. Aerobic exercise alleviates oxidative stress-induced apoptosis in kidneys of myocardial infarction mice by inhibiting ALCAT1 and activating FNDC5/Irisin signaling pathway. Free Radic. Biol. Med. 2020, 158, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Mao, C.; Zhou, E.; You, J.; Gao, E.; Han, Z.; Fan, Y.; He, Q.; Wang, C. MicroRNA-21 Mediates a Positive Feedback on Angiotensin II-Induced Myofibroblast Transformation. J. Inflamm. Res. 2020, 13, 1007–1020. [Google Scholar] [CrossRef]
- Nie, C.; Zou, R.; Pan, S.; Rong, A.; Gao, Y.; Yang, H.; Bai, J.; Xi, S.; Wang, X.; Hong, X.; et al. Hydrogen gas inhalation ameliorates cardiac remodelling and fibrosis by regulating NLRP3 inflammasome in myocardial infarction rats. J. Cell. Mol. Med. 2021, 25, 8997–9010. [Google Scholar] [CrossRef]
- Guo, J.L.; Yu, Y.; Jia, Y.Y.; Ma, Y.Z.; Zhang, B.Y.; Liu, P.Q.; Chen, S.R.; Jiang, J.M. Transient receptor potential melastatin 7 (TRPM7) contributes to H2O2-induced cardiac fibrosis via mediating Ca2+ influx and extracellular signal-regulated kinase 1/2 (ERK1/2) activation in cardiac fibroblasts. J. Pharmacol. Sci. 2014, 125, 184–192. [Google Scholar] [CrossRef] [Green Version]
- Song, Y.; Ding, J.; Jin, R.; Jung, J.; Li, S.; Yang, J.; Wang, A.; Li, Z. Expression and purification of FGF21 in Pichia pastoris and its effect on fibroblast-cell migration. Mol. Med. Rep. 2016, 13, 3619–3626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Y.; Kuang, Y.; Bo, W.; Liang, Q.; Zhu, W.; Cai, M.; Tian, Z. Exercise Training Alleviates Cardiac Fibrosis through Increasing Fibroblast Growth Factor 21 and Regulating TGF-β1-Smad2/3-MMP2/9 Signaling in Mice with Myocardial Infarction. Int. J. Mol. Sci. 2021, 22, 12341. https://doi.org/10.3390/ijms222212341
Ma Y, Kuang Y, Bo W, Liang Q, Zhu W, Cai M, Tian Z. Exercise Training Alleviates Cardiac Fibrosis through Increasing Fibroblast Growth Factor 21 and Regulating TGF-β1-Smad2/3-MMP2/9 Signaling in Mice with Myocardial Infarction. International Journal of Molecular Sciences. 2021; 22(22):12341. https://doi.org/10.3390/ijms222212341
Chicago/Turabian StyleMa, Yixuan, Yixin Kuang, Wenyan Bo, Qiaoqin Liang, Wenfei Zhu, Mengxin Cai, and Zhenjun Tian. 2021. "Exercise Training Alleviates Cardiac Fibrosis through Increasing Fibroblast Growth Factor 21 and Regulating TGF-β1-Smad2/3-MMP2/9 Signaling in Mice with Myocardial Infarction" International Journal of Molecular Sciences 22, no. 22: 12341. https://doi.org/10.3390/ijms222212341
APA StyleMa, Y., Kuang, Y., Bo, W., Liang, Q., Zhu, W., Cai, M., & Tian, Z. (2021). Exercise Training Alleviates Cardiac Fibrosis through Increasing Fibroblast Growth Factor 21 and Regulating TGF-β1-Smad2/3-MMP2/9 Signaling in Mice with Myocardial Infarction. International Journal of Molecular Sciences, 22(22), 12341. https://doi.org/10.3390/ijms222212341








