Transcriptome Differences in Response Mechanisms to Low-Nitrogen Stress in Two Wheat Varieties
Abstract
:1. Introduction
2. Results
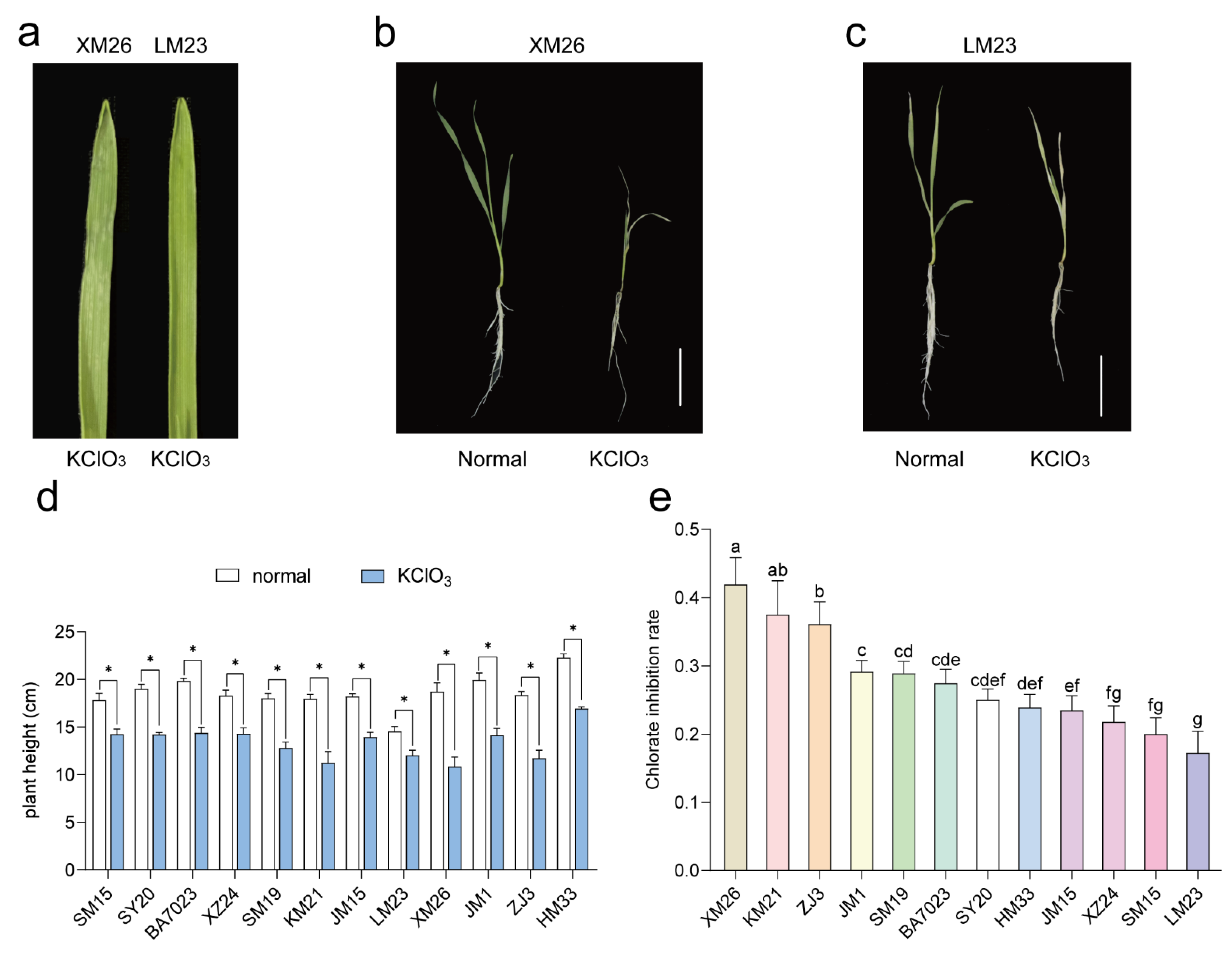
2.1. Screening of Wheat Varieties Based on Chlorate and Low-Nitrogen Stress-Tolerance Analyses
2.2. Transcriptome Analysis of the Two Wheat Varieties under Normal and Low-Nitrogen Conditions
2.3. GO and KEGG Enrichment Analyses of DEGs
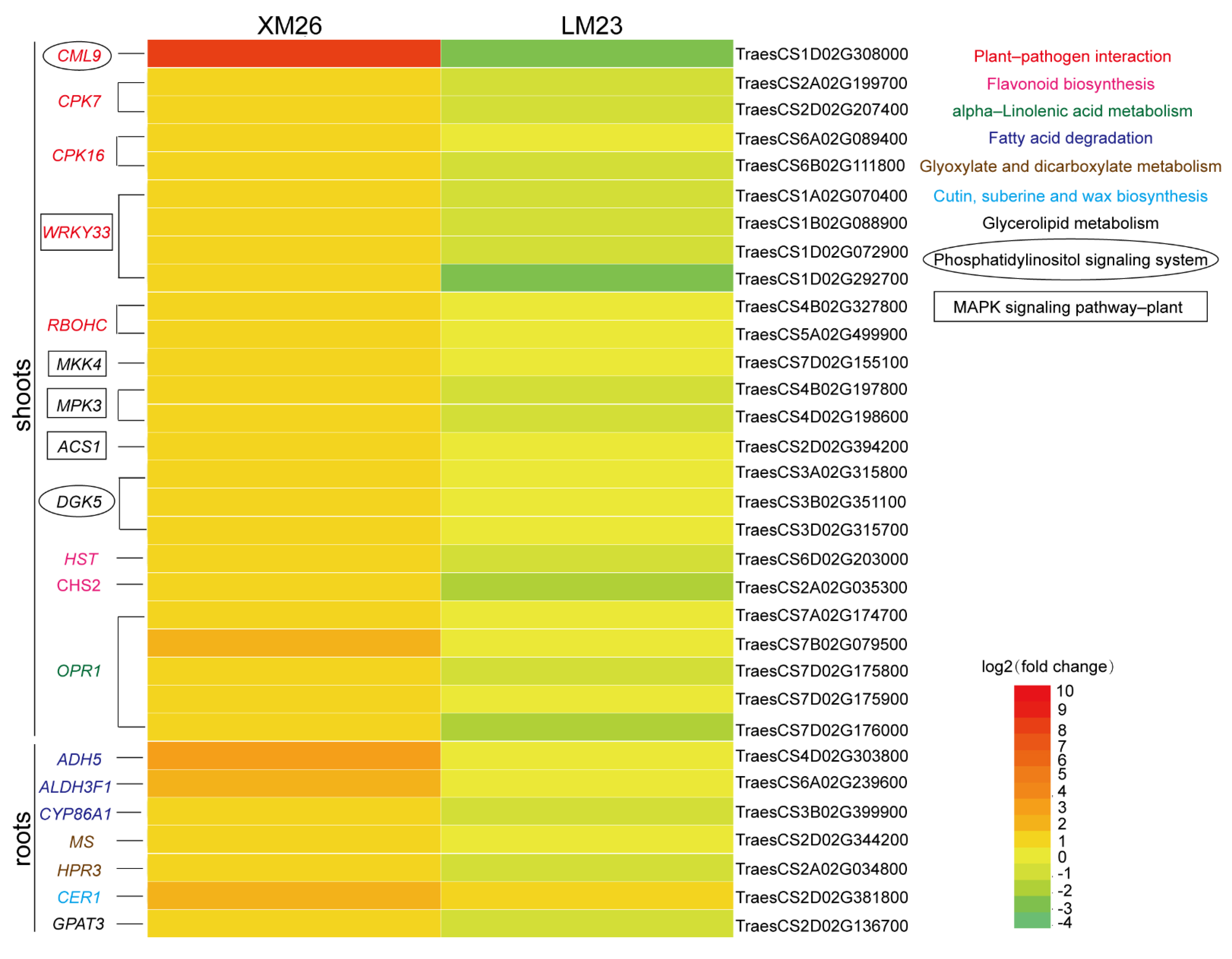
2.4. Validation of Some Key Genes Involved in Important Pathways
2.5. Transcription Factors among DEGs
3. Discussion
3.1. Calcium-Mediated Pathways Help Regulate Low-Nitrogen Stress Response between XM26 and LM23
3.2. Screening Low-Nitrogen-Tolerance Wheat Varieties Using the Chlorate Tolerance Analysis
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Chlorate Sensitivity Experiment
4.3. Transcriptome Analysis under Normal and Low-Nitrogen Conditions
4.4. RNA Extraction and Quantitative Reverse-Transcription PCR
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, J.; Wang, M.; Li, W.; He, X.; Teng, W.; Ma, W.; Zhao, X.; Hu, M.; Li, H.; Zhang, Y.; et al. Reducing expression of a nitrate-responsive bZIP transcription factor increases grain yield and N use in wheat. Plant Biotechnol. J. 2019, 17, 1823–1833. [Google Scholar] [CrossRef] [PubMed]
- Socolow, R. Nitrogen management and the future of food: Lessons from the management of energy and carbon. Proc. Natl. Acad. Sci. USA 1999, 96, 6001–6008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Curci, P.L.; Bergès, H.; Marande, W.; Maccaferri, M.; Tuberosa, R.; Sonnante, G. Asparagine synthetase genes (AsnS1 and AsnS2) in durum wheat: Structural analysis and expression under nitrogen stress. Euphytica 2018, 214, 1–13. [Google Scholar] [CrossRef]
- Wang, Q.; Su, Q.; Nian, J.; Zhang, J.; Guo, M.; Dong, G.; Hu, J.; Wang, R.; Wei, C.; Li, G.; et al. The Ghd7 transcription factor represses ARE1 expression to enhance nitrogen utilization and grain yield in rice. Mol. Plant 2021, 14, 1012–1023. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Song, K.; Sun, L.; Qin, Q.; Jiang, T.; Jiang, Q.; Xue, Y. Morpho-Physiological and transcriptome analysis provide insights into the effects of zinc application on nitrogen accumulation and metabolism in wheat (Triticum aestivum L.). Plant Physiol. Biochem. PPB 2020, 149, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhang, P.; Liu, Q.; Li, G.; Di, D.; Xia, G.; Kronzucker, H.J.; Fang, S.; Chu, J.; Shi, W. TaANR1-TaBG1 and TaWabi5-TaNRT2s/NARs Link ABA Metabolism and Nitrate Acquisition in Wheat Roots. Plant Physiol. 2020, 182, 1440–1453. [Google Scholar] [CrossRef] [Green Version]
- Crawford, N.; Forde, B. Molecular and developmental biology of inorganic nitrogen nutrition. Arab. Book 2002, 1, e0011. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Zhang, Q.; Cai, H.; Zhou, W.; Xu, F. H2O2 mediates nitrate-induced iron chlorosis by regulating iron homeostasis in rice. Plant Cell Environ. 2018, 41, 767–781. [Google Scholar] [CrossRef]
- Andrews, M.; Raven, J.A.; Lea, P.J. Do plants need nitrate? The mechanisms by which nitrogen form affects plants. Ann. Appl. Biol. 2013, 163, 174–199. [Google Scholar] [CrossRef]
- Oaks, A. Primary nitrogen assimilation in higher plants and its regulation. Can. J. Bot. 2011, 72, 739–750. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, H.; Li, S.; Li, J.; Yan, L.; Xia, L. Increasing yield potential through manipulating of an ARE1 ortholog related to nitrogen use efficiency in wheat by CRISPR/Cas9. J. Integr. Plant Biol. 2021, 63, 1649–1663. [Google Scholar] [CrossRef] [PubMed]
- Vidal, E.; Alvarez, J.; Araus, V.; Riveras, E.; Brooks, M.; Krouk, G.; Ruffel, S.; Lejay, L.; Crawford, N.; Coruzzi, G.; et al. Nitrate in 2020: Thirty Years from Transport to Signaling Networks. Plant Cell 2020, 32, 2094–2119. [Google Scholar] [CrossRef] [PubMed]
- Chopin, F.; Wirth, J.; Dorbe, M.; Lejay, L.; Krapp, A.; Gojon, A.; Daniel-Vedele, F. The Arabidopsis nitrate transporter AtNRT2.1 is targeted to the root plasma membrane. Plant Physiol. Biochem. PPB 2007, 45, 630–635. [Google Scholar] [CrossRef] [PubMed]
- Wirth, J.; Chopin, F.; Santoni, V.; Viennois, G.; Tillard, P.; Krapp, A.; Lejay, L.; Daniel-Vedele, F.; Gojon, A. Regulation of root nitrate uptake at the NRT2.1 protein level in Arabidopsis thaliana. J. Biol. Chem. 2007, 282, 23541–23552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Zhao, Y.; Sun, L.; Han, P.; Bai, X.; Lin, R.; Xiao, K. The N uptake-associated physiological processes at late growth stage in wheat (Triticum aestivum) under N deprivation combined with deficit irrigation condition. Plant Physiol. Biochem. 2021, 164, 160–172. [Google Scholar] [CrossRef]
- Liu, K.; Niu, Y.; Konishi, M.; Wu, Y.; Du, H.; Sun Chung, H.; Li, L.; Boudsocq, M.; McCormack, M.; Maekawa, S.; et al. Discovery of nitrate-CPK-NLP signalling in central nutrient-growth networks. Nature 2017, 545, 311–316. [Google Scholar] [CrossRef] [Green Version]
- Castro Marín, I.; Loef, I.; Bartetzko, L.; Searle, I.; Coupland, G.; Stitt, M.; Osuna, D. Nitrate regulates floral induction in Arabidopsis, acting independently of light, gibberellin and autonomous pathways. Planta 2011, 233, 539–552. [Google Scholar] [CrossRef] [Green Version]
- Tian, Q.; Chen, F.; Liu, J.; Zhang, F.; Mi, G. Inhibition of maize root growth by high nitrate supply is correlated with reduced IAA levels in roots. J. Plant Physiol. 2008, 165, 942–951. [Google Scholar] [CrossRef]
- Ondzighi-Assoume, C.; Chakraborty, S.; Harris, J. Environmental Nitrate Stimulates Abscisic Acid Accumulation in Arabidopsis Root Tips by Releasing It from Inactive Stores. Plant Cell 2016, 28, 729–745. [Google Scholar] [CrossRef] [Green Version]
- Tian, Q.; Sun, P.; Zhang, W. Ethylene is involved in nitrate-dependent root growth and branching in Arabidopsis thaliana. New Phytol. 2009, 184, 918–931. [Google Scholar] [CrossRef]
- Gaudinier, A.; Rodriguez-Medina, J.; Zhang, L.; Olson, A.; Liseron-Monfils, C.; Bågman, A.; Foret, J.; Abbitt, S.; Tang, M.; Li, B.; et al. Transcriptional regulation of nitrogen-associated metabolism and growth. Nature 2018, 563, 259–264. [Google Scholar] [CrossRef]
- Krouk, G.; Lacombe, B.; Bielach, A.; Perrine-Walker, F.; Malinska, K.; Mounier, E.; Hoyerova, K.; Tillard, P.; Leon, S.; Ljung, K.; et al. Nitrate-Regulated auxin transport by NRT1.1 defines a mechanism for nutrient sensing in plants. Dev. Cell 2010, 18, 927–937. [Google Scholar] [CrossRef]
- Varala, K.; Marshall-Colón, A.; Cirrone, J.; Brooks, M.; Pasquino, A.; Léran, S.; Mittal, S.; Rock, T.; Edwards, M.; Kim, G.; et al. Temporal transcriptional logic of dynamic regulatory networks underlying nitrogen signaling and use in plants. Proc. Natl. Acad. Sci. USA 2018, 115, 6494–6499. [Google Scholar] [CrossRef] [Green Version]
- Sinha, S.; Sevanthi V., A.M.; Chaudhary, S.; Tyagi, P.; Venkadesan, S.; Rani, M.; Mandal, P. Transcriptome Analysis of Two Rice Varieties Contrasting for Nitrogen Use Efficiency under Chronic N Starvation Reveals Differences in Chloroplast and Starch Metabolism-Related Genes. Genes 2018, 9, 206. [Google Scholar] [CrossRef] [Green Version]
- Scheible, W.; Morcuende, R.; Czechowski, T.; Fritz, C.; Osuna, D.; Palacios-Rojas, N.; Schindelasch, D.; Thimm, O.; Udvardi, M.; Stitt, M. Genome-wide reprogramming of primary and secondary metabolism, protein synthesis, cellular growth processes, and the regulatory infrastructure of Arabidopsis in response to nitrogen. Plant Physiol. 2004, 136, 2483–2499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Song, K.; Sun, L.; Qin, Q.; Sun, Y.; Pan, J.; Xue, Y. Morphological and Transcriptome Analysis of Wheat Seedlings Response to Low Nitrogen Stress. Plants 2019, 8, 98. [Google Scholar] [CrossRef] [Green Version]
- Tsay, Y.; Schroeder, J.; Feldmann, K.; Crawford, N. The herbicide sensitivity gene CHL1 of Arabidopsis encodes a nitrate-inducible nitrate transporter. Cell 1993, 72, 705–713. [Google Scholar] [CrossRef]
- Wang, X.; Scholl, R.; Feldmann, K. Characterization of a chlorate-hypersensitive, high nitrate reductase Arabidopsis thaliana mutant. Tag. Theor. Appl. Genet. Theor. Angew. Genet. 1986, 72, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Scholz, J. Inositol trisphosphate, a new "second messenger" for positive inotropic effects on the heart? Klin. Wochenschr. 1989, 67, 271–279. [Google Scholar] [CrossRef]
- Morris, P.C. MAP kinase signal transduction pathways in plants. New Phytol. 2001, 151, 67–89. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Wang, Z.; Ma, Z.; Song, W.; Lu, W.; Xiao, K. Characterization on TaMPK14, an MAPK family gene of wheat, in modulating N-starvation response through regulating N uptake and ROS homeostasis. Plant Cell Rep. 2020, 39, 1285–1299. [Google Scholar] [CrossRef]
- Mizoguchi, T.; Irie, K.; Hirayama, T.; Hayashida, N.; Yamaguchi-Shinozaki, K.; Matsumoto, K.; Shinozaki, K. A gene encoding a mitogen-activated protein kinase kinase kinase is induced simultaneously with genes for a mitogen-activated protein kinase and an S6 ribosomal protein kinase by touch, cold, and water stress in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 1996, 93, 765–769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riveras, E.; Alvarez, J.; Vidal, E.; Oses, C.; Vega, A.; Gutiérrez, R. The Calcium Ion Is a Second Messenger in the Nitrate Signaling Pathway of Arabidopsis. Plant Physiol. 2015, 169, 1397–1404. [Google Scholar] [CrossRef] [Green Version]
- Magnan, F.; Ranty, B.; Charpenteau, M.; Sotta, B.; Galaud, J.; Aldon, D. Mutations in AtCML9, a calmodulin-like protein from Arabidopsis thaliana, alter plant responses to abiotic stress and abscisic acid. Plant J. Cell Mol. Biol. 2008, 56, 575–589. [Google Scholar] [CrossRef]
- Tan, W.; Yang, Y.; Zhou, Y.; Huang, L.; Xu, L.; Chen, Q.; Yu, L.; Xiao, S. DIACYLGLYCEROL ACYLTRANSFERASE and DIACYLGLYCEROL KINASE Modulate Triacylglycerol and Phosphatidic Acid Production in the Plant Response to Freezing Stress. Plant Physiol. 2018, 177, 1303–1318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mauceri, A.; Abenavoli, M.; Toppino, L.; Panda, S.; Mercati, F.; Aci, M.; Aharoni, A.; Sunseri, F.; Rotino, G.; Lupini, A. Transcriptomics reveal new insights into molecular regulation of nitrogen use efficiency in Solanum melongena. J. Exp. Bot. 2021, 72, 4237–4253. [Google Scholar] [CrossRef]
- Shi, S.; Li, S.; Asim, M.; Mao, J.; Xu, D.; Ullah, Z.; Liu, G.; Wang, Q.; Liu, H. ArabidopsisThe Calcium-Dependent Protein Kinases (CDPKs) and Their Roles in Plant Growth Regulation and Abiotic Stress Responses. Int. J. Mol. Sci. 2018, 19, 1900. [Google Scholar] [CrossRef] [Green Version]
- Hu, B.; Wang, W.; Ou, S.; Tang, J.; Li, H.; Che, R.; Zhang, Z.; Chai, X.; Wang, H.; Wang, Y.; et al. Variation in NRT1.1B contributes to nitrate-use divergence between rice subspecies. Nat. Genet. 2015, 47, 834–838. [Google Scholar] [CrossRef] [PubMed]
- Quan, X.; Zeng, J.; Ye, L.; Chen, G.; Han, Z.; Shah, J.; Zhang, G. Transcriptome profiling analysis for two Tibetan wild barley genotypes in responses to low nitrogen. BMC Plant Biol. 2016, 16, 30–45. [Google Scholar] [CrossRef] [Green Version]
- Maccaferri, M.; El-Feki, W.; Nazemi, G.; Salvi, S.; Canè, M.; Colalongo, M.; Stefanelli, S.; Tuberosa, R. Prioritizing quantitative trait loci for root system architecture in tetraploid wheat. J. Exp. Bot. 2016, 67, 1161–1178. [Google Scholar] [CrossRef] [PubMed]
- Subudhi, P.K.; Garcia, R.S.; Coronejo, S.; Tapia, R. Comparative Transcriptomics of Rice Genotypes with Contrasting Responses to Nitrogen Stress Reveals Genes Influencing Nitrogen Uptake through the Regulation of Root Architecture. Int. J. Mol. Sci. 2020, 21, 5759. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Finn, R.; Thomas, P. TreeGrafter: Phylogenetic tree-based annotation of proteins with Gene Ontology terms and other annotations. Bioinformatics 2019, 35, 518–520. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Furumichi, M.; Sato, Y.; Ishiguro-Watanabe, M.; Tanabe, M. KEGG: Integrating viruses and cellular organisms. Nucleic Acids Res. 2021, 49, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Ye, J.; Yao, X.; Zhao, P.; Xuan, W.; Tian, Y.; Zhang, Y.; Xu, S.; An, H.; Chen, G.; et al. Genome-Wide associated study identifies NAC42-activated nitrate transporter conferring high nitrogen use efficiency in rice. Nat. Commun. 2019, 10, 5279–5289. [Google Scholar] [CrossRef] [PubMed] [Green Version]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, H.; Shi, H.; Hu, C.; Luo, M.; Xu, C.; Wang, S.; Li, N.; Tang, W.; Zhou, Y.; Wang, C.; et al. Transcriptome Differences in Response Mechanisms to Low-Nitrogen Stress in Two Wheat Varieties. Int. J. Mol. Sci. 2021, 22, 12278. https://doi.org/10.3390/ijms222212278
Yan H, Shi H, Hu C, Luo M, Xu C, Wang S, Li N, Tang W, Zhou Y, Wang C, et al. Transcriptome Differences in Response Mechanisms to Low-Nitrogen Stress in Two Wheat Varieties. International Journal of Molecular Sciences. 2021; 22(22):12278. https://doi.org/10.3390/ijms222212278
Chicago/Turabian StyleYan, Huishu, Huawei Shi, Chengmei Hu, Mingzhao Luo, Chengjie Xu, Shuguang Wang, Ning Li, Wensi Tang, Yongbin Zhou, Chunxiao Wang, and et al. 2021. "Transcriptome Differences in Response Mechanisms to Low-Nitrogen Stress in Two Wheat Varieties" International Journal of Molecular Sciences 22, no. 22: 12278. https://doi.org/10.3390/ijms222212278
APA StyleYan, H., Shi, H., Hu, C., Luo, M., Xu, C., Wang, S., Li, N., Tang, W., Zhou, Y., Wang, C., Xu, Z., Chen, J., Ma, Y., Sun, D., & Chen, M. (2021). Transcriptome Differences in Response Mechanisms to Low-Nitrogen Stress in Two Wheat Varieties. International Journal of Molecular Sciences, 22(22), 12278. https://doi.org/10.3390/ijms222212278







