Incretins in the Therapy of Diabetic Kidney Disease
Abstract
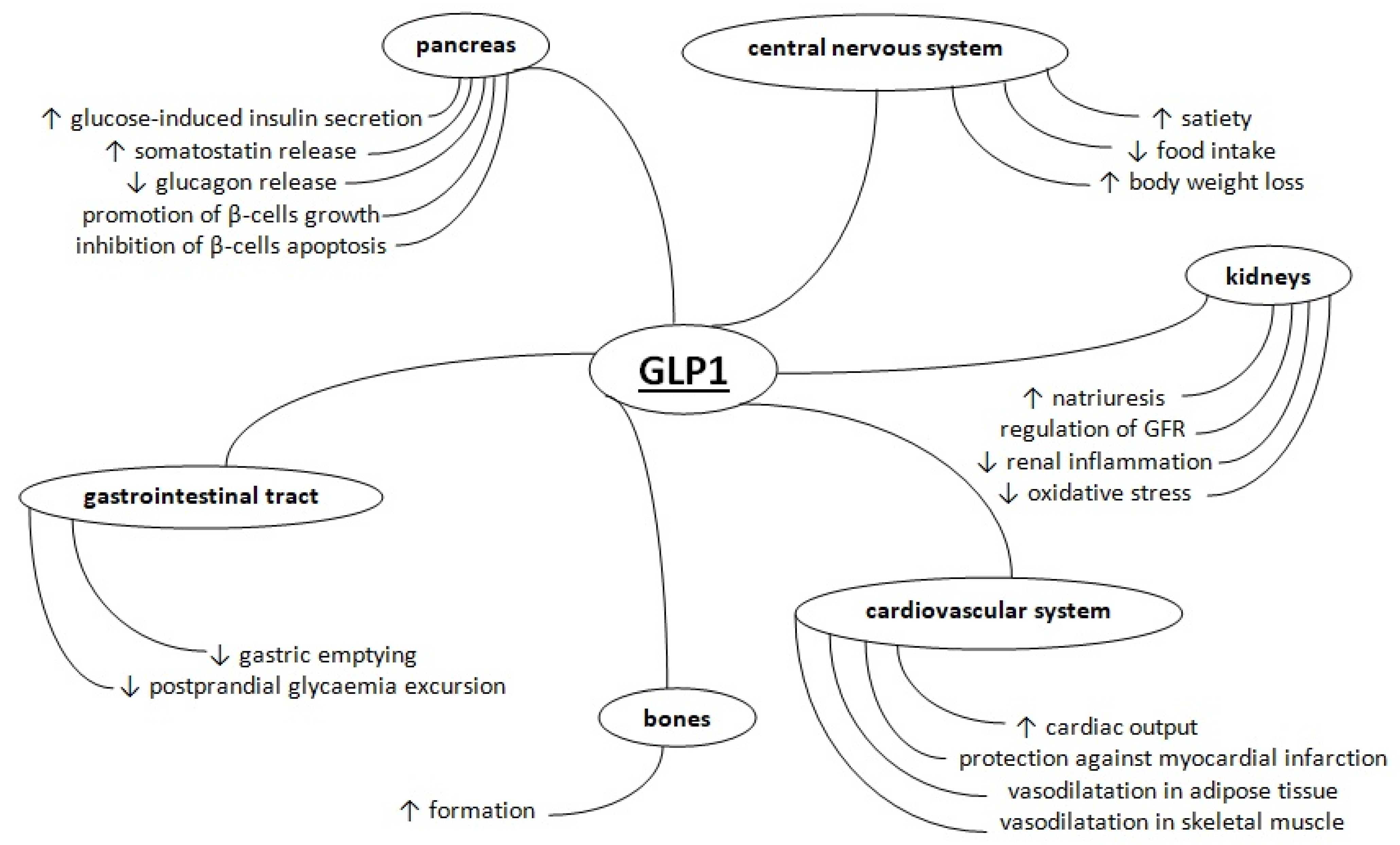
:1. Introduction
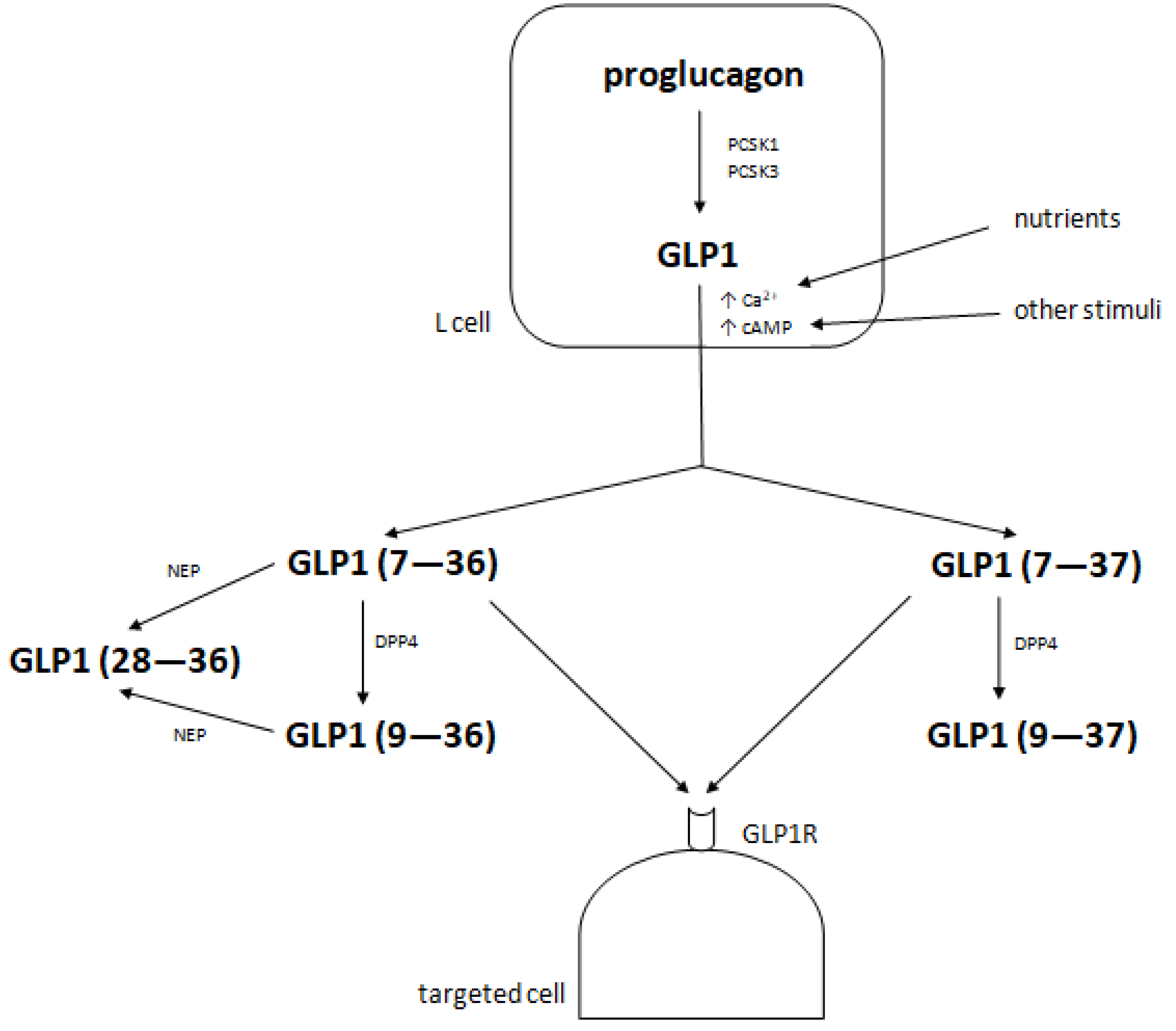
2. Metabolism of Glucagon-Like Peptide 1
2.1. Glucagon-Like Peptide 1 Receptor Agonists—Drugs Showing the Beneficial Effects of Native GLP1
2.2. Dipeptidyl Peptidase 4 Inhibitors—Drugs Promoting the Action of Native GLP1
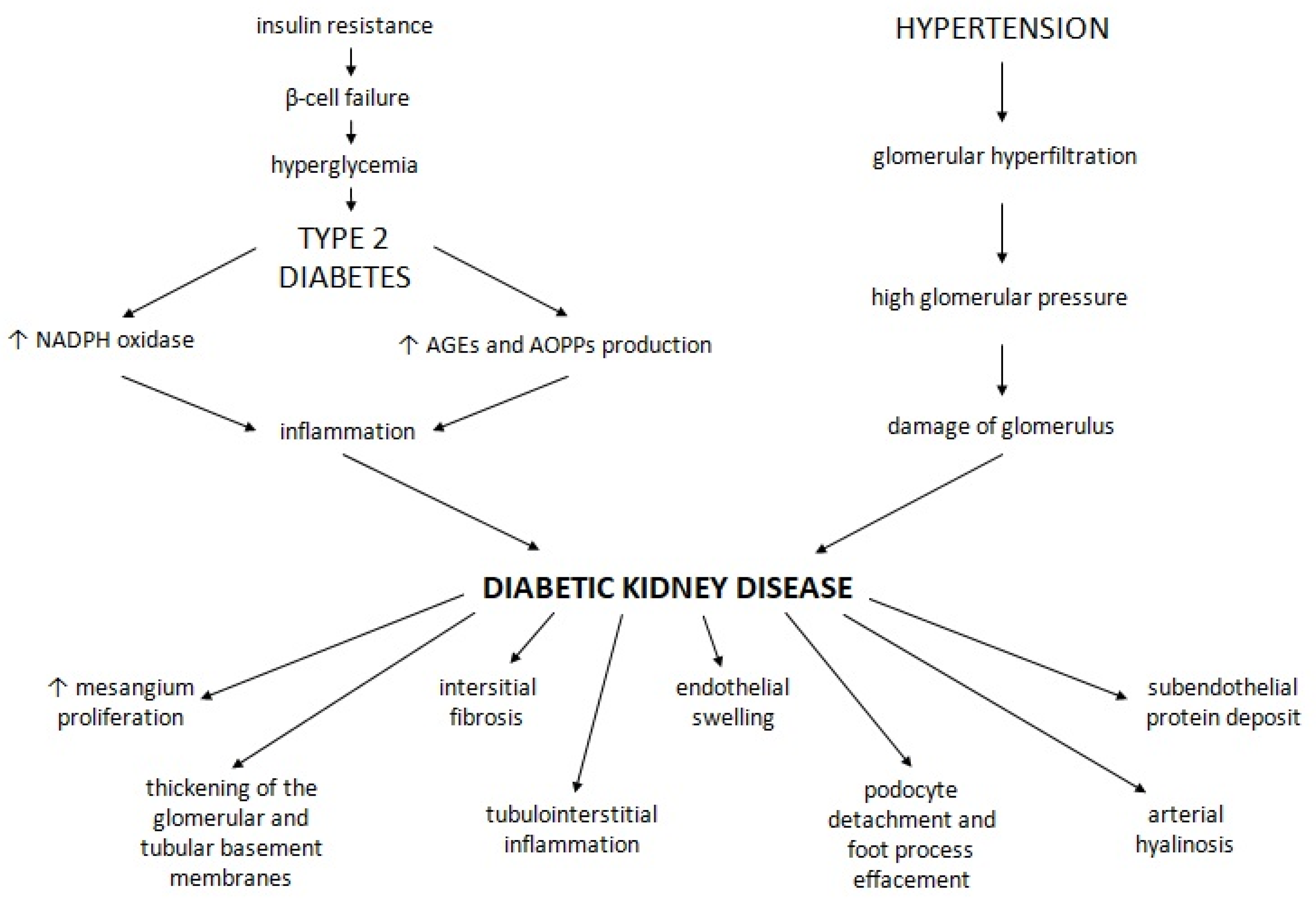
2.3. Effects of GLP1RAs and DPP4is in Diabetic Kidney Disease
2.4. Effects of Incretin-Based Therapy on the Kidneys
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| GIP | glucose-dependent insulinotropic polypeptide (previously known as gastric inhibitory peptide) |
| GLP1 | glucagon-like peptide 1 |
| T2D | type 2 diabetes |
| GLP1R | glucagon-like peptide 1 receptor |
| GLP1RA | glucagon-like peptide 1 receptor agonist |
| DPP4 | dipeptidyl peptidase 4 |
| DPP4i | dipeptidyl peptidase 4 inhibitor |
| DKD | diabetic kidney disease |
| T1D | type 1 diabetes |
| ESRD | end stage renal disease |
| GRF | glomerular filtration rate |
| KRT | kidney replacement therapy |
| PCSK1 | proprotein convertase subtilisin-kexin type 1 |
| PCSK3 | proprotein convertase subtilisin-kexin type 3 |
| cAMP | cyclic adenosine monophosphate |
| NEP | neutral endopeptidase (neprilysin) |
| Fc | fragment crystallizable |
| XR | extended release |
| SNAC | sodium N-(8-[2-hydroxybenzoyl]amino) caprylate |
| HbA1c | haemoglobin A1c |
| CVOT | cardiovascular outcome trial |
| LEADER trial | the Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results trial |
| eGFR | estimated glomerular filtration rate |
| SUSTAIN-6 trial | the Trial to Evaluate Cardiovascular and Other Long-Term Outcomes with Semaglutide in Subjects with Type 2 Diabetes trial |
| EXSCEL trial | the Exenatide Study of Cardiovascular Event Lowering trial |
| CARMELINA trial | the Cardiovascular and Renal Microvascular Outcome Study with Linagliptin trial |
| UACR | urine albumin-to-creatinine ratio |
| SAVOR-TIMI 53 trial | the Saxagliptin Assessment of Vascular Outcomes Recorded in Patients with Diabetes Mellitus—Thrombosis in Myocardial Infraction trial |
| AGE | advanced glycation end-product |
| AOPP | advanced oxidation protein product |
| NADPH | reduced form of nicotinamide adenine dinucleotide phosphate |
| NF-ĸB | nuclear factor ĸB |
| TGF-β | transformin growth factor-β |
| ERα | oestrogen receptor α |
| ERβ | oestrogen receptor β |
| GLUT4 | glucose transporter type 4 |
| NHE3 | sodium-hydrogen exchanger 3 |
| SDF1α | stromal cell-derived factor 1α |
| ICAM1 | intercellular adhesion molecule 1 |
| TLR2 | toll-like receptor 2 |
| TLR4 | toll-like receptor 4 |
| TNF | tumour necrosis factor |
| IL-1B | interleukin 1B |
| MAPK8 | mitogen-activated protein kinase 8 |
| SOCS3 | suppressor of cytokine signalling 3 |
| CCl2 | C-C motif chemokine ligand 2 |
| NGAL | neutrophil gelatinase-associated lipocalin |
| CRP | C reactive protein |
| IL-6 | interleukin 6 |
| IL-18 | interleukin 18 |
| LDL | low density lipoprotein |
| HDL | high density lipoprotein |
References
- Nauck, M.A.; Homberger, E.; Siegel, E.G.; Allen, R.C.; Eaton, R.P.; Ebert, R.; Creutzfeldt, W. Incretin effects of increasing glucose loads in man calculated from venous insulin and C-peptide responses. J. Clin. Endocrinol. Metab. 1986, 63, 492–498. [Google Scholar] [CrossRef] [PubMed]
- Vilsbøll, T.; Holst, J.J. Incretins, insulin secretion and Type 2 diabetes mellitus. Diabetologia 2004, 47, 357–366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kahn, S.E.; Cooper, M.E.; Del Prato, S. Pathophysiology and treatment of type 2 diabetes: Perspectives on the past, present, and future. Lancet 2014, 383, 1068–1083. [Google Scholar] [CrossRef] [Green Version]
- Nauck, M.A.; Heimesaat, M.M.; Orskov, C.; Holst, J.J.; Ebert, R.; Creutzfeldt, W. Preserved incretin activity of glucagon-like peptide 1 [7-36 amide] but not of synthetic human gastric inhibitory polypeptide in patients with type-2 diabetes mellitus. J. Clin. Investig. 1993, 91, 301–307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nauck, M.; Stöckmann, F.; Ebert, R.; Creutzfeldt, W. Reduced incretin effect in type 2 (non-insulin-dependent) diabetes. Diabetologia 1986, 29, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Johansen, K.L.; Chertow, G.M.; Foley, R.N.; Gilbertson, D.T.; Herzog, C.A.; Ishani, A.; Israni, A.K.; Ku, E.; Kurella Tamura, M.; Li, S.; et al. US Renal Data System 2020 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am. J. Kidney Dis. 2021, 77, A7–A8. [Google Scholar] [CrossRef] [PubMed]
- Reutens, A.T. Epidemiology of diabetic kidney disease. Med. Clin. N. Am. 2013, 97, 1–18. [Google Scholar] [CrossRef]
- Afkarian, M.; Sachs, M.C.; Kestenbaum, B.; Hirsch, I.B.; Tuttle, K.R.; Himmelfarb, J.; de Boer, I.H. Kidney disease and increased mortality risk in type 2 diabetes. J. Am. Soc. Nephrol. 2013, 24, 302–308. [Google Scholar] [CrossRef]
- Alicic, R.Z.; Rooney, M.T.; Tuttle, K.R. Diabetic Kidney Disease: Challenges, Progress, and Possibilities. Clin. J. Am. Soc. Nephrol. 2017, 12, 2032–2045. [Google Scholar] [CrossRef]
- Alicic, R.Z.; Cox, E.J.; Neumiller, J.J.; Tuttle, K.R. Incretin drugs in diabetic kidney disease: Biological mechanisms and clinical evidence. Nat. Rev. Nephrol. 2021, 17, 227–244. [Google Scholar] [CrossRef]
- Holst, J.J. The physiology of glucagon-like peptide 1. Physiol. Rev. 2007, 87, 1409–1439. [Google Scholar] [CrossRef]
- Holt, M.K.; Richards, J.E.; Cook, D.R.; Brierley, D.I.; Williams, D.L.; Reimann, F.; Gribble, F.M.; Trapp, S. Preproglucagon Neurons in the Nucleus of the Solitary Tract Are the Main Source of Brain GLP-1, Mediate Stress-Induced Hypophagia, and Limit Unusually Large Intakes of Food. Diabetes 2019, 68, 21–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boer, G.A.; Holst, J.J. Incretin Hormones and Type 2 Diabetes-Mechanistic Insights and Therapeutic Approaches. Biology 2020, 9, 473. [Google Scholar] [CrossRef]
- Worthington, J.J.; Reimann, F.; Gribble, F.M. Enteroendocrine cells-sensory sentinels of the intestinal environment and orchestrators of mucosal immunity. Mucosal Immunol. 2018, 11, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Bortolato, A.; Doré, A.S.; Hollenstein, K.; Tehan, B.G.; Mason, J.S.; Marshall, F.H. Structure of Class B GPCRs: New horizons for drug discovery. Br. J. Pharmacol. 2014, 171, 3132–3145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andersen, A.; Lund, A.; Knop, F.K.; Vilsbøll, T. Glucagon-like peptide 1 in health and disease. Nat. Rev. Endocrinol. 2018, 14, 390–403. [Google Scholar] [CrossRef] [PubMed]
- Baggio, L.L.; Drucker, D.J. Biology of incretins: GLP-1 and GIP. Gastroenterology 2007, 132, 2131–2157. [Google Scholar] [CrossRef] [PubMed]
- Gasbjerg, L.S.; Helsted, M.M.; Hartmann, B.; Jensen, M.H.; Gabe, M.B.N.; Sparre-Ulrich, A.H.; Veedfald, S.; Stensen, S.; Lanng, A.R.; Bergmann, N.C.; et al. Combined Glucometabolic Effects of Endogenous Glucose-Dependent Insulinotropic Polypeptide and Glucagon-like Peptide 1 in Healthy Individuals. Diabetes 2019, 68, 906–917. [Google Scholar] [CrossRef] [Green Version]
- Farilla, L.; Hui, H.; Bertolotto, C.; Kang, E.; Bulotta, A.; Di Mario, U.; Perfetti, R. Glucagon-like peptide-1 promotes islet cell growth and inhibits apoptosis in Zucker diabetic rats. Endocrinology 2002, 143, 4397–4408. [Google Scholar] [CrossRef]
- de Heer, J.; Rasmussen, C.; Coy, D.H.; Holst, J.J. Glucagon-like peptide-1, but not glucose-dependent insulinotropic peptide, inhibits glucagon secretion via somatostatin (receptor subtype 2) in the perfused rat pancreas. Diabetologia 2008, 51, 2263–2270. [Google Scholar] [CrossRef] [Green Version]
- Little, T.J.; Pilichiewicz, A.N.; Russo, A.; Phillips, L.; Jones, K.L.; Nauck, M.A.; Wishart, J.; Horowitz, M.; Feinle-Bisset, C. Effects of intravenous glucagon-like peptide-1 on gastric emptying and intragastric distribution in healthy subjects: Relationships with postprandial glycemic and insulinemic responses. J. Clin. Endocrinol. Metab. 2006, 91, 1916–1923. [Google Scholar] [CrossRef] [Green Version]
- Nauck, M.A.; Niedereichholz, U.; Ettler, R.; Holst, J.J.; Orskov, C.; Ritzel, R.; Schmiegel, W.H. Glucagon-like peptide 1 inhibition of gastric emptying outweighs its insulinotropic effects in healthy humans. Am. J. Physiol. 1997, 273, E981–E988. [Google Scholar] [CrossRef]
- Ten Kulve, J.S.; Veltman, D.J.; van Bloemendaal, L.; Groot, P.F.; Ruhé, H.G.; Barkhof, F.; Diamant, M.; Ijzerman, R.G. Endogenous GLP1 and GLP1 analogue alter CNS responses to palatable food consumption. J. Endocrinol. 2016, 229, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muskiet, M.H.A.; Tonneijck, L.; Smits, M.M.; van Baar, M.J.B.; Kramer, M.H.H.; Hoorn, E.J.; Joles, J.A.; van Raalte, D.H. GLP-1 and the kidney: From physiology to pharmacology and outcomes in diabetes. Nat. Rev. Nephrol. 2017, 13, 605–628. [Google Scholar] [CrossRef]
- Skov, J. Effects of GLP-1 in the kidney. Rev. Endocr. Metab. Disord. 2014, 15, 197–207. [Google Scholar] [CrossRef]
- Bose, A.K.; Mocanu, M.M.; Carr, R.D.; Brand, C.L.; Yellon, D.M. Glucagon-like peptide 1 can directly protect the heart against ischemia/reperfusion injury. Diabetes 2005, 54, 146–151. [Google Scholar] [CrossRef] [Green Version]
- Chai, W.; Zhang, X.; Barrett, E.J.; Liu, Z. Glucagon-like peptide 1 recruits muscle microvasculature and improves insulin’s metabolic action in the presence of insulin resistance. Diabetes 2014, 63, 2788–2799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asmar, A.; Asmar, M.; Simonsen, L.; Madsbad, S.; Holst, J.J.; Hartmann, B.; Sorensen, C.M.; Bülow, J. Glucagon-like peptide-1 elicits vasodilation in adipose tissue and skeletal muscle in healthy men. Physiol. Rep. 2017, 5, e13073. [Google Scholar] [CrossRef]
- Bergmann, N.C.; Lund, A.; Gasbjerg, L.S.; Jørgensen, N.R.; Jessen, L.; Hartmann, B.; Holst, J.J.; Christensen, M.B.; Vilsbøll, T.; Knop, F.K. Separate and Combined Effects of GIP and GLP-1 Infusions on Bone Metabolism in Overweight Men Without Diabetes. J. Clin. Endocrinol. Metab. 2019, 104, 2953–2960. [Google Scholar] [CrossRef] [PubMed]
- Maselli, D.B.; Camilleri, M. Effects of GLP-1 and Its Analogs on Gastric Physiology in Diabetes Mellitus and Obesity. Adv. Exp. Med. Biol. 2021, 1307, 171–192. [Google Scholar] [CrossRef] [PubMed]
- Röhrborn, D.; Wronkowitz, N.; Eckel, J. DPP4 in Diabetes. Front. Immunol. 2015, 6, 386. [Google Scholar] [CrossRef] [Green Version]
- Ohnuma, K.; Dang, N.H.; Morimoto, C. Revisiting an old acquaintance: CD26 and its molecular mechanisms in T cell function. Trends Immunol. 2008, 29, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Deacon, C.F. What do we know about the secretion and degradation of incretin hormones? Regul. Pept. 2005, 128, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Moellmann, J.; Klinkhammer, B.M.; Onstein, J.; Stöhr, R.; Jankowski, V.; Jankowski, J.; Lebherz, C.; Tacke, F.; Marx, N.; Boor, P.; et al. Glucagon-Like Peptide 1 and Its Cleavage Products Are Renoprotective in Murine Diabetic Nephropathy. Diabetes 2018, 67, 2410–2419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, B.; Ji, K.; Peng, A.; Yang, X.; Huang, K. GLP-1(28-36)amide, a Long Ignored Peptide Revisited. Open Biochem. J. 2014, 8, 107–111. [Google Scholar] [CrossRef]
- Asmar, A.; Simonsen, L.; Asmar, M.; Madsbad, S.; Holst, J.J.; Frandsen, E.; Moro, C.; Jonassen, T.; Bülow, J. Renal extraction and acute effects of glucagon-like peptide-1 on central and renal hemodynamics in healthy men. Am. J. Physiol. Endocrinol. Metab. 2015, 308, E641–E649. [Google Scholar] [CrossRef] [Green Version]
- Bhavsar, S.; Mudaliar, S.; Cherrington, A. Evolution of exenatide as a diabetes therapeutic. Curr. Diabetes Rev. 2013, 9, 161–193. [Google Scholar] [CrossRef] [Green Version]
- Antza, C.; Nirantharakumar, K.; Doundoulakis, I.; Tahrani, A.A.; Toulis, K.A. The development of an oral GLP-1 receptor agonist for the management of type 2 diabetes: Evidence to date. Drug Des. Devel. Ther. 2019, 13, 2985–2996. [Google Scholar] [CrossRef] [Green Version]
- Twarog, C.; Fattah, S.; Heade, J.; Maher, S.; Fattal, E.; Brayden, D.J. Intestinal Permeation Enhancers for Oral Delivery of Macromolecules: A Comparison between Salcaprozate Sodium (SNAC) and Sodium Caprate (C10). Pharmaceutics 2019, 11, 78. [Google Scholar] [CrossRef] [Green Version]
- 40Yaribeygi, H.; Sathyapalan, T.; Sahebkar, A. Molecular mechanisms by which GLP-1 RA and DPP-4i induce insulin sensitivity. Life Sci. 2019, 234, 116776. [Google Scholar] [CrossRef]
- Mari, A.; Del Prato, S.; Ludvik, B.; Milicevic, Z.; de la Peña, A.; Shurzinske, L.; Karanikas, C.A.; Pechtner, V. Differential effects of once-weekly glucagon-like peptide-1 receptor agonist dulaglutide and metformin on pancreatic β-cell and insulin sensitivity during a standardized test meal in patients with type 2 diabetes. Diabetes Obes. Metab. 2016, 18, 834–839. [Google Scholar] [CrossRef] [PubMed]
- Vilsboll, T. Liraglutide: A new treatment for type 2 diabetes. Drugs Today 2009, 45, 101–113. [Google Scholar] [CrossRef]
- Pratley, R.E.; Aroda, V.R.; Lingvay, I.; Lüdemann, J.; Andreassen, C.; Navarria, A.; Viljoen, A. SUSTAIN 7 investigators. Semaglutide versus dulaglutide once weekly in patients with type 2 diabetes (SUSTAIN 7): A randomised, open-label, phase 3b trial. Lancet Diabetes Endocrinol. 2018, 6, 275–286. [Google Scholar] [CrossRef]
- Verdich, C.; Flint, A.; Gutzwiller, J.P.; Näslund, E.; Beglinger, C.; Hellström, P.M.; Long, S.J.; Morgan, L.M.; Holst, J.J.; Astrup, A. A meta-analysis of the effect of glucagon-like peptide-1 (7-36) amide on ad libitum energy intake in humans. J. Clin. Endocrinol. Metab. 2001, 86, 4382–4389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raun, K.; von Voss, P.; Knudsen, L.B. Liraglutide, a once-daily human glucagon-like peptide-1 analog, minimizes food intake in severely obese minipigs. Obesity 2007, 15, 1710–1716. [Google Scholar] [CrossRef] [Green Version]
- Kolterman, O.G.; Buse, J.B.; Fineman, M.S.; Gaines, E.; Heintz, S.; Bicsak, T.A.; Taylor, K.; Kim, D.; Aisporna, M.; Wang, Y.; et al. Synthetic exendin-4 (exenatide) significantly reduces postprandial and fasting plasma glucose in subjects with type 2 diabetes. J. Clin. Endocrinol. Metab. 2003, 88, 3082–3089. [Google Scholar] [CrossRef]
- Lorenz, M.; Pfeiffer, C.; Steinsträsser, A.; Becker, R.H.; Rütten, H.; Ruus, P.; Horowitz, M. Effects of lixisenatide once daily on gastric emptying in type 2 diabetes--relationship to postprandial glycemia. Regul. Pept. 2013, 185, 1–8. [Google Scholar] [CrossRef]
- Drucker, D.J.; Buse, J.B.; Taylor, K.; Kendall, D.M.; Trautmann, M.; Zhuang, D.; Porter, L. DURATION-1 Study Group. Exenatide once weekly versus twice daily for the treatment of type 2 diabetes: A randomised, open-label, non-inferiority study. Lancet 2008, 372, 1240–1250. [Google Scholar] [CrossRef]
- Buse, J.B.; Rosenstock, J.; Sesti, G.; Schmidt, W.E.; Montanya, E.; Brett, J.H.; Zychma, M.; Blonde, L. LEAD-6 Study Group. Liraglutide once a day versus exenatide twice a day for type 2 diabetes: A 26-week randomised, parallel-group, multinational, open-label trial (LEAD-6). Lancet 2009, 374, 39–47. [Google Scholar] [CrossRef]
- Madsbad, S. Review of head-to-head comparisons of glucagon-like peptide-1 receptor agonists. Diabetes Obes. Metab. 2016, 18, 317–332. [Google Scholar] [CrossRef]
- Nauck, M.A.; Kemmeries, G.; Holst, J.J.; Meier, J.J. Rapid tachyphylaxis of the glucagon-like peptide 1-induced deceleration of gastric emptying in humans. Diabetes 2011, 60, 1561–1565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okemah, J.; Peng, J.; Quiñones, M. Addressing Clinical Inertia in Type 2 Diabetes Mellitus: A Review. Adv. Ther. 2018, 35, 1735–1745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deacon, C.F.; Danielsen, P.; Klarskov, L.; Olesen, M.; Holstm, J.J. Dipeptidyl peptidase IV inhibition reduces the degradation and clearance of GIP and potentiates its insulinotropic and antihyperglycemic effects in anesthetized pigs. Diabetes 2001, 50, 1588–1597. [Google Scholar] [CrossRef] [Green Version]
- Ahrén, B.; Hughes, T.E. Inhibition of dipeptidyl peptidase-4 augments insulin secretion in response to exogenously administered glucagon-like peptide-1, glucose-dependent insulinotropic polypeptide, pituitary adenylate cyclase-activating polypeptide, and gastrin-releasing peptide in mice. Endocrinology 2005, 146, 2055–2059. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gökçay Canpolat, A.; Şahin, M. Glucose Lowering Treatment Modalities of Type 2 Diabetes Mellitus. Adv. Exp. Med. Biol. 2021, 1307, 7–27. [Google Scholar] [CrossRef] [PubMed]
- Nauck, M.A. Incretin-based therapies for type 2 diabetes mellitus: Properties, functions, and clinical implications. Am. J. Med. 2011, 124, S3–S18. [Google Scholar] [CrossRef]
- Amori, R.E.; Lau, J.; Pittas, A.G. Efficacy and safety of incretin therapy in type 2 diabetes: Systematic review and meta-analysis. JAMA 2007, 298, 194–206. [Google Scholar] [CrossRef]
- Marso, S.P.; Daniels, G.H.; Brown-Frandsen, K.; Kristensen, P.; Mann, J.F.; Nauck, M.A.; Nissen, S.E.; Pocock, S.; Poulter, N.R.; Ravn, L.S.; et al. LEADER Steering Committee; LEADER Trial Investigators. Liraglutide and Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2016, 375, 311–322. [Google Scholar] [CrossRef] [Green Version]
- Mann, J.F.E.; Ørsted, D.D.; Brown-Frandsen, K.; Marso, S.P.; Poulter, N.R.; Rasmussen, S.; Tornøe, K.; Zinman, B.; Buse, J.B. LEADER Steering Committee and Investigators. Liraglutide and Renal Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2017, 377, 839–848. [Google Scholar] [CrossRef] [Green Version]
- Marso, S.P.; Bain, S.C.; Consoli, A.; Eliaschewitz, F.G.; Jódar, E.; Leiter, L.A.; Lingvay, I.; Rosenstock, J.; Seufert, J.; Warren, M.L.; et al. SUSTAIN-6 Investigators. Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N. Engl. J. Med. 2016, 375, 1834–1844. [Google Scholar] [CrossRef] [Green Version]
- Holman, R.R.; Bethel, M.A.; Mentz, R.J.; Thompson, V.P.; Lokhnygina, Y.; Buse, J.B.; Chan, J.C.; Choi, J.; Gustavson, S.M.; Iqbal, N.; et al. EXSCEL Study Group. Effects of Once-Weekly Exenatide on Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2017, 377, 1228–1239. [Google Scholar] [CrossRef] [PubMed]
- Bethel, M.A.; Mentz, R.J.; Merrill, P.; Buse, J.B.; Chan, J.C.; Goodman, S.G.; Iqbal, N.; Jakuboniene, N.; Katona, B.; Lokhnygina, Y.; et al. Microvascular and Cardiovascular Outcomes According to Renal Function in Patients Treated With Once-Weekly Exenatide: Insights From the EXSCEL Trial. Diabetes Care 2020, 43, 446–452. [Google Scholar] [CrossRef] [PubMed]
- Rosenstock, J.; Perkovic, V.; Johansen, O.E.; Cooper, M.E.; Kahn, S.E.; Marx, N.; Alexander, J.H.; Pencina, M.; Toto, R.D.; Wanner, C.; et al. CARMELINA Investigators. Effect of Linagliptin vs Placebo on Major Cardiovascular Events in Adults with Type 2 Diabetes and High Cardiovascular and Renal Risk: The CARMELINA Randomized Clinical Trial. JAMA 2019, 321, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Scirica, B.M.; Bhatt, D.L.; Braunwald, E.; Steg, P.G.; Davidson, J.; Hirshberg, B.; Ohman, P.; Frederich, R.; Wiviott, S.D.; Hoffman, E.B.; et al. SAVOR-TIMI 53 Steering Committee and Investigators. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N. Engl. J. Med. 2013, 369, 1317–1326. [Google Scholar] [CrossRef] [Green Version]
- Mosenzon, O.; Leibowitz, G.; Bhatt, D.L.; Cahn, A.; Hirshberg, B.; Wei, C.; Im, K.; Rozenberg, A.; Yanuv, I.; Stahre, C.; et al. Effect of Saxagliptin on Renal Outcomes in the SAVOR-TIMI 53 Trial. Diabetes Care 2017, 40, 69–76. [Google Scholar] [CrossRef] [Green Version]
- Duni, A.; Liakopoulos, V.; Roumeliotis, S.; Peschos, D.; Dounousi, E. Oxidative Stress in the Pathogenesis and Evolution of Chronic Kidney Disease: Untangling Ariadne’s Thread. Int. J. Mol. Sci. 2019, 20, 3711. [Google Scholar] [CrossRef] [Green Version]
- Akhtar, M.; Taha, N.M.; Nauman, A.; Mujeeb, I.B.; Al-Nabet, A.D.M.H. Diabetic Kidney Disease: Past and Present. Adv. Anat. Pathol. 2020, 27, 87–97. [Google Scholar] [CrossRef]
- Alicic, R.Z.; Johnson, E.J.; Tuttle, K.R. Inflammatory Mechanisms as New Biomarkers and Therapeutic Targets for Diabetic Kidney Disease. Adv. Chronic Kidney Dis. 2018, 25, 181–191. [Google Scholar] [CrossRef]
- Amatruda, M.; Gembillo, G.; Giuffrida, A.E.; Santoro, D.; Conti, G. The Aggressive Diabetic Kidney Disease in Youth-Onset Type 2 Diabetes: Pathogenetic Mechanisms and Potential Therapies. Medicina 2021, 57, 868. [Google Scholar] [CrossRef]
- Lane, P.H. Diabetic kidney disease: Impact of puberty. Am. J. Physiol. Renal Physiol. 2002, 283, F589–F600. [Google Scholar] [CrossRef]
- Ofosu, W.A.; Mohamed, D.; Corcoran, O.; Ojo, O.O. The Role of Oestrogen Receptor Beta (ERβ) in the Aetiology and Treatment of Type 2 Diabetes Mellitus. Curr. Diabetes Rev. 2019, 15, 100–104. [Google Scholar] [CrossRef]
- Hevener, A.L.; Zhou, Z.; Moore, T.M.; Drew, B.G.; Ribas, V. The impact of ERα action on muscle metabolism and insulin sensitivity—Strong enough for a man, made for a woman. Mol. Metab. 2018, 15, 20–34. [Google Scholar] [CrossRef] [PubMed]
- Barros, R.P.; Gabbi, C.; Morani, A.; Warner, M.; Gustafsson, J.A. Participation of ERalpha and ERbeta in glucose homeostasis in skeletal muscle and white adipose tissue. Am. J. Physiol. Endocrinol. Metab. 2009, 297, E124–E133. [Google Scholar] [CrossRef]
- Barros, R.P.; Machado, U.F.; Warner, M.; Gustafsson, J.A. Muscle GLUT4 regulation by estrogen receptors ERbeta and ERalpha. Proc. Natl. Acad. Sci. USA 2006, 103, 1605–1608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderberg, R.J.; Meek, R.L.; Hudkins, K.L.; Cooney, S.K.; Alpers, C.E.; Leboeuf, R.C.; Tuttle, K.R. Serum amyloid A and inflammation in diabetic kidney disease and podocytes. Lab. Investig. 2015, 95, 250–262, Erratum in: Lab. Investig. 2015, 95, 697. [Google Scholar] [CrossRef] [Green Version]
- Alicic, R.Z.; Neumiller, J.J.; Johnson, E.J.; Dieter, B.; Tuttle, K.R. Sodium-Glucose Cotransporter 2 Inhibition and Diabetic Kidney Disease. Diabetes 2019, 68, 248–257, Erratum in: Diabetes 2019, 68, 1094. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pichler, R.; Afkarian, M.; Dieter, B.P.; Tuttle, K.R. Immunity and inflammation in diabetic kidney disease: Translating mechanisms to biomarkers and treatment targets. Am. J. Physiol. Ren. Physiol. 2017, 312, F716–F731. [Google Scholar] [CrossRef] [Green Version]
- Tang, S.C.W.; Yiu, W.H. Innate immunity in diabetic kidney disease. Nat. Rev. Nephrol. 2020, 16, 206–222. [Google Scholar] [CrossRef]
- Jha, J.C.; Banal, C.; Okabe, J.; Gray, S.P.; Hettige, T.; Chow, B.S.M.; Thallas-Bonke, V.; De Vos, L.; Holterman, C.E.; Coughlan, M.T.; et al. NADPH Oxidase Nox5 Accelerates Renal Injury in Diabetic Nephropathy. Diabetes 2017, 66, 2691–2703. [Google Scholar] [CrossRef] [Green Version]
- Sourris, K.C.; Yao, H.; Jerums, G.; Cooper, M.E.; Ekinci, E.I.; Coughlan, M.T. Can Targeting the Incretin Pathway Dampen RAGE-Mediated Events in Diabetic Nephropathy? Curr. Drug Targets 2016, 17, 1252–1264. [Google Scholar] [CrossRef]
- Thomson, S.C.; Kashkouli, A.; Liu, Z.Z.; Singh, P. Renal hemodynamic effects of glucagon-like peptide-1 agonist are mediated by nitric oxide but not prostaglandin. Am. J. Physiol. Renal Physiol. 2017, 313, F854–F858. [Google Scholar] [CrossRef] [PubMed]
- Tonneijck, L.; Muskiet, M.H.A.; Smits, M.M.; Hoekstra, T.; Kramer, M.H.H.; Danser, A.H.J.; Diamant, M.; Joles, J.A.; van Raalte, D.H. Postprandial renal haemodynamic effect of lixisenatide vs once-daily insulin-glulisine in patients with type 2 diabetes on insulin-glargine: An 8-week, randomised, open-label trial. Diabetes Obes. Metab. 2017, 19, 1669–1680. [Google Scholar] [CrossRef]
- Tonneijck, L.; Muskiet, M.H.A.; Blijdorp, C.J.; Smits, M.M.; Twisk, J.W.; Kramer, M.H.H.; Danser, A.H.J.; Diamant, M.; Joles, J.A.; Hoorn, E.J.; et al. Renal tubular effects of prolonged therapy with the GLP-1 receptor agonist lixisenatide in patients with type 2 diabetes mellitus. Am. J. Physiol. Ren. Physiol. 2019, 316, F231–F240. [Google Scholar] [CrossRef] [PubMed]
- van Baar, M.J.B.; van der Aart, A.B.; Hoogenberg, K.; Joles, J.A.; Heerspink, H.J.L.; van Raalte, D.H. The incretin pathway as a therapeutic target in diabetic kidney disease: A clinical focus on GLP-1 receptor agonists. Ther. Adv. Endocrinol. Metab. 2019, 10, 2042018819865398. [Google Scholar] [CrossRef] [PubMed]
- Girardi, A.C.; Fukuda, L.E.; Rossoni, L.V.; Malnic, G.; Rebouças, N.A. Dipeptidyl peptidase IV inhibition downregulates Na+-H+ exchanger NHE3 in rat renal proximal tubule. Am. J. Physiol. Ren. Physiol. 2008, 294, F414–F422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Girardi, A.C.; Knauf, F.; Demuth, H.U.; Aronson, P.S. Role of dipeptidyl peptidase IV in regulating activity of Na+/H+ exchanger isoform NHE3 in proximal tubule cells. Am. J. Physiol. Cell Physiol. 2004, 287, C1238–C1245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rieg, T.; Gerasimova, M.; Murray, F.; Masuda, T.; Tang, T.; Rose, M.; Drucker, D.J.; Vallon, V. Natriuretic effect by exendin-4, but not the DPP-4 inhibitor alogliptin, is mediated via the GLP-1 receptor and preserved in obese type 2 diabetic mice. Am. J. Physiol. Ren. Physiol. 2012, 303, F963–F971. [Google Scholar] [CrossRef] [Green Version]
- Takashima, S.; Fujita, H.; Fujishima, H.; Shimizu, T.; Sato, T.; Morii, T.; Tsukiyama, K.; Narita, T.; Takahashi, T.; Drucker, D.J.; et al. Stromal cell-derived factor-1 is upregulated by dipeptidyl peptidase-4 inhibition and has protective roles in progressive diabetic nephropathy. Kidney Int. 2016, 90, 783–796. [Google Scholar] [CrossRef]
- Hendarto, H.; Inoguchi, T.; Maeda, Y.; Ikeda, N.; Zheng, J.; Takei, R.; Yokomizo, H.; Hirata, E.; Sonoda, N.; Takayanagi, R. GLP-1 analog liraglutide protects against oxidative stress and albuminuria in streptozotocin-induced diabetic rats via protein kinase A-mediated inhibition of renal NAD(P)H oxidases. Metabolism 2012, 61, 1422–1434. [Google Scholar] [CrossRef]
- Sancar-Bas, S.; Gezginci-Oktayoglu, S.; Bolkent, S. Exendin-4 attenuates renal tubular injury by decreasing oxidative stress and inflammation in streptozotocin-induced diabetic mice. Growth Factors 2015, 33, 419–429. [Google Scholar] [CrossRef]
- Kodera, R.; Shikata, K.; Kataoka, H.U.; Takatsuka, T.; Miyamoto, S.; Sasaki, M.; Kajitani, N.; Nishishita, S.; Sarai, K.; Hirota, D.; et al. Glucagon-like peptide-1 receptor agonist ameliorates renal injury through its anti-inflammatory action without lowering blood glucose level in a rat model of type 1 diabetes. Diabetologia 2011, 54, 965–978. [Google Scholar] [CrossRef] [Green Version]
- Katagiri, D.; Hamasaki, Y.; Doi, K.; Okamoto, K.; Negishi, K.; Nangaku, M.; Noiri, E. Protection of glucagon-like peptide-1 in cisplatin-induced renal injury elucidates gut-kidney connection. J. Am. Soc. Nephrol. 2013, 24, 2034–2043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, H.; Li, H.; Wang, Z.; Shi, Y.; Jiang, G.; Zeng, F. Exendin-4 ameliorates renal ischemia-reperfusion injury in the rat. J. Surg. Res. 2013, 185, 825–832. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, A.J.; Lamarche, B.; Deacon, C.F.; Weisnagel, S.J.; Couture, P. Effects of sitagliptin therapy on markers of low-grade inflammation and cell adhesion molecules in patients with type 2 diabetes. Metabolism 2014, 63, 1141–1148. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, A.; Ghanim, H.; Vora, M.; Sia, C.L.; Korzeniewski, K.; Dhindsa, S.; Makdissi, A.; Dandona, P. Exenatide exerts a potent antiinflammatory effect. J. Clin. Endocrinol. Metab. 2012, 97, 198–207. [Google Scholar] [CrossRef] [Green Version]
- Moschovaki Filippidou, F.; Kirsch, A.H.; Thelen, M.; Kétszeri, M.; Artinger, K.; Aringer, I.; Schabhüttl, C.; Mooslechner, A.A.; Frauscher, B.; Pollheimer, M.; et al. Glucagon-Like Peptide-1 Receptor Agonism Improves Nephrotoxic Serum Nephritis by Inhibiting T-Cell Proliferation. Am. J. Pathol. 2020, 190, 400–411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.; Cui, M.; Wei, Y.; Kong, X.; Tang, L.; Xu, D. Inhibition of the expression of TGF-β1 and CTGF in human mesangial cells by exendin-4, a glucagon-like peptide-1 receptor agonist. Cell Physiol. Biochem. 2012, 30, 749–757. [Google Scholar] [CrossRef] [PubMed]
- Akarte, A.S.; Srinivasan, B.P.; Gandhi, S.; Sole, S. Chronic DPP-IV inhibition with PKF-275-055 attenuates inflammation and improves gene expressions responsible for insulin secretion in streptozotocin induced diabetic rats. Eur. J. Pharm. Sci. 2012, 47, 456–463. [Google Scholar] [CrossRef]
- Tian, L.; Gao, J.; Hao, J.; Zhang, Y.; Yi, H.; O’Brien, T.D.; Sorenson, R.; Luo, J.; Guo, Z. Reversal of new-onset diabetes through modulating inflammation and stimulating beta-cell replication in nonobese diabetic mice by a dipeptidyl peptidase IV inhibitor. Endocrinology 2010, 151, 3049–3060. [Google Scholar] [CrossRef]
- Tahrani, A.A.; Barnett, A.H.; Bailey, C.J. Pharmacology and therapeutic implications of current drugs for type 2 diabetes mellitus. Nat. Rev. Endocrinol. 2016, 12, 566–592. [Google Scholar] [CrossRef] [Green Version]
- Bunck, M.C.; Diamant, M.; Eliasson, B.; Cornér, A.; Shaginian, R.M.; Heine, R.J.; Taskinen, M.R.; Yki-Järvinen, H.; Smith, U. Exenatide affects circulating cardiovascular risk biomarkers independently of changes in body composition. Diabetes Care 2010, 33, 1734–1737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, F.; Wu, S.; Guo, S.; Yu, K.; Yang, Z.; Li, L.; Zhang, Y.; Quan, X.; Ji, L.; Zhan, S. Impact of GLP-1 receptor agonists on blood pressure, heart rate and hypertension among patients with type 2 diabetes: A systematic review and network meta-analysis. Diabetes Res. Clin. Pract. 2015, 110, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Wu, S.; Wang, J.; Guo, S.; Chai, S.; Yang, Z.; Li, L.; Zhang, Y.; Ji, L.; Zhan, S. Effect of glucagon-like peptide-1 receptor agonists on lipid profiles among type 2 diabetes: A systematic review and network meta-analysis. Clin. Ther. 2015, 37, 225–241.e8. [Google Scholar] [CrossRef] [PubMed]
- Monami, M.; Lamanna, C.; Desideri, C.M.; Mannucci, E. DPP-4 inhibitors and lipids: Systematic review and meta-analysis. Adv. Ther. 2012, 29, 14–25. [Google Scholar] [CrossRef] [PubMed]
| Type of GLP1RA | Short-Acting GLP1RAs | Long-Acting GLP1RAs |
|---|---|---|
| Agents | exenatide lixisenatide | liraglutide exenatide XR albiglutide dulaglutide semaglutide |
| Activation of GLP1R | intermittent | continuous |
| Gastric emptying | delaying | no influence due to tachyphylaxis |
| Postprandial glucose excursion | superior impact | inferior impact |
| Fasting glucose levels and HbA1c | inferior influence | superior influence |
| Bodyweight reduction | comparable effect | comparable effect |
| Trial | Agent | Enrolled Patients | Renal Outcomes | Results | References |
|---|---|---|---|---|---|
| LEADER | liraglutide | 9340 patients with T2D and high cardiovascular risk |
|
| [58,59] |
| SUSTAIN-6 | semaglutide | 3297 patients with T2D, of whom 2735 had established cardiovascular disease, chronic kidney disease or both | new-onset or worsening nephropathy is defined as:
|
| [60] |
| EXSCEL | exenatide XR | 14,752 patients with T2D, of whom 10,782 had previous cardiovascular disease |
|
| [61,62] |
| CARMELINA | linagliptin | 6991 patients with T2D and high cardiovascular or chronic kidney disease risk |
|
| [63] |
| SAVOR-TIMI 53 | saxagliptin | 16,492 patients with T2D who had a history of, or were at risk for, cardiovascular events |
|
| [64,65] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Przezak, A.; Bielka, W.; Pawlik, A. Incretins in the Therapy of Diabetic Kidney Disease. Int. J. Mol. Sci. 2021, 22, 12312. https://doi.org/10.3390/ijms222212312
Przezak A, Bielka W, Pawlik A. Incretins in the Therapy of Diabetic Kidney Disease. International Journal of Molecular Sciences. 2021; 22(22):12312. https://doi.org/10.3390/ijms222212312
Chicago/Turabian StylePrzezak, Agnieszka, Weronika Bielka, and Andrzej Pawlik. 2021. "Incretins in the Therapy of Diabetic Kidney Disease" International Journal of Molecular Sciences 22, no. 22: 12312. https://doi.org/10.3390/ijms222212312
APA StylePrzezak, A., Bielka, W., & Pawlik, A. (2021). Incretins in the Therapy of Diabetic Kidney Disease. International Journal of Molecular Sciences, 22(22), 12312. https://doi.org/10.3390/ijms222212312






