Transcriptomic Changes in Internode Explants of Stinging Nettle during Callogenesis
Abstract
:1. Introduction
2. Results and Discussion
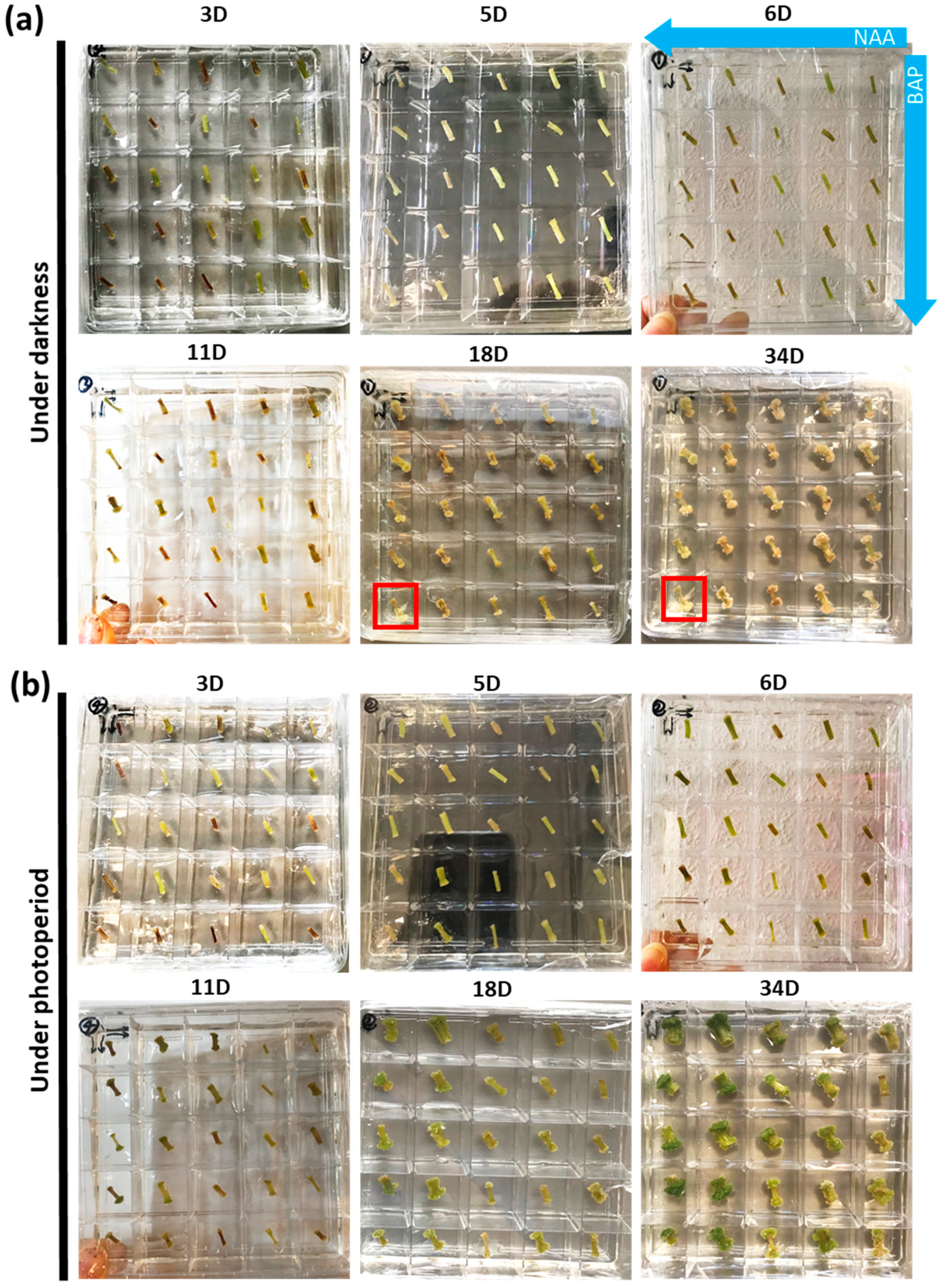
2.1. Identification of Optimal Concentrations of Plant Growth Regulators Promoting Callogenesis
2.2. Selection of the Most Representative Time-Points of Nettle Callogenesis via qPCR
2.3. Gene Ontology Categories Characterizing Callogenesis at Different Time Points in Nettle
2.3.1. Ontologies Characterizing the Advanced Stage of Callogenesis (Cluster C1)
Iron Deficiency Response
Scavenging of ROS
Pathogenesis-Related Transcripts
2.3.2. Ontologies Characterizing the Intermediate Stage of Callogenesis (Clusters C2–C3)
Immune Response
Phenylpropanoid Pathway- and Hypoxia-Related Transcripts
Transcripts Involved in the Metabolism of Brassinosteroids and Auxins
2.3.3. Ontologies Characterizing the Early Stages of Callogenesis (Clusters C4, C5, C6 and C7)
Transcripts Involved in Cell Wall Loosening
Transcripts Related with the Metabolism of Organic Acids and Cytokinins
Transcripts Involved in Base Excision Repair, Cell Wall Biosynthesis and Photosynthesis
3. Materials and Methods
3.1. Plant Materials and Growth Conditions
3.2. Optimal Concentrations and Combinations of Plant Growth Regulators for Callus Induction
3.3. Sampling, RNA Extraction, qPCR and Statistics
3.4. Preparation of the Libraries, RNA-Seq Analysis and Validation with qPCR
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fehér, A. Callus, Dedifferentiation, Totipotency, Somatic Embryogenesis: What These Terms Mean in the Era of Molecular Plant Biology? Front. Plant Sci. 2019, 10, 536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Florentin, A.; Damri, M.; Grafi, G. Stress Induces Plant Somatic Cells to Acquire Some Features of Stem Cells Accompanied by Selective Chromatin Reorganization. Dev. Dyn. 2013, 242, 1121–1133. [Google Scholar] [CrossRef] [PubMed]
- Krasteva, G.; Georgiev, V.; Pavlov, A. Recent Applications of Plant Cell Culture Technology in Cosmetics and Foods. Eng. Life Sci. 2021, 21, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Eibl, R.; Meier, P.; Stutz, I.; Schildberger, D.; Hühn, T.; Eibl, D. Plant Cell Culture Technology in the Cosmetics and Food Industries: Current State and Future Trends. Appl. Microbiol. Biotechnol. 2018, 102, 8661–8675. [Google Scholar] [CrossRef] [Green Version]
- Santos, R.B.; Abranches, R.; Fischer, R.; Sack, M.; Holland, T. Putting the Spotlight Back on Plant Suspension Cultures. Front. Plant Sci. 2016, 7, 297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kregiel, D.; Pawlikowska, E.; Antolak, H. Urtica spp.: Ordinary Plants with Extraordinary Properties. Molecules 2018, 23, 1664. [Google Scholar] [CrossRef] [Green Version]
- Bourgeois, C.; Leclerc, É.A.; Corbin, C.; Doussot, J.; Serrano, V.; Vanier, J.-R.; Seigneuret, J.-M.; Auguin, D.; Pichon, C.; Lainé, É.; et al. Nettle (Urtica dioica L.) as a Source of Antioxidant and Anti-Aging Phytochemicals for Cosmetic Applications. Comptes Rendus Chim. 2016, 19, 1090–1100. [Google Scholar] [CrossRef]
- Xu, X.; Guignard, C.; Renaut, J.; Hausman, J.-F.; Gatti, E.; Predieri, S.; Guerriero, G. Insights into Lignan Composition and Biosynthesis in Stinging Nettle (Urtica dioica L.). Molecules 2019, 24, 3863. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Backes, A.; Legay, S.; Berni, R.; Faleri, C.; Gatti, E.; Hausman, J.-F.; Cai, G.; Guerriero, G. Cell Wall Composition and Transcriptomics in Stem Tissues of Stinging Nettle (Urtica dioica L.): Spotlight on a Neglected Fibre Crop. Plant Direct 2019, 3, e00151. [Google Scholar] [CrossRef] [Green Version]
- Behr, M.; Faleri, C.; Hausman, J.-F.; Planchon, S.; Renaut, J.; Cai, G.; Guerriero, G. Distribution of Cell-Wall Polysaccharides and Proteins during Growth of the Hemp Hypocotyl. Planta 2019, 250, 1539–1556. [Google Scholar] [CrossRef] [Green Version]
- Suryawan, I.G.P.A.; Suardana, N.P.G.; Winaya, I.N.S.; Suyasa, I.W.B.; Nindhia, T.G.T. Study of Stinging Nettle (Urtica dioica L.) Fibers Reinforced Green Composite Materials: A Review. IOP Conf. Ser. Mater. Sci. Eng. 2017, 201, 012001. [Google Scholar] [CrossRef] [Green Version]
- Suomela, J.A.; Vajanto, K.; Räisänen, R. Seeking Nettle Textiles—Utilizing a Combination of Microscopic Methods for Fibre Identification. Stud. Conserv. 2018, 63, 412–422. [Google Scholar] [CrossRef] [Green Version]
- Shah, S.S.; Qasem, M.A.A.; Berni, R.; Del Casino, C.; Cai, G.; Contal, S.; Ahmad, I.; Siddiqui, K.S.; Gatti, E.; Predieri, S.; et al. Physico-Chemical Properties and Toxicological Effects on Plant and Algal Models of Carbon Nanosheets from a Nettle Fibre Clone. Sci. Rep. 2021, 11, 6945. [Google Scholar] [CrossRef]
- Guerriero, G.; Berni, R.; Muñoz-Sanchez, J.A.; Apone, F.; Abdel-Salam, E.M.; Qahtan, A.A.; Alatar, A.A.; Cantini, C.; Cai, G.; Hausman, J.-F.; et al. Production of Plant Secondary Metabolites: Examples, Tips and Suggestions for Biotechnologists. Genes 2018, 9, 309. [Google Scholar] [CrossRef] [Green Version]
- Atta, R.; Laurens, L.; Boucheron-Dubuisson, E.; Guivarc’h, A.; Carnero, E.; Giraudat-Pautot, V.; Rech, P.; Chriqui, D. Pluripotency of Arabidopsis Xylem Pericycle Underlies Shoot Regeneration from Root and Hypocotyl Explants Grown in Vitro. Plant J. 2009, 57, 626–644. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, K.; Jiao, Y.; Meyerowitz, E.M. Arabidopsis Regeneration from Multiple Tissues Occurs via a Root Development Pathway. Dev. Cell 2010, 18, 463–471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ikeuchi, M.; Sugimoto, K.; Iwase, A. Plant Callus: Mechanisms of Induction and Repression. Plant Cell 2013, 25, 3159–3173. [Google Scholar] [CrossRef] [Green Version]
- Schaller, G.E.; Bishopp, A.; Kieber, J.J. The Yin-Yang of Hormones: Cytokinin and Auxin Interactions in Plant Development. Plant Cell 2015, 27, 44–63. [Google Scholar] [CrossRef] [Green Version]
- Swarup, R.; Kargul, J.; Marchant, A.; Zadik, D.; Rahman, A.; Mills, R.; Yemm, A.; May, S.; Williams, L.; Millner, P.; et al. Structure-Function Analysis of the Presumptive Arabidopsis Auxin Permease AUX1. Plant Cell 2004, 16, 3069–3083. [Google Scholar] [CrossRef] [Green Version]
- Dindas, J.; Scherzer, S.; Roelfsema, M.R.G.; von Meyer, K.; Müller, H.M.; Al-Rasheid, K.A.S.; Palme, K.; Dietrich, P.; Becker, D.; Bennett, M.J.; et al. AUX1-Mediated Root Hair Auxin Influx Governs SCFTIR1/AFB-Type Ca2+ Signaling. Nat. Commun. 2018, 9, 1174. [Google Scholar] [CrossRef]
- Hutchison, C.E.; Li, J.; Argueso, C.; Gonzalez, M.; Lee, E.; Lewis, M.W.; Maxwell, B.B.; Perdue, T.D.; Schaller, G.E.; Alonso, J.M.; et al. The Arabidopsis Histidine Phosphotransfer Proteins Are Redundant Positive Regulators of Cytokinin Signaling. Plant Cell 2006, 18, 3073–3087. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, K.H.; Ha, C.V.; Nishiyama, R.; Watanabe, Y.; Leyva-González, M.A.; Fujita, Y.; Tran, U.T.; Li, W.; Tanaka, M.; Seki, M.; et al. Arabidopsis Type B Cytokinin Response Regulators ARR1, ARR10, and ARR12 Negatively Regulate Plant Responses to Drought. Proc. Natl. Acad. Sci. USA 2016, 113, 3090–3095. [Google Scholar] [CrossRef] [Green Version]
- Okushima, Y.; Overvoorde, P.J.; Arima, K.; Alonso, J.M.; Chan, A.; Chang, C.; Ecker, J.R.; Hughes, B.; Lui, A.; Nguyen, D.; et al. Functional Genomic Analysis of the AUXIN RESPONSE FACTOR Gene Family Members in Arabidopsis thaliana: Unique and Overlapping Functions of ARF7 and ARF19. Plant Cell 2005, 17, 444–463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tiwari, S.B.; Wang, X.-J.; Hagen, G.; Guilfoyle, T.J. AUX/IAA Proteins Are Active Repressors, and Their Stability and Activity Are Modulated by Auxin. Plant Cell 2001, 13, 2809–2822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gälweiler, L.; Guan, C.; Müller, A.; Wisman, E.; Mendgen, K.; Yephremov, A.; Palme, K. Regulation of Polar Auxin Transport by AtPIN1 in Arabidopsis Vascular Tissue. Science 1998, 282, 2226–2230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, Z.; Wang, B.; Moreno, I.; Dupláková, N.; Simon, S.; Carraro, N.; Reemmer, J.; Pěnčík, A.; Chen, X.; Tejos, R.; et al. ER-Localized Auxin Transporter PIN8 Regulates Auxin Homeostasis and Male Gametophyte Development in Arabidopsis. Nat. Commun. 2012, 3, 941. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbez, E.; Kubeš, M.; Rolčík, J.; Béziat, C.; Pěnčík, A.; Wang, B.; Rosquete, M.R.; Zhu, J.; Dobrev, P.I.; Lee, Y.; et al. A Novel Putative Auxin Carrier Family Regulates Intracellular Auxin Homeostasis in Plants. Nature 2012, 485, 119–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dewitte, W.; Scofield, S.; Alcasabas, A.A.; Maughan, S.C.; Menges, M.; Braun, N.; Collins, C.; Nieuwland, J.; Prinsen, E.; Sundaresan, V.; et al. Arabidopsis CYCD3 D-Type Cyclins Link Cell Proliferation and Endocycles and Are Rate-Limiting for Cytokinin Responses. Proc. Natl. Acad. Sci. USA 2007, 104, 14537–14542. [Google Scholar] [CrossRef] [Green Version]
- Carpenter, E.J.; Matasci, N.; Ayyampalayam, S.; Wu, S.; Sun, J.; Yu, J.; Jimenez Vieira, F.R.; Bowler, C.; Dorrell, R.G.; Gitzendanner, M.A.; et al. Access to RNA-Sequencing Data from 1,173 Plant Species: The 1000 Plant Transcriptomes Initiative (1KP). GigaScience 2019, 8, giz126. [Google Scholar] [CrossRef]
- Leebens-Mack, J.H.; Barker, M.S.; Carpenter, E.J.; Deyholos, M.K.; Gitzendanner, M.A.; Graham, S.W.; Grosse, I.; Li, Z.; Melkonian, M.; Mirarab, S.; et al. One Thousand Plant Transcriptomes and the Phylogenomics of Green Plants. Nature 2019, 574, 679–685. [Google Scholar] [CrossRef] [Green Version]
- Benková, E.; Michniewicz, M.; Sauer, M.; Teichmann, T.; Seifertová, D.; Jürgens, G.; Friml, J. Local, Efflux-Dependent Auxin Gradients as a Common Module for Plant Organ Formation. Cell 2003, 115, 591–602. [Google Scholar] [CrossRef] [Green Version]
- Xu, K.; Liu, J.; Fan, M.; Xin, W.; Hu, Y.; Xu, C. A Genome-Wide Transcriptome Profiling Reveals the Early Molecular Events during Callus Initiation in Arabidopsis Multiple Organs. Genomics 2012, 100, 116–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siwinska, J.; Siatkowska, K.; Olry, A.; Grosjean, J.; Hehn, A.; Bourgaud, F.; Meharg, A.A.; Carey, M.; Lojkowska, E.; Ihnatowicz, A. Scopoletin 8-Hydroxylase: A Novel Enzyme Involved in Coumarin Biosynthesis and Iron-Deficiency Responses in Arabidopsis. J. Exp. Bot. 2018, 69, 1735–1748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajniak, J.; Giehl, R.F.H.; Chang, E.; Murgia, I.; von Wirén, N.; Sattely, E.S. Biosynthesis of Redox-Active Metabolites in Response to Iron Deficiency in Plants. Nat. Chem. Biol. 2018, 14, 442–450. [Google Scholar] [CrossRef] [PubMed]
- Hindt, M.N.; Guerinot, M.L. Getting a Sense for Signals: Regulation of the Plant Iron Deficiency Response. Biochim. Biophys. Acta 2012, 1823, 1521–1530. [Google Scholar] [CrossRef] [Green Version]
- Fourcroy, P.; Sisó-Terraza, P.; Sudre, D.; Savirón, M.; Reyt, G.; Gaymard, F.; Abadía, A.; Abadia, J.; Álvarez-Fernández, A.; Briat, J.-F. Involvement of the ABCG37 Transporter in Secretion of Scopoletin and Derivatives by Arabidopsis Roots in Response to Iron Deficiency. New Phytol. 2014, 201, 155–167. [Google Scholar] [CrossRef]
- Vanholme, R.; Sundin, L.; Seetso, K.C.; Kim, H.; Liu, X.; Li, J.; De Meester, B.; Hoengenaert, L.; Goeminne, G.; Morreel, K.; et al. COSY Catalyses Trans–Cis Isomerization and Lactonization in the Biosynthesis of Coumarins. Nat. Plants 2019, 5, 1066–1075. [Google Scholar] [CrossRef]
- Murgia, I.; Tarantino, D.; Soave, C.; Morandini, P. Arabidopsis CYP82C4 Expression Is Dependent on Fe Availability and Circadian Rhythm, and Correlates with Genes Involved in the Early Fe Deficiency Response. J. Plant Physiol. 2011, 168, 894–902. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.R.; Kenzior, A.; Willmot, D.; Scanlon, S.; Chen, Z.; Topin, A.; He, S.H.; Acevedo, A.; Folk, W.R. Altered Expression of Plant Lysyl TRNA Synthetase Promotes TRNA Misacylation and Translational Recoding of Lysine. Plant J. 2007, 50, 627–636. [Google Scholar] [CrossRef]
- Thimm, O.; Essigmann, B.; Kloska, S.; Altmann, T.; Buckhout, T.J. Response of Arabidopsis to Iron Deficiency Stress as Revealed by Microarray Analysis. Plant Physiol. 2001, 127, 1030–1043. [Google Scholar] [CrossRef]
- Giritch, A.; Herbik, A.; Balzer, H.J.; Ganal, M.; Stephan, U.W.; Bäumlein, H. A Root-Specific Iron-Regulated Gene of Tomato Encodes a Lysyl-TRNA-Synthetase-like Protein. Eur. J. Biochem. 1997, 244, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Brevet, A.; Chen, J.; Lévêque, F.; Plateau, P.; Blanquet, S. In Vivo Synthesis of Adenylylated Bis(5′-Nucleosidyl) Tetraphosphates (Ap4N) by Escherichia coli Aminoacyl-TRNA Synthetases. Proc. Natl. Acad. Sci. USA 1989, 86, 8275–8279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, P.C.; Bochner, B.R.; Ames, B.N. Diadenosine 5′,5″′-P1,P4-Tetraphosphate and Related Adenylylated Nucleotides in Salmonella typhimurium. J. Biol. Chem. 1983, 258, 6827–6834. [Google Scholar] [CrossRef]
- Baltzinger, M.; Ebel, J.P.; Remy, P. Accumulation of Dinucleoside Polyphosphates in Saccharomyces cerevisiae under Stress Conditions. High Levels Are Associated with Cell Death. Biochimie 1986, 68, 1231–1236. [Google Scholar] [CrossRef]
- Pietrowska-Borek, M.; Nuc, K.; Zielezińska, M.; Guranowski, A. Diadenosine Polyphosphates (Ap3A and Ap4A) Behave as Alarmones Triggering the Synthesis of Enzymes of the Phenylpropanoid Pathway in Arabidopsis thaliana. FEBS Open Bio. 2011, 1, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernández-Morales, R.; Becerra, A.; Lazcano, A. Alarmones as Vestiges of a Bygone RNA World. J. Mol. Evol. 2019, 87, 37–51. [Google Scholar] [CrossRef]
- He, D.; Zhang, H.; Yang, P. The Mitochondrion-Located Protein OsB12D1 Enhances Flooding Tolerance during Seed Germination and Early Seedling Growth in Rice. Int. J. Mol. Sci. 2014, 15, 13461–13481. [Google Scholar] [CrossRef] [Green Version]
- Zheng, L.; Huang, F.; Narsai, R.; Wu, J.; Giraud, E.; He, F.; Cheng, L.; Wang, F.; Wu, P.; Whelan, J.; et al. Physiological and Transcriptome Analysis of Iron and Phosphorus Interaction in Rice Seedlings. Plant Physiol. 2009, 151, 262–274. [Google Scholar] [CrossRef] [Green Version]
- Long, T.A.; Tsukagoshi, H.; Busch, W.; Lahner, B.; Salt, D.E.; Benfey, P.N. The BHLH Transcription Factor POPEYE Regulates Response to Iron Deficiency in Arabidopsis Roots. Plant Cell 2010, 22, 2219–2236. [Google Scholar] [CrossRef] [Green Version]
- Siwinska, J.; Kadzinski, L.; Banasiuk, R.; Gwizdek-Wisniewska, A.; Olry, A.; Banecki, B.; Lojkowska, E.; Ihnatowicz, A. Identification of QTLs Affecting Scopolin and Scopoletin Biosynthesis in Arabidopsis thaliana. BMC Plant Biol. 2014, 14, 280. [Google Scholar] [CrossRef] [Green Version]
- Kanai, M.; Hirai, M.; Yoshiba, M.; Tadano, T.; Higuchi, K. Iron Deficiency Causes Zinc Excess in Zea Mays. Soil Sci. Plant Nutr. 2009, 55, 271–276. [Google Scholar] [CrossRef] [Green Version]
- Santos, C.S.; Ozgur, R.; Uzilday, B.; Turkan, I.; Roriz, M.; Rangel, A.O.S.S.; Carvalho, S.M.P.; Vasconcelos, M.W. Understanding the Role of the Antioxidant System and the Tetrapyrrole Cycle in Iron Deficiency Chlorosis. Plants 2019, 8, 348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsukagoshi, H.; Busch, W.; Benfey, P.N. Transcriptional Regulation of ROS Controls Transition from Proliferation to Differentiation in the Root. Cell 2010, 143, 606–616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Passardi, F.; Penel, C.; Dunand, C. Performing the Paradoxical: How Plant Peroxidases Modify the Cell Wall. Trends Plant Sci. 2004, 9, 534–540. [Google Scholar] [CrossRef] [PubMed]
- Wagner, U.; Edwards, R.; Dixon, D.P.; Mauch, F. Probing the Diversity of the Arabidopsis Glutathione S-Transferase Gene Family. Plant Mol. Biol. 2002, 49, 515–532. [Google Scholar] [CrossRef]
- Ugalde, J.M.; Lamig, L.; Herrera-Vásquez, A.; Fuchs, P.; Müller-Schüssele, S.J.; Meyer, A.J.; Holuigue, L. GSTU7 Affects Growth Performance and Acts as an Antagonist of Oxidative Stress Induced by Methyl Viologen. bioRxiv 2020. [Google Scholar] [CrossRef]
- Sewelam, N.; Jaspert, N.; Van Der Kelen, K.; Tognetti, V.B.; Schmitz, J.; Frerigmann, H.; Stahl, E.; Zeier, J.; Van Breusegem, F.; Maurino, V.G. Spatial H2O2 Signaling Specificity: H2O2 from Chloroplasts and Peroxisomes Modulates the Plant Transcriptome Differentially. Mol. Plant 2014, 7, 1191–1210. [Google Scholar] [CrossRef] [Green Version]
- Herrera-Vásquez, A.; Fonseca, A.; Ugalde, J.M.; Lamig, L.; Seguel, A.; Moyano, T.C.; Gutiérrez, R.A.; Salinas, P.; Vidal, E.A.; Holuigue, L. Transcription Factor TGA2 Is Essential for UV-B Stress Tolerance Controlling Oxidative Stress in Arabidopsis. bioRxiv 2020. [Google Scholar] [CrossRef]
- Lee, K.; Park, O.-S.; Seo, P.J. RNA-Seq Analysis of the Arabidopsis Transcriptome in Pluripotent Calli. Mol. Cells 2016, 39, 484–494. [Google Scholar] [CrossRef] [Green Version]
- Pičmanová, M.; Neilson, E.H.; Motawia, M.S.; Olsen, C.E.; Agerbirk, N.; Gray, C.J.; Flitsch, S.; Meier, S.; Silvestro, D.; Jørgensen, K.; et al. A Recycling Pathway for Cyanogenic Glycosides Evidenced by the Comparative Metabolic Profiling in Three Cyanogenic Plant Species. Biochem. J. 2015, 469, 375–389. [Google Scholar] [CrossRef] [Green Version]
- Oracz, K.; El-Maarouf-Bouteau, H.; Kranner, I.; Bogatek, R.; Corbineau, F.; Bailly, C. The Mechanisms Involved in Seed Dormancy Alleviation by Hydrogen Cyanide Unravel the Role of Reactive Oxygen Species as Key Factors of Cellular Signaling during Germination. Plant Physiol. 2009, 150, 494–505. [Google Scholar] [CrossRef] [Green Version]
- Caarls, L.; Van der Does, D.; Hickman, R.; Jansen, W.; Verk, M.C.V.; Proietti, S.; Lorenzo, O.; Solano, R.; Pieterse, C.M.J.; Van Wees, S.C.M. Assessing the Role of Ethylene Response Factor Transcriptional Repressors in Salicylic Acid-Mediated Suppression of Jasmonic Acid-Responsive Genes. Plant Cell Physiol. 2017, 58, 266–278. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Zhang, Y.; Li, Y.; Zhang, Q.; Ding, Y.; Zhang, Y. ChIP-Seq Reveals Broad Roles of SARD1 and CBP60g in Regulating Plant Immunity. Nat. Commun. 2015, 6, 10159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Brader, G.; Palva, E.T. The WRKY70 Transcription Factor: A Node of Convergence for Jasmonate-Mediated and Salicylate-Mediated Signals in Plant Defense. Plant Cell 2004, 16, 319–331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, M.; Lu, Y.; Bethke, G.; Harrison, B.T.; Hatsugai, N.; Katagiri, F.; Glazebrook, J. WRKY70 Prevents Axenic Activation of Plant Immunity by Direct Repression of SARD1. New Phytol. 2018, 217, 700–712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lecourieux, D.; Mazars, C.; Pauly, N.; Ranjeva, R.; Pugin, A. Analysis and Effects of Cytosolic Free Calcium Increases in Response to Elicitors in Nicotiana plumbaginifolia Cells. Plant Cell 2002, 14, 2627–2641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Truman, W.; Sreekanta, S.; Lu, Y.; Bethke, G.; Tsuda, K.; Katagiri, F.; Glazebrook, J. The CALMODULIN-BINDING PROTEIN60 Family Includes Both Negative and Positive Regulators of Plant Immunity. Plant Physiol. 2013, 163, 1741–1751. [Google Scholar] [CrossRef] [Green Version]
- Lian, K.; Gao, F.; Sun, T.; van Wersch, R.; Ao, K.; Kong, Q.; Nitta, Y.; Wu, D.; Krysan, P.; Zhang, Y. MKK6 Functions in Two Parallel MAP Kinase Cascades in Immune Signaling. Plant Physiol. 2018, 178, 1284–1295. [Google Scholar] [CrossRef] [Green Version]
- Fukunaga, S.; Sogame, M.; Hata, M.; Singkaravanit-Ogawa, S.; Piślewska-Bednarek, M.; Onozawa-Komori, M.; Nishiuchi, T.; Hiruma, K.; Saitoh, H.; Terauchi, R.; et al. Dysfunction of Arabidopsis MACPF Domain Protein Activates Programmed Cell Death via Tryptophan Metabolism in MAMP-Triggered Immunity. Plant J. 2017, 89, 381–393. [Google Scholar] [CrossRef]
- Ge, X.; Li, G.-J.; Wang, S.-B.; Zhu, H.; Zhu, T.; Wang, X.; Xia, Y. AtNUDT7, a Negative Regulator of Basal Immunity in Arabidopsis, Modulates Two Distinct Defense Response Pathways and Is Involved in Maintaining Redox Homeostasis. Plant Physiol. 2007, 145, 204–215. [Google Scholar] [CrossRef] [Green Version]
- Bacete, L.; Mélida, H.; Miedes, E.; Molina, A. Plant Cell Wall-Mediated Immunity: Cell Wall Changes Trigger Disease Resistance Responses. Plant J. 2018, 93, 614–636. [Google Scholar] [CrossRef] [PubMed]
- He, Z.H.; Cheeseman, I.; He, D.; Kohorn, B.D. A Cluster of Five Cell Wall-Associated Receptor Kinase Genes, Wak1-5, Are Expressed in Specific Organs of Arabidopsis. Plant Mol. Biol. 1999, 39, 1189–1196. [Google Scholar] [CrossRef] [PubMed]
- Kohorn, B.D.; Johansen, S.; Shishido, A.; Todorova, T.; Martinez, R.; Defeo, E.; Obregon, P. Pectin Activation of MAP Kinase and Gene Expression Is WAK2 Dependent. Plant J. 2009, 60, 974–982. [Google Scholar] [CrossRef] [Green Version]
- Hartmann, M.; Zeier, J. N-Hydroxypipecolic Acid and Salicylic Acid: A Metabolic Duo for Systemic Acquired Resistance. Curr. Opin. Plant Biol. 2019, 50, 44–57. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, M.; Zeier, T.; Bernsdorff, F.; Reichel-Deland, V.; Kim, D.; Hohmann, M.; Scholten, N.; Schuck, S.; Bräutigam, A.; Hölzel, T.; et al. Flavin Monooxygenase-Generated N-Hydroxypipecolic Acid Is a Critical Element of Plant Systemic Immunity. Cell 2018, 173, 456–469.e16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yadav, V.; Wang, Z.; Wei, C.; Amo, A.; Ahmed, B.; Yang, X.; Zhang, X. Phenylpropanoid Pathway Engineering: An Emerging Approach towards Plant Defense. Pathogens 2020, 9, 312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ismond, K.P.; Dolferus, R.; De Pauw, M.; Dennis, E.S.; Good, A.G. Enhanced Low Oxygen Survival in Arabidopsis through Increased Metabolic Flux in the Fermentative Pathway. Plant Physiol. 2003, 132, 1292–1302. [Google Scholar] [CrossRef] [Green Version]
- Kerpen, L.; Niccolini, L.; Licausi, F.; van Dongen, J.T.; Weits, D.A. Hypoxic Conditions in Crown Galls Induce Plant Anaerobic Responses That Support Tumor Proliferation. Front. Plant Sci. 2019, 10, 56. [Google Scholar] [CrossRef]
- Maruta, T.; Yonemitsu, M.; Yabuta, Y.; Tamoi, M.; Ishikawa, T.; Shigeoka, S. Arabidopsis Phosphomannose Isomerase 1, but Not Phosphomannose Isomerase 2, Is Essential for Ascorbic Acid Biosynthesis. J. Biol. Chem. 2008, 283, 28842–28851. [Google Scholar] [CrossRef] [Green Version]
- Turk, E.M.; Fujioka, S.; Seto, H.; Shimada, Y.; Takatsuto, S.; Yoshida, S.; Denzel, M.A.; Torres, Q.I.; Neff, M.M. CYP72B1 Inactivates Brassinosteroid Hormones: An Intersection between Photomorphogenesis and Plant Steroid Signal Transduction. Plant Physiol. 2003, 133, 1643–1653. [Google Scholar] [CrossRef] [Green Version]
- Mudgil, Y.; Uhrig, J.F.; Zhou, J.; Temple, B.; Jiang, K.; Jones, A.M. Arabidopsis N-MYC DOWNREGULATED-LIKE1, a Positive Regulator of Auxin Transport in a G Protein–Mediated Pathway. Plant Cell 2009, 21, 3591–3609. [Google Scholar] [CrossRef] [Green Version]
- Urbanowicz, B.R.; Bennett, A.B.; del Campillo, E.; Catalá, C.; Hayashi, T.; Henrissat, B.; Höfte, H.; McQueen-Mason, S.J.; Patterson, S.E.; Shoseyov, O.; et al. Structural Organization and a Standardized Nomenclature for Plant Endo-1,4-β-Glucanases (Cellulases) of Glycosyl Hydrolase Family 9. Plant Physiol. 2007, 144, 1693–1696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shani, Z.; Dekel, M.; Roiz, L.; Horowitz, M.; Kolosovski, N.; Lapidot, S.; Alkan, S.; Koltai, H.; Tsabary, G.; Goren, R.; et al. Expression of Endo-1,4-Beta-Glucanase (Cel1) in Arabidopsis thaliana Is Associated with Plant Growth, Xylem Development and Cell Wall Thickening. Plant Cell Rep. 2006, 25, 1067–1074. [Google Scholar] [CrossRef] [PubMed]
- Tsabary, G.; Shani, Z.; Roiz, L.; Levy, I.; Riov, J.; Shoseyov, O. Abnormal `wrinkled’ Cell Walls and Retarded Development of Transgenic Arabidopsis thaliana Plants Expressing Endo-1,4-β-Glucanase (Cell) Antisense. Plant Mol. Biol. 2003, 51, 213–224. [Google Scholar] [CrossRef]
- Majda, M.; Robert, S. The Role of Auxin in Cell Wall Expansion. Int. J. Mol. Sci. 2018, 19, 951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahashi, E.; Ma, J.F.; Miyake, Y. The Possibility of Silicon as an Essential Element for Higher Plants. Comments Agric. Food Chem. 1990, 2, 99–102. [Google Scholar]
- Spartz, A.K.; Ren, H.; Park, M.Y.; Grandt, K.N.; Lee, S.H.; Murphy, A.S.; Sussman, M.R.; Overvoorde, P.J.; Gray, W.M. SAUR Inhibition of PP2C-D Phosphatases Activates Plasma Membrane H+-ATPases to Promote Cell Expansion in Arabidopsis. Plant Cell 2014, 26, 2129–2142. [Google Scholar] [CrossRef] [Green Version]
- Cosgrove, D.J. Cell Wall Loosening by Expansins1. Plant Physiol. 1998, 118, 333–339. [Google Scholar] [CrossRef] [Green Version]
- Wolf, S.; Mouille, G.; Pelloux, J. Homogalacturonan Methyl-Esterification and Plant Development. Mol. Plant 2009, 2, 851–860. [Google Scholar] [CrossRef]
- Sénéchal, F.; Wattier, C.; Rustérucci, C.; Pelloux, J. Homogalacturonan-Modifying Enzymes: Structure, Expression, and Roles in Plants. J. Exp. Bot. 2014, 65, 5125–5160. [Google Scholar] [CrossRef] [Green Version]
- Kanter, U.; Usadel, B.; Guerineau, F.; Li, Y.; Pauly, M.; Tenhaken, R. The Inositol Oxygenase Gene Family of Arabidopsis Is Involved in the Biosynthesis of Nucleotide Sugar Precursors for Cell-Wall Matrix Polysaccharides. Planta 2005, 221, 243–254. [Google Scholar] [CrossRef] [Green Version]
- Lorence, A.; Chevone, B.I.; Mendes, P.; Nessler, C.L. Myo-Inositol Oxygenase Offers a Possible Entry Point into Plant Ascorbate Biosynthesis. Plant Physiol. 2004, 134, 1200–1205. [Google Scholar] [CrossRef] [Green Version]
- Ivanov Kavkova, E.; Blöchl, C.; Tenhaken, R. The Myo-inositol Pathway Does Not Contribute to Ascorbic Acid Synthesis. Plant Biol. 2019, 21, 95–102. [Google Scholar] [CrossRef] [Green Version]
- Campbell, R.E.; Sala, R.F.; van de Rijn, I.; Tanner, M.E. Properties and Kinetic Analysis of UDP-Glucose Dehydrogenase from Group A Streptococci. Irreversible Inhibition by UDP-Chloroacetol. J. Biol. Chem. 1997, 272, 3416–3422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cruz-Valderrama, J.E.; Gómez-Maqueo, X.; Salazar-Iribe, A.; Zúñiga-Sánchez, E.; Hernández-Barrera, A.; Quezada-Rodríguez, E.; Gamboa-deBuen, A. Overview of the Role of Cell Wall DUF642 Proteins in Plant Development. Int. J. Mol. Sci. 2019, 20, 3333. [Google Scholar] [CrossRef] [Green Version]
- Zúñiga-Sánchez, E.; Soriano, D.; Martínez-Barajas, E.; Orozco-Segovia, A.; Gamboa-deBuen, A. BIIDXI, the At4g32460 DUF642 Gene, Is Involved in Pectin Methyl Esterase Regulation during Arabidopsis thaliana Seed Germination and Plant Development. BMC Plant Biol. 2014, 14, 338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garza-Caligaris, L.E.; Avendaño-Vázquez, A.O.; Alvarado-López, S.; Zúñiga-Sánchez, E.; Orozco-Segovia, A.; Pérez-Ruíz, R.V.; Gamboa-deBuen, A. At3g08030 Transcript: A Molecular Marker of Seed Ageing. Ann. Bot. 2012, 110, 1253–1260. [Google Scholar] [CrossRef] [Green Version]
- Zell, M.B.; Fahnenstich, H.; Maier, A.; Saigo, M.; Voznesenskaya, E.V.; Edwards, G.E.; Andreo, C.; Schleifenbaum, F.; Zell, C.; Drincovich, M.F.; et al. Analysis of Arabidopsis with Highly Reduced Levels of Malate and Fumarate Sheds Light on the Role of These Organic Acids as Storage Carbon Molecules. Plant Physiol. 2010, 152, 1251–1262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galuszka, P.; Popelková, H.; Werner, T.; Frébortová, J.; Pospíšilová, H.; Mik, V.; Köllmer, I.; Schmülling, T.; Frébort, I. Biochemical Characterization of Cytokinin Oxidases/Dehydrogenases from Arabidopsis thaliana Expressed in Nicotiana tabacum L. J. Plant Growth Regul. 2007, 26, 255–267. [Google Scholar] [CrossRef]
- Kurakawa, T.; Ueda, N.; Maekawa, M.; Kobayashi, K.; Kojima, M.; Nagato, Y.; Sakakibara, H.; Kyozuka, J. Direct Control of Shoot Meristem Activity by a Cytokinin-Activating Enzyme. Nature 2007, 445, 652–655. [Google Scholar] [CrossRef] [PubMed]
- Balcerowicz, D.; Schoenaers, S.; Vissenberg, K. Cell Fate Determination and the Switch from Diffuse Growth to Planar Polarity in Arabidopsis Root Epidermal Cells. Front. Plant Sci. 2015, 6, 1163. [Google Scholar] [CrossRef] [Green Version]
- Kuczak, M.; Kurczyńska, E. Cell Wall Composition as a Marker of the Reprogramming of the Cell Fate on the Example of a Daucus carota (L.) Hypocotyl in Which Somatic Embryogenesis Was Induced. Int. J. Mol. Sci. 2020, 21, 8126. [Google Scholar] [CrossRef] [PubMed]
- Roldán-Arjona, T.; Ariza, R.R.; Córdoba-Cañero, D. DNA Base Excision Repair in Plants: An Unfolding Story With Familiar and Novel Characters. Front. Plant Sci. 2019, 10, 1055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shim, S.; Lee, H.G.; Park, O.-S.; Shin, H.; Lee, K.; Lee, H.; Huh, J.H.; Seo, P.J. Dynamic Changes in DNA Methylation Occur in TE Regions and Affect Cell Proliferation during Leaf-to-Callus Transition in Arabidopsis. Epigenetics 2021, 1–18. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Chen, X.; Huang, H.; Xu, L. Reprogramming of H3K27me3 Is Critical for Acquisition of Pluripotency from Cultured Arabidopsis Tissues. PLoS Genet. 2012, 8, e1002911. [Google Scholar] [CrossRef] [Green Version]
- Schumann, U.; Lee, J.M.; Smith, N.A.; Zhong, C.; Zhu, J.-K.; Dennis, E.S.; Millar, A.A.; Wang, M.-B. DEMETER Plays a Role in DNA Demethylation and Disease Response in Somatic Tissues of Arabidopsis. Epigenetics 2019, 14, 1074–1087. [Google Scholar] [CrossRef]
- Taylor, N.G.; Laurie, S.; Turner, S.R. Multiple Cellulose Synthase Catalytic Subunits Are Required for Cellulose Synthesis in Arabidopsis. Plant Cell 2000, 12, 2529–2540. [Google Scholar] [CrossRef] [Green Version]
- Tucker, M.R.; Lou, H.; Aubert, M.K.; Wilkinson, L.G.; Little, A.; Houston, K.; Pinto, S.C.; Shirley, N.J. Exploring the Role of Cell Wall-Related Genes and Polysaccharides during Plant Development. Plants 2018, 7, 42. [Google Scholar] [CrossRef] [Green Version]
- Grafi, G. How Cells Dedifferentiate: A Lesson from Plants. Dev. Biol. 2004, 268, 1–6. [Google Scholar] [CrossRef]
- Harikrishna, K.; Darby, R.; Draper, J. Chloroplast Dedifferentiation in Mechanically Isolated Asparagus Cells during Culture Initiation. Plant Physiol. 1992, 100, 1177–1183. [Google Scholar] [CrossRef] [Green Version]
- Backes, A.; Behr, M.; Xu, X.; Gatti, E.; Legay, S.; Predieri, S.; Hausman, J.-F.; Deyholos, M.K.; Cai, G.; Guerriero, G. Sucrose Synthase Gene Expression Analysis in the Fibre Nettle (Urtica dioica L.) Cultivar “Clone 13”. Ind. Crop. Prod. 2018, 123, 315–322. [Google Scholar] [CrossRef] [Green Version]
- Mortazavi, A.; Williams, B.A.; McCue, K.; Schaeffer, L.; Wold, B. Mapping and Quantifying Mammalian Transcriptomes by RNA-Seq. Nat. Methods 2008, 5, 621–628. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A Software Environment for Integrated Models of Biomolecular Interaction Networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
- Bindea, G.; Mlecnik, B.; Hackl, H.; Charoentong, P.; Tosolini, M.; Kirilovsky, A.; Fridman, W.-H.; Pagès, F.; Trajanoski, Z.; Galon, J. ClueGO: A Cytoscape Plug-in to Decipher Functionally Grouped Gene Ontology and Pathway Annotation Networks. Bioinformatics 2009, 25, 1091–1093. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ge, S.X.; Son, E.W.; Yao, R. IDEP: An Integrated Web Application for Differential Expression and Pathway Analysis of RNA-Seq Data. BMC Bioinform. 2018, 19, 534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eisen, M.B.; Spellman, P.T.; Brown, P.O.; Botstein, D. Cluster Analysis and Display of Genome-Wide Expression Patterns. Proc. Natl. Acad. Sci. USA 1998, 95, 14863–14868. [Google Scholar] [CrossRef] [Green Version]
- Saldanha, A.J. Java Treeview—Extensible Visualization of Microarray Data. Bioinformatics 2004, 20, 3246–3248. [Google Scholar] [CrossRef] [Green Version]



| Sequence Reference | Gene | Forward Primer (5′→3′) | Reverse Primer (5′→3′) | Efficiency % |
|---|---|---|---|---|
| contig_19482 | AHP4 | TTCGTGGAGGAAATCGCTAC | CTTTCAGCTGGTGCATGAAG | 109.9 |
| WKCY_scaffold_2048943 | AHP5 | CGAGGCTCAGAATTTGGAAG | GCTCAAGCATGAACAGTTGG | 99.6 |
| contig_1005 | ARF2B | ATGAGGGCGATATGATGCTC | GTCTCCATGCGAATTTAGGG | 100.3 |
| contig_3166 | ARF19 | CTGAAGCGTCATCTGAGCATAC | CGTCCATGAACATGAACACC | 90.6 |
| WKCY_scaffold_2007870 | ARF19-1 | TTGCATGCCGATACAGAGAC | AAAACTCGGTGGGTTGTCTG | 101.3 |
| contig_1723 | ARR12 | TGCTCTTCGTCCTCTTTTCC | ATGGGTTTCATCGCAGTCTC | 97.3 |
| contig_2471 | AUX1 | CGAATGCCCAATACACACAG | GAGATTATGCACGCGATGTG | 92.0 |
| contig_3855 | IAA22 | TTTTGGTGGCCGAAGTAGTC | CTCCGTGTTCAAGCATTTCC | 100.5 |
| contig_15219 | IAA21 | TCGTTCTCCTGAACATGCTG | TGTCCACCTCCCTTTTTCAG | 89.5 |
| WKCY_scaffold_2013249 | CYCD3 | TTGCACTGGGAGTTTCTGTG | TGATCACGAGGAGCATTGTC | 94.7 |
| contig_25826 | CYCD3.1 | TCTTGCTGGAACAAGACCTG | ATGCTCTTCGTCTTCCTTGG | 92.1 |
| contig_20694 | PIN8 | CAGGCTGTGATGCGTAATTC | GAACCAGCAATGTTCGATCC | 97.2 |
| contig_29151/WKCY_scaffold_2010532 | PIN1a | TGGGATGGCCATGTTTAGTC | AAACAACGCCACTGAGTTCC | 95.2 |
| contig_8278 | PIN4 | TCTCCAGAAGCTCATCATGC | TGGAGAGGGAGAAGATGGTG | 95.8 |
| contig_5072 | PILS7 | ACGCTGTTTGAAGTGGCATC | CGAAGTTGCCAAGAAAGCTC | 99.7 |
| Component | Pathways | e-Value |
|---|---|---|
| PC1 | Generation of precursor metabolites and energy | 1 × 10−2 |
| Cell wall polysaccharide metabolic process | 5 × 10−2 | |
| Photosynthesis | 2 × 10−12 | |
| Photosynthesis, light reaction | 1 × 10−5 | |
| Protein-containing complex subunit organization | 2 × 10−2 | |
| Protein-containing complex assembly | 2 × 10−2 | |
| PC2 | Regulation of response to biotic stimulus | 5 × 10−3 |
| Defense reponse | 8 × 10−3 | |
| Response to wounding | 5 × 10−3 | |
| Secondary metabolic process | 9 × 10−3 | |
| Regulation of response to external stimulus | 2 × 10−3 | |
| Regulation of response to stress | 8 × 10−3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, X.; Legay, S.; Berni, R.; Hausman, J.-F.; Guerriero, G. Transcriptomic Changes in Internode Explants of Stinging Nettle during Callogenesis. Int. J. Mol. Sci. 2021, 22, 12319. https://doi.org/10.3390/ijms222212319
Xu X, Legay S, Berni R, Hausman J-F, Guerriero G. Transcriptomic Changes in Internode Explants of Stinging Nettle during Callogenesis. International Journal of Molecular Sciences. 2021; 22(22):12319. https://doi.org/10.3390/ijms222212319
Chicago/Turabian StyleXu, Xuan, Sylvain Legay, Roberto Berni, Jean-Francois Hausman, and Gea Guerriero. 2021. "Transcriptomic Changes in Internode Explants of Stinging Nettle during Callogenesis" International Journal of Molecular Sciences 22, no. 22: 12319. https://doi.org/10.3390/ijms222212319
APA StyleXu, X., Legay, S., Berni, R., Hausman, J.-F., & Guerriero, G. (2021). Transcriptomic Changes in Internode Explants of Stinging Nettle during Callogenesis. International Journal of Molecular Sciences, 22(22), 12319. https://doi.org/10.3390/ijms222212319










