Chymase as a Novel Therapeutic Target in Acute Pancreatitis
Abstract
:1. Introduction
2. Results
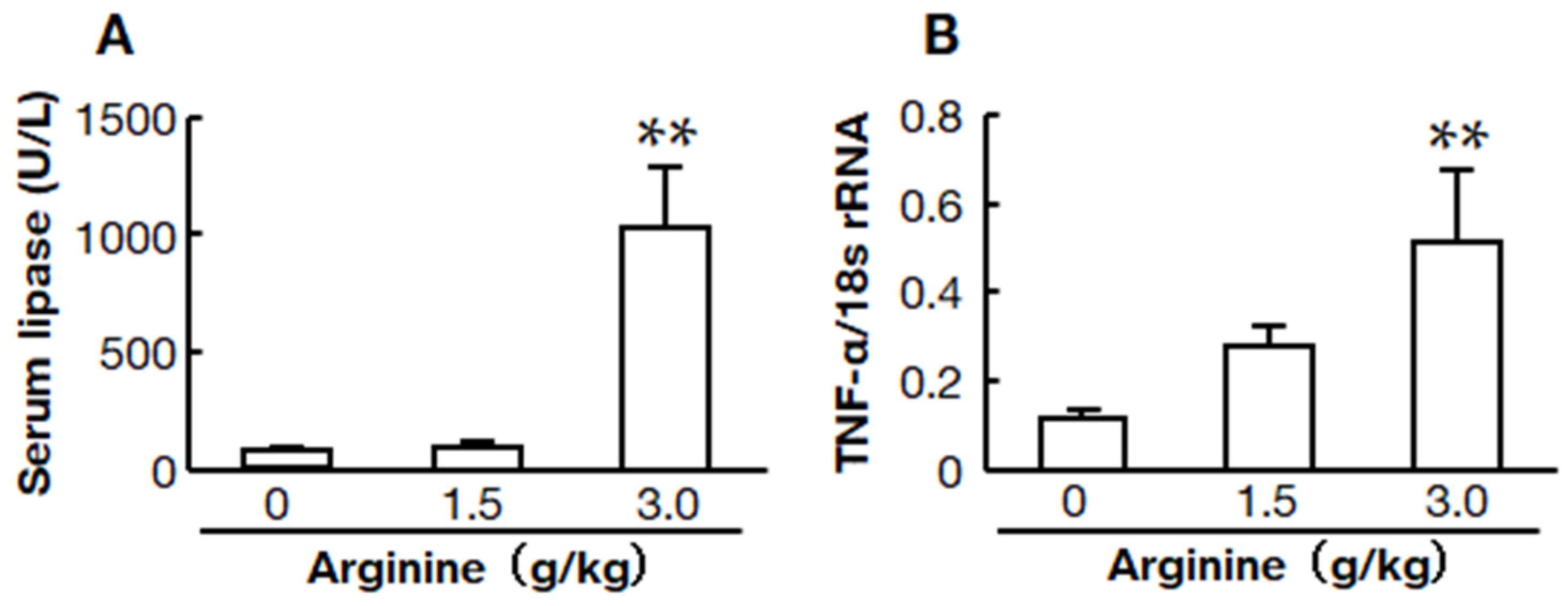
2.1. Two Doses of L-Arginine-Induced Acute Pancreatitis Models
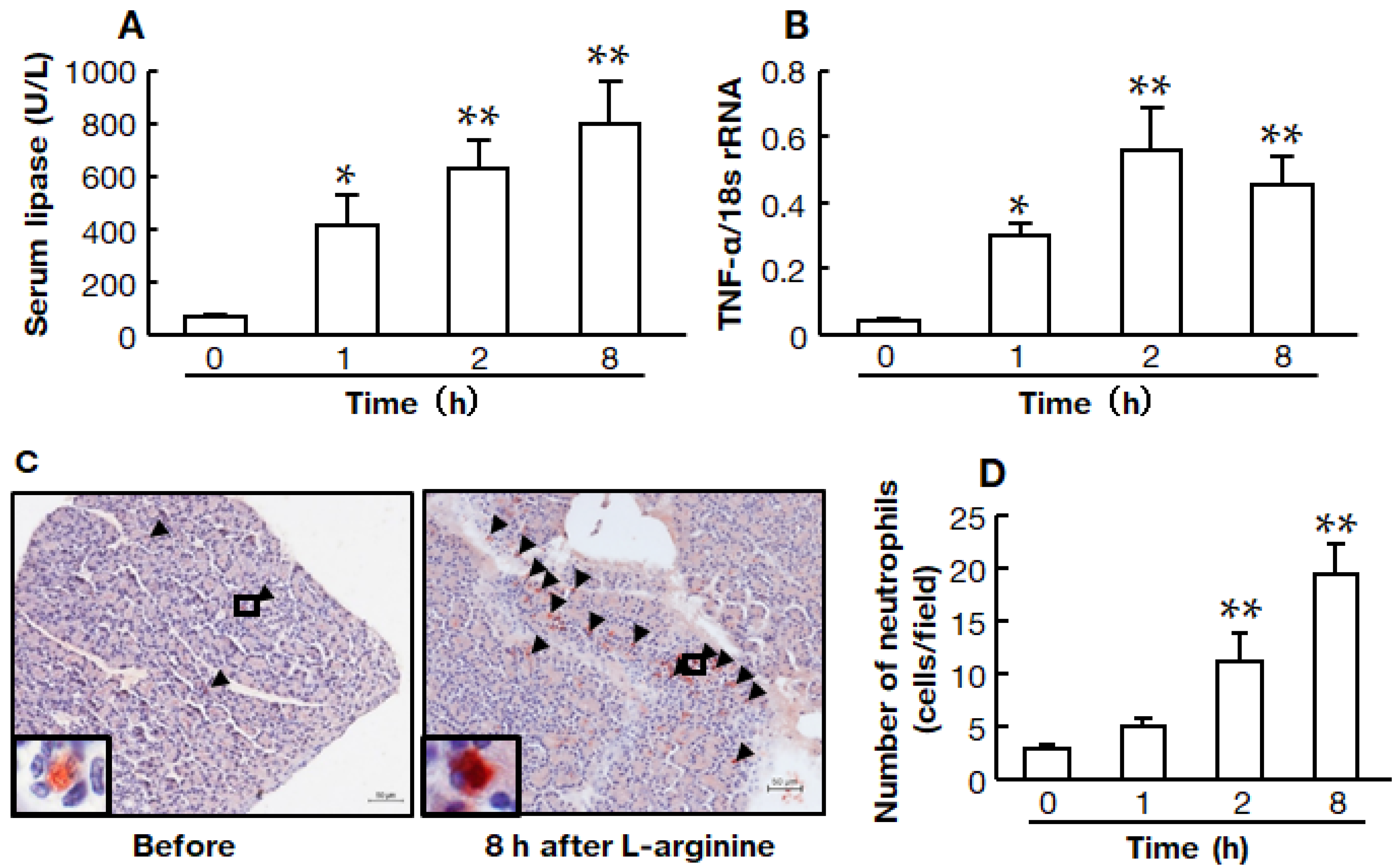
2.2. Time Course of the High-Dose L-Arginine-Induced Model of Acute Pancreatitis
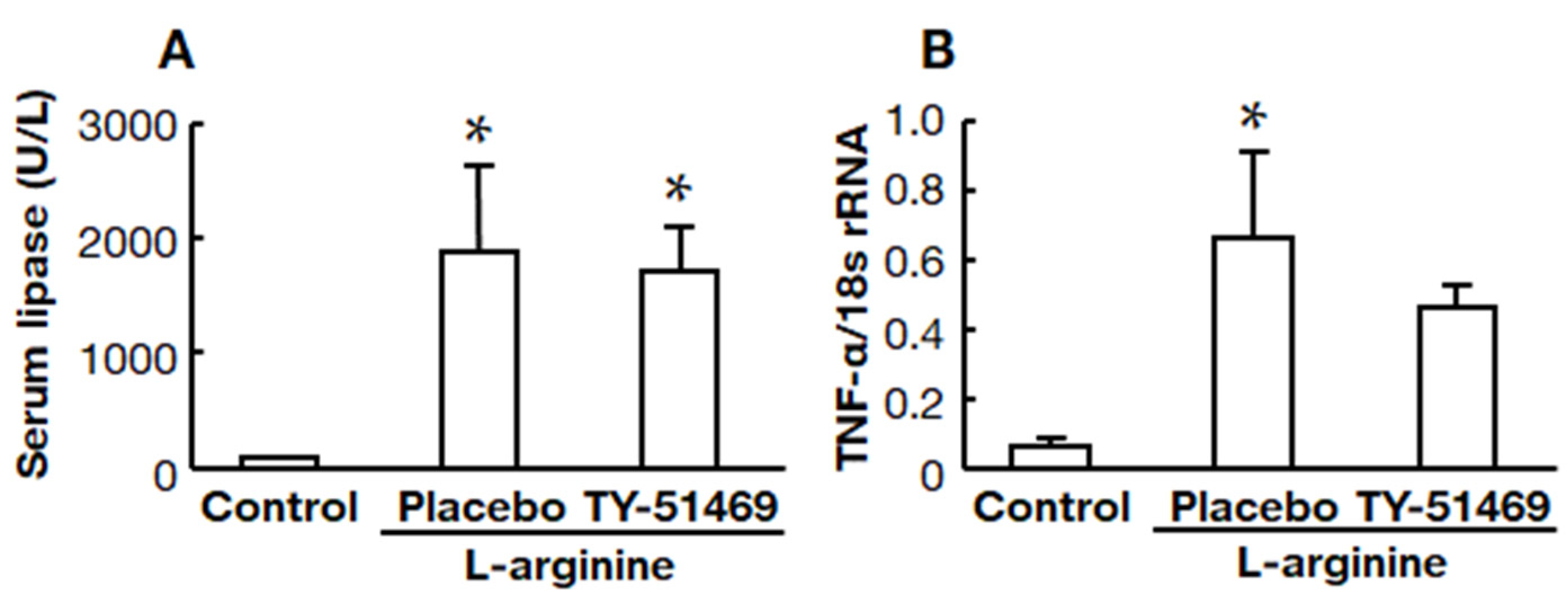
2.3. Effects of Chymase Inhibitor on Serum Lipase and Pancreatic TNF-α mRNA Levels
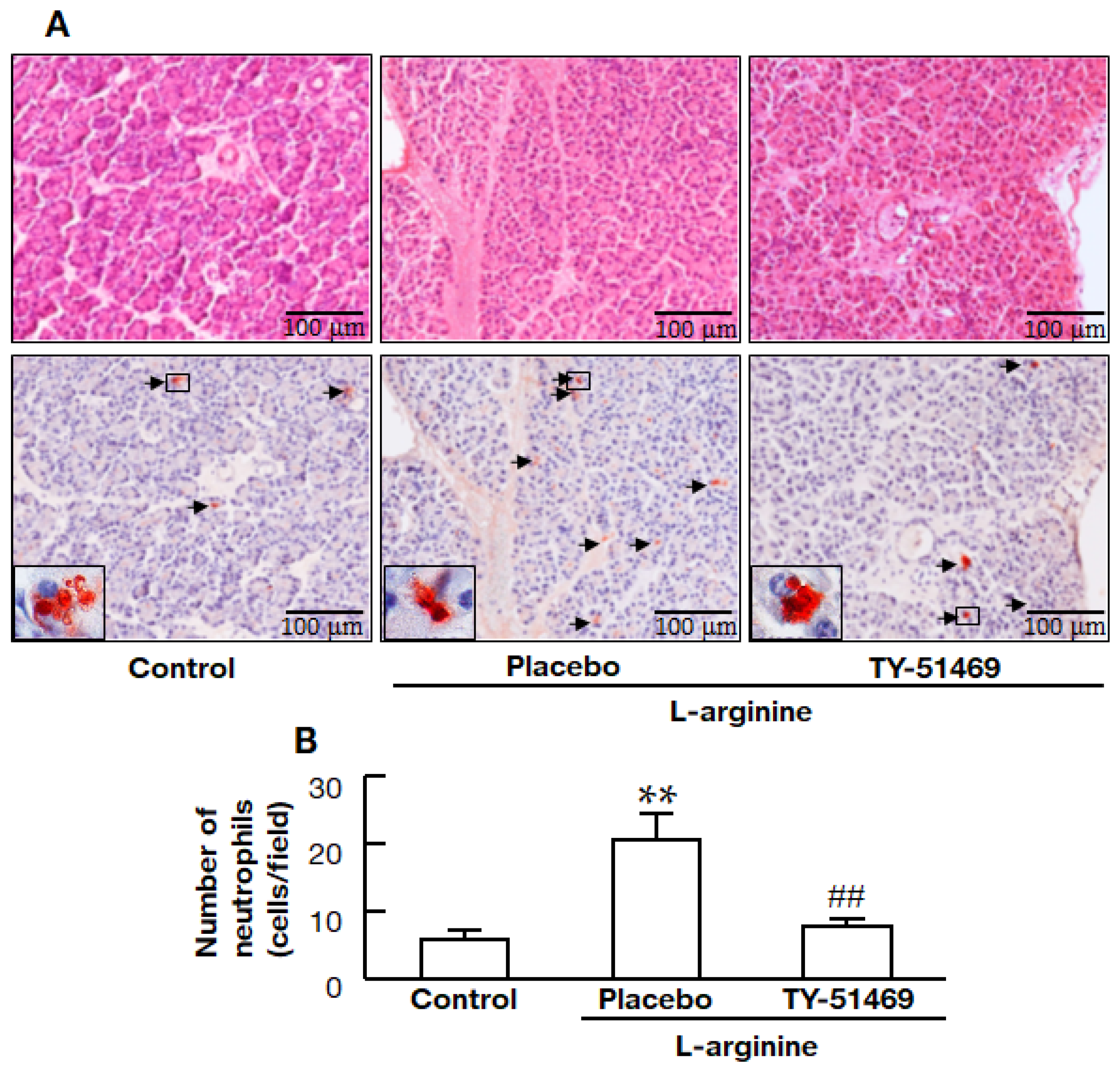
2.4. Chymase Inhibitor TY-51469 Decreases Immune Cell Infiltration into the Pancreas
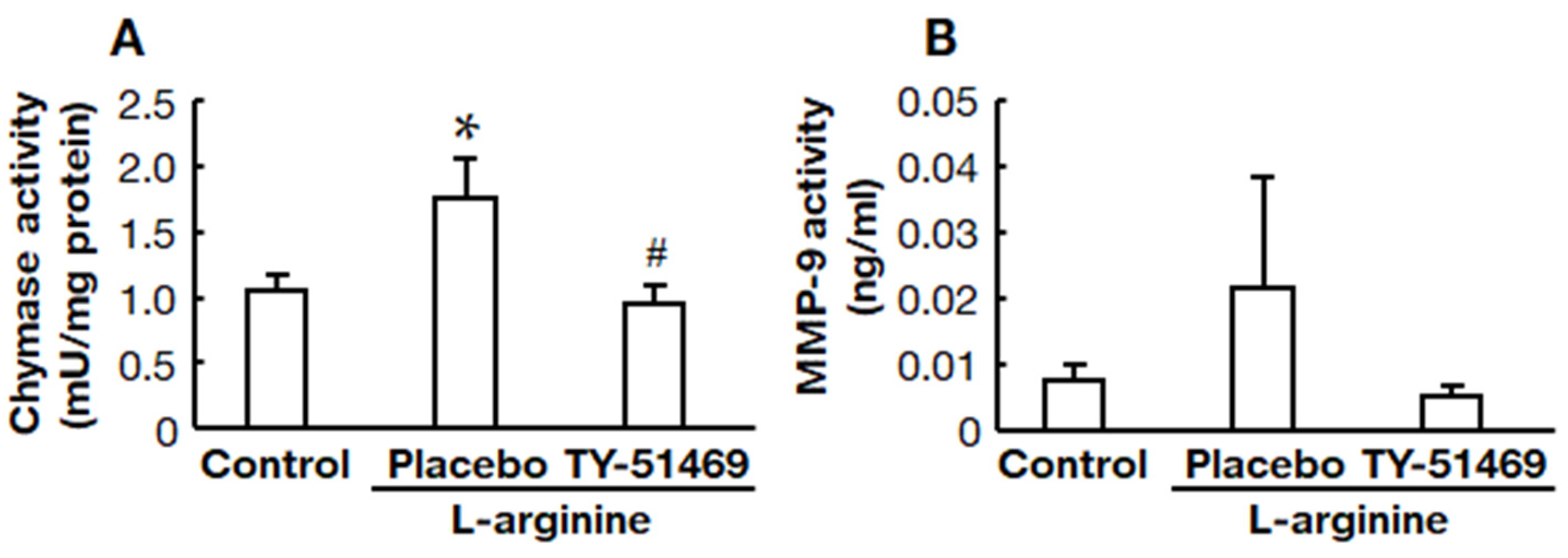
2.5. Chymase Inhibitor TY-51469 Suppressed Pancreatic Chymase and MMP-9 Activities
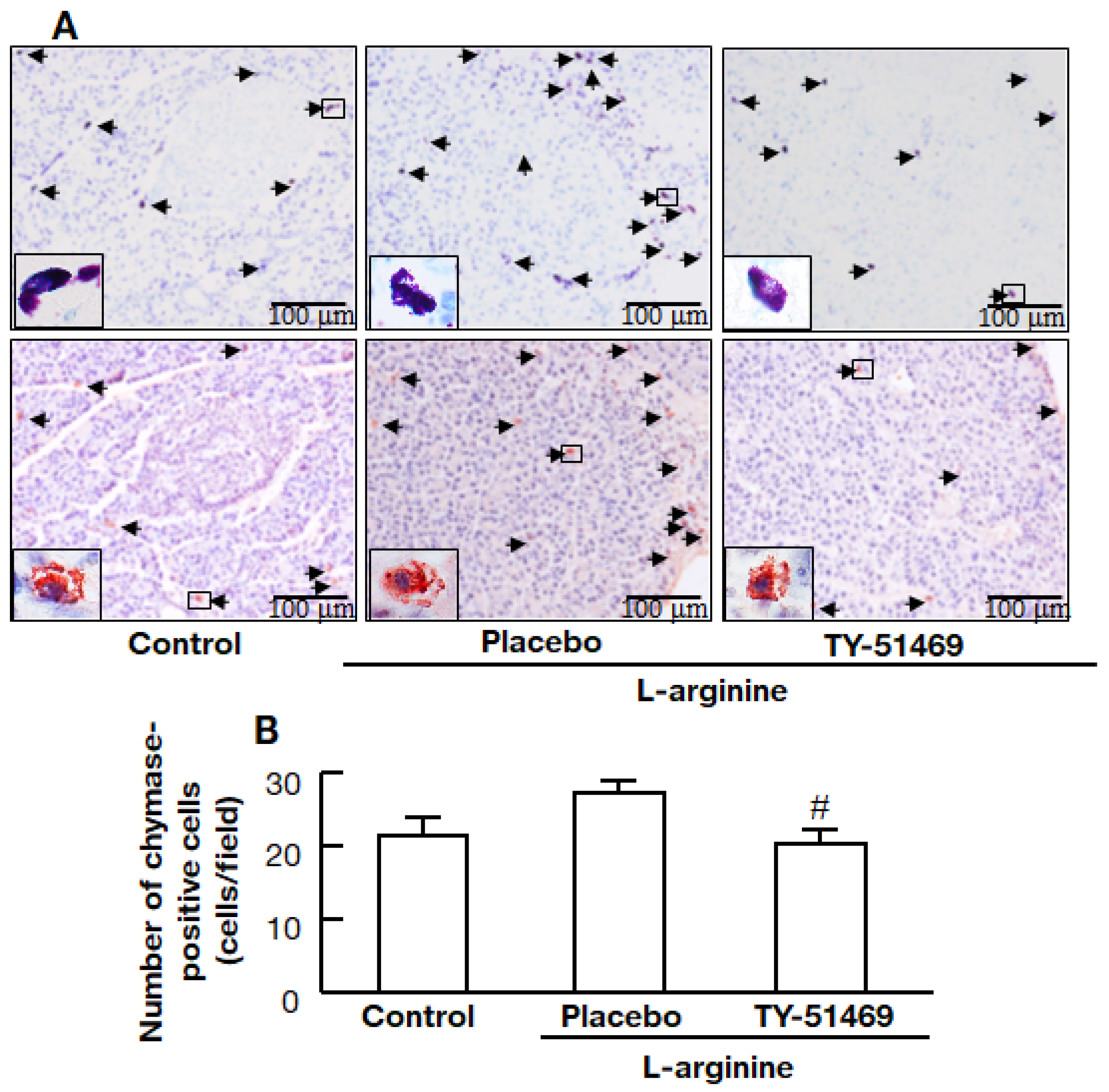
2.6. Chymase Inhibitor TY-51469 Decreases the Number of Chymase-Positive Cells in the Pancreas
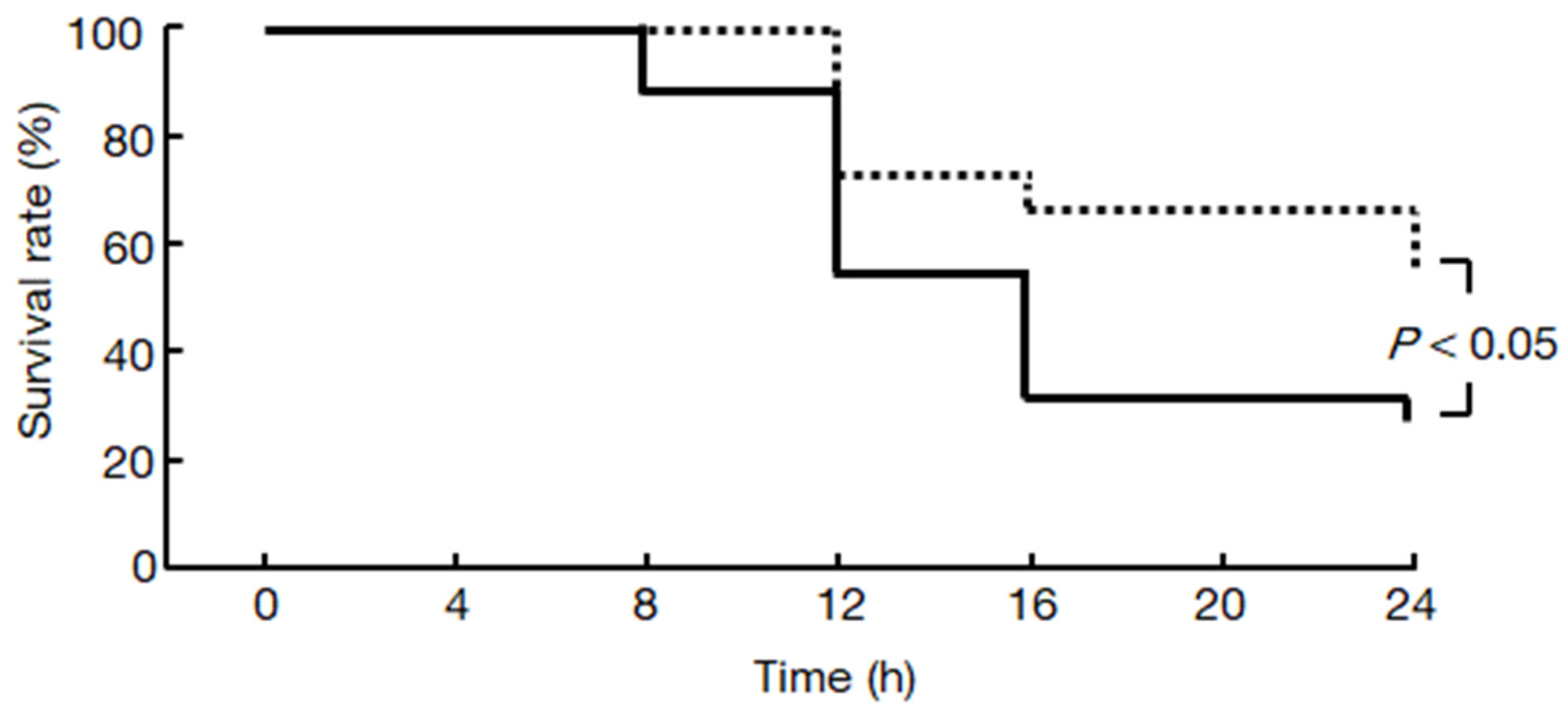
2.7. Chymase Inhibitor TY-51469 Increases the Survival Rate of Hamsters
3. Discussion
4. Material and Methods
4.1. Drug
4.2. Animals and Experimental Design
4.3. Serum Lipase
4.4. Preparation of the Pancreas
4.5. Histological Analysis
4.6. Real-Time Reverse Transcription Polymerase Chain Reaction (RT-PCR)
4.7. Enzyme Activities
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vege, S.S.; Di Magno, M.J.; Forsmark, C.E.; Martel, M.; Barkun, A.N. Initial Medical Treatment of Acute Pancreatitis: American Gastroenterological Association Institute Technical Review. Gastroenterology 2018, 154, 1103–1139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yokoe, M.; Takada, T.; Mayumi, T.; Yoshida, M.; Isaji, S.; Wada, K.; Itoi, T.; Sata, N.; Gabata, T.; Igarashi, H.; et al. Japanese Guidelines for the Management of Acute Pancreatitis: Japanese Guidelines 2015. J. Hepato-Biliary-Pancreat. Sci. 2015, 22, 405–432. [Google Scholar] [CrossRef] [PubMed]
- Crockett, S.D.; Wani, S.; Gardner, T.B.; Falck-Ytter, Y.; Barkun, A.N.; Crockett, S.; Falck-Ytter, Y.; Feuerstein, J.; Flamm, S.; Gellad, Z.; et al. American Gastroenterological Association Institute Guideline on Initial Management of Acute Pancreatitis. Gastroenterology 2018, 154, 1096–1101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tenner, S.; Baillie, J.; DeWitt, J.; Vege, S.S. American College of Gastroenterology Guideline: Management of Acute Pancreatitis. Am. J. Gastroenterol. 2013, 108, 1400–1415. [Google Scholar] [CrossRef]
- Lopez-Font, I.; Gea-Sorlí, S.; de-Madaria, E.; Gutiérrez, L.M.; Pérez-Mateo, M.; Closa, D. Pancreatic and pulmonary mast cells activation during experimental acute pancreatitis. World J. Gastroenterol. 2010, 16, 3411–3417. [Google Scholar] [CrossRef] [Green Version]
- Yönetçi, N.; Oruç, N.; Ozütemiz, A.O.; Celik, H.A.; Yüce, G. Effects of mast-cell stabilization in cerulein-induced acute pancreatitis in rats. Int. J. Pancreatol. 2001, 29, 163–171. [Google Scholar] [CrossRef]
- Dib, M.; Zhao, X.; Wang, X.D.; Andersson, R. Role of mast cells in the development of pancreatitis-induced multiple organ dysfunction. Br. J. Surg. 2002, 89, 172–178. [Google Scholar] [CrossRef]
- Takai, S.; Jin, D.; Miyazaki, M. Multiple mechanisms for the action of chymase inhibitors. J. Pharmacol. Sci. 2012, 118, 311–316. [Google Scholar] [CrossRef] [Green Version]
- Imai, Y.; Takai, S.; Jin, D.; Komeda, K.; Tashiro, K.; Li, Z.-L.; Otsuki, Y.; Okamura, H.; Hayashi, M.; Uchiyama, K. Chymase inhibition attenuates lipopolysaccharide/d-galactosamine-induced acute liver failure in hamsters. Pharmacology 2014, 93, 47–56. [Google Scholar] [CrossRef]
- Okunishi, H.; Oka, Y.; Shiota, N.; Kawamoto, T.; Song, K.; Miyazaki, M. Marked species-difference in the vascular angiotensin II-forming pathways: Humans versus rodents. Jpn. J. Pharmacol. 1993, 62, 207–210. [Google Scholar] [CrossRef] [Green Version]
- Kakimoto, K.; Takai, S.; Murano, M.; Ishida, K.; Yoda, Y.; Inoue, T.; Jin, D.; Umegaki, E.; Higuchi, K. Significance of chymase-dependent matrix metalloproteinase-9 activation on indomethacin-induced small intestinal damages in rats. J. Pharmacol. Exp. Ther. 2010, 332, 684–689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madhi, R.; Rahman, M.; Taha, D.; Mörgelin, M.; Thorlacius, H. Targeting peptidylarginine deiminase reduces neutrophil extracellular trap formation and tissue injury in severe acute pancreatitis. J. Cell Physiol. 2019, 234, 11850–11860. [Google Scholar] [CrossRef]
- Awla, D.; Abdulla, A.; Syk, I.; Jeppsson, B.; Regnér, S.; Thorlacius, H. Neutrophil-derived matrix metalloproteinase-9 is a potent activator of trypsinogen in acinar cells in acute pancreatitis. J. Leukoc. Biol. 2012, 91, 711–719. [Google Scholar] [CrossRef]
- Wan, M.H.; Huang, W.; Latawiec, D.; Jiang, K.; Booth, D.M.; Elliott, V.; Mukherjee, R.; Xia, Q. Review of experimental animal models of biliary acute pancreatitis and recent advances in basic research. HPB 2012, 14, 73–81. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.H.; Kim, H. Oxidative stress and inflammatory signaling in cerulein pancreatitis. World J. Gastroenterol. 2014, 20, 17324–17329. [Google Scholar] [CrossRef]
- Mizunuma, T.; Kawamura, S.; Kishino, Y. Effects of injecting excess arginine on rat pancreas. J. Nutr. 1984, 114, 467–471. [Google Scholar] [CrossRef]
- Wang, Y.; Kayoumu, A.; Lu, G.; Xu, P.; Qiu, X.; Chen, L.; Qi, R.; Huang, S.; Li, W.; Wang, Y.; et al. Experimental models in syrian golden hamster replicate human acute pancreatitis. Sci. Rep. 2016, 6, 28014. [Google Scholar] [CrossRef]
- Takai, S.; Shiota, N.; Yamamoto, D.; Okunishi, H.; Miyazaki, M. Purification and characterization of angiotensin II-generating chymase from hamster cheek pouch. Life Sci. 1996, 58, 591–597. [Google Scholar] [CrossRef]
- Takai, S.; Sakaguchi, M.; Jin, D.; Yamada, M.; Kirimura, K.; Miyazaki, M. Different angiotensin II-forming pathways in human and rat vascular tissues. Clin. Chim. Acta 2001, 305, 191–195. [Google Scholar] [CrossRef]
- El-Ashmawy, N.E.; Khedr, N.F.; El-Bahrawy, H.A.; Hamada, O.B. Anti-inflammatory and antioxidant effects of captopril compared to methylprednisolone in L-arginine-induced acute pancreatitis. Dig. Dis. Sci. 2018, 63, 1497–1505. [Google Scholar] [CrossRef]
- Takai, S.; Jin, D.; Ohzu, M.; Tanaka, K.; Miyazaki, M. Chymase inhibition provides pancreatic islet protection in hamsters with streptozotocin-induced diabetes. J. Pharmacol. Sci. 2009, 110, 459–465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitchell, R.M.; Byrne, M.F.; Baillie, J. Pancreatitis. Lancet 2003, 361, 1447–1455. [Google Scholar] [CrossRef]
- Furubayashi, K.; Takai, S.; Jin, D.; Miyazaki, M.; Katsumata, T.; Inagaki, S.; Kimura, M.; Tanaka, K.; Nishimoto, M.; Fukumoto, H. Chymase activates promatrix metalloproteinase-9 in human abdominal aortic aneurysm. Clin. Chim. Acta 2008, 388, 214–216. [Google Scholar] [CrossRef] [PubMed]
- Vandooren, J.; Van den Steen, P.E.; Opdenakker, G. Biochemistry and molecular biology of gelatinase B or matrix metalloproteinase-9 (MMP-9): The next decade. Crit. Rev. Biochem. Mol. Biol. 2013, 48, 222–272. [Google Scholar] [CrossRef] [PubMed]
- De Young, M.B.; Nemeth, E.F.; Scarpa, A. Measurement of the internal pH of mast cell granules using microvolumetric fluorescence and isotopic techniques. Arch. Biochem. Biophys. 1987, 254, 222–233. [Google Scholar] [CrossRef]
- Longley, B.J.; Tyrrell, L.; Ma, Y.; Williams, D.A.; Halaban, R.; Langley, K.; Lu, H.S.; Schechter, N.M. Chymase cleavage of stem cell factor yields a bioactive, soluble product. Proc. Natl. Acad. Sci. USA 1997, 94, 9017–9021. [Google Scholar] [CrossRef] [Green Version]
- Hughes, C.B.; Gaber, L.W.; Mohey el-Din, A.B.; Grewal, H.P.; Kotb, M.; Mann, L.; Gaber, A.O. Inhibition of TNF α improves survival in an experimental model of acute pancreatitis. Am. Surg. 1996, 62, 8–13. [Google Scholar]
- Muhs, B.E.; Patel, S.; Yee, H.; Marcus, S.; Shamamian, P. Increased matrix metalloproteinase expression and activation following experimental acute pancreatitis. J. Surg. Res. 2001, 101, 21–28. [Google Scholar] [CrossRef]
- Bísaro de Lorenc, L.; Ramos, A.M.; Sánchez, M.C.; Montenegero, R.; Chiabrando, G.A. Structural evaluation of plasma α2-macroglobulin in acute pancreatitis. Clin. Chem. Lab. Med. 2005, 43, 1183–1189. [Google Scholar] [CrossRef]
- Pejler, G. Novel insight into the in vivo function of mast cell chymase: Lessons from knockouts and inhibitors. J. Innate Immun. 2020, 12, 357–372. [Google Scholar] [CrossRef]
- Ishida, K.; Takai, S.; Murano, M.; Nishikawa, T.; Inoue, T.; Murano, N.; Inoue, N.; Jin, D.; Umegaki, E.; Higuchi, K.; et al. Role of chymase-dependent matrix metalloproteinase-9 activation in mice with dextran sodium sulfate-induced colitis. J. Pharmacol. Exp. Ther. 2008, 324, 422–426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watase, K.; Jin, D.; Terai, K.; Kanemiya, T.; Nakakura, H.; Shibahara, N.; Arima, S.; Takai, S. Possible roles of periostin in the formation of hemodialysis vascular access stenosis after polytetrafluoroethylene graft implantation in dogs. Int. J. Mol. Sci. 2020, 21, 3251. [Google Scholar] [CrossRef]
- Miyaoka, Y.; Jin, D.; Tashiro, K.; Komeda, K.; Masubuchi, S.; Hirokawa, F.; Hayashi, M.; Takai, S.; Uchiyama, K. Chymase inhibitor prevents the development and progression of non-alcoholic steatohepatitis in rats fed a high-fat and high-cholesterol diet. J. Pharmacol. Sci. 2017, 134, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Miyaoka, Y.; Jin, D.; Tashiro, K.; Masubuchi, S.; Ozeki, M.; Hirokawa, F.; Hayashi, M.; Takai, S.; Uchiyama, K. A novel hamster nonalcoholic steatohepatitis model induced by a high-fat and high-cholesterol diet. Exp. Anim. 2018, 67, 239–247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuramoto, T.; Jin, D.; Komeda, K.; Taniguchi, K.; Hirokawa, F.; Takai, S.; Uchiyama, K. Chymase as a Novel Therapeutic Target in Acute Pancreatitis. Int. J. Mol. Sci. 2021, 22, 12313. https://doi.org/10.3390/ijms222212313
Kuramoto T, Jin D, Komeda K, Taniguchi K, Hirokawa F, Takai S, Uchiyama K. Chymase as a Novel Therapeutic Target in Acute Pancreatitis. International Journal of Molecular Sciences. 2021; 22(22):12313. https://doi.org/10.3390/ijms222212313
Chicago/Turabian StyleKuramoto, Toru, Denan Jin, Koji Komeda, Kohei Taniguchi, Fumitoshi Hirokawa, Shinji Takai, and Kazuhisa Uchiyama. 2021. "Chymase as a Novel Therapeutic Target in Acute Pancreatitis" International Journal of Molecular Sciences 22, no. 22: 12313. https://doi.org/10.3390/ijms222212313
APA StyleKuramoto, T., Jin, D., Komeda, K., Taniguchi, K., Hirokawa, F., Takai, S., & Uchiyama, K. (2021). Chymase as a Novel Therapeutic Target in Acute Pancreatitis. International Journal of Molecular Sciences, 22(22), 12313. https://doi.org/10.3390/ijms222212313





