Hyperandrogenism? Increased 17, 20-Lyase Activity? A Metanalysis and Systematic Review of Altered Androgens in Boys and Girls with Autism
Abstract
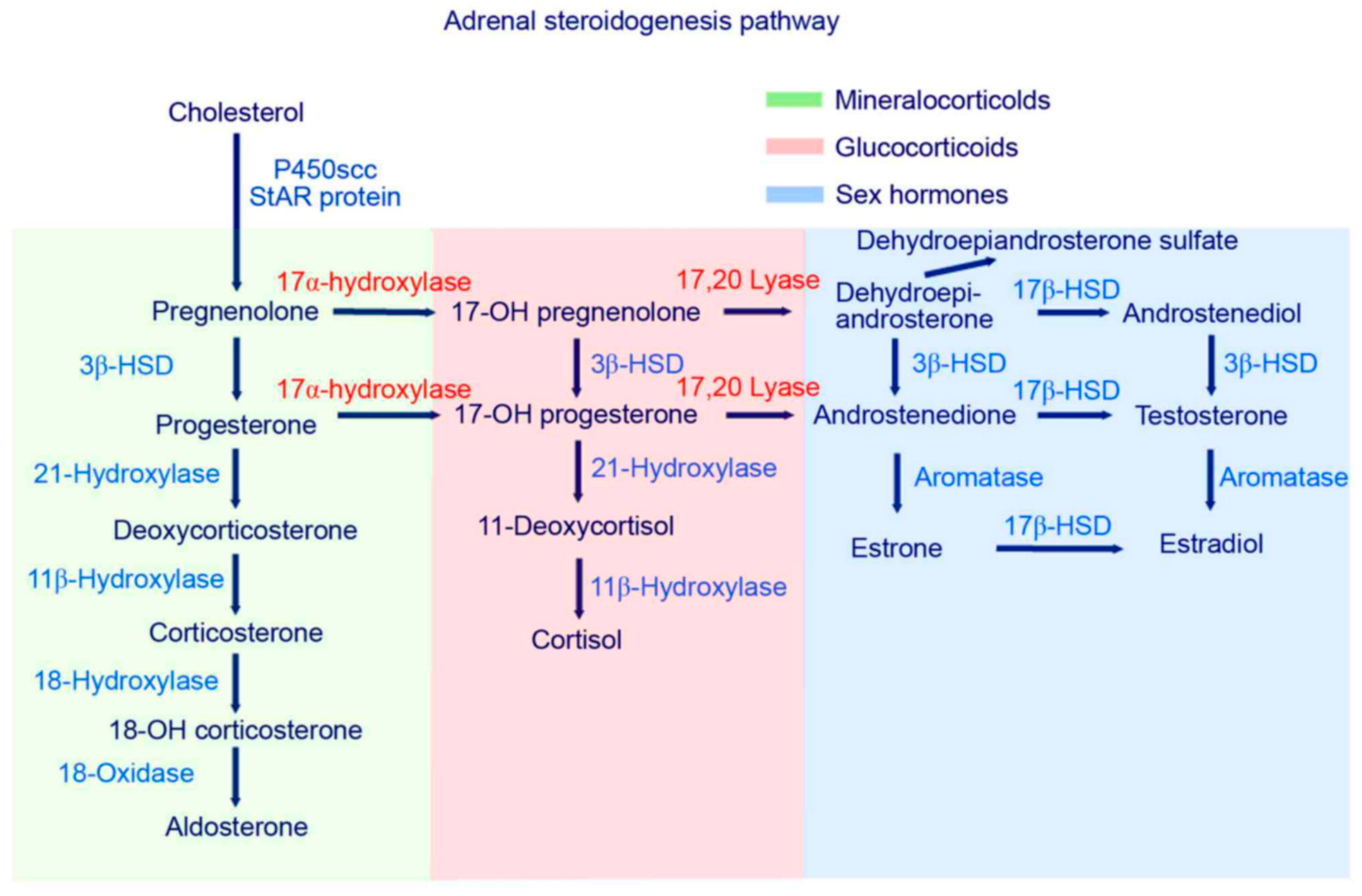
:1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Study Eligibility Criteria
2.3. Data Collection and Analysis
2.4. Outcomes
2.5. Publication Bias
2.6. Statistical Analysis
3. Results and Discussion
4. Conclusions
5. Ethics and Dissemination Policy
5.1. Developmental STAGE
5.2. Severity of Impairment
5.3. Gender
5.4. Comorbidities and Medication Use
5.5. Diagnostic Differences throughout History and between Samples
5.6. Sample Size
5.7. Methodical Problems
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Asperger, H. Die “Autistischen Psychopathen” im Kindesalter. Arch. Psychiatr. Nervenkrankh. 1944, 117, 76–136. [Google Scholar] [CrossRef]
- Gillberg, C.; Fernell, E.; Kočovská, E.; Minnis, H.; Bourgeron, T.; Thompson, L.; Allely, C.S. The role of cholesterol metabolism and various steroid abnormalities in autism spectrum disorders: A hypothesis paper. Autism Res. 2017, 10, 1022–1044. [Google Scholar] [CrossRef] [PubMed]
- Tordjman, S.; Anderson, G.M.; McBride, P.A.; Hertzig, M.E.; Snow, M.E.; Hall, L.M.; Ferrari, P.; Cohen, D.J. Plasma androgens in autism. J. Autism Dev. Disord. 1995, 25, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Gasser, B.A. The Case of Hellmuth in The Autistic Psychopathy—Suffering from Cushing Syndrome? Glob. J. Intellect. Dev. Disabil. 2018, 4, 555643. [Google Scholar] [CrossRef]
- Baron-Cohen, S.; Auyeung, B.; Nørgaard-Pedersen, B.; Hougaard, D.M.; Abdallah, M.W.; Melgaard, L.; Cohen, A.S.; Chakrabarti, B.; Ruta, L.; Lombardo, M.V. Elevated fetal steroidogenic activity in autism. Mol. Psychiatry 2014, 20, 369–376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janšáková, K.; Hill, M.; Čelárová, D.; Celušáková, H.; Repiská, G.; Bičíková, M.; Máčová, L.; Ostatníková, D. Alteration of the steroidogenesis in boys with autism spectrum disorders. Transl. Psychiatry 2020, 10, 1–15. [Google Scholar] [CrossRef]
- Gasser, B.A.; Kurz, J.; Dick, B.; Mohaupt, M.G. Steroid Metabolites Support Evidence of Autism as a Spectrum. Behav. Sci. 2019, 9, 52. [Google Scholar] [CrossRef] [Green Version]
- Gasser, B.A.; Kurz, J.; Dick, B.; Mohaupt, M.G. A reply to high androgens in autism. A reply to ‘Alteration of steroidogenesis in boys with autism spectrum disorders’. Nat. Transl. Psychiatry 2021, 11, 278. [Google Scholar] [CrossRef]
- Xu, X.-J.; Shou, X.-J.; Li, J.; Jia, M.-X.; Zhang, J.-S.; Guo, Y.; Wei, Q.-Y.; Zhang, X.-T.; Han, S.-P.; Zhang, R.; et al. Mothers of Autistic Children: Lower Plasma Levels of Oxytocin and Arg-Vasopressin and a Higher Level of Testosterone. PLoS ONE 2013, 8, e74849. [Google Scholar] [CrossRef] [Green Version]
- Ingudomnukul, E.; Baron-Cohen, S.; Wheelwright, S.J.; Knickmeyer, R. Elevated rates of testosterone-related disorders in women with autism spectrum conditions. Horm. Behav. 2007, 51, 597–604. [Google Scholar] [CrossRef]
- Takagishi, H.; Takahashi, T.; Yamagishi, T.; Shinada, M.; Inukai, K.; Tanida, S.; Mifune, N.; Horita, Y.; Hashimoto, H.; Yang, Y.; et al. Salivary testosterone levels and autism-spectrum quotient in adults. Neuroendocrinol. Lett. 2010, 31, 837–841. [Google Scholar]
- Palomba, S.; Marotta, R.; Di Cello, A.; Russo, T.; Falbo, A.; Orio, F.; Tolino, A.; Zullo, F.; Esposito, R.; La Sala, G.B. Pervasive developmental disorders in children of hyperandrogenic women with polycystic ovary syndrome: A longitudinal case-control study. Clin. Endocrinol. 2012, 77, 898–904. [Google Scholar] [CrossRef]
- Saenz, J.; Alexander, G.M. Postnatal testosterone levels and disorder relevant behavior in the second year of life. Biol. Psychol. 2013, 94, 152–159. [Google Scholar] [CrossRef]
- Taylor, J.L.; Corbett, B.A. A review of rhythm and responsiveness of cortisol in individuals with autism spectrum disorders. Psychoneuroendocrinology 2014, 49, 207–228. [Google Scholar] [CrossRef] [Green Version]
- Iwata, K.; Matsuzaki, H.; Miyachi, T.; Shimmura, C.; Suda, S.; Tsuchiya, K.J.; Matsumoto, K.; Suzuki, K.; Iwata, Y.; Nakamura, K.; et al. Investigation of the serum levels of anterior pituitary hormones in male children with autism. Mol. Autism 2011, 2, 16. [Google Scholar] [CrossRef] [Green Version]
- Bitsika, V.; Sharpley, C.; Sweeney, J.A.; McFarlane, J.R. HPA and SAM axis responses as correlates of self- vs parental ratings of anxiety in boys with an Autistic Disorder. Physiol. Behav. 2014, 127, 1–7. [Google Scholar] [CrossRef]
- Brosnan, M.; Turner-Cobb, J.; Munro-Naan, Z.; Jessop, D. Absence of a normal Cortisol Awakening Response (CAR) in adolescent males with Asperger Syndrome (AS). Psychoneuroendocrinology 2009, 34, 1095–1100. [Google Scholar] [CrossRef]
- Marinović-Ćurin, J.; Marinović-Terzić, I.; Bujas-Petković, Z.; Zekan, L.; Škrabić, V.; Đogaš, Z.; Terzić, J. Slower cortisol response during ACTH stimulation test in autistic children. Eur. Child Adolesc. Psychiatry 2007, 17, 39–43. [Google Scholar] [CrossRef]
- Hoshino, Y.; Yokoyama, F.; Watanabe, M.; Murata, S.; Kaneko, M.; Kumashiro, H. The diurnal variation and response to dexamethasone suppression test of saliva cortisol level in autistic children. Psychiatry Clin. Neurosci. 1987, 41, 227–235. [Google Scholar] [CrossRef]
- Hollocks, M.; Howlin, P.; Papadopoulos, A.S.; Khondoker, M.; Simonoff, E. Differences in HPA-axis and heart rate responsiveness to psychosocial stress in children with autism spectrum disorders with and without co-morbid anxiety. Psychoneuroendocrinology 2014, 46, 32–45. [Google Scholar] [CrossRef]
- Hamza, R.T.; Hewedi, D.H.; Ismail, M.A. Basal and Adrenocorticotropic Hormone Stimulated Plasma Cortisol Levels Among Egyptian Autistic Children: Relation to Disease Severity. Ital. J. Pediatr. 2010, 36, 71. [Google Scholar] [CrossRef] [Green Version]
- Curin, J.M.; Terzić, J.; Petković, Z.B.; Zekan, L.; Terzić, I.M.; Susnjara, I.M. Lower cortisol and higher ACTH levels in individuals with autism. J. Autism Dev. Disord. 2003, 33, 443–448. [Google Scholar] [CrossRef]
- Ruta, L.; Ingudomnukul, E.; Taylor, K.; Chakrabarti, B.; Baron-Cohen, S. Increased serum androstenedione in adults with autism spectrum conditions. Psychoneuroendocrinology 2011, 36, 1154–1163. [Google Scholar] [CrossRef]
- Majewska, M.D. Neurosteroids: Endogenous bimodal modulators of the GABAA receptor. Mechanism of action and physiological significance. Prog. Neurobiol. 1992, 38, 379–395. [Google Scholar] [CrossRef]
- Hu, V.W.; Nguyen, A.; Kim, K.S.; Steinberg, M.E.; Sarachana, T.; Scully, M.A.; Soldin, S.J.; Luu, T.; Lee, N.H. Gene expression profiling of lymphoblasts from autistic and nonaffected sib pairs: Altered pathways in neuronal development and steroid biosynthesis. PLoS ONE 2009, 4, e5775. [Google Scholar] [CrossRef]
- Hu, V.; Sarachana, T.; Sherrard, R.M.; Kocher, K. Investigation of sex differences in the expression of RORA and its transcriptional targets in the brain as a potential contributor to the sex bias in autism. Mol. Autism 2015, 6, 7. [Google Scholar] [CrossRef] [Green Version]
- Sarachana, T.; Xu, M.; Wu, R.C.; Hu, V.W. Sex hormones in autism: Androgens and estrogens differentially and reciprocally regulate RORA, a novel candidate gene for autism. PLoS ONE 2011, 6, e17116. [Google Scholar] [CrossRef]
- Sarachana, T.; Hu, V. Differential recruitment of coregulators to the RORA promoter adds another layer of complexity to gene (dys) regulation by sex hormones in autism. Mol. Autism 2013, 4, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Broek, J.A.C.; Brombacher, E. Stelzhammer, V.; Guest, P.C.; Rahmoune, H.; Bahn, S. The need for a comprehensive molecular characterization of autism spectrum disorders. Int. J. Neuropsychopharmacol. 2014, 17, 651–673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bezdickova, M.; Molikova, R.; Bebarova, L.; Kolar, Z. Distribution ofnuclear receptors for steroid hormones in the human brain: A pre-liminary study. Biomed. Pap. Med Fac. Palacky Univ. Olomouc 2007, 151, 69–71. [Google Scholar] [CrossRef] [Green Version]
- Quartier, A.; Chatrousse, L.; Redin, C.; Keime, C.; Haumesser, N.; Maglott-Roth, A.; Brino, L.; Le Gras, S.; Benchoua, A.; Mandel, J.L.; et al. Genes and Pathways Regulated by Androgens in Human Neural Cells, Potential Candidates for the Male Excess in Autism Spectrum Disorder. Biol. Psychiatry 2018, 84, 239–252. [Google Scholar] [CrossRef] [PubMed]
- Simerly, R.B.; Chang, C.; Muramatsu, M.; Swanson, L.W. Distributionof androgen and estrogen receptor mRNA-containing cells in the ratbrain: An in situ hybridization study. J. Comp. Neurol. 1990, 294, 76–95. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Coats, J.K.; Yang, C.F.; Wang, A.; Ahmed, O.M.; Alvarado, M.; Izumi, T.; Shah, N.M. Modular genetic control of sexually dimorphic behaviors. Cell 2012, 148, 596–607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hammond, J.; Le, Q.; Goodyer, C.; Gelfand, M.; Trifiro, M.; LeBlanc, A. Testosterone-mediated neuroprotection through the androgen re-ceptor in human primary neurons. J. Neurochem. 2001, 77, 1319–1326. [Google Scholar] [CrossRef]
- Pike, C.J.; Nguyen, T.V.; Ramsden, M.; Yao, M.; Murphy, M.P.; Rosario, E.R. Androgen cell signaling pathways involved in neuroprotectiveactions. Horm. Behav. 2008, 53, 693–705. [Google Scholar] [CrossRef] [Green Version]
- Hatanaka, Y.; Mukai, H.; Mitsuhashi, K.; Hojo, Y.; Murakami, G.; Komatsuzaki, Y.; Sato, R.; Kawato, S. Androgen rapidly increases dendriticthorns of CA3 neurons in male rat hippocampus. Biochem. Biophys. Res. Commun. 2009, 381, 728–732. [Google Scholar] [CrossRef]
- Lustig, R.H. Sex hormone modulation of neural developmentin vitro. Horm. Behav. 1994, 28, 383–395. [Google Scholar] [CrossRef]
- Popper, K.R. Logik Der Forschung; Mohr Siebeck: Tübingen, Germany, 1969. [Google Scholar]
- Gevi, F.; Zolla, L.; Gabriele, S.; Persico, A.M. Urinary metabolomics of young Italian autistic children supports abnormal tryptophan and purine metabolism. Mol. Autism 2016, 7, 47. [Google Scholar] [CrossRef] [Green Version]
- Egger, M.; Smith, G.D.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef] [Green Version]
- Hedges, L.V. Distribution Theory for Glass’s Estimator of Effect Size and Related Estimators. J. Educ. Stat. 1981, 6, 107–128. [Google Scholar] [CrossRef]
- Langan, D.; Higgins, J.P.; Simmonds, M. Comparative performance of heterogeneity variance estimators in meta-analysis: A review of simulation studies. Res. Synth. Methods 2017, 8, 181–198. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Geier, D.A.; Geier, M.R. A clinical and laboratory evaluation of methionine cycle-transsulfuration and androgen pathway markers in children with autistic disorders. Horm. Res. 2006, 66, 182–188. [Google Scholar] [CrossRef]
- Geier, D.A.; Geier, M.R. A prospective assessment of androgen levels in patients with autistic spectrum disorders: Biochemical underpinnings and suggested therapies. Neuroendocrinol. Lett. 2007, 28, 565–573. [Google Scholar]
- El-Baz, F.; Hamza, R.T.; Ayad, M.S.; Mahmoud, N.H. Hyperandrogenemia in male autistic children and adolescents: Relation to disease severity. Int. J. Adolesc. Med. Health 2014, 26, 79–84. [Google Scholar] [CrossRef]
- Croonenberghs, J.; Van Grieken, S.; Wauters, A.; Van West, D.; Brouw, L.; Maes, M.; Deboutte, D. Serum testosterone con centration in male autistic youngsters. Neuroendocrinol. Lett. 2010, 31, 483–488. [Google Scholar]
- Majewska, M.D.; Hill, M.; Urbanowicz, E.; Rok-Bujko, P.; Bieńkowski, P.; Namysłowska, I.; Mierzejewski, P. Marked elevation of adrenal steroids, especially androgens, in saliva of prepubertal autistic children. Eur. Child Adolesc. Psychiatry 2013, 23, 485–498. [Google Scholar] [CrossRef] [Green Version]
- Gasser, B.A.; Kurz, J.; Dick, B.; Mohaupt, M.G. Are steroid hormones dysregulated in autistic girls. Diseases 2020, 8, 6. [Google Scholar] [CrossRef] [Green Version]
- Zhu, W.; Han, B.; Fan, M.; Wang, N.; Wang, H.; Zhu, H.; Cheng, T.; Zhao, S.; Song, H.; Qiao, J. Oxidative stress increases the 17,20-lyase-catalyzing activity of adrenal P450c17 through p38α in the development of hyperandrogenism. Mol. Cell. Endocrinol. 2019, 484, 25–33. [Google Scholar] [CrossRef]
- Miller, W.L.; Tee, M.K. The post-translational regulation of 17,20 lyase activity. Mol. Cell. Endocrinol. 2015, 408, 99–106. [Google Scholar] [CrossRef]
- Miller, W.L.; Auchus, R.J. The Molecular Biology, Biochemistry, and Physiology of Human Steroidogenesis and Its Disorders. Endocr. Rev. 2011, 32, 579. [Google Scholar] [CrossRef] [Green Version]
- McCarthy, M.M. How it’s made: Organisational effects of hormones on the developing brain. J. Neuroendocrinol. 2010, 22, 736–742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phoenix, C.H.; Goy, R.W.; Geralerall, A.A.; Young, W.C. Organizing action of prenatally administered testosterone propionate on the tissues mediating mating behavior in the female guinea pig. Endocrinology 1959, 65, 369–382. [Google Scholar] [CrossRef] [PubMed]
- Monnet, F.P.; Mahé, V.; Robel, P.; Baulieu, E.E. Neurosteroids, via sigma receptors, modulate the [3H]norepinephrine release evoked by N-methyl-D-aspartate in the rat hippocampus. Proc. Natl. Acad. Sci. USA 1995, 92, 3774–3778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, P. Neuroactive steroid regulation of neurotransmitter release in the CNS: Action, mechanism and possible significance. Prog. Neurobiol. 2009, 89, 134–152. [Google Scholar] [CrossRef]
- Rossignol, D.A.; Frye, R.E. Evidence linking oxidative stress, mitochondrial dysfunction, and inflammation in the brain of individuals with autism. Front. Physiol. 2014, 5, 150. [Google Scholar] [CrossRef] [Green Version]
- Gasser, B.A.; Kurz, J.; Escher, G.; Senn, W.; Mohaupt, M.G. Stress-induced alterations of social behavior is reversible by antagonism of steroid hormones in C57/BL6 mice. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2020, 394, 127–135. [Google Scholar] [CrossRef]







| Study/Author | Number of Patients and Description of Inclusion Criteria | Methods and Measured Outcome | Major Findings | Notes | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Boys | Girls | ||||||||||
| Autism | Control | Autism | Control | ||||||||
| Prepubertal | Postpubertal | Prepubertal | Postpubertal | Prepubertal | Postpubertal | Prepubertal | Postpubertal | ||||
| TORDJMAN ET AL. 1995 | 31N/A | 10N/A | 8 healthy children | 11 healthy children | Blood (plasma), Testosterone and DHEA-S | No alterations of Testosterone and DHEA-S | no significant increase neither in the prepubertal nor in the postpubertal group of children with autism as compared to ten healthy controls | ||||
| 14 Sixteen consecutive pre-pubertal age children (</=11 years old; mean +/- SD: 5.9 +/- 2.1 years old) | Age- and sex- specific reference values from LabCorp | 2 Sixteen consecutive pre-pubertal age children (</=11 years old; mean +/- SD: 5.9 +/- 2.1 years old) | Age- and sex- specific reference values from LabCorp | Blood samples | Significantly increased levels of serum/plasma DHEA and serum total Testosterone relative to the age- and sex-specific normal laboratory reference ranges were observed. | There was no control group—results were reported as percent of mean reference value | |||||
| 59 children 10.8 ± 4.1 (34 with Autism and 36 with Asperger syndrome and PDD-NOS according to DSM-IV criteria, >/= 6 years-old) | Age- and sex- specific reference values from LabCorp | 11 children 10.8 ± 4.1 (34 with Autism and 36 with Asperger syndrome and PDD-NOS according to DSM-IV criteria, >/= 6 years-old) | Age- and sex- specific reference values from LabCorp | Blood samples, serum Testosterone, serum free Testosterone, % free Testosterone, DHEA, Androstendione, morning blood samples collected afer overnight fast | Affected subjects showed significantly increased relative mean levels for: serum Testosterone (158%), serum free Testosterone (214%), percent free Testosterone (121%), DHEA (192%), and Androstenedione (173%). Additionally, at least one of the androgen attributes examined exceeded its recognized laboratory age- and sex-specific reference range in 81.4% (57 of 70) of the patients examined. With respect to their age- and sex-specific reference ranges, females had significantly higher overall mean relative Testosterone and relative free Testosterone levels than males. | There was no control group—results were reported as percent of mean reference value | |||||
| CROONENBERGHS ET AL. 2010 | 18 DSM-IV criteria to make the diagnosis of autism. | 22 healthy volunteers | Blood samples, the serum Testosterone concentration on 9 consecutives time points between 08.00 AM and 12.00 AM | The total Testosterone concentration was significantly lower in the autism group compared to the group of healthy controls. | |||||||
| MAJEWSKA ET AL. 2013 | 23 age group 3–4 years DSM-IV | 19 age group 7–9 years | 20 age group 3–4 years DSM-IV | 17 age group 7–9 years | 22 age group 3–4 years DSM-IV | 13 age group 7–9 years | 16 age group 3–4 years DSM-IV | 18 age group 7–9 years | salivary levels of 22 steroids | Children with autism had significantly higher Androstenediol, DHEA, Androsterone and their polar conjugates), indicative of precocious adrenarche and predictive of early puberty | |
| EL-BAZ ET AL. 2014 | 30 (DSM-IV), 12 (40%) had mild to moderate autism and 18 (60%) had severe autism. | 20 sex- and pubertal-stage-matched children and adolescents | Blood (serum), serum free Testosterone, DHEA, Δ4-Androstenedione (Δ4-A). | 11 showed higher free Testosterone levels, 9 had high DHEA, 12 had high Δ4-A and 8 children showed an elevation of all androgen levels, an association was detected between disease severity and androgen levels. | |||||||
| GASSER ET AL. 2019 | 41 20 boys with Asperger syndrome, 21 boys with Kanner’s syndrome | 41 matched for age, weight, and height | comprehensive steroid hormone metabolite analysis via gas chromatography–mass spectrometry from urine probes controlled for creatinine excretion | Higher levels of most steroid metabolites were detected in boys with Kanner’s syndrome and Asperger syndrome compared to their matched controls. These differences were more pronounced in affected individuals with Kanner’s syndrome versus Asperger syndrome. | A specific and unique pattern of alteration of Androsterone, Etiocholanolone, Progesterone, Tetrahydrocortisone, and Tetrahydrocortisol was identified in boys with Kanner’s syndrome and Asperger syndrome. | ||||||
| GASSER ET AL. 2020 | 16 Sixteen autistic girls (BMI 17.4 ± 2.8; average age 14.3 + 4.2 years) | 16 matched control cohort for age, weight and height (BMI 16.8 ± 2.4; average age 14.4 ± 4 years) | Urine—MS-GC | ||||||||
| JANSAKOVA ET AL. 2020 | 86 DSM-V | 24 age and sex-matched neurotypical control group | Blood—MS-GC | ||||||||
| TOTAL PER CATEGORY | 170 | 161 | 94 | 69 | 35 | 29 | 16 | 34 | |||
| TOTAL PER CLASS | 331 | 163 | 64 | 50 | |||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gasser, B.A.; Buerki, S.F.; Kurz, J.; Mohaupt, M.G. Hyperandrogenism? Increased 17, 20-Lyase Activity? A Metanalysis and Systematic Review of Altered Androgens in Boys and Girls with Autism. Int. J. Mol. Sci. 2021, 22, 12324. https://doi.org/10.3390/ijms222212324
Gasser BA, Buerki SF, Kurz J, Mohaupt MG. Hyperandrogenism? Increased 17, 20-Lyase Activity? A Metanalysis and Systematic Review of Altered Androgens in Boys and Girls with Autism. International Journal of Molecular Sciences. 2021; 22(22):12324. https://doi.org/10.3390/ijms222212324
Chicago/Turabian StyleGasser, Benedikt A., Samuel F. Buerki, Johann Kurz, and Markus G. Mohaupt. 2021. "Hyperandrogenism? Increased 17, 20-Lyase Activity? A Metanalysis and Systematic Review of Altered Androgens in Boys and Girls with Autism" International Journal of Molecular Sciences 22, no. 22: 12324. https://doi.org/10.3390/ijms222212324
APA StyleGasser, B. A., Buerki, S. F., Kurz, J., & Mohaupt, M. G. (2021). Hyperandrogenism? Increased 17, 20-Lyase Activity? A Metanalysis and Systematic Review of Altered Androgens in Boys and Girls with Autism. International Journal of Molecular Sciences, 22(22), 12324. https://doi.org/10.3390/ijms222212324






