The Two-Way Interaction between the Molecules That Cause Vaginal Malodour and Lactobacilli: An Opportunity for Probiotics
Abstract
:1. Introduction
2. Results
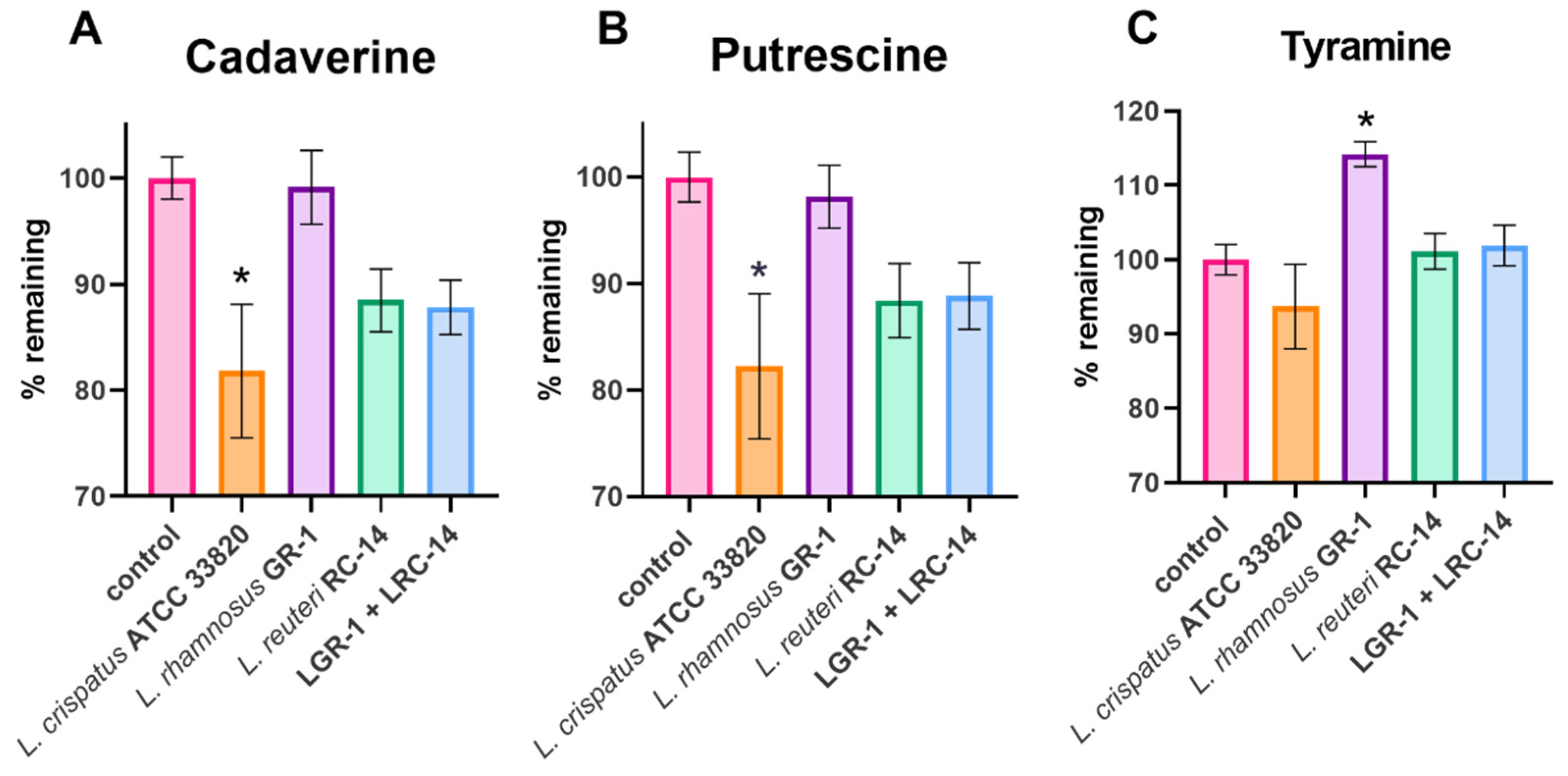
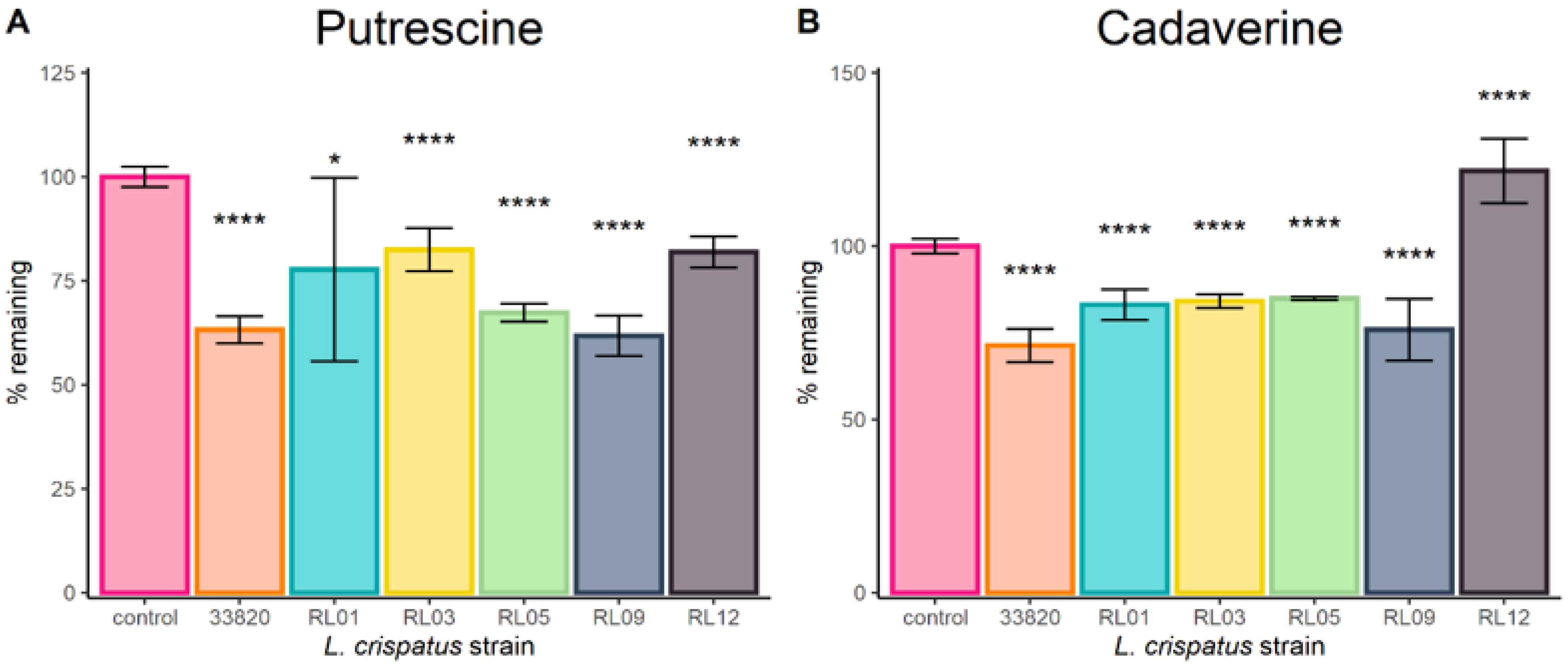
2.1. Lactobacilli Growth in Biogenic Amines
2.2. Effect of Biogenic Amines and pH on the Growth of Lactobacillus Crispatus
3. Discussion
4. Materials and Methods
4.1. Lactobacillus Crispatus Clinical Strains
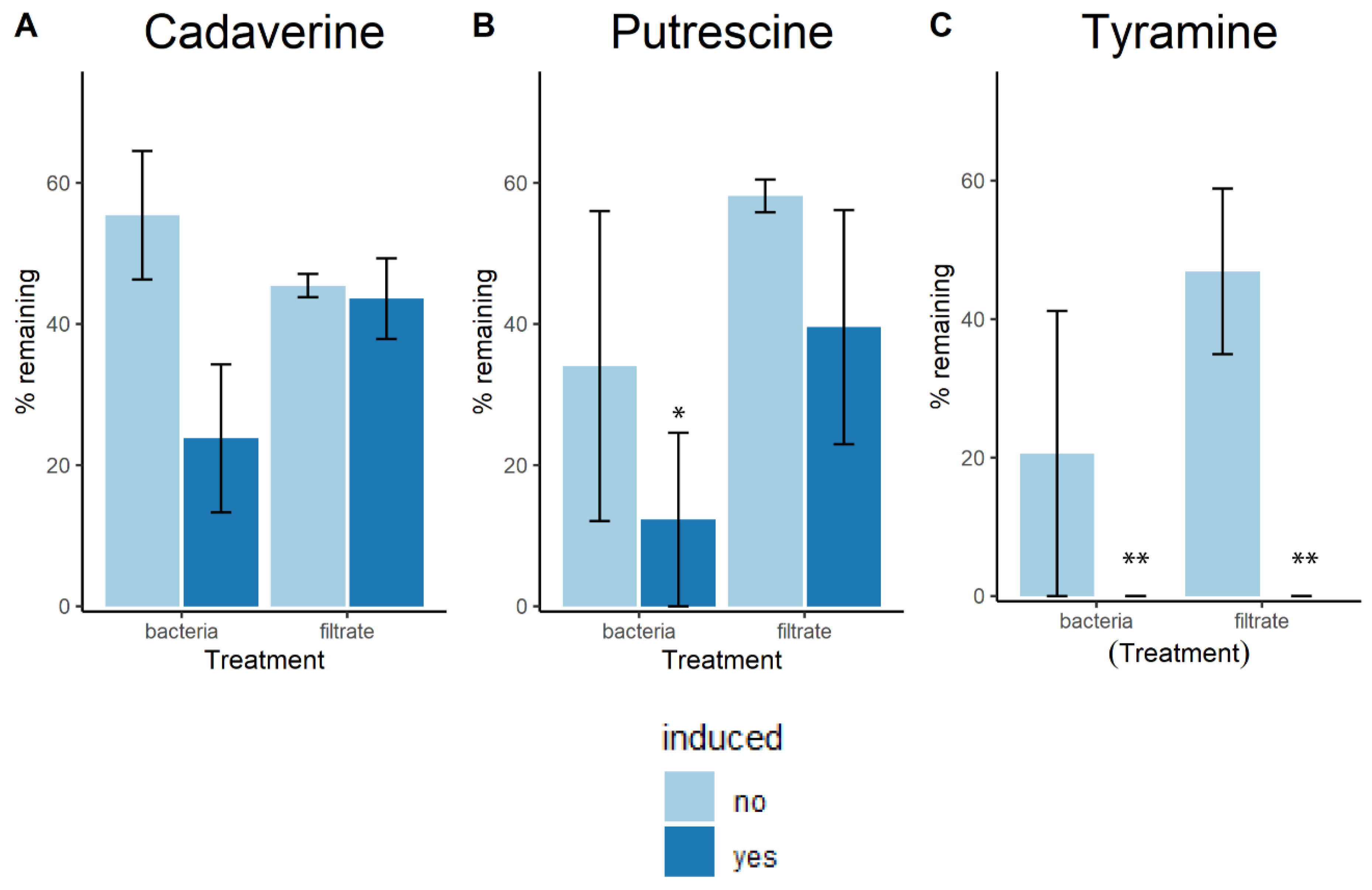
4.2. Biogenic Amine Reduction Experiments
4.3. Induction Assays
4.4. LC–MS/MS Protocol
4.5. HPLC–UV Analysis
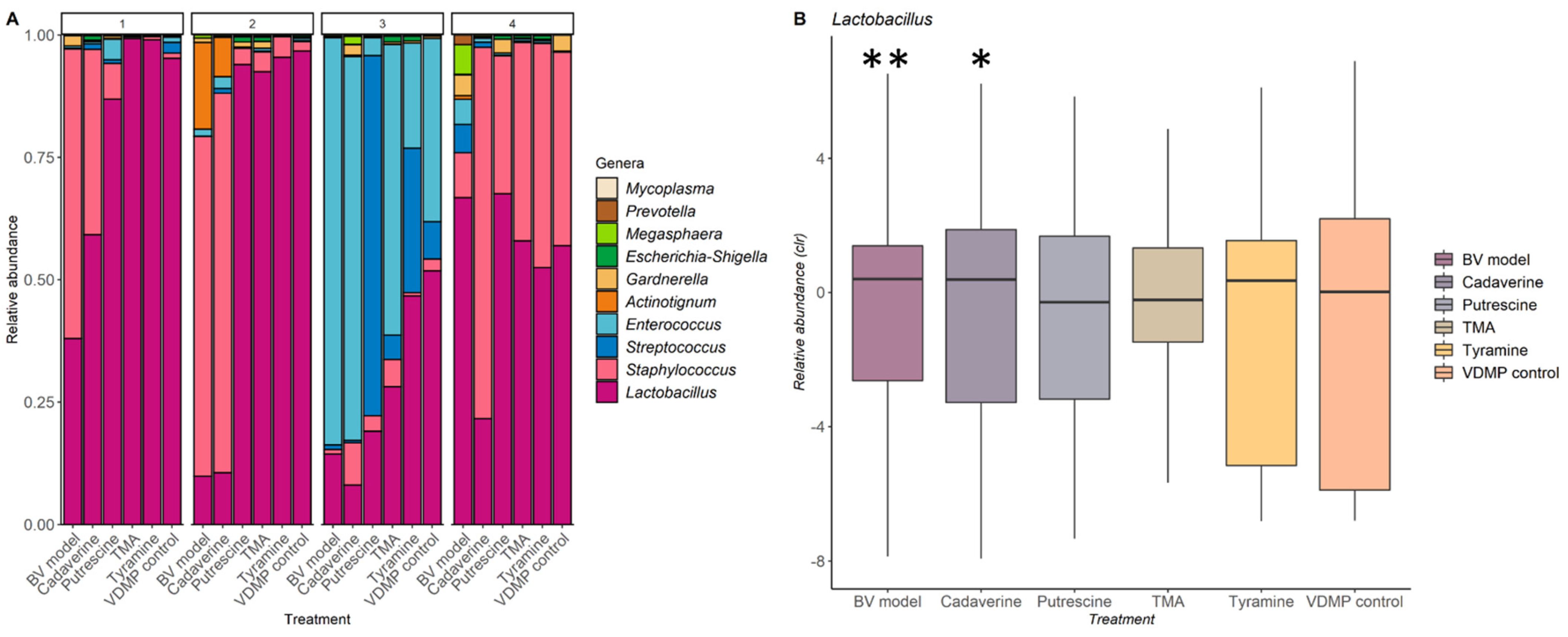
4.6. Analysis of the Impact of Biogenic Amines on the Vaginal Microbiota
4.7. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McMillan, A.; Rulisa, S.; Sumarah, M.; Macklaim, J.M.; Renaud, J.; Bisanz, J.E.; Gloor, G.B.; Reid, G. A multi-platform metabolomics approach identifies highly specific biomarkers of bacterial diversity in the vagina of pregnant and non-pregnant women. Sci. Rep. 2015, 5, 14174. [Google Scholar] [CrossRef]
- Subramanian, C.; Nyirjesy, P.; Sobel, J.D. Genital malodor in women: A modern reappraisal. J. Low. Genit. Tract Dis. 2012, 16, 49–55. [Google Scholar] [CrossRef]
- FAO/WHO Working Group. Report of a Joint FAO/WHO Expert Consultation on Evaluation of Health and Nutrition Properties of Probiotics in Food Including Powder Milk with Live Lactic acid Bacteria; FAO: Cordoba, Argentina, 2002; ISSN 0254-4725. Available online: http://www.fao.org/3/a0512e/a0512e.pdf (accessed on 1 June 2021).
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petrova, M.I.; Reid, G.; Ter Haar, J.A. Lacticaseibacillus rhamnosus GR-1, a.k.a. Lactobacillus rhamnosus GR-1: Past and future perspectives. Trends Microbiol. 2021, 8, 747–761. [Google Scholar] [CrossRef] [PubMed]
- Petrova, M.I.; Macklaim, J.M.; Wuyts, S.; Verhoeven, T.; Vanderleyden, J.; Gloor, G.B.; Lebeer, S.; Reid, G. Comparative genomic and phenotypic analysis of the vaginal probiotic Lactobacillus rhamnosus GR-1. Front. Microbiol. 2018, 9, 1278. [Google Scholar] [CrossRef]
- Martinez, R.; Franceschini, S.A.; Patta, M.C.; Quintana, S.M.; Candido, R.C.; Ferreira, J.C.; De Martinis, E.C.P.; Reid, G. Improved treatment of vulvovaginal candidiasis with fluconazole plus probiotic Lactobacillus rhamnosus GR-1 and Lactobacillus reuteri RC-14. Lett. Appl. Microbiol. 2009, 48, 269–274. [Google Scholar] [CrossRef]
- Vujic, G.; Jajac Knez, A.; Despot Stefanovic, V.; Kuzmic Vrbanovic, V. Efficacy of orally applied probiotic capsules for bacterial vaginosis and other vaginal infections: A double-blind, randomized, placebo-controlled study. Eur. J. Obstet. Gynecol. Reprod. Biol. 2013, 168, 75–79. [Google Scholar] [CrossRef]
- Reid, G. Probiotic use in an infectious disease setting. Expert Rev. Anti-Infect. Ther. 2017, 15, 449–455. [Google Scholar] [CrossRef]
- Macklaim, J.M.; Clemente, J.C.; Knight, R.; Gloor, G.B.; Reid, G. Changes in vaginal microbiota following antimicrobial and probiotic therapy. Microb. Ecol. Health Dis. 2015, 26, 27799. [Google Scholar] [CrossRef]
- Borgogna, J.-L.C.; Shardell, M.D.; Grace, S.G.; Santori, E.K.; Americus, B.; Li, Z.; Ulanov, A.; Forney, L.; Nelson, T.M.; Brotman, R.M.; et al. Biogenic amines increase the odds of bacterial vaginosis and affect the growth of and lactic acid production by vaginal Lactobacillus spp. Appl. Environ. Microbiol. 2021, 87, e03068-20. [Google Scholar] [CrossRef] [PubMed]
- Nelson, T.M.; Borgogna, J.-L.C.; Brotman, R.M.; Ravel, J.; Walk, S.T.; Yeoman, C.J. Vaginal biogenic amines: Biomarkers of bacterial vaginosis or precursors to vaginal dysbiosis? Front. Physiol. 2015, 6, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Puebla-Barragan, S.; Watson, E.; van der Veer, C.; Chmiel, J.A.; Carr, C.; Burton, J.P.; Sumarah, M.; Kort, R.; Reid, G. Interstrain variability of human vaginal lactobacillus crispatus for metabolism of biogenic amines and antimicrobial activity against urogenital pathogens. Molecules 2021, 26, 4538. [Google Scholar] [CrossRef]
- Anukam, K.C.; Osazuwa, E.; Osemene, G.I.; Ehigiagbe, F.; Bruce, A.W.; Reid, G. Clinical study comparing probiotic Lactobacillus GR-1 and RC-14 with metronidazole vaginal gel to treat symptomatic bacterial vaginosis. Microbes Infect. 2006, 8, 2772–2776. [Google Scholar] [CrossRef] [PubMed]
- Reid, G.; Charbonneau, D.; Erb, J.; Kochanowski, B.; Beuerman, D.; Poehner, R.; Bruce, A.W. Oral use of Lactobacillus rhamnosus GR-1 and L. fermentum RC-14 significantly alters vaginal flora: Randomized, placebo-controlled trial in 64 healthy women. FEMS Immunol. Med. Microbiol. 2003, 35, 131–134. [Google Scholar] [CrossRef] [Green Version]
- Martinez, R.; Seney, S.; Summers, K.; Nomizo, A.; De Martinis, E.; Reid, G. Effect of Lactobacillus rhamnosus GR-1 and Lactobacillus reuteri RC-14 on the ability of Candida albicans to infect cells and induce inflammation. Microbiol. Immunol. 2009, 53, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Reid, G.; Bruce, A.W.; Fraser, N.; Heinemann, C.; Owen, J.; Henning, B. Oral probiotics can resolve urogenital infections. FEMS Immunol. Med. Microbiol. 2001, 30, 49–52. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, M.; Scherbak, N.; Khalaf, H.; Olsson, P.E.; Jass, J. Substances released from probiotic Lactobacillus rhamnosus GR-1 potentiate NF-ΚB activity in Escherichia coli-stimulated urinary bladder cells. FEMS Immunol. Med. Microbiol. 2012, 66, 147–156. [Google Scholar] [CrossRef] [Green Version]
- Rimaux, T.; Rivière, A.; Illeghems, K.; Weckx, S.; De Vuyst, L.; Leroy, F. Expression of the arginine deiminase pathway genes in Lactobacillus sakei is strain dependent and is affected by the environmental pH. Appl. Environ. Microbiol. 2012, 78, 4874–4883. [Google Scholar] [CrossRef] [Green Version]
- Narendranath, N.V.; Power, R. Relationship between pH and medium dissolved solids in terms of growth and metabolism of lactobacilli and Saccharomyces cerevisiae during ethanol production. Appl. Environ. Microbiol. 2005, 71, 2239–2243. [Google Scholar] [CrossRef] [Green Version]
- Corcoran, B.M.; Stanton, C.; Fitzgerald, G.F.; Ross, R.P. Survival of probiotic lactobacilli in acidic environments is enhanced in the presence of metabolizable sugars. Appl. Environ. Microbiol. 2005, 71, 3060–3067. [Google Scholar] [CrossRef] [Green Version]
- Noguchi, T.; Sado, T.; Naruse, K.; Kobayashi, H. Vaginal fluid pH and buffer capacity for predicting false preterm labor in Japanese women. Int. J. Gynecol. Obstet. 2016, 134, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Collins, S.L.; McMillan, A.; Seney, S.; van der Veer, C.; Kort, R.; Sumarah, M.W.; Reid, G. Promising prebiotic candidate established by evaluation of lactitol, lactulose, raffinose, and oligofructose for maintenance of a Lactobacillus-dominated vaginal microbiota. Appl. Environ. Microbiol. 2017, 84, e02200-17. [Google Scholar] [CrossRef] [Green Version]
- van der Veer, C.; Hertzberger, R.Y.; Bruisten, S.M.; Tytgat, H.L.P.; Swanenburg, J.; de Kat Angelino-Bart, A.; Schuren, F.; Molenaar, D.; Reid, G.; de Vries, H.; et al. Comparative genomics of human Lactobacillus crispatus isolates reveals genes for glycosylation and glycogen degradation: Implications for in vivo dominance of the vaginal microbiota. Microbiome 2019, 7, 49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geshnizgani, A.M.; Onderdonk, A.B. Defined medium simulating genital tract secretions for growth of vaginal microflora. J. Clin. Microbiol. 1992, 30, 1323–1326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nugent, R.; Krohn, M.; Hillier, S. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of Gram stain interpretation. J. Clin. Microbiol. 1991, 29, 297–301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spiegel, C.; Amsel, R.; Holmes, K. Diagnosis of bacterial vaginosis by direct Gram stain of vaginal fluid. J. Clin. Microbiol. 1983, 18, 170–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolrath, H.; Forsum, U.; Larsson, P.G.; Borén, H. Analysis of bacterial vaginosis-related amines in vaginal fluid by gas chromatography and mass spectrometry. J. Clin. Microbiol. 2001, 39, 4026. [Google Scholar] [CrossRef] [Green Version]
- Sprouffske, K. Simple Metrics to Summarize Growth Curves. R Package Version 0.3.1. 2020. Available online: https://cran.r-project.org/package=growthcurver/index.html (accessed on 25 July 2021).
- Dziarkowska, K.; Jönsson, J.Ǻ.; Wieczorek, P.P. Single hollow fiber SLM extraction of polyamines followed by tosyl chloride derivatization and HPLC determination. Anal. Chim. Acta 2008, 606, 184–193.b. [Google Scholar] [CrossRef]
- Al, K.F.; Denstedt, J.D.; Daisley, B.A.; Bjazevic, J.; Welk, B.K.; Pautler, S.E.; Gloor, G.B.; Reid, G.; Razvi, H.; Burton, J.P. Ureteral stent microbiota is associated with patient comorbidities but not antibiotic exposure. Cell Rep. Med. 2020, 1, 100094. [Google Scholar] [CrossRef]
- Parada, A.E.; Needham, D.M.; Fuhrman, J.A. Every base matters: Assessing small subunit rRNA primers for marine microbiomes with mock communities, time series and global field samples. Environ. Microbiol. 2016, 18, 1403–1414. [Google Scholar] [CrossRef]
- Gloor, G.B.; Macklaim, J.M.; Fernandes, A.D. Displaying variation in large datasets: Plotting a visual summary of effect sizes. J. Comput. Graph. Stat. 2016, 25, 971–979. [Google Scholar] [CrossRef]
- Fernandes, A.D.; Reid, J.N.; Macklaim, J.M.; McMurrough, T.A.; Edgell, D.R.; Gloor, G.B. Unifying the analysis of high-throughput sequencing datasets: Characterizing RNA-seq, 16S rRNA gene sequencing and selective growth experiments by compositional data analysis. Microbiome 2014, 2, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandes, A.D.; Macklaim, J.M.; Linn, T.G.; Reid, G.; Gloor, G.B. ANOVA-like differential expression (ALDEx) analysis for mixed population RNA-seq. PLoS ONE 2013, 8, e67019. [Google Scholar] [CrossRef] [PubMed]
- Oksanen, J.; Blanchet, G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.; O’Hara, R.; Simpson, G.; Solymos, P.; et al. Vegan: Community Ecology Package. 2020. Available online: http://cran.r-project.org/web/packages/vegan/index.html (accessed on 25 July 2021).
- Kassambara, A. Rstatix: Pipe-Friendly Framework for Basic Statistical Tests. R Package Version 0.7.0. 2021. Available online: http://cran.r-project.org/web/packages/rstatix/index.html (accessed on 25 July 2021).
- Lenth, R. Emmeans: Estimated Marginal Means, Aka Least-Squares Means. R Package Version 1.6.0. 2021. Available online: http://cran.r-project.org/web/packages/emmeans/index.html (accessed on 25 July 2021).
- Zeileis, A. Econometric computing with HC and HAC covariance matrix estimators. J. Stat. Softw. 2004, 11, 1–17. [Google Scholar] [CrossRef]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016; ISBN 978-0-387-98140-6. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puebla-Barragan, S.; Akouris, P.P.; Al, K.F.; Carr, C.; Lamb, B.; Sumarah, M.; van der Veer, C.; Kort, R.; Burton, J.; Reid, G. The Two-Way Interaction between the Molecules That Cause Vaginal Malodour and Lactobacilli: An Opportunity for Probiotics. Int. J. Mol. Sci. 2021, 22, 12279. https://doi.org/10.3390/ijms222212279
Puebla-Barragan S, Akouris PP, Al KF, Carr C, Lamb B, Sumarah M, van der Veer C, Kort R, Burton J, Reid G. The Two-Way Interaction between the Molecules That Cause Vaginal Malodour and Lactobacilli: An Opportunity for Probiotics. International Journal of Molecular Sciences. 2021; 22(22):12279. https://doi.org/10.3390/ijms222212279
Chicago/Turabian StylePuebla-Barragan, Scarlett, Polycronis Paul Akouris, Kait F. Al, Charles Carr, Britney Lamb, Mark Sumarah, Charlotte van der Veer, Remco Kort, Jeremy Burton, and Gregor Reid. 2021. "The Two-Way Interaction between the Molecules That Cause Vaginal Malodour and Lactobacilli: An Opportunity for Probiotics" International Journal of Molecular Sciences 22, no. 22: 12279. https://doi.org/10.3390/ijms222212279
APA StylePuebla-Barragan, S., Akouris, P. P., Al, K. F., Carr, C., Lamb, B., Sumarah, M., van der Veer, C., Kort, R., Burton, J., & Reid, G. (2021). The Two-Way Interaction between the Molecules That Cause Vaginal Malodour and Lactobacilli: An Opportunity for Probiotics. International Journal of Molecular Sciences, 22(22), 12279. https://doi.org/10.3390/ijms222212279







